Abstract
Breathlessness and impaired quality of life are prominent features in patients with severe emphysema even when conventional methods of treatment are optimal. Lung volume reduction using endobronchial management for emphysema has emerged as a new method to relieve symptoms and improve lung function tests in this group. The endobronchial valves (EBVs) are the most widely used treatment. This article outlines current criteria of patients’ selection with literature review and evidence of efficacy. Complications of EBV insertion as well as current shortfalls of this method of treatment are also discussed.
Keywords: Emphysema, COPD, FEV1, volume reduction, valves
Background
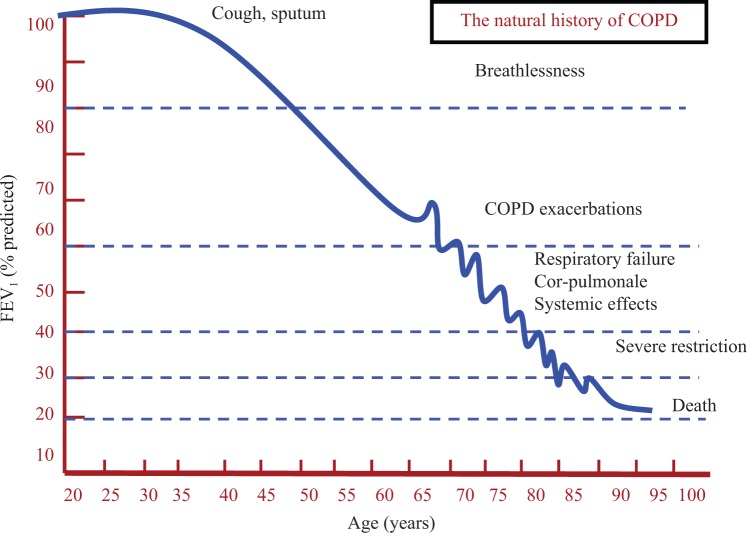
Chronic obstructive pulmonary disease (COPD) is a leading cause of disability and death affecting approximately 1.8% of population.1 The disease is caused mainly by cigarette smoking. In susceptible smokers, COPD is a progressive disease in which lung function and the disease burden increases with advancing age (Figure 1). Exacerbations occur more frequently as the disease gets worse, although in a subgroup of patients exacerbations occur in the earlier stages of the disease.2–4
Figure 1.
A schematic adaptation of FEV1/age graph in susceptible smokers with COPD. The progressive decline in FEV1 is associated with increased tendency for lung exacerbations. The ECLIPSE study2 found that a group of patients can have exacerbations at higher level of FEV1. The dashed blue lines are the level of FEV1 at which each set of symptoms/complication of COPD are broadly occur. FEV1: forced expiratory volume in 1 second; COPD: chronic obstructive pulmonary disease.
The current modalities of treatment consist of smoking cessation, inhaled bronchodilators, pulmonary rehabilitation, annual influenza vaccination, and oxygen therapy.
The cost of the disease is high owing to the cost of lifelong drugs and to the high utilization of hospital and community health-care services. The available modalities of treatment have improved the quality of life in patients with mild to moderate disease; however, these are less effective in advanced disease.5
Emphysema-predominant COPD
Emphysema is a component of COPD. It is characterized by destruction of the lung parenchyma distal to respiratory bronchioles. Emphysema is thought to be the result of an imbalance between proteases released from neutrophils and anti-proteases made in the liver.6
Emphysema causes breathlessness that does not ordinarily respond to bronchodilators which work on the smooth muscles on the bronchial tree. As emphysema represents an irreparable destruction of the lung tissues, the disease has been faced with nihilism.
The stereotypical picture of patients with advanced emphysema depicted as thin breathless patients with pursed lip breathing with elongated chest wall. The chest radiographic images typically show increased distance of the lung fields and flat hemi-diaphragm (Figure 2).
Figure 2.
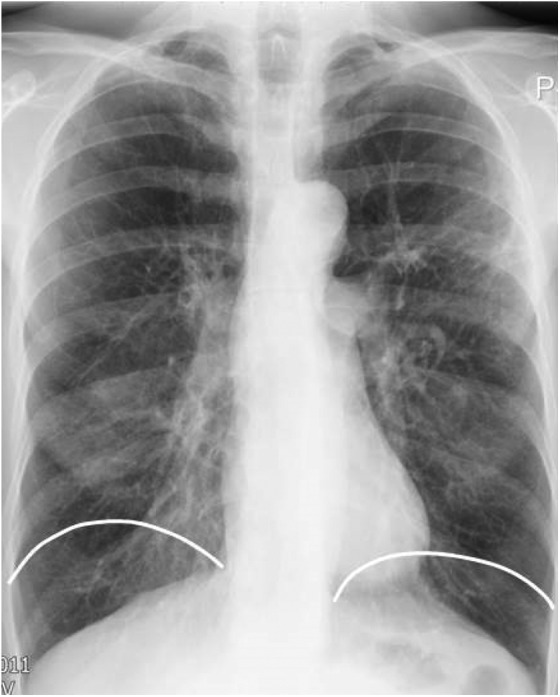
A chest radiograph of a patient with emphysema showing hyperinflated lung fields and flat diaphragm. The white lines represent the position of diaphragm in normal lungs.
The mechanism of breathlessness in emphysema is complex. The airway is easily collapsible due to destruction of alveolar tethering of the small airways. Collapsibility of the airways results in a significant air trapping which exacerbate breathlessness.
The hyperinflated lungs in emphysema render the thoracic cage less compliant. It becomes unable to respond to the normal change in lung volume during the work of breathing. Therefore, the chest wall mechanics are impaired in particular during activities – a process that is better known as dynamic hyperinflation.
A recent study7 using quantification of emphysema on a high-resolution (HR) CT scan found that the presence and the extent of emphysema increases in the lung fields as lung function declines (Figure 3). This important observation suggests that the decline in lung function in COPD is more likely to be due to increase in lung destruction rather than due to progressive airways narrowing which is the marker of chronic bronchitis. This study also suggests that in patients where the forced expiratory volume in 1 second (FEV1) fall below the 40% of predicted value, it is likely that the degree of emphysematous destruction is great which would merit investigating patients for lung volume reduction as one of the ranges of the therapeutic methods.
Figure 3.
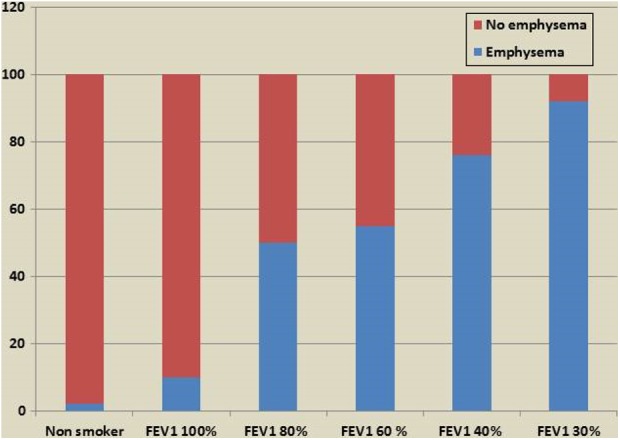
Radiological emphysema as a proportion of all lung fields (y axis) on HRCT scan imaging according to progressive severity of impairment of lung function tests (x axis). HRCT: high-resolution computed tomography.7
Heterogeneity of emphysema
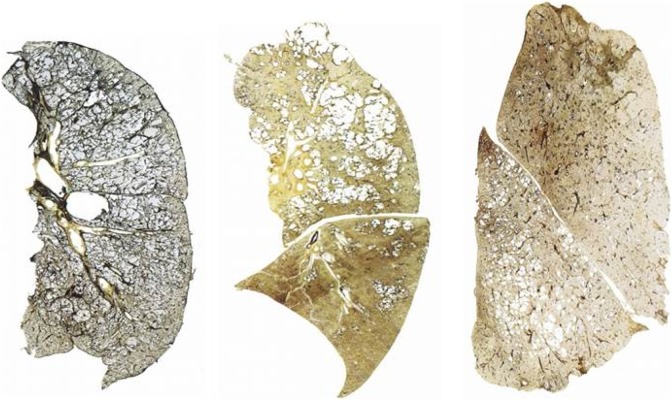
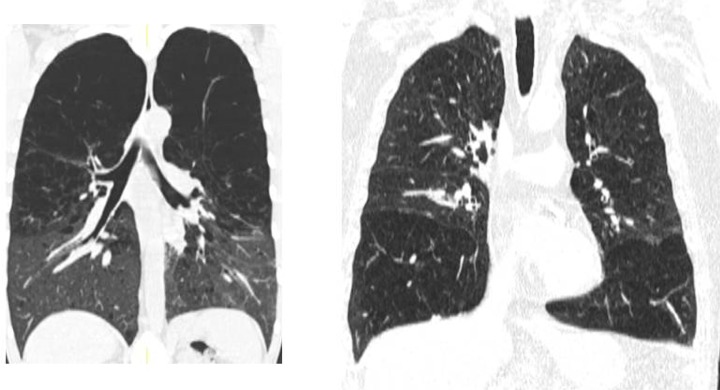
It has long been noted that the degree and distribution of emphysema in lung lobes differed among patients (Figure 4). Figure 5 confirms the advent of thin cut using the HRCT scan. The heterogeneity of emphysema has become relevant due to its impact on therapeutic trials. Emphysema has been defined as lung density below −910 Hounsfield units.7
Figure 4.
Types of emphysema on section of the lungs at post-mortem from coal miners. (a) Homogenous emphysema; (b) predominantly upper lobe emphysema; and (c) predominantly lower lobe emphysema.
Figure 5.
Upper lobes emphysema on coronal images of HRCT (left) and lower lobe emphysema (right). HRCT: high-resolution computed tomography.
The results of the National Emphysema Treatment Trial (NETT)8 showed that high impact, heterogeneous emphysema with upper lobe distribution would benefit from upper lobe surgical removal. Several computer packages have been developed to quantify the percentage of emphysema in lobes and lungs on chest CT scan. A difference of 15% of emphysema prevalence index between lobes was generally agreed as a marker of heterogeneity.
Although emphysema can be divided into heterogeneous emphysema with lobe predominance and homogenous emphysema where most lung lobes are affected, in many patients, the emphysema could be patchy with small or large bulla formation. These conditions represent a challenge for endobronchial management.
Interventional management of emphysema-predominant COPD
Lung volume reduction surgery
As stated preciously, lung volume reduction (LVR) surgery has been shown to be effective in selected patients with emphysema. The NETT8 was a carefully designed multicentre interventional trial. The trial prospectively allocated patients to those patients undergoing surgical removal of the most affected lung lobe or to best usual care. Adequate numbers were included for outcome analyses.
The study found no difference in survival or in improvement of exercise capacity between the two study arms for all patients. When analysing subsets of patients, however, a group of patients appeared to benefit from volume reduction surgery. This group were those with heterogeneous upper lobe emphysema and reduced exercise capacity (Figure 6).
Figure 6.
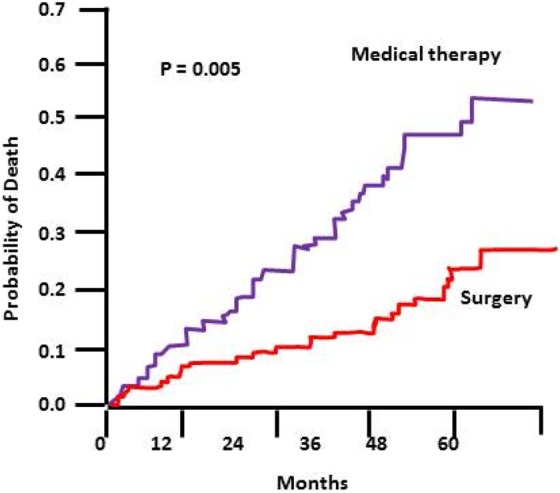
The 5-year probability of death in a subset of patients in the NETT trial – patients included in this graph were those with poor exercise capacity and upper lobe predominant disease. NETT: National Emphysema Treatment Trial.8
Despite this trial, the rate of surgical management of patients with emphysema remained low. This was probably due to increased post-operative mortality rate and an inherent degree of scepticism in the minds of the surgeons.
A recent resurgence of interest in surgery for emphysema has been reported by changing the attitude towards LVR surgery.9 This is owing to improvement in stapling technique after lobe resection, the improved safety of the less invasive procedure through video-assisted thoracoscopy (VATS), adapting unilateral LVR resection of emphysema lobes which is associated with reduced morbidity and mortality and increased confidence of thoracic surgeons.9
Other factor is the widely acceptable formation of advanced emphysema multidisciplinary team meetings for bronchoscopic LVR procedures in which thoracic surgeons join respiratory physicians and thoracic radiologists in selecting best management methods for patients with complex and advanced emphysema.
Endobronchial LVR
Endobronchial LVR is a minimally invasive procedure that uses methods and devices aimed at reducing the lung volume by targeting most affected lobes by the emphysema process as identified by HRCT scan of the chest.
Criteria for LVR are broadly outlined in Table 1. Endobronchial valve insertion is the most widely used procedure. Other methods include endobronchial coils, polymer sealants and hot water vapour. Evidence of efficacy and safety for endobronchial valve (EBV) only is outlined in this article.
Table 1.
Criteria for insertion of EBVs in the author’s unit.
| FEV1 | <50% of Expected values |
| RV | >170% of Expected values |
| Modified MRC Breathlessness Score | >2 |
| COPD Assessment Test score | >15 |
| Pulmonary artery pressure | <50 mm Hg |
| Stopped smoking | |
| Undergone pulmonary rehabilitation programme | |
| No major co-morbid conditions |
EBVs: endobronchial valves; RV: residual volume; MRC: Medical Research Council; COPD: chronic obstructive pulmonary disease.
Endobronchial valves
EBV is a one-way valve inserted in all bronchi leading to the target lobe. The aim is to collapse that lobe by blocking inspired air from entering but allows out air and secretions during expiration. The procedure is on the whole well tolerated and is done under light anaesthesia or heavy sedation. Removal of the valves is possible in the event of complications or unsatisfactory insertion.
Unlike LVR surgery,8 upper and lower lobes can be used as the target.10 In addition, some of these procedures can be used, albeit with probably a more modest efficacy, in patients with homogenous emphysema.11
Two types of valves are primarily available, the Zephyr valve (Pulmonx, Redwood City, California, USA) and Spiration valve (Olympus, Japan). The majority of recent literature has been produced by the former device. This article discusses studies using the Zephyr valve only.
There is a modest variation in the way the procedure is done in different centres. Rigid and fibre-optic bronchoscopes are used. General anaesthesia, gentle sedation and heavy sedation are all successfully used.
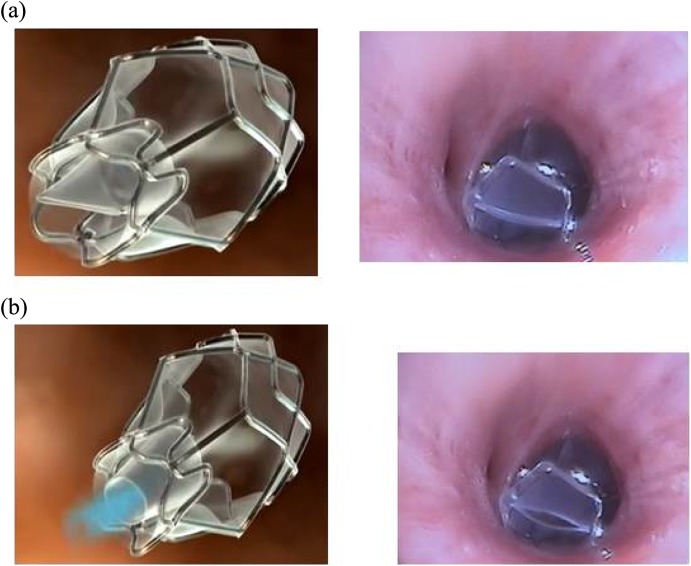
Figures 7 and 8 illustrate the sizes and the function of the Zephyr valves and figure 9 shows an example of a patient who received valves for left upper lobe emphysema.
Figure 7.

The three sizes of the Zephyr EBVs. From left to right: 5.5 mm wide, 4.0 mm wide and 4.0 mm wide with short length (low profile). EBVs: endobronchial valves.
Figure 8.
Zephyr EBV during inspiration (a) and expiration (b). The left panel is a schematic presentation (Pulmonx with permission). The right panel are valves in situ. Please note that the ‘duckbill’ is closed during inspiration and open during expiration to allow air and secretions out. EBV: endobronchial valve.
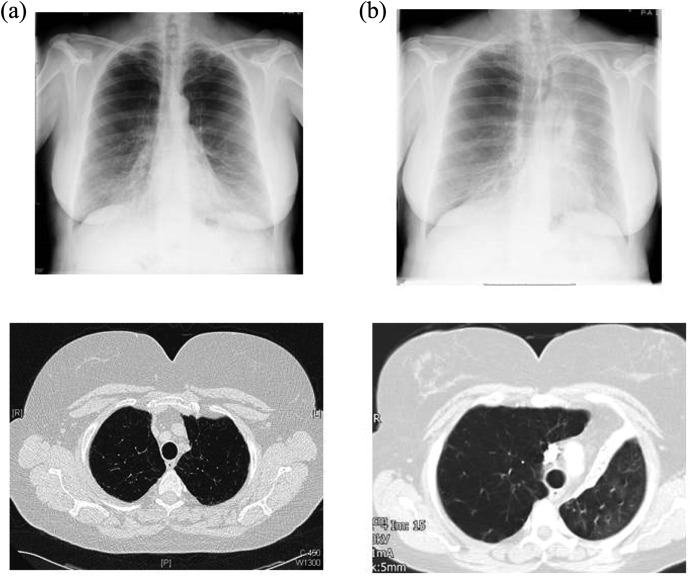
Figure 9.
A 64-year-old patient with upper lobe emphysema prior to valve insertion (a) and 4 weeks after valve insertion (b). Please note the collapse in the left upper lobe and the elevation of the left hemi-diaphragm (b).
Assessment of lobe isolation and CV
The initial clinical trial on EBV (the VENT study10) yielded disappointing results. Only a modest improvement in FEV1 of 6.8% was achieved. The change in 6-minute walk distance was smaller at 5.8% in the group receiving EBV compared with the control group.
Subgroup analyses of the degree of emphysema heterogeneity and the completeness of the lobe fissure showed more favourable results (shown subsequently).
As a result, selection of best responders from EBV on observing the lung HRCT has undergone a significant change. To put it simply, the more heterogeneous the disease is and the more complete the fissure is, the greater the benefit from the treatment.
This principle, however, needs a careful qualification in order not to deprive patients with homogeneous emphysema from useful therapy. The percentage improvement for FEV1 in the VENT study8 was similar to those with difference in emphysema destruction index of 6%–15% compared with 15%–25% subgroup. The emphysema destruction index is defined as the percentage of lobe with low attenuation of −910 Hounsfield unit or lower between the target lobe and the adjacent lobe. Therefore, the 15% heterogeneity cut-off marker was chosen to distinguish between homogenous disease (destruction index below 15%) and heterogeneous disease (index of 15% or more).
The results of an ongoing prospective trial in EBV management of homogenous emphysema are awaited.
To accurately capture these two concepts, software analysis of the extent of emphysema was developed. Also methods to assess the presence of collateral ventilation (CV) and fissure completeness are available.
Collateral ventilation
Collateral channels are anatomical links between the alveolar spaces between lung lobes. Whether these channels have any physiological value remains unclear. The presence or absence of CV has become important in predicting the outcome of EBV insertion. Patients with CV between the target lobe and the adjacent lobe (better known as CV positive) responds less to EBV insertion to those without CV – or CV negative lobes. Collateral channels within one lobe do not affect the response to EBV treatment which is aimed at inducing collapse in the entire target lobe.
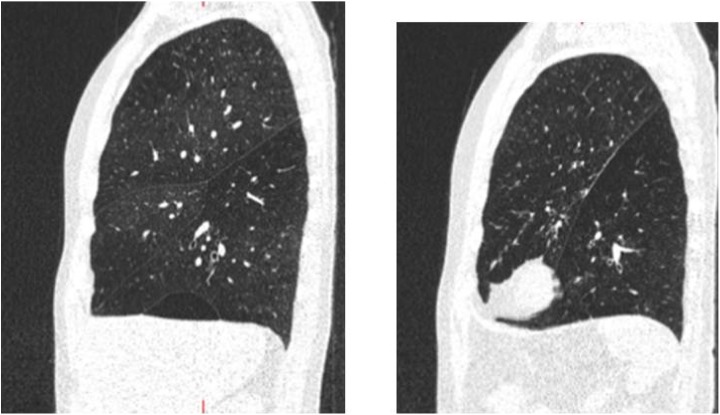
CV can be suspected to be present when the inter-lobar fissures are not complete on HRCT scan (Figure 11). It can be confirmed by measurement of expiratory flow over time using a balloon catheter (Chartis® catheter, Pulmonx) inserted through a bronchoscope and blocking the main bronchus of the target lobe.
Figure 11.
Incomplete fissure between the left upper and the left lower lobe (right). Incomplete horizontal fissure between the right upper lobe and the right middle lobe (left). The second patient is not likely to respond to insertion of an EBV in the right upper lobe but may respond to treatment of combined right upper lobe and right middle lobe. EBV: endobronchial valve.
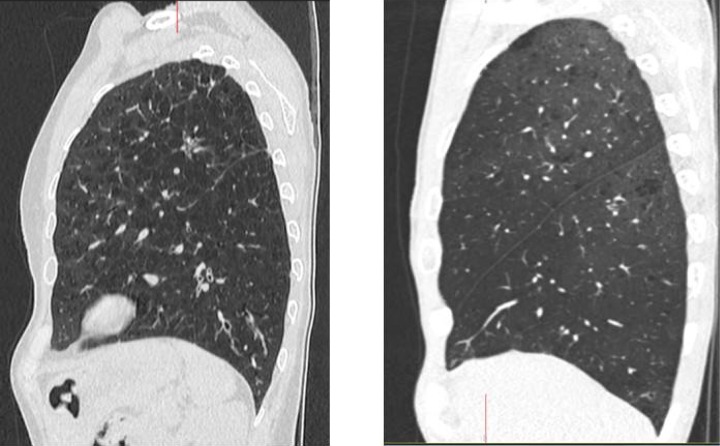
Assessment of inter-lobar fissure completeness: This is best assessed by three-dimensional contiguous thoracic CT scan. Eyeballing is the commonest practice. However, studies have demonstrated a significant inter-observer disagreement on the presence or absence and on the degree of fissure completeness.11 The same study also demonstrated that inter-observer agreements were greater when over 70% fissure completeness or less than 50% fissure completeness were present. Software packages have been developed to help with estimating fissure completeness and are used in some units. However, there is currently no evidence that their use result in increased efficacy of EBV insertion. Figures 10 and 11 show complete and incomplete fissures on chest CT scans.
Figure 10.
Complete (intact) interlobar fissures on the right lung (left) and the left lung (right). The patient is likely to respond to insertion of EBV. EBV: endobronchial valve.
As stated previously, the inclusion of patients with incomplete fissure in the original VENT study has accounted for the overall modest improvement in FEV1 and in the 6-minute walk distance.10
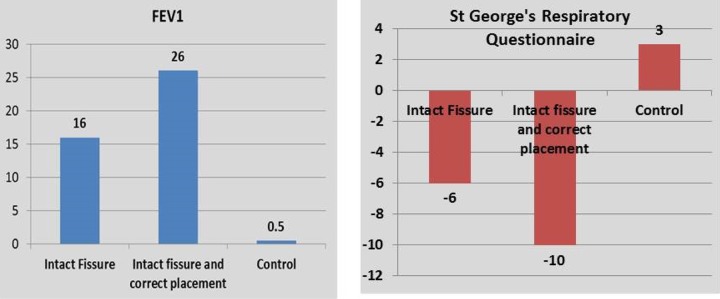
Further sub-analysis of the VENT study on the European patients was made taking into account the following two factors: fissure completeness and atelectasis in the target lobe as a marker of correct valve placement. These sub-analyses demonstrated markedly better response to valve insertion in terms of improvement in lung function tests and in health-related quality of life as assessed by St George’s Respiratory Questionnaire (SGRQ).12
The different outcomes for various measures of EBV insertion in the original VENT study and various subsequent analyses have been summarized in a review article by Shah and Herth13 (Figures 12 and 13). In short, the presence of intact fissures and a successful valve insertion resulting in volume reduction were associated with best outcome after EBV insertion.
Figure 12.
Change in FEV1 and SGRQ at 6 months in a subgroup of patients enrolled in the European VENT study. Please note the marked improvement in both outcome measures in patients with intact fissure and when there is a volume reduction (correct placement) in the target lobe after the procedure. FEV1: forced expiratory volume in 1 second; SGRQ: St George’s Respiratory Questionnaire.12,13
Figure 13.
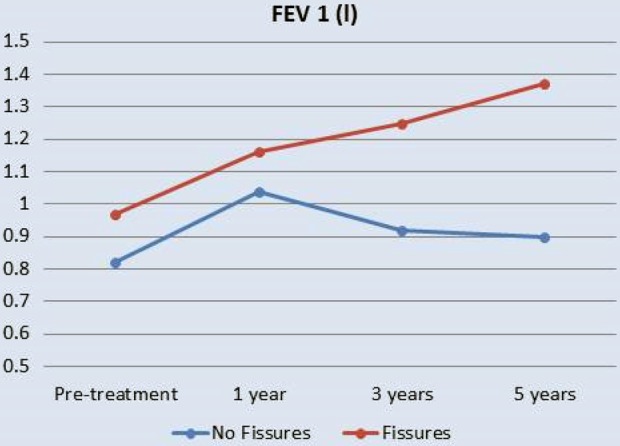
Long-term (5 years) change in FEV1 in those with complete interlobar fissures (fissures) compared to those with incomplete fissures (no fissure). FEV1: forced expiratory volume in 1 second.14
Further two independent analyses confirmed another outcome of successful insertion of EBV and of fissure completeness – increased survival. A non-randomized study14 found that valve insertion in patients with fissure completeness resulted in a 5-year survival rate of 83.3% in those with complete fissure compared with 24% survival in those with incomplete fissure. The rate of lobe atelectasis in an earlier observational study had a clear survival benefit. The study found a 100% survival after 5 years in patients with post-valve lobar atelectasis compared with 43% survival rate in patients where atelectasis was not achieved.15
There is currently no prospective evaluation on the degree of fissure completeness necessary to proceed for valve insertion. However, a recent analysis by Oliveira et al.16 (published as abstract only) reported that a clinically important volume reduction likelihood of the target lobe occurred in 90.5% of patients with fissure completeness of 90% and in 70% when completeness was 75%–90%. The study went on to show that volume reduction did not happen in any patient when fissure completeness was less than 75%. This study concluded that where fissure completeness was between 75% and 90%, a flow measurement using the Chartis catheter should be used to confirm or rule out CV prior to valve insertion.
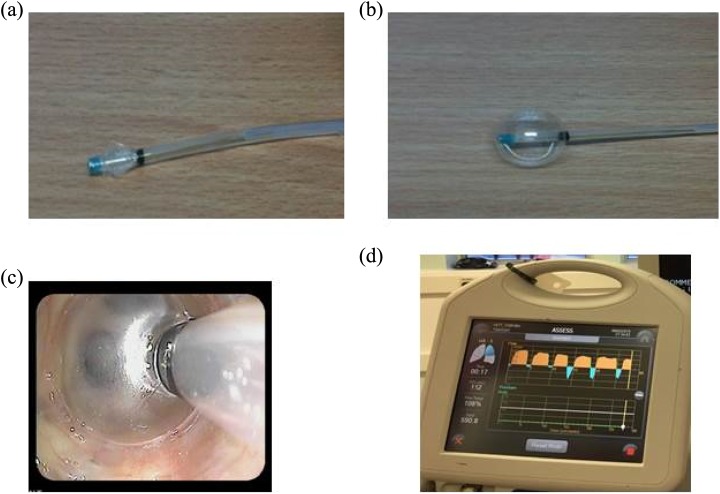
2. Assessment of CV using endobronchial flow catheter (Chartis): The Chartis system (Pulmonx; Figure 14) consists of a balloon catheter attached to a sensor based in a console. The catheter is inserted through the working channel of the bronchoscope and inflated to occlude the orifice of the target lobe.
Figure 14.
The Chartis catheter before (a) and after (b) balloon inflation. The inflated balloon is occluding the left upper lobe (c). The Chartis console showing positive collateral ventilation trace (d).
A standard Chartis graph contains information displayed as a time flow graph. Patterns of Chartis graphs are illustrated in Figure 15.
Figure 15.
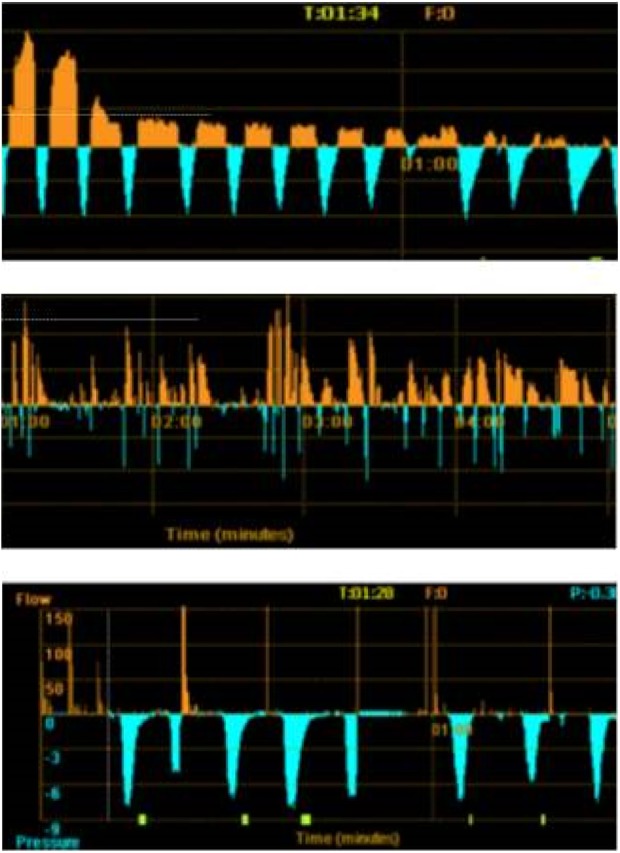
Three patterns of recordings of flow using the Chartis catheter. Decline in flow with time (orange recording) seen on the upper panel representing CV negative. The flow is unchanged with time on the middle panel representing CV positive pattern. The absence of flow typically seen in highly destructed lobes is demonstrated in the lower panel. The blue recording represents the pressure in the target lobe and helps to identify an adequate balloon seal. CV: collateral ventilation.
A CV negative graph shows typically a gradual reduction in the flow to the point of becoming invisible after few minutes of lobe occlusion. Four patterns of Chartis graphs have been described (Hubner R – personal communication): the CV negative graph, the CV positive graph, the low plateau graph, and the low-flow graph. All types but the first pattern have been suggested to represent positive CV that would predict unsuccessful outcome after valve insertion.
The usefulness of the Chartis was reported in a study by Herth and colleagues.17 In this analysis, a clinically important volume loss after valve insertion was regarded to be 350 ml on the three-dimensional volumetric chest CT scan. The study found that volume reduction occurred in the majority of patients in whom the Chartis recordings showed CV negative pattern. In contrast, when the pattern was shown to be CV positive post valve volume reduction occurred in the minority of patients as shown in Figure 16.
Figure 16.
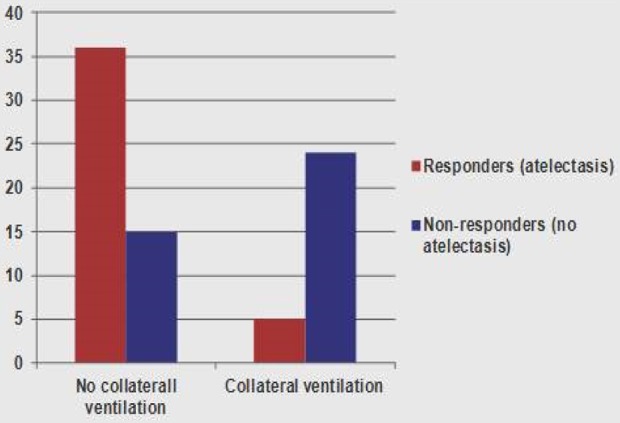
Proportion of patients in whom target lobe volume reduction of over 350 ml (responders) when CV was not detected compared to those in whom CV was detected using the Chartis catheter. Adapted from Herth et al.17. CV: collateral ventilation.
Differences in the likelihood of lung lobes being CV negative, and therefore may predict response to valve insertion, differ between the two lungs. The likelihood of CV negative pattern is greatest in the left upper lobe and the left lower lobe, followed by the right lower lobe. The least likely lobe to show CV negative pattern is the right upper lobe.
The reason for the presence of CV in the right upper lobe is the natural anatomical connection with the right middle lobe. The right horizontal fissure between the right upper lobe and the right middle lobe is often incomplete. Many EBV therapists consider the right middle lobe for treatment when treating the right upper lobe to ensure post-treatment LVR.
Complications of EBV insertion
Several side effects and complications are expected after insertion of EBV. Patients ought to be informed of the rate and significance of these side effects. The rates of side effects are derived from the experience in the author’s unit and from Pulmonx data file.
Infections and COPD exacerbations: Temporary increase in COPD exacerbation has been reported in up to 20% of the cases. These may represent true exacerbations or a reaction to the insertion of foreign body. Prevention of exacerbations is attempted by prescribing prednisolone and/or antibiotics prior to and after the procedure. However, there are no studies to support this practice.
Pneumonia distal to the valve is seen in 3.2% of patients (Figure 17). This should be managed in the usual way. Occasionally, abscess formation in the emphysema spaces is seen.
Temporary haemoptysis is also encountered and often does not require any treatment. In the author’s unit, anti-coagulation and clopidogril (but not aspirin) are withheld 7–10 days prior to valve insertion and restarted 72 h after the procedure.
Pneumothorax is one of the most serious complications of EBV insertion (Figures 18 and 19). The mechanism is thought to be a rapid expansion of the lobe adjacent to the target lobe after the collapse of the treated lobe. In some patients, adhesions were observed on VATS between the visceral and parietal pleura.18 Tearing of these adhesions upon expansion of the lobe has also been proposed as a mechanism of pneumothorax.
Figure 17.
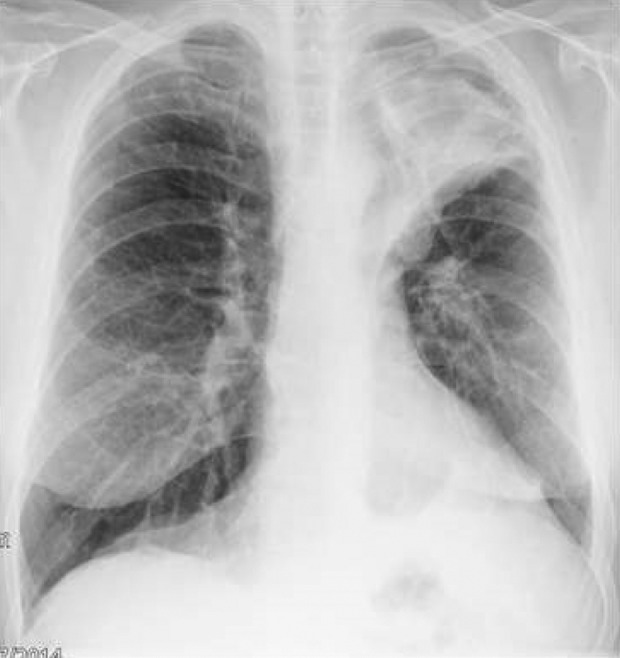
Left upper lobe pneumonia in a patient with EBV in the left upper lobe bronchus. Note the reduction in the left lung volume and the pulling up of the left hemi-diaphragm. The patient was treated successfully with intravenous then oral antibiotics. Following the treatment, the patient experienced a good clinical response to EBV insertion. EBV: endobronchial valve.
Figure 18.
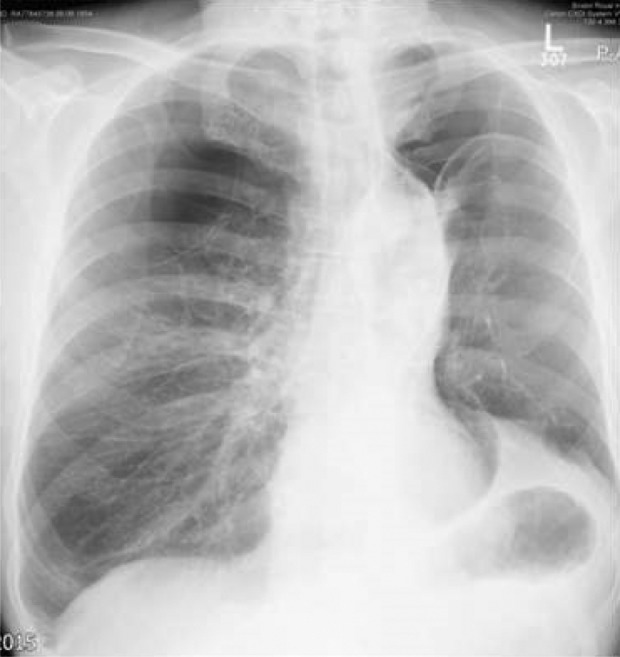
Left-sided stable pneumothorax. Please note a successful valve insertion with collapse in the left upper lobe and elevation of the left hemi-diaphragm.
Figure 19.
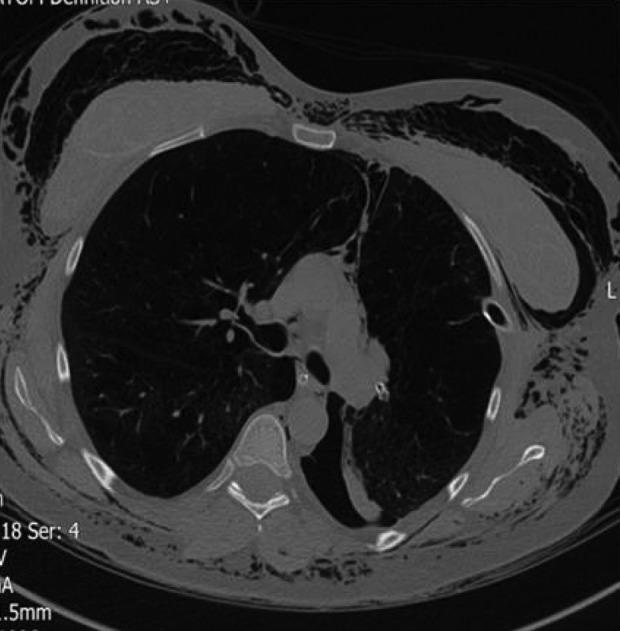
Severe pneumothorax and surgical emphysema 2 days after insertion of EBVs in the left upper lobe of a 42-year-old lady. The valves are visible in the left hilum successfully occluding the left upper lobe. The lower aspect of a collapsed left upper lobe is also seen. Pneumothorax in this patient did not respond to the intercostal chest drain due to the formation of bronchopleural fistula. Valves needed to be removed. EBV: endobronchial valve.
Pneumothorax rate differs between units. Figures ranging between 15% and 25% of patients have been reported.18 It almost always occur in patients with complete fissure and a successful collapse of the target lobe. The median onset of pneumothorax after the procedure was 2 days.18,19 In the author centre, all pneumothoraces occurred within few hours from valve insertion. For that reason, hospitalization for 3–5 days with a daily chest X-ray after valve insertion is recommended. A retrospective observation by Gompelmann and colleagues found that pneumothorax was associated with target lobe collapse and was a marker of good response to valve insertion.18
The management of pneumothorax depends on its size, its clinical impact and the presence of bronchopleural fistula.19 Observation is sufficient for patient with a stable small pneumothorax with little symptoms and normal oxygen.
A chest drain is inserted when the pneumothorax is severe or persistent. Less frequently, broncho-pleural fistula with continuous large leak may occur. In this group, surgical management of pneumothorax may be indicated. Removal of one or all valve may be necessary to stop pneumothorax in some patients.
Reinsertion of EBVs after pneumothorax is possible and does not necessarily result in recurring pneumothorax. Some physicians however chose not to reinsert valves especially when the initial pneumothorax is severe.
Strategies to reduce the incidence of pneumothorax after valve insertion have been the subject of interest. In a recent study, modifying post-operative care by bed rest and cough prevention medications were found to cause a significant reduction in the rate of pneumothorax. Pneumothorax occurred in 2 of 40 patients who had the modified care compared to 8 of 32 in those with standard care, p = 0.02.20
-
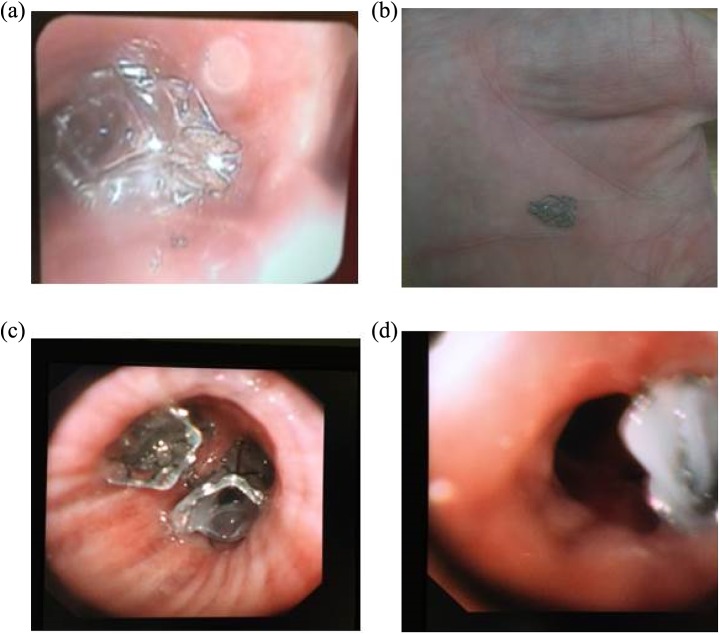
5. Valve migration and dislodgement: Valves could be displaced after insertion leaving a gap and air leak that obviates the desired collapse of the target lobe. In the minority of patients, the valves are dislodged and coughed up (Figure 20).
The reason for that is thought to be either incorrect insertion of the valves or choosing a smaller size valve. The latter can easily occur because bronchial oedema occurs during the Chartis procedure or during bronchoscopy. Oedema can reduce the calibre of the airways when measured. This may result in choosing a smaller valve size. In some patients, valve migration can occur for no obvious reason. Migrated and dislodged valves can be removed and new valves are inserted. The procedure is technically easy and can result in good response.
6. Formation of granulation tissue: Valves are foreign, partly metallic, bodies. Formation of benign granulation in the bronchial mucosa is common although the exact rate is not clear. Granulation is observed during bronchoscopy. Granulation tissue may be responsible for haemoptysis or loss of efficacy of inserted valves by changing the architecture of the bronchi (Figure 21). Valve removal and reinsertion after few weeks can restore the function of the valves.
7. Death can occur after EBVs in up to 3%. Patients who need EBVs are challenging. They have severe emphysema and often have other co-morbid conditions including cardiac disease induced by previous cigarette smoking and other co-morbid conditions. Despite a good selection process that takes into account clinical status as well as co-morbid conditions, death occurs at a rate of 2.5% in the author’s unit. The causes of death are ventilatory failure, pneumothorax, pneumonia, systemic sepsis, or massive haemoptysis. Careful observation and working closely with an intensive care unit would probably reduce but do not totally obviate death.
Figure 20.
Dislodging of valves. (a) A valve dislodged shortly after insertion and situated near the main carina; (b) a coughing up valve in a 42-year-old patient; (c) two well-placed valves in the right lower lobe bronchi in a 72-year-old patient; and (d) a displaced valve with mucous impact on the valve inserted in the apical segment of the left lower lobe bronchus.
Figure 21.
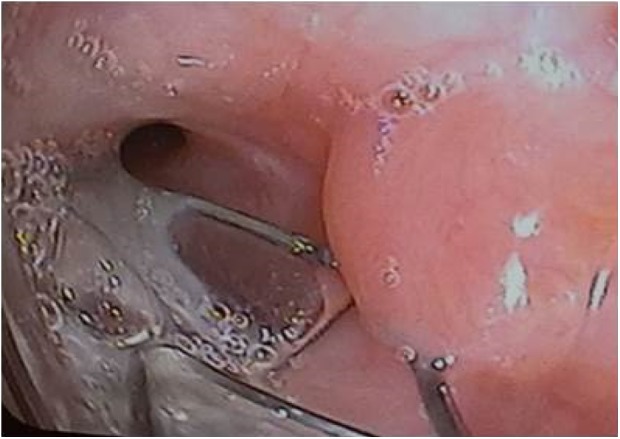
Granulation tissue causing leak around the valve. Valve was removed, and another valve was successfully inserted 2 months later after healing of tissue granulation.
Evidence for increasing efficacy of EBV insertion
The effectiveness of the valves has improved with improved selection process. As stated previously, the initial VENT trial10 found a small improvement in FEV1 and in exercise tolerance in all patients compared with the control arm. The outcome improved in subgroup of patients with highly heterogeneous disease and fissure integrity.
A further sub-analysis of the European patients of the VENT trial12 found marked improvement in FEV1 and quality of life as assessed by SGRQ when EBV was placed in patients where there is fissure completeness between the target and the adjacent lobes. Further improvement was found when there were signs of lobe exclusion (collapse) after valve insertion.
More recently the results of two prospective clinical trials are published. The first study21 was the first double-blind placebo-controlled trial comparing EBV insertion with a sham procedure. The sham arm consisted of bronchoscopy and Chartis balloon assessment. Of note, the selection criteria were based on fissure integrity only. Although Chartis assessment was made, this did not influence the selection of patients. The primary outcome was change in FEV1.
A total of 25 patients from each arm were recruited. At 3 months, the between-group difference for FEV1 was 20.9% (95% confidence interval [4.3, 37.5]). There was a decrease of 400 ml for residual volume (RV) and 5.06 point reductions in SGRQ and 33 m increase in the 6-minute walk distance. The rate of total collapse in the target lobe in this study was approximately 50%.
In another single-centre study, 68 patients were selected only when favourable data from both Chartis and fissure completeness rate on CT scan. The 6 months results were impressive.22
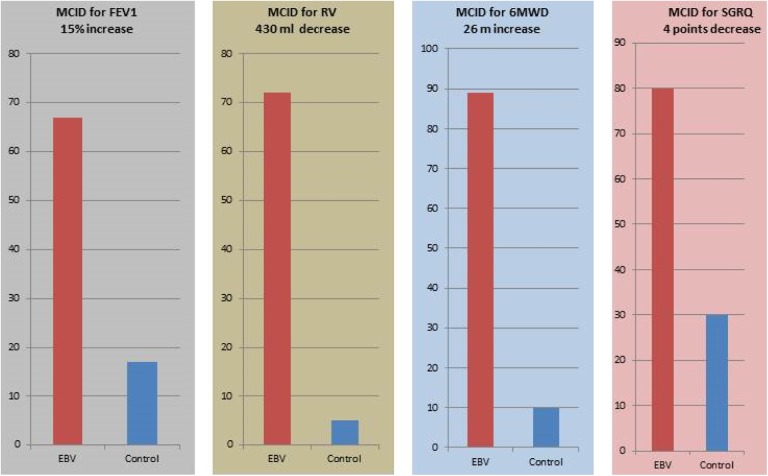
Compared with the control group, there was a marked difference in the number of patients achieving minimally important difference in all parameters in the valve-treated group compared with the control group. The results included improvement in objective measurements (FEV1 and RV), and in subjective markers (SGRQ) and 6-minute walk distance (Figure 22). No placebo arm was used in this study.
Figure 22.
Percentage of patients attaining the MCID 6 months after EBV insertion compared with the control group. MCID: minimal clinically important difference; EBV: endobronchial valve; RV: residual volume; 6MWD: 6-minute walk distance; SGRQ: St George’s Respiratory Questionnaire.22
Clinical, ethical and economic considerations
The rapidly accumulating literature indicates that LVR using EBV is a credible method in selected patients with advanced emphysema.
The findings are consistent in case series studies and in prospective clinical trials.
Careful patients selection is essential and better done by a multidisciplinary group of respiratory physicians, thoracic radiologists and thoracic surgeons.
It is important that EBV should be part of a COPD management programme. Enforcing the basic management including pulmonary rehabilitation and smoking cessation as prerequisite to further treatment can help improving the outlook of COPD management.
The long-term outcome of treatment remains uncertain, although long-term survival benefit has been reported in a 5-year case series. Similarly, the outcome of managing homogeneous and heterogeneous emphysema needs clarification.
Less clear results are available, and more work needs to be done for patients with emphysema due to α1 anti-trypsin deficiency. This group of patients tend to present at younger age and a more severe disease with different underlying mechanism to conventional emphysema. A retrospective report by Hillerdal and Mindus23 showed favourable results in 12 of 15 patients with homozygote α1 anti-trypsin patients with emphysema.
Managing patients’ expectation is important. Many patients referred for treatment suffer from severe breathlessness and already are on maximum inhaled therapy. The publicity drawn from super-responders can be misleading. Patients’ expectations of significant improvement after valve insertion need to be made realistic. Frank discussion on the fact that some patients may not respond is important to avoid disappointment, complaints and unnecessary patients’ resentment. In the author’s unit, patients are advised that up to 40% of patients may not respond to EBV treatment.
The procedure is attractive to respiratory physicians, and on the whole, the techniques are not difficult to master. However, a minimal level of competency is needed for identifying therapist. The procedure is probably better concentrated in institution where thoracic surgery is present. This is important for managing complications.
Finally, the procedure is expensive. Depending on local commercial deals, the cost of valves, the Chartis catheter and delivery system for one lobe treatment range from £7000 to £8000 (8200–9200 Euros). This does not include the cost of operators, operating theatres and the cost of managing complications.
Making this treatment more affordable in developing countries as well as in developed countries is a challenge that both health systems and valve-producing companies have to face.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Global initiative for Chronic Obstructive Pulmonary Disease (GOLD initiative), http://www.goldcopd.org/
- 2. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363: 1128–1138. [DOI] [PubMed] [Google Scholar]
- 3. Donaldson GC, Wedzicha JA. COPD exaerbations – 1. Epidemiology. Thorax 2006; 61: 164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. Eur Resp J 2007; 29: 1224–1238. [DOI] [PubMed] [Google Scholar]
- 5. Kelly JL, Bamsey O, Smith C, et al. Health status assessment in routine clinical practice: the chronic obstructive pulmonary disease assessment test score in outpatients. Respiration 2012; 84: 193–199. [DOI] [PubMed] [Google Scholar]
- 6. Abboud RT, Vimalanathan S. Pathogenesis of COPD. Part I. The role of protease-antiprotease imbalance in emphysema [State of the Art Series]. Chronic obstructive pulmonary disease in high- and low-income countries. Int J Tuberc Lung Dis 2008; 12(4): 361–367. [PubMed] [Google Scholar]
- 7. Schroeder JD, McKenzie AS, Zach JA, et al. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. Am J Radiol 2013; 201: 460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume–reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003; 348: 2059–2073. [DOI] [PubMed] [Google Scholar]
- 9. McNulty W, Jordan S, Hopkinson NS. Attitudes and access to lung volume reduction surgery for COPD: a survey by the British Thoracic Society. BMJ Open Resp Res 2014; 1: e000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sciurba F, Ernst A, Herth F, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010; 363: 1233–1244. [DOI] [PubMed] [Google Scholar]
- 11. Koenigkam Santos M, Puderback M, Gompelmann D, et al. Inclomplete Fissures in severe emphysematous patients evaluated with MDCT: incidence and interobserver aagreement among radiologists and pneumologists. Eur J Radiol 2012; 81: 4161–4166. [DOI] [PubMed] [Google Scholar]
- 12. Herth FJ, Noppen M, Valipour A. Efficacy predictors of lung volume reduction with Zepher valves in a European Cohort. Eur Respir J 2012; 39: 1334–1342. [DOI] [PubMed] [Google Scholar]
- 13. Shah P, Herth FJF. Current status of bronchoscopic lung volume reduction with endobronchial valves. Thorax 2014; 69: 280–286. [DOI] [PubMed] [Google Scholar]
- 14. Venuta F, Anile M, Diso D, et al. Long-term follow-up after bronchoscopiclung volume reduction in patients with emphysema. Eur Respir J 2012; 39: 1084–1089. [DOI] [PubMed] [Google Scholar]
- 15. Hopkinson NS, Kemp SV, Toma TP, et al. Atelectasis and survival after bronchoscopic lung volume reduction for COPD. Eur Respir J 2011; 37: 1346–1351. [DOI] [PubMed] [Google Scholar]
- 16. Oliveira HG, Rambo R, Oliveira S, et al. Semi-automated CT fissure integrity assessment as a guide to decision making in bronchoscopic emphysema treatment using one-way Zephyr® valves. Am J Crit Care Respi Med 2015; 191: A1146. [Google Scholar]
- 17. Herth FJF, Eberhardt R, Gomplemann D, et al. Radiological and clinical outcomes of using Chartis™ to plan endobronchial valve treatment. Eur Respir J 2013; 41: 302–308. [DOI] [PubMed] [Google Scholar]
- 18. Gompelmann D, Herth FJF, Slebos DJ, et al. Pneumothorax following endo-bronchial valve therapy and impact on clinical outcomes in severe emphysema. Respiration 2014; 87: 485–491. [DOI] [PubMed] [Google Scholar]
- 19. Valipour A, Slebos DJ, de Oliveira HG, et al. Expert statement: pneumothorax associated with endoscopic valve therapy for emphysema – potential mechanisms, treatment algorithm, and case examples. Respiration 2014; 87: 513–521. [DOI] [PubMed] [Google Scholar]
- 20. Herzog D, Poellinger A, Doellinger F, et al. Modifying post-operative medical care after EBV implant may reduce pneumothorax incidence. Plos One 2015; 10(5): e0128097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davey C, Zoumot Z, Jordan S, et al. Bronchoscopiclung volume reduction withendobronchial valvesfor patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet 2015; 386: 1066–1073. [DOI] [PubMed] [Google Scholar]
- 22. Klooster K, Ten Hacken NHT, Hartman JE, et al. Endobronchial valve treatment versus standard medical care in patients with emphysema without interlobar collateral ventilation (The STELVIO-Trial). Am J Resp Crit Care Med 2015; 191: A6312. [Google Scholar]
- 23. Hillerdal G, Mindus S. One- to four-year follow-up of endobronchial lung volume reduction in alpha-1-antitrypsin deficiency patients: a case series. Respiration 2014; 88(4): 320–328. [DOI] [PubMed] [Google Scholar]