Abstract
The Leicester Cough Questionnaire (LCQ) has been validated in non-cystic fibrosis bronchiectasis (NCFBC). The present study aimed to create and validate a Spanish version of the LCQ (LCQ-Sp) in NCFBC. The LCQ-Sp was developed following a standardized protocol. For reliability, we assessed internal consistency and the change in score over a 15-day period in stable state. For responsiveness, we assessed the change in scores between visit 1 and the first exacerbation. For validity, we evaluated convergent validity through correlation with the Saint George’s Respiratory Questionnaire (SGRQ) and discriminant validity. Two hundred fifty-nine patients (118 mild bronchiectasis, 90 moderate bronchiectasis and 47 severe bronchiectasis) were included. Internal consistency was high for the total scoring and good for the different domains (Cronbach’s α: 0.86–0.91). The test–retest reliability shows an intraclass correlation coefficient of 0.87 for the total score. The mean LCQ-Sp score at visit 1 decreased at the beginning of an exacerbation (15.13 ± 4.06 vs. 12.24 ± 4.64; p < 0.001). The correlation between LCQ-Sp and SGRQ scores was −0.66 (p < 0.01). The differences in the LCQ-Sp total score between the different groups of severity were significant (p < 0.001). The LCQ-Sp discriminates disease severity, is responsive to change when faced with exacerbations and is reliable for use in bronchiectasis.
Keywords: Bronchiectasis, cough, quality of life, patient-reported outcome, questionnaire
Introduction
Patients with bronchiectasis suffer from cough, daily expectoration and exacerbations. The main objectives of treatment are clinical improvement and to halt the pulmonary damage progression.1 Pulmonary damage may be measured through high-resolution computed tomography (HRCT) and pulmonary function tests. However, unlike patients with cystic fibrosis (CF), these explorations, especially the pulmonary function test, are insufficiently sensitive to monitor the changes.2 Clinical monitoring could reveal minor changes but its measurement is more subjective. Health-Related Quality of Life (HRQoL) Questionnaires allow the impact of disease or of certain symptoms to be measured, and these are being used ever more frequently in research.
Two English HRQoL Questionnaires initially designed for other diseases – the St. George’s Respiratory Questionnaire (SGRQ)3,4 and the Leicester Cough Questionnaire (LCQ)5,6 – have been validated in non-CF bronchiectasis (NCFBC).4,6,7 The SGRQ was designed for chronic obstructive pulmonary disease (COPD) and the LCQ5 for chronic cough. Other cough questionnaires have been designed but validation has not been performed in bronchiectasis.8–10
Given that coughing is one of the most frequent symptoms of patients with bronchiectasis11and that the LCQ6 is included as a variable in the studies of these patients,2,12–16 and, furthermore, that no Spanish version of the LCQ (LCQ-Sp) has previously been available, the objectives of this study were to create a LCQ-Sp17,18 and to validate it in patients with NCFBC.
Methods
Adaptation into Spanish of the LCQ
After obtaining the permission of the authors of the LCQ, we followed a standardized protocol for the Spanish adaptation (translation and back translation by bilingual speakers in consultation with a professional committee and a lay panel18,19). A first translation was produced by a bilingual speaker whose mother tongue was Spanish. This translation was reviewed by a committee of professionals composed of two pneumologists and a respiratory physiotherapist, who rated the equivalence between this first forward translation and the original version. After changing three terms to ones that seemed to give greater equivalence to the English original, the new version was back translated by a bilingual speaker whose mother tongue was English for comparison. A harmonization meeting was convened to reconcile differences between the original LCQ and the translated Spanish version. No further changes were made. A panel of six patients with NCFBC were asked to respond to the questionnaire and give their opinion regarding possible difficulties in understanding the questions and answers. These discussions led to the structure of question number 2 being improved and to one adjective being changed for a near synonym that was considered more appropriate.
The third version resulting from the completion of these tasks was pilot tested with 20 NCFBC patients (12 females, mean age 63.75 ± 12.31 years). This third version proved to be understandable and easy to complete and so was adopted as the final version.
Study design and participants
A prospective, longitudinal multicentre study to validate the (LCQ-Sp) in bronchiectasis was performed. Consecutive adult patients with bronchiectasis diagnosed by clinical and HRCT, in stable phase (defined as no exacerbation1 in the previous month), attending the bronchiectasis clinics of six hospitals between April 2011 and April 2012, were invited to participate in the study. Patients were required to have had an HRCT in the previous 5 years.
Exclusion criteria: CF, current smokers, COPD patients with a predicted forced expiratory volume in 1 s (FEV1) of <60%, patients with asthma, patients with previous pulmonary resection or unable to respond to the questionnaire or who had suffered an exacerbation in the previous month.1
The study was approved by the Ethical Committee of the Dr. Trueta Hospital (registration number 2011053). All patients gave their written informed consent before inclusion.
Study protocol
In order to validate the LCQ-Sp, an analysis of its feasibility, reliability, validity and responsiveness was undertaken.20 All patients were invited to fill in the LCQ-Sp, the SGRQ and the modified Medical Research Council (mMRC) dyspnoea scale21(visit 1). The variables recorded were sputum colour,22 sputum volume in the previous 24 h and number of exacerbations and admissions in the previous 6 months. If the patient continued in stable phase at 15 days and there had been no modification to their treatment, they were asked to fill in the LCQ-Sp again (visit 2) to analyse the reliability. The rest of the demographic variables, aetiology, microbiological data, respiratory function (the last obtained in the stable phase during the previous 18 months) and HRCT studies were obtained from the investigators’ databases. To predict the severity of bronchiectasis, two validated severity scales in NCFBC – the FACED score23 and the Bronchiectasis Severity Index (BSI)24 – were used. A categorization of the patients using variables that were thought likely to be related with the cough was performed to complete the validity analysis.
Patients were also asked to fill in the LCQ-Sp again in the case that they presented an exacerbation1 in the following 6 months (visit 3) to analyse responsiveness.
Questionnaires
The LCQ5 is a 19-item questionnaire that measures the impact of coughing on the quality of life in the 2 weeks prior to completion in three domains: physical (8 items), psychological (7 items) and social (4 items). The total severity score ranges 3–21, with a lower score indicating greater impairment of health status due to cough. The minimal clinically important difference (MCID) for the total LCQ score is 1.3.25
The SGRQ3 consisted of 50 items grouped in three domains: symptoms (8 items), activity (16 items) and impacts (26 items). The total score ranges 0–100 with zero indicating no impairment to quality of life. The MCID is 4.26
The mMRC21 dyspnoea scale is a questionnaire that consists of five statements about perceived breathlessness.
Statistics
The sample size of internal consistency for the Cronbach’s α was calculated using Bonnett’s Formula.27 Expecting a Cronbach’s α of 0.80 for the LCQ-Sp and setting a required level of 0.70 in a two-sided test at α = 0.05, power of 0.80 and assuming a 20% of missing data rate, a sample size of 216 subjects would be required. This sample size is valid for the global questionnaire (k = 19 items, n = 171) and also for the three domains (physical: k = 8 items, n = 186; psychological: k = 7 items, n = 190 and social: k = 4 items; n = 216).
Feasibility was analysed calculating the percentage of patients without a response for the total score and for each domain of the LCQ-Sp in visit 1. The percentage of patients obtaining the lowest possible score (floor effect) and highest possible score (ceiling effect) was analysed.
For the assessment of reliability,28 internal consistency was estimated for the total number of items and for each of the domains at visit 1 using Cronbach’s α. Test–retest reliability to analyse changes in the LCQ-Sp score between visits 1 and 2 was estimated through the intraclass correlation coefficient (ICC) using a two-way mixed effects model and type consistency.29 The commonly accepted minimal standard for reliability coefficients is 0.70.20 A graphical analysis was also performed using the Bland and Altman method.30 The effect size using Cohen’s criteria has been calculated.31
The construct validity was analysed using convergent validity and discriminant validity. For convergent validity, correlation of LCQ-Sp and SGRQ at visit 1 was analysed by Spearman’s rank correlation. Discriminant validity was analysed comparing the means of LCQ-Sp with the FACED score23 and BSI24 using analysis of variance (ANOVA). Relative measurement precision (RMP) of LCQ-Sp for detecting group differences was calculated by computing the ratio of pairwise F statistics (F-FACED divided by F-LCQ-Sp and F-BSI divided by F-LCQ-Sp). This ratio indicates, as a proportion, how much more (or less) precise are FACED and BSI compared with the LCQ-Sp at detecting group differences.32 Furthermore, to complete the validity analyses, known-groups validity approach was performed to compare means of LCQ-Sp across groups with different cough severity by using one-way ANOVA with Bonferroni test for post hoc comparisons. In order to define the grade of severity of cough, a score from 0 to 2 was assigned to previously selected variables that were thought likely to be related with the cough: sputum volume (0: ≤10 mL; 1: 11–29 mL; 2: ≥30 mL), sputum colour (0: mucous; 1: mucopurulent; 2: purulent), chronic bronchial colonisation1 (0: no evidence; 1: by microorganisms other than Pseudomonas aeruginosa; 2: by P. aeruginosa), predicted FEV1% (0: ≥70%; 1: 69–31%; 2: ≤30%), mMRC dyspnoea scale (0: 0; 1: 1–2; 2: 3–4) and the number of lobes affected (the lingula was counted as separate lobe; 0: 1 lobe; 1: 2–3; 2: >3). Variables showing significant differences in LCQ-Sp using ANOVA were used to establish groups of cough severity of equal dimensions (mild, moderate and severe).
For responsiveness, the mean change in total score between visit 1 and the beginning of the first exacerbation was compared using the paired Student’s t-test.
A two-tailed p value of <0.05 was statistically significant. Statistical analysis was performed using SPSS for Windows version 22.0 (IBM Corp., Armonk, New York, USA) and Stata/IC 13.1 (StataCorp. 2013, Stata Statistical Software: Release 13, StataCorp LP, College Station, Texas, USA).
Results
Feasibility
A total of 259 patients were included in the study and responded to the LCQ-Sp during visit 1 (Table 1). The response rate of these patients was 100% both for the total score and the three domains. No floor or ceiling effect was observed (Table 2).
Table 1.
Baseline characteristics of recruited patients.a
| All patients | Mild | Moderate | Severe | |
|---|---|---|---|---|
| n | 259 | 118 (46) | 90 (35) | 47 (18) |
| Females | 154 (59) | 72 (61) | 51 (56) | 28 (59) |
| Age (years) | 58.37 ± 18.10 | 55.91 ± 17.74 | 58.71 ± 19.53 | 63.89 ± 14.73 |
| Ex-smokers | 78 (30) | 33 (28) | 34 (37) | 10 (21) |
| Aetiology | ||||
| Post-infective | 94 (36.3) | 42 (35.6) | 34 (37.7) | 19 (40.4) |
| Idiopathic | 78 (30.4) | 34 (28.8) | 26 (28.9) | 18 (38.3) |
| Ciliary dyskinesia | 26 (10.1) | 12 (10.2) | 6 (6.7) | 1 (2.1) |
| Immune defect | 21 (7.8) | 10 (8.4) | 7 (7.8) | 3 (6.4) |
| Connective pathologies | 7 (2.7) | 2 (1.7) | 3 (3.3) | 1 (2.1) |
| Aspiration or GE reflux | 2 (0.8) | 0 | 0 | 2 (4.3) |
| Other | 31 (11.7) | 18 (15.3) | 14 (15.4) | 3 (6.3) |
| BMI (kg/m2) | 25.45 ± 5.21 | 24.78 ± 4.6 | 25.80 ± 6.05 | 26.48 ± 5.02 |
| Exacerbations in the preceding 6 months | 0.83 ± 0.98 | 0.69 ± 0.86 | 0.82 ± 1.07 | 1.23 ± 1 |
| Hospitalizations in the preceding 6 months | 0.12 ± 0.38 | 0.07 ± 0.25 | 0.08 ± 0.3 | 0.36 ± 0.6 |
| 24 h Sputum volume (mL) | 22.35 ± 25.69 | 8.08 ± 10.92 | 22.55 ± 17.62 | 57.34 ± 31.55 |
| Sputum colour | ||||
| No expectoration | 41 (16) | 35 (29.7) | 6 (6.7) | 0 |
| Mucous | 77 (30) | 56 (47.5) | 20 (22.2) | 0 |
| Mucopurulent | 97 (37.7) | 24 (20.3) | 50 (55.6) | 22 (46.8) |
| Purulent | 42 (16.3) | 3 (2.5) | 14 (15.6) | 25 (53.2) |
| Chronic colonization | ||||
| None | 98 (38) | 71 (60.2) | 23 (25.6) | 2 (4.3) |
| Other than Pseudomonas | 88 (34.1) | 38 (32.2) | 38 (42.2) | 12 (25.5) |
| Pseudomonas aeruginosa | 72 (27.9) | 9 (7.6) | 29 (32.2) | 33 (70.2) |
| FEV1 % predicted | 65.9 ± 22.1 | 76.6 ± 18.2 | 61.8 ± 22.1 | 47.1 ± 15.3 |
| FVC % predicted | 74.5 ± 18.7 | 82.3 ± 15.5 | 71.4 ± 19.1 | 61.1 ± 16.4 |
| mMRC dyspnoea score | 1 ± 0.96 | 0.54 ± 0.64 | 1.16 ± 0.96 | 1.85 ± 0.99 |
| Number of affected lobes | 3 ± 1.49 | 2.8 ± 1.5 | 2.9 ± 1.3 | 3.5 ± 1.5 |
FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; GE: gastroesophageal; mMRC: modified Medical Research Council; BMI: body mass index.
aData are presented as mean ± SD or n (%), unless otherwise stated.
Table 2.
Feasibility and reliability of the LCQ-Sp.
| Domain | Items (N) | No response (%) | Mean ± SD | Floor (%) | Ceiling (%) | Cronbach’s α | ICC (95% CI) | Effect size |
|---|---|---|---|---|---|---|---|---|
| Physical | 8 | 0 | 4.96 ± 1.37 | 1.50 (0.8) | 7 (4.2) | 0.870 | 0.87 (0.84–0.90) | −0.08365 |
| Psychological | 7 | 0 | 4.99 ± 1.50 | 1.42 (0.4) | 7 (9.7) | 0.874 | 0.82 (0.77–0.86) | −0.00996 |
| Social | 4 | 0 | 5.34 ± 1.49 | 1 (0.4) | 7 (18.5) | 0.860 | 0.79 (0.73–0.84) | 0.000604 |
| Total | 19 | 0 | 15.26 ± 4.07 | 5.67 (0.4) | 21 (3.1) | 0.911 | 0.84 (0.79–0.87) | −0.04416 |
SD: standard deviation, ICC: intraclass correlation coefficient; LCQ: Leicester Cough Questionnaire; LCQ-Sp: Spanish version of the LCQ.
Reliability
The internal consistency of the LCQ-Sp of the 259 patients at visit 1 was high for the total score and good for the different domains, with Cronbach’s α values ranging 0.86–0.91 (Table 2).
The test–retest reliability was calculated with the scores of the LCQ-Sp of the 199 patients who filled in the questionnaire again at 15 days (Figure 1) with an ICC that indicates excellent stability for the total score and for the three domains, with ICC values ranging 0.79–0.87. Using Cohen’s criteria, a small size for difference in means has been obtained for the total score and each domain (Table 2). A Bland–Altman plot of the difference between repeat total scores for the LCQ-Sp is shown in Figure 2.
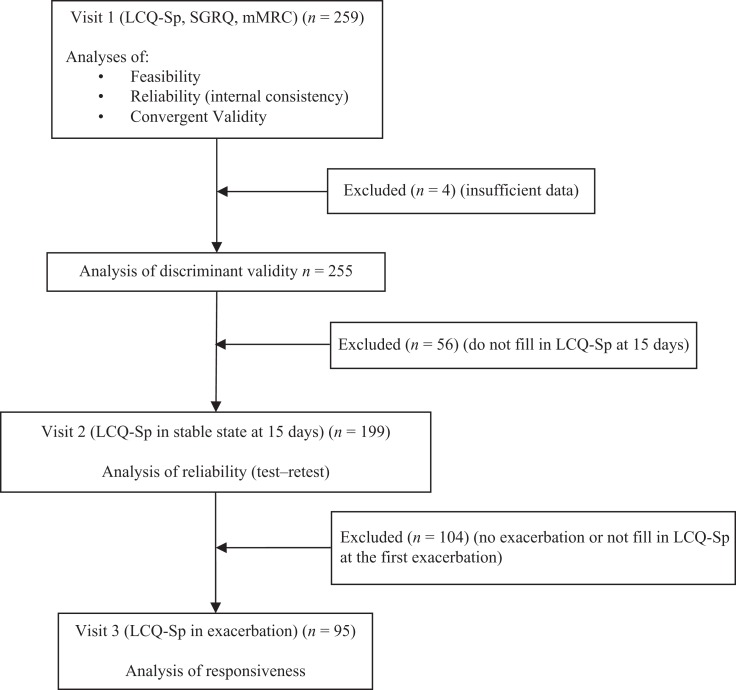
Figure 1.
Flow chart of the study. LCQ-Sp: Spanish version of the Leicester Cough Questionnaire; SGRQ: Saint Georges Respiratory Questionnaire; mMRC: modified Medical Research Council dyspnoea scale.
Figure 2.
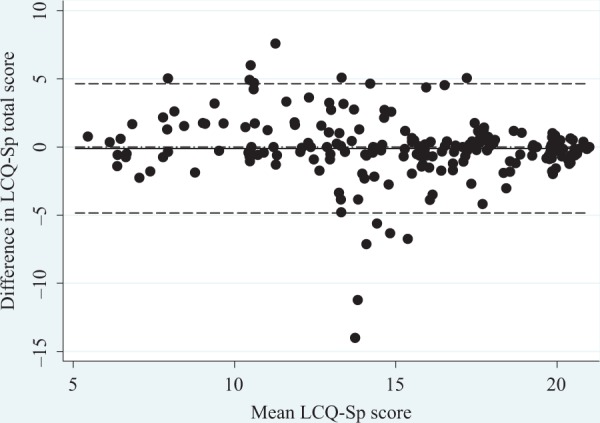
Bland–Altman plot of LCQ-Sp total score repeated over 15 days in 199 patients with stable bronchiectasis. —: Mean difference between the two scores (−0.10); ------: 95% limits of agreement (−4.8 to 4.6). LCQ: Leicester Cough Questionnaire; LCQ-Sp: Spanish version of the LCQ.
Validity
In the analysis of convergent validity, the LCQ-Sp had a significant moderate inverse correlation with the SGRQ at visit 1 for the total score and for the three domains, with scores ranging from −0.59 to −0.67 (Table 3).
Table 3.
Correlation of the LCQ-Sp with the SGRQ at visit 1, convergent validity.
| SGRQ | ||||
|---|---|---|---|---|
| LCQ-Sp | Symptoms | Activity | Impacts | Total |
| Physical | −0.55a | −0.54a | −0.65a | −0.67a |
| Psychological | −0.46a | −0.45a | −0.64a | −0.59a |
| Social | −0.52a | −0.53 | −0.66a | −0.65a |
| Total | −0.52a | −0.53a | −0.68a | −0.66a |
LCQ: Leicester Cough Questionnaire; LCQ-Sp: Spanish version of the LCQ; SGRQ: Saint George’s Respiratory Questionnaire.
aSignificant Spearman correlation: p < 0.01 (bilateral).
For the analysis of the discriminant validity, we selected the 255 patients for whom we had all of the data of the chosen variables (Figure 1). Significant differences for the FACED score between mild and severe bronchiectasis (p = 0.001) and significant differences for the BSI between mild and moderate (p = 0.001) and mild and severe bronchiectasis (p < 0.001) were observed (Table 4). RMP analysis shows that the FACED and the BSI are 21.70% and 29.34% as precise, respectively, as the LCQ-Sp in detecting three-group differences.
Table 4.
Discriminant ability of the LCQ-Sp to detect differences in disease severity.a
| Mild | Moderate | Severe | ANOVA | Comparison group | Post hoc | RMP (%) | |
|---|---|---|---|---|---|---|---|
| n | n | n | p-value | ||||
| FACED score25 | 16.13±3.83 | 14.57±4.15 | 13.24±4.20 | F = 8.459 | mild vs. moderate | 0.19 | 21.70% |
| 146 | 74 | 30 | p < 0.001 | mild vs. severe | 0.001 | ||
| moderate vs. severe | 0.366 | ||||||
| Bronchiectasis Severity Index26 | 16.76±3.59 | 14.69±4.09 | 13.84±4.17 | F = 11.434 | mild vs. moderate | 0.001 | 29.34% |
| 99 | 101 | 48 | p < 0.001 | mild vs. severe | <0.001 | ||
| moderate vs. severe | 0.653 | ||||||
| LCQ-Sp | 17.13±3.36 | 14.68±3.78 | 11.81±3.73 | F = 38.966 | mild vs. moderate | <0.001 | 100% |
| 118 | 90 | 47 | p < 0.001 | mild vs. severe | <0.001 | ||
| moderate vs. severe | <0.001 |
LCQ-Sp: Spanish version of the Leicester Cough Questionnaire; RMP: Relative measurement precision; ANOVA: analysis of variance. aData are presented as mean ± SD.
In the preliminary analysis of the chosen variables, it was observed that all variables except the extent of bronchiectasis were significantly associated with the LCQ-Sp, and so this variable was excluded from the categorization of cough severity. On dividing the possible range of values of the total score of the variables into three groups of equal dimensions, mild cough was defined as a score of ≤3, moderate as 4–6 and severe as ≥7. With this categorization, 118 patients were classified as having mild cough with an LCQ-Sp total score of 17.13 ± 3.36, 90 patients as having moderate cough with an LCQ-Sp total score of 14.68 ± 3.78 and 47 patients as having severe cough with an LCQ-Sp total score of 11.81 ± 3.73. The differences in the mean of the LCQ-Sp total score between the three groups (mild vs. moderate; mild vs. severe and moderate vs. severe; p < 0.001) were significant and greater than the previously established MCID for the LCQ (>1.3) (Table 4).
Responsiveness
The mean total score at visit 1 decreased in the 96 patients that filled in the questionnaire at the beginning of the first exacerbation (15.13 ± 4.06 vs. 12.24 ± 4.64, respectively; p < 0.001). The magnitude of the difference was higher than the MCID of 1.3. The mean score of the individual domains also decreased significantly (physical 4.82 ± 1.44 vs. 3.89 ± 1.49; psychological 4.97 ± 1.45 vs. 4.09 ± 1.64 and social 5.32 ± 1.47 vs. 4.24 ± 1.71; p < 0.001).
Discussion
We have created a LCQ-Sp and have validated it in adult NCFBC patients in a multicentre study. This version is shown to be able to discriminate disease severity, responsive to change and reliable for use in NCFBC.
The analysis of feasibility shows that all of the patients completed all of the answers, suggesting that patients do not find it difficult to respond to. The absence of a floor or ceiling effect indicates that the results of the questionnaire were not affected by extreme values.
With regard to reliability, good internal consistency for all of the domains as well as for the total score were obtained, with a high Cronbach’s α, a good repeatability, a high ICC and a low effect size. These results are similar to those obtained in the original version of the questionnaire.5 With regard to Murray,6 in patients with bronchiectasis, no data were available regarding internal consistency and the repeatability was analysed 6 months after the completion of the initial questionnaire in patients in stable phase whose treatment had not been modified.
In the case of the validity, as with the two previous studies,5,6 it was observed that the LCQ-Sp total score had a significant negative correlation with the total SGRQ score. In the three studies, the correlation was only moderate, probably because the LCQ and the SGRQ are providing information on different aspects of the impact of bronchiectasis on the HRQoL.5,6 With regard to the analysis of discriminant validity, we have used two classifications on the severity of bronchiectasis – the FACED score23 and the BSI24 – which classify severity according to its prognosis and have recently been published. The analysis proves the capacity of the LCQ-Sp to discriminate disease severity. Before having these classifications available to us, we had categorized patients into mild, moderate and severe cough using variables that we considered could be related to the severity of the cough. Therefore, in addition to the criteria of Murray et al.6 (sputum colour, chronic colonization and extension in HRCT), we also considered sputum volume, FEV1 and dyspnoea. The extension of bronchiectasis was initially considered but finally excluded when we failed to find an association of this with the LCQ-Sp. The analysis showed that with this categorization the questionnaire was able to identify different cough severities.
It has been shown that the LCQ is responsive to change after treatment. Birring et al.5 tested the instrument before the performance of different treatments to improve cough and at 2 months of the interventions in nine patients with an effect size for change in the total LCQ score of 1.68. Murray et al.6 tested the questionnaire in 32 patients with bronchiectasis with an exacerbation prior to treatment and 1 week following its completion, showing a significant improvement in both the individual domain and total scores between the start and end of treatment with a median improvement in the total score of 4.6 (3.2–7.2). Unlike earlier studies, we analysed responsiveness at the beginning of an exacerbation and a significant worsening was detected across all domains and in the total score. This suggests that the LCQ can also detect changes in health status as a result of an exacerbation and might be a useful outcome measure in assessing such changes. The utility of this questionnaire in monitoring changes has been shown in several studies of bronchiectasis, in which it has been used to detect changes in cough status resulting from different interventions.2,12–16 Two of these studies have even taken the LCQ as the primary end point.12,16
The LCQ has been also validated in other languages in chronic cough33–35 and in COPD.36,37 The results in repeatability and concurrent validity measured with different questionnaires are similar to our results.33–37 Responsiveness has been studied at 2 or 6 months after improvement or treatment but not at the beginning of an exacerbation. No data about feasibility, discriminant validity were reported.
Regarding other questionnaires for chronic cough, only the Cough Quality of Life Questionnaire8 has been validated and compared with the LCQ in chronic cough, COPD, asthma and bronchiectasis.38 No significant differences in the total scores of the two questionnaires were observed between groups. However, differences in the analysis of subdomains with the CQLQ were observed, suggesting that both questionnaires can each provide important additional information concerning the impact of cough.38
In conclusion, the LCQ-Sp is able to discriminate disease severity, is responsive to change in the event of exacerbations and is reliable.
Acknowledgements
We would like to thank Dr Nicholas John Kelleher (ICO-Haematology, Dr. Josep Trueta University Hospital) for his help in the translation of the questionnaire and Andrew Hughes for assistance in drafting the manuscript in English.
Authors’ Note: GM and MV contributed to the conception and design of the study, acquisition, analysis, interpretation of the data and drafting of the manuscript. Both authors are the guarantor of the manuscript, taking responsibility for the integrity of the word as a whole from inception to published article. MB contributed to the statistical analysis and critical revision of the manuscript. JG, CO, M-A M-G, RG, EP and AA contributed to acquisition of the data and critical revision of the manuscript. SSB contributed to the critical revision of the manuscript. Beatriz Herrero RPT (Department of Pneumology, Hospital Clínic, Barcelona) and Francisco Espildora (Pneumology, Malaga Regional University Hospital) have also participated in this study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is included in the PII of Bronchiectasis of the SEPAR and supported by FIS PI12/01551.
References
- 1. Vendrell M, de Gracia J, Olveira C, et al. Diagnosis and treatment of bronchiectasis. Spanish society of pneumology and thoracic surgery. Arch Bronconeumol 2008; 44: 629–640. [DOI] [PubMed] [Google Scholar]
- 2. Murray MP, Govan JRW, Doherty CJ, et al. A randomized controlled trial of nebulized gentamicin in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2011; 183: 491–499. [DOI] [PubMed] [Google Scholar]
- 3. Jones PW, Quirk FH, Baveystock CM, et al. A self-completed measure for chronic airflow limitation: the St George’s respiratory questionnaire. Am Rev Respir Dis 1992; 145: 1321–1327. [DOI] [PubMed] [Google Scholar]
- 4. Wilson CB, Paul WJ, O’leary CJ, et al. Validation of the St. George’s respiratory questionnaire in bronchiectasis. Am J Respir Crit Care Med 1997; 156: 536–541. [DOI] [PubMed] [Google Scholar]
- 5. Birring SS, Prudon B, Carr AJ, et al. Development of a symptom specific health status measure for patients with chronic cough: leicester cough questionnaire (LCQ). Thorax 2003; 58: 339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murray MP, Turnbull K, MacQuarrie S, et al. Validation of the leicester cough questionnaire in non-cystic fibrosis bronchiectasis. Eur Respir J 2009; 34: 125–131. [DOI] [PubMed] [Google Scholar]
- 7. Martínez García MA, Perpiñá Tordera M, Román Sánchez P, et al. Consistencia interna y validez de la versión española del St. George’s respiratory questionnaire para su uso en pacientes afectados de bronquiectasias clínicamente estables. Arch Bronconeumol 2005; 41: 110–117. [DOI] [PubMed] [Google Scholar]
- 8. French CT, Irwin RS, Fletcher KE, et al. Evaluation of a cough-specific quality of-life questionnaire. Chest 2002; 121: 1123–1131. [DOI] [PubMed] [Google Scholar]
- 9. Baiardini I, Braido F, Fassio O, et al. A new tool to assess and monitor the burden of chronic cough on quality of life: chronic cough impact questionnaire. Allergy 2005; 60: 482–488. [DOI] [PubMed] [Google Scholar]
- 10. Crawford B, Monz B, Hohlfeld J, et al. Development and validation of a cough and sputum assessment questionnaire. Respir Med 2008; 102: 1545–1555. [DOI] [PubMed] [Google Scholar]
- 11. King PT, Holdsworth SR, Freezer NJ, et al. Characterisation of the onset and presenting clinical features of adult bronchiectasis. Respir Med 2006; 100: 2183–2189. [DOI] [PubMed] [Google Scholar]
- 12. Murray MP, Pentland JL, Hill AT. Randomised crossover trial of chest physiotherapy in non-cystic fibrosis bronchiectasis. Eur Respir J 2009; 34: 1086–1092. [DOI] [PubMed] [Google Scholar]
- 13. Mutalithas K, Watkin G, Willig B, et al. Improvement in health status following bronchopulmonary hygiene physical therapy in patients with bronchiectasis. Respir Med 2008; 102: 1140–1144. [DOI] [PubMed] [Google Scholar]
- 14. Mandal P, Sidhu MK, Kope L, et al. A pilot study of pulmonary rehabilitation and chest physiotherapy versus chest physiotherapy alone in bronchiectasis. Resp Med 2012; 106: 1647–1654. [DOI] [PubMed] [Google Scholar]
- 15. Torrego A, Haque RA, Nguyen LT, et al. Capsaicin cough sensitivity in bronchiectasis. Thorax 2006; 61: 706–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mandal P, Chalmers JD, Graham C, et al. Atorvastatin as a stable treatment in bronchiectasis: a randomised controlled trial. Lancet Respir Med 2014; 2: 455–463. [DOI] [PubMed] [Google Scholar]
- 17. Berkanovic E. The effect of inadequate language translation on Hispanics’ responses to healthsurveys. Am J Public Health 1980; 70: 1273–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hunt SM, Alonso J, Bucquet D, et al. Cross cultural adaptation of health measures. Health Policy 1991; 19: 33–34. [DOI] [PubMed] [Google Scholar]
- 19. Ferrer M, Alonso J, Prieto L, et al. Validity and reliability of the St George’s respiratory questionnaire after adaptation to a different language and culture: the Spanish example. Eur Respir J 1996; 9: 1160–1166. [DOI] [PubMed] [Google Scholar]
- 20. Aaronson N, Alonso J, Burnam A, et al. Assessing health status and quality-of-life instruments: attributes and review criteria. Qual Life Res 2002; 11: 193–205. [DOI] [PubMed] [Google Scholar]
- 21. Brooks SM. Surveillance for respiratory hazards. ATS News 1982; 8: 12–16. [Google Scholar]
- 22. Murray MP, Pentland JL, Turnbull K, et al. Sputum colour: a useful clinical tool in non-cystic fibrosis bronchiectasis. Eur Respir J 2009; 34: 361–364. [DOI] [PubMed] [Google Scholar]
- 23. Martínez MA, de Gracia J, Vendrell M, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis. The FACED score. Eur Respir J 2014; 43: 1357–1367. [DOI] [PubMed] [Google Scholar]
- 24. Chalmers J, Goeminne P, Aliberti S, et al. The bronchiectasis severity index: an international derivation and validation study. Am J Respir Crit Care Med 2014; 189: 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raj AA, Pavord DI, Birring SS. Clinical cough IV: what is the minimal important difference for the leicester cough questionnaire? Handb Exp Pharmacol 2009; 187: 311–320. [DOI] [PubMed] [Google Scholar]
- 26. Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J 2002; 19: 398–404. [DOI] [PubMed] [Google Scholar]
- 27. Bonett DG. Sample size requirements for testing and estimating coefficient alpha. J Educ Behav Stat 2002; 27: 335–340. [Google Scholar]
- 28. Kirshner B, Guyatt G. A methodological framework for assessing health indices. J Chronic Dis 1985; 38: 27–36. [DOI] [PubMed] [Google Scholar]
- 29. McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods 1996; 1: 30–46. [Google Scholar]
- 30. Bland J, Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–310. [PubMed] [Google Scholar]
- 31. Cohen J. Statistical power analysis for the behavioral sciences, 2nd ed New Jersey: Lawrence Erlbaum, 1988. [Google Scholar]
- 32. Hobart J, Kalkers N, Barkhof F, et al. Outcome measures for multiple sclerosis clinical trials: relative measurement precision of the expanded disability status scale and multiple sclerosis functional composite. Mult Scler 2004; 10: 41–46. [DOI] [PubMed] [Google Scholar]
- 33. Huisman AN, Wu MZ, Uil SM, et al. Reliability and validity of a dutch version of the leicester cough questionnaire. Cough 2007; 3: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Han JM, Jung IC, Kang W, et al. Reliability and validity of leicester cough questionnaire Korean version. Chron Respir Dis 2014; 1: 147–152. [DOI] [PubMed] [Google Scholar]
- 35. Ma W, Yu L, Wang Y, et al. Changes in health-related quality of life and clinical implications in Chinese patients with chronic cough. Cough 2009; 5: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Berkhof FF, Boom LN, ten Hertog NE, et al. The validity and precision of the Leicester Cough Questionnaire in COPD patients with chronic cough. Health Qual Life Outcomes 2012; 10: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gregory R, Mathilde S, Antoine F, et al. Validation of the French version of the Leicester Cough Questionnaire in chronic obstructive pulmonary disease. Chron Respir Dis. Epub ahead of print 7 September 2015. DOI: 10.1177/1479972315602618 [DOI] [PubMed] [Google Scholar]
- 38. Polley L, Yaman N, Heaney L, et al. Impact of cough across different chronic respiratory diseases: comparison of two cough specific health-related quality of life questionnaires. Chest 2008; 134: 295–302. [DOI] [PubMed] [Google Scholar]