Abstract
Nutritional status has been associated with clinical outcome in chronic airflow limitation (CAL), but epidemiological studies are scarce. We aimed to assess the relationship between body mass index (BMI) and CAL, taking into account confounding factors. 18,606 participants (49% male, 21% smokers, mean age: 55.8 ± 11.2 years, mean BMI: 26.7 ± 5.5 kg/m2) of the BOLD initiative from 26 sites in 23 countries were included. CAL was defined as post-bronchodilator forced expiratory volume in the first second/forced vital capacity < lower limit of normal. Low and obese BMI were defined as <21 kg/m2 and ≥30 kg/m2, respectively. Multivariate logistic regression analysis controlled for confounders age, sex and smoking, and meta-analysis of between-site heterogeneity and clustering. Prevalence of low and obese BMI, smoking history and prevalence of CAL were highly variable between sites. After adjustment for confounders, the meta-analysis of all sites showed that compared to subjects without CAL, low BMI was more frequent, (adjusted odds ratio (OR): 2.23 (95% confidence interval: 1.75, 2.85)) and conversely, obesity was less frequent in subjects with CAL (adjusted OR: 0.78 (0.65, 0.94)). In a worldwide population sample, CAL was associated with lower BMI, even after adjusting for confounding factors age, gender, smoking and between-site heterogeneity. These results indicate a CAL-specific association with body composition.
Keywords: Chronic airflow limitation, chronic obstructive pulmonary disease, body mass index, burden of obstructive lung disease (BOLD)
Introduction
Low body weight has been observed in 10–20% of outpatients with mild-to-moderate chronic obstructive pulmonary disease (COPD)1,2 and in 37% of patients with severe disease referred for pulmonary rehabilitation.3 Moreover, low body mass index (BMI) is related to increased mortality in these patients,4–7 independent of disease severity.6 Also, in the general population, increased mortality in subjects with low to normal BMI is mainly due to respiratory diseases.8 This inverse association is much stronger for smokers than for non-smokers.8 Nevertheless, the association between low body weight and chronic airflow limitation (CAL) remains incompletely understood as it has only been studied in selected patient populations.1,7 Earlier studies did not account for the impact of smoking,9 gender10 and age11 on body weight.
On the other hand, obesity is increasingly recognized as a risk factor for respiratory symptoms12 and functional limitation13 in COPD. Furthermore, while obesity may be associated with low mortality in patients with severe airflow obstruction, a higher mortality rate was seen in mild-to-moderate COPD patients with obesity.3,6 Epidemiologic data on obesity in subjects with CAL in general population samples are scarce and conflicting.14,15
The burden of obstructive lung disease (BOLD) initiative is an international population-based study implementing rigorous standardized methods for estimating CAL prevalence (high-quality postbronchodilator spirometry) and its risk factors. While BMI has already been described in the context of other possible risk factors for the presence of airflow limitation in BOLD,16,17 the principal objective of the present study was to study in detail the relation between low and obese BMI and the presence of CAL in a general worldwide population, taking into account the impact of gender, smoking status, age and site-to-site heterogeneity.
Methods
Study design and participants
The design and rationale for the BOLD initiative and prevalence data have been previously published.18,19 Participants were recruited with use of population-based sampling plans. The following sampling strategies were used: simple random sample, stratified random sample, and cluster sample. The sampling methodology of each site has been reviewed and approved by the operations center. Representative samples of the non-institutionalized population over the age of 39 years were selected for all study sites. As of February 2014, 26 sites (in 23 countries) had completed data collection and are included in this analysis: Adana (Turkey), Annaba (Algeria), Bergen (Norway), Cape Town (South Africa), Fes (Morocco), Guangzhou (China), Hannover (Germany), Ife (Nigeria), Krakow (Poland), Lexington (USA), Lisbon (Portugal), London (England), Maastricht (the Netherlands), Manila (Philippines), Mumbai (India), Nampicuan Talugtug (Philippines), Pune (India), Reykjavik (Iceland), Salzburg (Austria), Sousse (Tunisia), Srinagar (India), Sydney (Australia), Tartu (Estonia), Tirana (Albania), Uppsala (Sweden), and Vancouver (Canada).
Each participating site aimed to recruit a population-based sample of at least 600 adults (300 men and 300 women) living in a well-defined administrative area in which the total population exceeded 150,000. Approval was obtained from each local ethics committee and written informed consent was obtained from each participant. All participants included in this analysis performed post-bronchodilator spirometry.
Spirometry testing
Spirometry was performed according to the American Thoracic Society (ATS) criteria20 by trained and certified technicians using the nddEasyOne spirometer (ndd Medical Technologies; Zurich, Switzerland) with participants in a seated position. Separate measurements of forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) were made before and at least 15 min after two puffs of salbutamol (200 μg) administered with a metered dose inhaler with Volumatic spacer (GlaxoSmithKline; Uxbridge, England). Spirometry data were sent electronically to the Pulmonary Function Quality Control Center in Salt Lake City, Utah, or London, where each spirogram was reviewed and graded using ATS guidelines.20
Definitions
Chronic airflow limitation
The definition of CAL was based on the post-bronchodilator lung function and defined according to lower limit of normal (LLN), which is beneath the 5th percentile of the population distribution for the FEV1/FVC ratio. Non-obstructive lung function was defined as a post-bronchodilator FEV1/FVC ratio ≥ LLN. We used the term CAL to describe our results as well to refer to other population-based studies. We used the term COPD to refer to clinical studies, evaluating patients with previously diagnosed COPD.
Smoking status
A current or ex-smoker was defined as a person who had smoked >20 packs of cigarettes in a lifetime or >1 cigarette/day for a year. An ex-smoker was further defined by a self-report of having stopped smoking.
Body mass index
Body height was measured to the nearest 0.5 cm. Body weight was assessed to the nearest 0.1 kg after emptying the bladder and with the subjects standing barefoot and wearing light indoor clothing. BMI was calculated as body weight/height2 (kg/m2). A priori, BMI was categorized into low (<21 kg/m2)6, normal (21–24.9 kg/m2), high (25–29.9 kg/m2),21 and obese (≥30 kg/m2).21
Statistical analysis
Statistics were performed using SAS version 9.3. Results are mainly expressed as mean ± standard deviation for quantitative variables. Independent samples Student’s t-test and the Wilcoxon–Mann–Whitney test were used to investigate the differences in mean BMI between subjects with and without CAL. Kernel density estimation (KDE), a non-parametric way to estimate the probability density function of a random variable, was used to create the distribution figures. To test whether two or more distributions are different, the χ 2 test was used.
The meta-analyses of the calculated adjusted odds ratios (ORs) and 95% confidence intervals were calculated using the Cochrane meta-analysis software RevMan 5.1. The v2-based Cochran’s Q statistic and the I 2 metric were used to quantify between-study heterogeneity. A p < 0.10 for the Q statistic was considered significant. An I2 metric of >25% and >50% was considered indicating moderate and large heterogeneity, respectively. Since significant between-study heterogeneity was found, random effects meta-analysis was used to combine data across sites.
Results
General characteristics
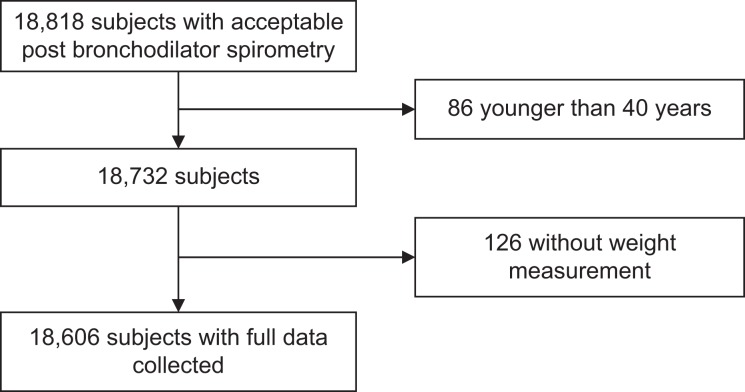
A total of 18,606 subjects ≥40 years had acceptable post-bronchodilator spirometry, weight and height measurement, and available information on smoking status (Figure 1).
Figure 1.
Flowchart of the study population.
In general, subjects were overweight with a mean age of 56 ± 11 years. CAL was present in 11% of subjects. Prevalence of CAL ranged from 6.2% in Pune, India, to 18.9% in Cape Town, South Africa. More than half of the subjects never smoked and almost a quarter were current smokers.
Characteristics for each site are shown in Table 1. Important differences in BMI were observed between sites. For example, in Uppsala, Sweden, low BMI was only seen in 3% of subjects while in Nampicuan Talugtug, the Philippines, this percentage was up to 47%. On the other hand, obesity was present in only 3% of Chinese subjects, but was present in 47% of participants from Lexington, USA.
Table 1.
General characteristics of study participants at each site and overall.
| Gender (%) | Age (years) | CAL (%) | BMI | Smoking history | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country (Site) | N | Male | Mean ± SD | Yes | No | Mean ± SD (kg/m2) | Low (%) | Normal (%) | High (%) | Obese (%) | Current (%) | Never (%) | Ex (%) |
| Albania (Tirana) | 928 | 49.9 | 55 ± 11 | 8.6 | 91.4 | 28.1 ± 4.7 | 4.4 | 22.3 | 43.3 | 30.0 | 21.8 | 62.9 | 15.3 |
| Algeria (Annaba) | 862 | 49.8 | 53 ± 10 | 7.0 | 93 | 28.3 ± 5.7 | 8.2 | 21.2 | 35.7 | 34.8 | 16.7 | 61.4 | 21.9 |
| Australia (Sidney) | 425 | 49.7 | 59 ± 12 | 10.1 | 89.9 | 28.0 ± 5.1 | 5.2 | 25.4 | 38.4 | 31.1 | 14.8 | 48.2 | 37.0 |
| Austria (Salzburg) | 1258 | 54.5 | 58 ± 11 | 15.8 | 84.2 | 26.4 ± 4.2 | 6.9 | 32.7 | 42.9 | 17.5 | 19.2 | 47.3 | 33.5 |
| Canada (Vancouver) | 825 | 41.6 | 56 ± 12 | 12.4 | 87.6 | 26.7 ± 5.2 | 8.9 | 34.2 | 35.4 | 21.6 | 13.7 | 47.9 | 38.4 |
| China (Ghuangzhou) | 472 | 50.0 | 54 ± 11 | 7.8 | 92.2 | 23.3 ± 3.3 | 25.2 | 46.2 | 25.2 | 3.4 | 29.9 | 56.1 | 14.0 |
| Estonia (Tartu) | 613 | 50.2 | 61 ± 12 | 7.0 | 93 | 28.5 ± 5.3 | 5.1 | 21.9 | 39.3 | 33.8 | 18.1 | 52.5 | 29.4 |
| Germany (Hannover) | 683 | 51.1 | 58 ± 11 | 8.9 | 91.1 | 27.3 ± 4.6 | 5.1 | 26.5 | 46.0 | 22.4 | 20.6 | 40.1 | 39.3 |
| Iceland (Reykjavik) | 756 | 53.2 | 56 ± 12 | 11.0 | 89 | 27.9 ± 4.9 | 4.2 | 24.2 | 43.5 | 28.0 | 18.4 | 39.0 | 42.6 |
| India (Mumbai) | 440 | 62.5 | 51 ± 9 | 6.8 | 93.2 | 23.8 ± 4.0 | 26.4 | 39.1 | 27.0 | 7.5 | 6.6 | 90.2 | 3.2 |
| India (Pune) | 844 | 59.5 | 52 ± 10 | 6.2 | 93.8 | 22.1 ± 3.8 | 42.3 | 36.6 | 18.3 | 2.8 | 9.0 | 88.0 | 3.0 |
| India (Srinagar) | 738 | 54.9 | 52 ± 10 | 16.5 | 83.5 | 22.4 ± 3.6 | 38.6 | 40.1 | 16.8 | 4.5 | 10.2 | 87.9 | 1.9 |
| Morocco (Fes) | 760 | 46.1 | 55 ± 10 | 9.6 | 90.4 | 27.9 ± 5.3 | 8.7 | 22.1 | 38.2 | 31.1 | 8.6 | 72.8 | 18.7 |
| Netherlands (Maastricht) | 588 | 50.9 | 57 ± 11 | 18.2 | 81.8 | 27.4 ± 4.5 | 5.4 | 23.8 | 47.6 | 23.1 | 23.0 | 34.7 | 42.3 |
| Nigeria (Ife) | 864 | 39.1 | 56 ± 12 | 6.9 | 93.1 | 25.4 ± 5.4 | 22.1 | 30.7 | 28.5 | 18.8 | 2.5 | 89.4 | 8.1 |
| Norway (Bergen) | 657 | 49.2 | 60 ± 13 | 12.5 | 87.5 | 26.5 ± 4.3 | 7.2 | 31.1 | 43.5 | 18.3 | 26.2 | 37.3 | 36.5 |
| Philippines (Manilla) | 885 | 42.6 | 52 ± 10 | 8.5 | 91.5 | 24.9 ± 3.9 | 19.3 | 34.9 | 32.9 | 12.9 | 32.5 | 47.6 | 19.9 |
| Philippines (Nampicuan–Talugtug) | 719 | 49.4 | 54 ± 11 | 14.2 | 85.8 | 21.6 ± 3.9 | 46.6 | 37.8 | 14.2 | 1.4 | 36.0 | 47.6 | 16.4 |
| Poland (Krakow) | 520 | 50.8 | 56 ± 12 | 13.9 | 86.1 | 27.8 ± 4.7 | 5.6 | 23.7 | 42.5 | 28.3 | 29.4 | 38.7 | 31.9 |
| Portugal (Lisbon) | 710 | 46.6 | 63 ± 11 | 11.6 | 88.4 | 28.2 ± 4.6 | 3.4 | 22.3 | 43.8 | 30.6 | 13.2 | 60.0 | 26.8 |
| South Africa (Cape Town) | 843 | 36.9 | 54 ± 11 | 18.9 | 81.1 | 27.9 ± 7.5 | 19.0 | 18.0 | 27.2 | 35.8 | 46.5 | 32.4 | 21.1 |
| Sweden (Uppsala) | 546 | 51.8 | 58 ± 11 | 9.3 | 90.7 | 27.0 ± 4.4 | 2.9 | 33.0 | 42.5 | 21.6 | 14.3 | 42.7 | 43.0 |
| Tunisia (Sousse) | 660 | 46.8 | 53 ± 9 | 5.0 | 95 | 29.3 ± 5.6 | 5.5 | 17.6 | 35.8 | 41.2 | 26.7 | 60.2 | 13.2 |
| Turkey (Adana) | 806 | 48.2 | 54 ± 10 | 14.3 | 85.7 | 29.6 ± 5.3 | 3.4 | 16.1 | 35.6 | 44.9 | 34.9 | 45.3 | 19.8 |
| United Kingdom (London) | 677 | 47.7 | 58 ± 12 | 16.0 | 84 | 27.1 ± 5.0 | 8.4 | 29.7 | 38.3 | 23.6 | 21.0 | 38.1 | 40.9 |
| USA (Lexington) | 507 | 40.4 | 57 ± 12 | 15.2 | 84.8 | 30.8 ± 6.8 | 3.8 | 14.2 | 35.1 | 46.9 | 26.4 | 39.6 | 33.9 |
| Total | 18,606 | 48.8 | 56 ± 11 | 11.3 | 88.7 | 26.7 ± 5.5 | 13.3 | 27.9 | 35.30 | 23.6 | 20.8 | 54.5 | 24.7 |
CAL: chronic airflow limitation; BMI: body mass index.
Distribution of BMI in the pooled data set
Subjects with CAL had lower mean BMI compared to subjects without CAL (mean BMI, kg/m2: CAL: 25.4 ± 5.5; non-CAL: 26.8 ± 5.4; p < 0.0001) (Figure 2). Likewise, in subjects with CAL compared to subjects without CAL, a higher proportion with a low BMI (21.8% vs. 12.3%; p < 0.001) and lower proportion with an obese BMI (17.6% vs. 24.3%; p < 0.001) was found. Even when gender-, age-, and smoking-specific strata were investigated, the presence of CAL was consistently associated with a shift of the distribution towards lower BMI, when compared to subjects without CAL (Supplemental Figures S1–S3).
Figure 2.
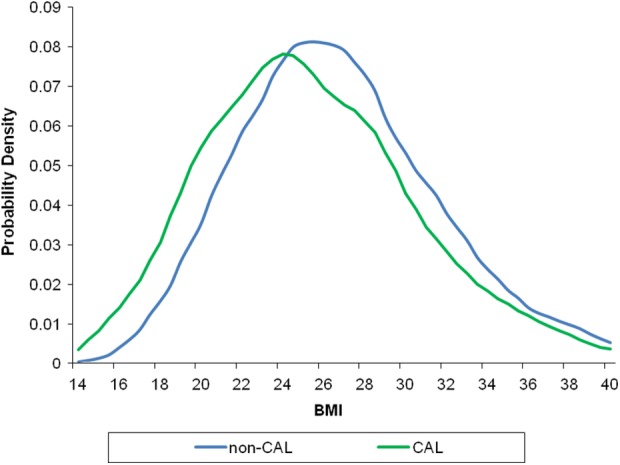
Distribution of BMI in subjects with and without CAL. Legend: mean BMI (kg/m2): CAL: 25.4 ± 5.5 versus non-CAL: 26.8 ± 5.4, p < 0.001; difference in KDE: p < 0.001. BMI: body mass index; KDE: Kernel density estimation; CAL: chronic airflow limitation.
Effects of smoking in the pooled data set
BMI was significantly different between current, former and never smokers (25.5 ± 5.2; 26.9 ± 5.2, and 26.8 ± 5.6 kg/m2; p < 0.001, respectively) and was lowest in current smokers. The prevalence of low BMI in current smokers with CAL (28.8%) was significantly increased compared to current smokers without CAL (16.7%; p < 0.001) as also compared to never smokers with CAL (21.1%; p < 0.001). Never smokers without CAL had the lowest proportion of subjects with a low BMI (13.1%; Figure 3).
Figure 3.
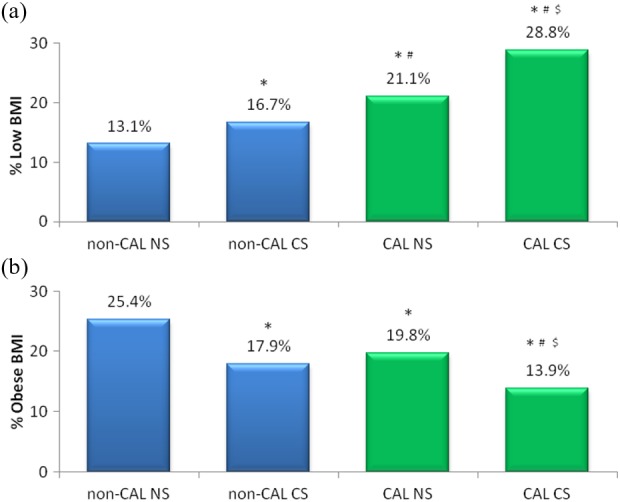
Prevalence of low BMI (a) and obese BMI (b) in patients with and without CAL according to smoking status. *p < 0.01: compared to non-CAL NS; # p < 0.01: compared to non-CAL CS; $ p < 0.01: compared to CAL NS; CS: current smoker; NS: never smoker; BMI: body mass index; CAL: chronic airflow limitation.
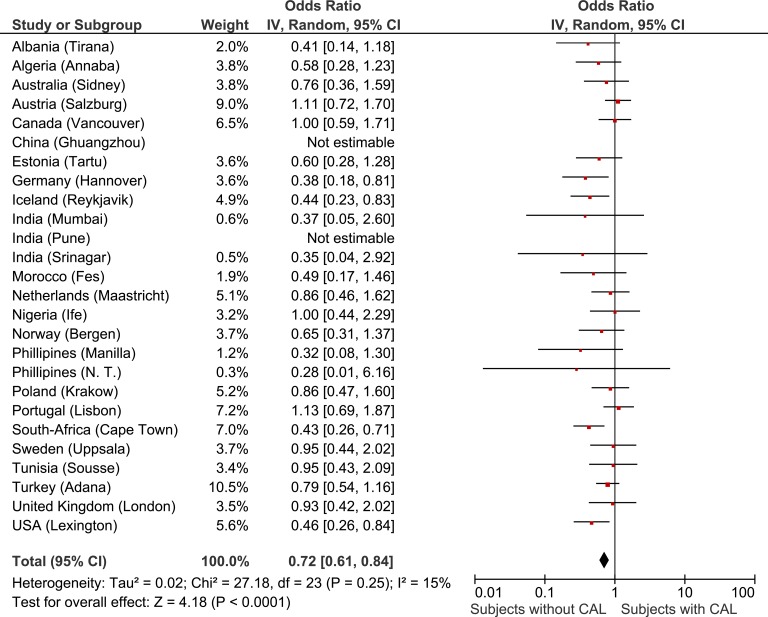
Both in subjects with CAL (19.8% vs. 13.9%; p < 0.001) and in subjects without CAL (25.4% vs.17.9%, p < 0.001), obesity was more frequent in never smokers compared to current smokers. The lowest prevalence of obesity was in smokers with CAL, which was significantly lower compared to smokers without CAL (respectively 13.9% vs. 17.9%; p = 0.009). (Figure 5).
Figure 5.
Forest plot showing the meta-analysis of adjusted ORs for age, gender and smoking, for obese BMI in subjects with CAL compared to subjects without CAL. OR was not estimable in China (Ghuangzhou) and India (Pune) because no subjects with CAL had obese BMI. N.T.: Nampicuan–Talugtug; BMI: body mass index; CAL: chronic airflow limitation; OR: odds ratio.
A meta-analytical approach
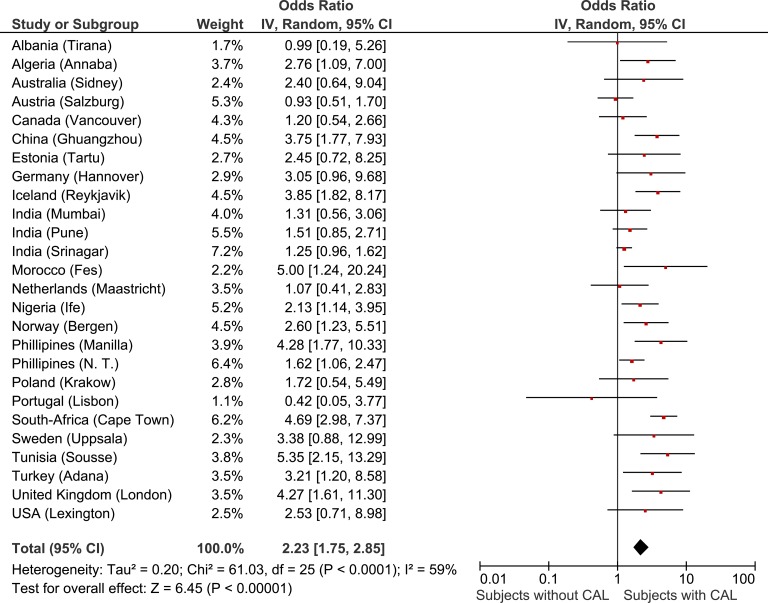
The present study consists of different surveys in multiple countries. In order to adjust for confounding by site and also to take into account the clustering of data within sites we performed a meta-analysis of the site-specific ORs relating BMI with CAL, adjusting for age, gender, and smoking history using multivariate logistic regression. The weighted meta-analyses of adjusted ORs for low BMI and obese BMI in subjects with CAL compared to subjects without CAL are shown in Figures 4 and 5, respectively. For low body weight, a large heterogeneity between sites was found (I 2: 59%). But even when taking into account the site-to-site heterogeneity of our data and adjusting for age, gender, and smoking status, the OR for low BMI was increased in subjects with CAL, compared to subjects without CAL. Secondly, overall a lower adjusted OR was found for the presence of obesity in CAL subjects. Here, the association seems consistent across sites (I2: 15%).
Figure 4.
Forest plot showing the meta-analysis of age, gender and smoking adjusted site-specific ORs for low BMI in subjects with CAL compared to subjects without CAL. BMI: body mass index; CAL: chronic airflow limitation; OR: odds ratio.
Differences in mean BMI between subjects with and without CAL for each site are shown in supplemental Table S1.
Discussion
This study is the first to evaluate BMI in subjects with and without CAL as defined by rigorously standardized post-bronchodilator spirometry in a worldwide population-based sample of adults, taking into account the possible impact of age, gender, smoking status, and geography. The results of our study indicate a positive association between the presence of CAL and low BMI and a negative association between CAL and obesity. Given the cross sectional nature of our data, the direction of this relationship remains unclear.
Evaluating data from the BOLD cohort, Lamprecht et al.16 showed that low BMI was a risk factor for the presence of CAL in never smokers. Hooper et al.17 showed that subjects with obese BMI, compared to those with normal body weight, had lower likelihood to have CAL. The current manuscript extends this knowledge by studying the association between both low and obese BMI and the presence of airflow limitation evaluating the impact of gender, smoking status, age, and site-to-site heterogeneity.
Only two other population-based studies have reported on BMI in relation to CAL and compared results to control subjects. In elderly subjects from the general population participating in the “health ABC” study, no difference in mean BMI was observed between those with pre-bronchodilator-defined airflow limitation and smokers without airflow limitation.11 Secondly, the South-American population-based PLATINO study reported a 7% prevalence of spirometrically defined CAL in subjects with BMI <20 kg/m2, which was significantly higher compared to non-CAL subjects, but no adjustments or stratification for smoking or other confounding factors were done.15 Hence, although low body weight is commonly observed in COPD or CAL, the relationship between airflow limitation and BMI per se has not been well studied and the impact of confounding factors has remained unclear.
The presence of a low body weight in patients with COPD is commonly recognized. A great variation of the reported prevalence of low BMI in COPD was seen in previous studies, depending on the setting and population studied. In an outpatient population of moderately severe COPD patients, low BMI was seen in 10% of males and 18% of females.1 Similarly, 15% of patients at first diagnosis of COPD had a low BMI.2 In the National Institutes of Health-Intermittent Positive Breathing trial, 25% of 779 male patients with stable but severe COPD had a body weight <90% of their ideal body weight and body weight was positively associated with FEV1.4 For 255 COPD patients entering an inpatient pulmonary rehabilitation program, Schols et al. showed that the prevalence of underweight was 37% and increased in parallel with the severity of airflow obstruction and the presence of hypoxia.3 These studies showed that underweight is occurring frequently in subjects with COPD and that it is related to the severity of the disease. However, these studies were uncontrolled for the effects of age, gender, and smoking, and data were not compared to subjects without airflow limitation. More recently, in a sub-analysis on body composition of the ECLIPSE study, the prevalence of a low BMI was 14% in COPD, compared to 7% in controls.22 However, the control group was significantly younger, had smoked fewer pack-years, and consisted of significantly more current smokers compared to the COPD subjects (61% vs. 36%, p < 0.001).
The present study identified subjects with and without CAL using guideline-based post-bronchodilator spirometry. In addition, the standardized assessment of smoking status by interviewed questionnaires allowed for stratification. Smoking is not only an important risk factor in the development of COPD but also may adversely affect body composition.9 Nicotine has appetite suppressant effects in the brain resulting in lower nutrient intake in smokers.23 Also, one of the acute systemic effects of smoking is an increase in resting energy metabolism.24 It is well known that quitting is frequently followed by a rapid weight gain.25,26 Also in the present study, smokers had a lower BMI compared to ex- and never smokers. However, the present study clearly demonstrated that smoking does only partly account for the high prevalence of low BMI in subjects with CAL.
CAL is importantly associated with older age as years of exposure to tobacco smoke precede the development of CAL.27 Older people tend to weigh less than younger adults and old age is also associated with a tendency to lose weight.11,28 However, the present study showed that even after stratification for age, subjects with CAL had a higher prevalence of low BMI compared to subjects with normal lung function. Neither gender nor site-related BMI differences influenced these results. Hence, we conclude that the relationship between the presence of CAL and the presence of low BMI is independent of age, gender, and smoking.
Having excluded smoking and age as possible reasons for subjects with CAL being more frequently underweight, two possibilities remain: either (at least some) subjects with CAL lose weight or thin subjects are at higher risk of developing CAL.
Weight loss occurs when energy expenditure exceeds energy intake and this balance can be disturbed on both sides in patients with CAL. A decreased appetite and low dietary intake have been associated with aggravation of disease symptoms related to exacerbations of COPD.29 On the other hand, an elevated resting energy expenditure has been reported in patients with COPD and has been associated with weight loss.30 Also, increased energy expenditure related to physical activities has been reported in COPD. This is likely due to a decreased oxidative capacity of the peripheral muscle, as a consequence of a shift from type 1 to type 2 fibres, which likely results in decreased mechanical efficiency when performing activity.31 Also, the increased oxygen cost of breathing might contribute to the elevated energy expenditure during activities in subjects with COPD.32 A low BMI in COPD is commonly associated with low fat-free mass.3 A loss of muscle bulk is a common complication in patients with COPD, among other things related to physical inactivity.
In the present cross-sectional study, data on weight loss, energy balance, exacerbations, and physical inactivity were not collected. Therefore, it is not possible to attribute the increased prevalence of underweight in subjects with CAL to any of these factors. However, since the present study sample is population-based and not a selected patient population, the frequency of ‘disease exacerbations’ is low.
It has been suggested that subjects with a thin phenotype are at higher risk of developing CAL. A large general population study showed that increased mortality in people with low BMI was mainly associated with respiratory diseases.8 Although this observed association might be attributable to reverse causation.33 More specifically, the Baltimore Longitudinal Study of Aging showed that, at least in men, a low BMI was an independent risk factor for developing CAL.34 Also in a previous report of the BOLD study, low BMI was associated with the presence of CAL in never smokers.16 In addition, studies have suggested that malnutrition and starvation may contribute to the development of emphysema. Autopsies undertaken on starved patients during World War II revealed signs of emphysema in relatively young individuals.35 In addition, early levels of emphysema have been detected in patients with anorexia nervosa.36 Due to the cross-sectional nature of the present data we cannot determine whether the low BMI preceded the CAL or was a consequence of the disease.
In the present study, on average, only one in five subjects with CAL had low body weight. Together with the worldwide obesity pandemic, also obesity in COPD is increasingly recognized and importantly contributes to the disease expression of patients with COPD.37 Referring to the historical description of the pink puffer (emphysematous type with underweight) and the blue bloater (chronic bronchitis type with overweight) body weight might discriminate different phenotypes within the umbrella disease COPD.38 More recently, low and obese body weight also showed to differentiate different clusters of comorbidities in COPD.39
However, data on the prevalence of obesity in COPD or CAL compared to subjects without COPD or CAL are scarce and have been conflicting, probably as a result of differences in CAL-specific and global risk factors for obesity between studied populations. In the population-based Canadian National Health Survey, the prevalence of obesity was increased in subjects with self-reported COPD (24.6%) compared to non-COPD subjects (17.1%),40 while the South-American population-based PLATINO study reported a lower prevalence of obesity in subjects with CAL, compared to subjects without CAL (23% vs. 30%).15 In the patient cohort ECLIPSE, obesity prevalence was not different in COPD subjects compared to smoking/former smoking controls (respectively 22% vs. 20%).22 The present study showed that the prevalence of obesity is lower in subjects with CAL compared to subjects without CAL, also when adjusted for gender, smoking status, and age. The present study showed that the influence of smoking history is greater than the influence of CAL on the presence of obesity.
Next to the overall mean effect in this study, the international comparisons in baseline characteristics and forest plots are intriguing. There is clearly quite a lot of variation between countries. For example, in Asian countries, overweight and obesity were only present in a minority. In China and India, no OR could be calculated for obesity because no obese subjects were identified. At the same time, almost half of the subjects in the US site was obese. Also regarding smoking prevalence, important differences were noted. For example, only 2.5% were current smokers in Nigeria, and almost half of subjects were current smokers in South Africa. Given the differences in the forest plots, it is also reasonable to anticipate that cultural and socio-economic effects in smoking and non-smoking populations are important.
In conclusion, this study was the first to demonstrate that in a worldwide general population, the presence of objectively assessed CAL was associated with increased risk of low BMI and a reduced risk of obese BMI, independent of age, gender, smoking history and geography.
These findings suggest a direct association between CAL and low BMI, although the direction of this association cannot be established from the present cross-sectional data. Both low BMI as a risk factor for development and progression of CAL as well as (mechanisms of) weight loss in CAL need exploration in future studies. In the meantime, clinicians and dieticians should stay aware of what is already known, in fact that low BMI is related to poor outcome in subjects with COPD or CAL and should be appropriately managed.
Acknowledgements
A Sonia Buist, William Vollmer, Suzanne Gillespie, MaryAnn McBurnie (BOLD Co-ordinating Centre, Kaiser Permanente, Portland, USA), Peter GJ Burney, Louisa Gnatiuc, Bernet Kato, Sonia Coton, Hadia Azhar, Jaymini Patel, Anamika Jithoo, Risha Dudhaiya (BOLD Co-ordinating Centre, Imperial College London, UK), NanShanZhong (principal investigator (PI)), Shengming Liu, Jiachun Lu, Pixin Ran, Dali Wang, Jingping Zheng, Yumin Zhou (Guangzhou Institute of Respiratory Diseases, Guangzhou Medical College, Guangzhou, China); Ali Kocabaş (PI), Attila Hancioglu, Ismail Hanta, SedatKuleci, Ahmet Sinan Turkyilmaz, SemaUmut, TurgayUnalan (Cukurova University School of Medicine, Department of Chest Diseases, Adana, Turkey); Michael Studnicka (PI), Torkil Dawes, Bernd Lamprecht, Lea Schirhofer (Paracelsus Medical University, Department of Pulmonary Medicine, Salzburg Austria); Eric Bateman (PI), Anamika Jithoo (PI), Desiree Adams, Edward Barnes, Jasper Freeman, Anton Hayes, SiphoHlengwa, Christine Johannisen, Mariana Koopman, Innocentia Louw, Ina Ludick, Alta Olckers, Johanna Ryck, Janita Storbeck, (University of Cape Town Lung Institute, Cape Town, South Africa); Thorarinn Gislason (PI), Bryndis Benedikdtsdottir, Kristin Jörundsdottir, Lovisa Gudmundsdottir, Sigrun Gudmundsdottir, Gunnar Gundmundsson, (Landspitali University Hospital, Department of Allergy, Respiratory Medicine and Sleep, Reykjavik, Iceland); EwaNizankowska-Mogilnicka (PI), Jakub Frey, RafalHarat, Filip Mejza, Pawel Nastalek, Andrzej Pajak, WojciechSkucha, Andrzej Szczeklik, Magda Twardowska, (Division of Pulmonary Diseases, Department of Medicine, Jagiellonian University School of Medicine, Cracow, Poland); Tobias Welte (PI), Isabelle Bodemann, Henning Geldmacher, Alexandra Schweda-Linow (Hannover Medical School, Hannover, Germany); Amund Gulsvik (PI), Tina Endresen, Lene Svendsen (Department of Thoracic Medicine, Institute of Medicine, University of Bergen, Bergen, Norway); Wan C Tan (PI), Wen Wang (iCapture Center for Cardiovascular and Pulmonary Research, University of British Columbia, Vancouver, BC, Canada); David M Mannino (PI), John Cain, Rebecca Copeland, Dana Hazen, Jennifer Methvin, (University of Kentucky, Lexington, Kentucky, USA); Renato B Dantes (PI), Lourdes Amarillo, Lakan U Berratio, Lenora C Fernandez, Norberto A Francisco, Gerard S Garcia, Teresita S de Guia, Luisito F Idolor, Sullian S Naval, Thessa Reyes, Camilo C Roa, Jr, Ma. Flordeliza Sanchez, Leander P Simpao (Philippine College of Chest Physicians, Manila, Philippines); Christine Jenkins (PI), Guy Marks (PI), Tessa Bird, Paola Espinel, Kate Hardaker, Brett Toelle (Woolcock Institute of Medical Research, Sydney, Australia), Peter GJ Burney (PI), Caron Amor, James Potts, Michael Tumilty, Fiona McLean (National Heart and Lung Institute, Imperial College, London), EFM Wouters, GJ Wesseling (Maastricht University Medical Center, Maastricht, the Netherlands), Cristina Bárbara (PI), Fátima Rodrigues, Hermínia Dias, João Cardoso, João Almeida, Maria João Matos, Paula Simão, Moutinho Santos, Reis Ferreira (The Portuguese Society of Pneumology, Lisbon, Portugal), Christer Janson (PI), Inga Sif Olafsdottir, Katarina Nisser, Ulrike Spetz-Nyström, GunillaHägg and Gun-Marie Lund (Department of Medical Sciences: Respiratory Medicine &Allergology, Uppsala University, Sweden), Rain Jõgi (PI), Hendrik Laja, KatrinUlst, VappuZobel, Toomas-Julius Lill (Lung Clinic, Tartu University Hospital), Parvaiz A Koul (PI), Sajjad Malik, Nissar A Hakim, Umar Hafiz Khan (Sher-i-Kashmir Institute of Medical Sciences, Srinagar, Jammu and Kashmir, India); Rohini Chowgule (PI) Vasant Shetye, Jonelle Raphael, RoselAlmeda, Mahesh Tawde, Rafiq Tadvi, Sunil Katkar, Milind Kadam, Rupesh Dhanawade, Umesh Ghurup (Indian Institute of Environmental Medicine, Mumbai, India); Imed Harrabi (PI), Myriam Denguezli, ZouhairTabka, Hager Daldoul, Zaki Boukheroufa, Firas Chouikha, Wahbi Belhaj Khalifa (Faculté de Médecine, Sousse, Tunisia); Luisito F Idolor (PI), Teresita S de Guia, Norberto A Francisco, Camilo C. Roa, Fernando G. Ayuyao, Cecil Z.Tady, Daniel T. Tan, Sylvia Banal-Yang, Vincent M Balanag, Jr, Maria Teresita N Reyes, Renato B Dantes (Lung Centre of the Philippines, Philippine General Hospital, Nampicuan and Talugtug, Philippines); Sundeep Salvi (PI), Sundeep Salvi (PI), Siddhi Hirve, Bill Brashier, Jyoti Londhe, Sapna Madas, Somnath Sambhudas, Bharat Chaidhary, Meera Tambe, Savita Pingale, Arati Umap, Archana Umap, Nitin Shelar, Sampada Devchakke, Sharda Chaudhary, Suvarna Bondre, Savita Walke, Ashleshsa Gawhane, Anil Sapkal, Rupali Argade, Vijay Gaikwad (Vadu HDSS, KEM Hospital Research Centre Pune, Chest Research Foundation (CRF), Pune, India); Mohamed C Benjelloun (PI), ChakibNejjari, Mohamed Elbiaze, Karima El Rhazi (Laboratoired’épidémiologie, Recherche Clinique et Santé Communautaire, Fès, Morroco); Daniel Obaseki (PI), Gregory Erhabor, Olayemi Awopeju, Olufemi Adewole (Obafemi Awolowo University, Ile-Ife, Nigeria), Hasan Hafizi (PI), Anila Aliko, Donika Bardhi, Holta Tafa, Natasha Thanasi, Arian Mezini, Alma Teferici, Dafina Todri, Jolanda Nikolla, RezartaKazasi (Tirana University Hospital “ShefqetNdroqi”, Albania), Hamid Hacene Cherkaski (PI), Amira Bengrait, Tabarek Haddad, Ibtissem Zgaoula, Maamar Ghit, Abdelhamid Roubhia, Soumaya Boudra, Feryal Atoui, Randa Yakoubi, Rachid Benali, Abdelghani Bencheikh, Nadia Ait-Khaled (Faculté de Médecine Annaba, SEMEP Elhadjar, Algérie)
In the first phase of the study Dr R O Crapo, Dr R L Jensen (LDS Hospital, Pulmonary Division, Salt Lake City, Utah, USA) and Dr A Jithoo (Imperial College London, UK) were responsible for quality assurance of lung function; they and Dr Paul Enright (The University of Arizona, Tucson, AZ, USA) and Georg Harnoncourt (nddMedizintechnik AG, Zurich, Switzerland) assisted with training lung function technicians. Dr R Hooper (Imperial College London, UK) was responsible for design of data systems at the Co-ordinating center in London.
Footnotes
Authors’ Note: BoehringerIngelheim China (GuangZhou, China); Turkish Thoracic Society, Boehringer-Ingelheim, and Pfizer (Adana, Turkey); Altana, Astra-Zeneca, Boehringer-Ingelheim, GlaxoSmithKline, Merck Sharpe & Dohme, Novartis, SalzburgerGebietskrankenkasse and Salzburg Local Government (Salzburg, Austria); Research for International Tobacco Control, the International Development Research Centre, the South African Medical Research Council, the South African Thoracic Society GlaxoSmithKline Pulmonary Research Fellowship, and the University of Cape Town Lung Institute (Cape Town, South Africa); and Landspítali-University Hospital-Scientific Fund, GlaxoSmithKline Iceland and AstraZeneca Iceland (Reykjavik, Iceland); GlaxoSmithKline Pharmaceuticals, Polpharma, Ivax Pharma Poland, AstraZeneca Pharma Poland, ZF Altana Pharma, PlivaKraków, Adamed, Novartis Poland, Linde GazPolska, LekPolska, TarchomińskieZakładyFarmaceutycznePolfa, StarostwoProszowice, Skanska, Zasada, AgencjaMieniaWojskowego w Krakowie, TelekomunikacjaPolska, Biernacki, Biogran, AmplusBucki, Skrzydlewski, Sotwin, and Agroplon (Krakow, Poland); Boehringer-Ingelheim, and Pfizer Germany (Hannover, Germany); the Norwegian Ministry of Health’s Foundation for Clinical Research, and Haukeland University Hospital’s Medical Research Foundation for Thoracic Medicine (Bergen, Norway); AstraZeneca, Boehringer-Ingelheim, Pfizer, and GlaxoSmithKline (Vancouver, Canada); Marty Driesler Cancer Project (Lexington, Kentucky, USA); Altana, BoehringerIngelheim (Phil), GlaxoSmithKline, Pfizer, Philippine College of Chest Physicians, Philippine College of Physicians, and United Laboratories (Phil) (Manila, Philippines); Air Liquide Healthcare P/L, AstraZeneca P/L, BoehringerIngelheim P/L, GlaxoSmithKline Australia P/L, Pfizer Australia P/L (Sydney, Australia), Department of Health Policy Research Programme, Clement Clarke International (London, United Kingdom); BoehringerIngelheim and Pfizer (Lisbon, Portugal), Swedish Heart and Lung Foundation, The Swedish Association against Heart and Lung Diseases, Glaxo Smith Kline (Uppsala, Sweden); GlaxoSmithKline, Astra Zeneca, EestiTeadusfond (Estonian Science Foundation) (Tartu, Estonia); AstraZeneca, CIRO HORN (Maastricht, The Netherlands); Sher-i-Kashmir Institute of Medical Sciences, Srinagar, J&K (Srinagar, India); Foundation for Environmental Medicine, Kasturba Hospital, Volkart Foundation (Mumbai, India); BoehringerIngelheim (Sousse, Tunisia); BoehringerIngelheim (Fes, Morocco); Philippines College of Physicians, Philippines College of Chest Physicians, AstraZeneca, BoehringerIngelheim, GlaxoSmithKline, Orient Euro Pharma, Otsuka Pharma, United laboratories Phillipines (Nampicuan&Talugtug, Philippines); National Heart and Lung Institute, Imperial College, London (Pune, India); The Wellcome Trust, National Population Commission, Ile-Ife, Osun State, Nigeria (Ile-Ife, Nigeria), GlaxoSmithKline (Tirana, Albania), BoehringerIngelheim (Annaba, Algérie)
Some of the results of this study have been previously reported in the form of an abstract at the European Respiratory Society 2013 annual conference on 10 September in Barcelona. LEGWV, EFMW and FMEF were involved in conception and design. LEGWV, BL, MS, PB, LG, EFMW and FMEF were involved in drafting of the manuscript. LEGWV, BK, BL and MS performed acquisition and analysis of data. LEGWV, BL, MS, BK, PB and FMEF performed analysis and interpretation of data. All authors were involved in drafting the manuscript for important intellectual content. All co-authors critically revised the article and gave final approval of this version to be published. The sponsors of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The BOLD Study is currently funded by a grant from the Wellcome Trust (085790/Z/08/Z). The initial BOLD programme was funded in part by unrestricted educational grants to the Operations Center in Portland, Oregon, from ALTANA, Aventis, AstraZeneca, Boehringer-Ingelheim, Chiesi, GlaxoSmithKline, Merck, Novartis, Pfizer, Schering-Plough, Sepracor, and the University of Kentucky.
Supplemental material: The online [appendices/data supplements/etc] are available at http://crd.sagepub.com/supplemental
References
- 1. Vermeeren MA, Creutzberg EC, Schols AM, et al. Prevalence of nutritional depletion in a large out-patient population of patients with COPD. Respir Med 2006; 100: 1349–1355. [DOI] [PubMed] [Google Scholar]
- 2. Engelen MP, Schols AM, Baken WC, et al. Nutritional depletion in relation to respiratory and peripheral skeletal muscle function in out-patients with COPD. Eur Respir J 1994; 7: 1793–1797. [DOI] [PubMed] [Google Scholar]
- 3. Schols AM, Soeters PB, Dingemans AM, et al. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis 1993; 147: 1151–1156. [DOI] [PubMed] [Google Scholar]
- 4. Wilson DO, Rogers RM, Wright EC, et al. Body weight in chronic obstructive pulmonary disease. The National Institutes of Health Intermittent Positive-Pressure Breathing Trial. Am Rev Respir Dis 1989; 139: 1435–1438. [DOI] [PubMed] [Google Scholar]
- 5. Schols AM, Slangen J, Volovics L, et al. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157: 1791–1797. [DOI] [PubMed] [Google Scholar]
- 6. Landbo C, Prescott E, Lange P, et al. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 160: 1856–1861. [DOI] [PubMed] [Google Scholar]
- 7. Chailleux E, Laaban JP, Veale D. Prognostic value of nutritional depletion in patients with COPD treated by long-term oxygen therapy: data from the ANTADIR observatory. Chest 2003; 123: 1460–1466. [DOI] [PubMed] [Google Scholar]
- 8. Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009; 373: 1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wack JT, Rodin J. Smoking and its effects on body weight and the systems of caloric regulation. Am J Clin Nutr 1982; 35: 366–380. [DOI] [PubMed] [Google Scholar]
- 10. Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011; 377: 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van den Borst B, Koster A, Yu B, et al. Is age-related decline in lean mass and physical function accelerated by obstructive lung disease or smoking? Thorax 2011; 66: 961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cecere LM, Littman AJ, Slatore CG, et al. Obesity and COPD: associated symptoms, health-related quality of life, and medication use. COPD 2011; 8: 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramachandran K, McCusker C, Connors M, et al. The influence of obesity on pulmonary rehabilitation outcomes in patients with COPD. Chron Respir Dis 2008; 5: 205–209. [DOI] [PubMed] [Google Scholar]
- 14. Vozoris NT, O’Donnell DE. Prevalence, risk factors, activity limitation and health care utilization of an obese, population-based sample with chronic obstructive pulmonary disease. Can Respir J 2012; 19: e18–e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Montes de Oca M, Talamo C, Perez-Padilla R, et al. Chronic obstructive pulmonary disease and body mass index in five Latin America cities: the PLATINO study. Respir Med 2008; 102: 642–650. [DOI] [PubMed] [Google Scholar]
- 16. Lamprecht B, McBurnie MA, Vollmer WM, et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest 2011; 139: 752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hooper R, Burney P, Vollmer WM, et al. Risk factors for COPD spirometrically defined from the lower limit of normal in the BOLD project. Eur Respir J 2012; 39: 1343–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buist AS, Vollmer WM, Sullivan SD, et al. The Burden of Obstructive Lung Disease Initiative (BOLD): rationale and design. COPD 2005; 2: 277–283. [PubMed] [Google Scholar]
- 19. Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007; 370: 741–750. [DOI] [PubMed] [Google Scholar]
- 20. Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 1995; 152: 1107–1136. [DOI] [PubMed] [Google Scholar]
- 21. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363: 157–163. [DOI] [PubMed] [Google Scholar]
- 22. Rutten EP, Calverley PM, Casaburi R, et al. Changes in body composition in patients with chronic obstructive pulmonary disease: do they influence patient-related outcomes? Ann Nutr Metab 2013; 63: 239–247. [DOI] [PubMed] [Google Scholar]
- 23. Mineur YS, Abizaid A, Rao Y, et al. Nicotine decreases food intake through activation of POMC neurons. Science 2011; 332: 1330–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perkins KA, Epstein LH, Stiller RL, et al. Acute effects of nicotine on resting metabolic rate in cigarette smokers. Am J Clin Nutr 1989; 50: 545–550. [DOI] [PubMed] [Google Scholar]
- 25. Aubin HJ, Farley A, Lycett D, et al. Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ 2012; 345: e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chinn S, Jarvis D, Melotti R, et al. Smoking cessation, lung function, and weight gain: a follow-up study. Lancet 2005; 365: 1629–1635; discussion 1600-1621. [DOI] [PubMed] [Google Scholar]
- 27. Vanfleteren LE, Franssen FM, Wesseling G, et al. The prevalence of chronic obstructive pulmonary disease in Maastricht, the Netherlands. Respir Med 2012; 106: 871–874. [DOI] [PubMed] [Google Scholar]
- 28. Soenen S, Chapman IM. Body weight, anorexia, and undernutrition in older people. J Am Med Dir Assoc 2013; 14(9): 642–648. [DOI] [PubMed] [Google Scholar]
- 29. Vermeeren MA, Schols AM, Wouters EF. Effects of an acute exacerbation on nutritional and metabolic profile of patients with COPD. Eur Respir J 1997; 10: 2264–2269. [DOI] [PubMed] [Google Scholar]
- 30. Schols AM, Fredrix EW, Soeters PB, et al. Resting energy expenditure in patients with chronic obstructive pulmonary disease. Am J Clin Nutr 1991; 54: 983–987. [DOI] [PubMed] [Google Scholar]
- 31. Franssen FM, Wouters EF, Baarends EM, et al. Arm mechanical efficiency and arm exercise capacity are relatively preserved in chronic obstructive pulmonary disease. Med Sci Sports Exerc 2002; 34: 1570–1576. [DOI] [PubMed] [Google Scholar]
- 32. Levison H, Cherniack RM. Ventilatory cost of exercise in chronic obstructive pulmonary disease. J Appl Physiol 1968; 25: 21–27. [DOI] [PubMed] [Google Scholar]
- 33. Kivimaki M, Shipley MJ, Bell JA, et al. Underweight as a risk factor for respiratory death in the Whitehall cohort study: exploring reverse causality using a 45-year follow-up. Thorax 2016; 71: 84–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harik-Khan RI, Fleg JL, Wise RA. Body mass index and the risk of COPD. Chest 2002; 121: 370–376. [DOI] [PubMed] [Google Scholar]
- 35. Fliederbaum J. Clinical aspects of hunger disease in adults In: Winick M. (ed) Hunger disease: studies by the Jewish physicians in the Warsaw Ghetto. New York: John Wiley & Sons, 1979, pp. 11–36. [Google Scholar]
- 36. Coxson HO, Chan IH, Mayo JR, et al. Early emphysema in patients with anorexia nervosa. Am J Respir Crit Care Med 2004; 170: 748–752. [DOI] [PubMed] [Google Scholar]
- 37. O’Donnell DE, Deesomchok A, Lam YM, et al. Effects of BMI on static lung volumes in patients with airway obstruction. Chest 2011; 140: 461–468. [DOI] [PubMed] [Google Scholar]
- 38. Filley GF, Beckwitt HJ, Reeves JT, et al. Chronic obstructive bronchopulmonary disease. II. Oxygen transport in two clinical types. Am J Med 1968; 44: 26–38. [DOI] [PubMed] [Google Scholar]
- 39. Vanfleteren LE, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013; 187: 728–735. [DOI] [PubMed] [Google Scholar]
- 40. Vozoris NT, O’Donnell DE. Prevalence, risk factors, activity limitation and health care utilization of an obese, population-based sample with chronic obstructive pulmonary disease. Can Respir J 2012; 19: e18–e24. [DOI] [PMC free article] [PubMed] [Google Scholar]