Abstract
This study is aimed to (i) compare both the magnitude of impairment in exercise capacity and exercise responses measured during the six-minute walk test (6MWT) and the cardiopulmonary exercise test (CPET) and (ii) investigate the effect of test repetition on six-minute walk distance (6MWD) in people following curative intent treatment for non-small cell lung cancer (NSCLC). Twenty participants (67 ± 10 years; 14 females), 6–10 weeks following lobectomy, underwent a CPET and two 6MWTs. Peak exercise responses, dyspnoea and leg fatigue, as well as heart rate (HR) and oxygen saturation (SpO2) during the 6MWT, were compared to those during the CPET. Compared with exercise capacity when expressed as peak rate of oxygen consumption (%pred) measured during the CPET, exercise capacity when expressed as 6MWD (%pred) was less impaired (81 ± 10 vs. 63 ± 15 %pred; p < 0.001). Compared with the CPET, the 6MWT elicited lower peak HR (119 ± 15 vs. 128 ± 18 beats minute−1; p = 0.02), lower SpO2 (93 ± 2 vs. 95 ± 3%; p < 0.05), less dyspnoea (3.1 ± 1.6 vs. 6.9 ± 2.6; p < 0.01) and less leg fatigue (2.0 ± 1.9 vs. 6.8 ± 2.4; p < 0.01). The 6MWD increased 19 ± 19 metre (4 ± 4%) with test repetition (p < 0.001). In people following curative intent treatment for NSCLC, the 6MWT appears to elicit sub-maximal exercise responses when compared with the CPET. There is a significant effect of test repetition on 6MWD.
Keywords: Exercise test, carcinoma, non-small cell, exercise tolerance
Introduction
Lung cancer is the most common cause of death from malignancy in males and females.1 For people diagnosed with early stage non-small cell lung cancer (NSCLC) (i.e. stages I–IIIA), surgical resection of the tumour (with or without adjuvant chemotherapy) offers a chance of cure.2 Although the overall 5-year survival rate for people with lung cancer is approximately 15%,1,3 for those with early stage NSCLC who complete curative intent treatment, the 5-year survival rate can be as high as 80%.2 Therefore, in people following curative intent treatment for early stage NSCLC interest is increasing in the impact such treatment has on functional status, as well as the role rehabilitative strategies, such as exercise training, may have in optimizing recovery. Specifically, earlier work has demonstrated that people who have completed curative intent treatment for NSCLC have substantial impairments in exercise capacity.4–10 Compared to preoperative values, the peak rate of oxygen uptake (VO2peak) following lung resection is reduced by 10% to 28%.8–10 Further, there have been some randomized controlled trials, and a recent Cochrane review, that have investigated whether this impairment in exercise capacity can be ameliorated with exercise training.11–13
Although there is interest in exercise capacity and the role of exercise training in this population, data exploring the responses to exercise in people following curative intent treatment for NSCLC are scarce. The cardiopulmonary exercise test (CPET) is a laboratory-based assessment, which provides a global assessment of an individuals’ response to exercise.14 Even though several protocols exist, those which impose a progressive increase in work rate are most common. The CPET is accepted as the gold standard method to quantify exercise capacity, and the results can be used to prescribe the initial intensity for exercise training.14 It is, however, expensive to perform and requires access to specialized equipment and personnel. Therefore, the six-minute walk test (6MWT), in which an individual is asked to walk as far as possible in 6 minutes, is commonly used in pulmonary rehabilitation programmes to quantify exercise capacity and prescribe the initial intensity for walking-based exercise training.15–17
Studies in people with moderate-to-severe chronic obstructive pulmonary disease (COPD) that have compared exercise responses during the 6MWT to those during the CPET18–23 have shown no difference in VO2peak 18,19,21 and either minimal22 or no difference18–21,23 in peak heart rate (HR) between the two tests. In people with COPD, although the 6MWT has been shown to elicit similar peak responses as the CPET, walking-based exercise training is often prescribed and tolerated at a high percentage (e.g. 80%) of the average speed achieved during the 6MWT.24,25 In people following curative intent treatment for NSCLC, no studies have compared the exercise responses measured during the CPET and the 6MWT. Hence, it is unknown whether the 6MWT elicits peak or sub-maximal exercise responses, relative to the CPET. Such information will assist clinicians to decide whether it is appropriate to prescribe walking-based exercise training at 80% of the average speed achieved during the 6MWT or whether a higher initial intensity may be tolerated in this population.
The European Respiratory Society/American Thoracic Society (ERS/ATS) technical standard on field walking tests26 recommends that the 6MWT should be performed twice to account for any effect test repetition may have on six-minute walk distance (6MWD). However, this recommendation is based largely on studies that recruited people with COPD.26,27 No studies have explored the effect of test repetition on 6MWD in people following curative intent treatment for NSCLC.
Therefore, in people following curative intent treatment for NSCLC, the aims of this study were to (i) compare the magnitude of impairment in exercise capacity and exercise responses measured during the 6MWT and CPET and (ii) investigate the effect of test repetition on 6MWD. As we collected several cardiorespiratory variables during the CPET, we also speculated on the mechanisms of exercise limitation in this population.
Methods
Study design and participants
This was a cross-sectional and observational study. Data collection was performed between February 2012 and April 2014. People were included if they were 6 to 10 weeks following lobectomy for NSCLC (stages I–IIIA) or, for those who required adjuvant chemotherapy following surgery, 4 to 8 weeks following their last treatment. Exclusion criteria comprised presence of co-morbid conditions that may compromise safety during the assessments, severe neuromusculoskeletal limitations or the inability to understand spoken or written English. All participants gave written informed consent. The study was approved by the Ethics Committees of two tertiary hospitals (approval numbers 2011/105 and RA-11/033) and Curtin University (HR-178/2011).
Protocol
Measures were collected over 2 assessment days, separated by at least 7 days. On the first day, age, height and weight were recorded, and functional limitation resulting from dyspnoea was assessed by the modified Medical Research Council dyspnoea scale (MMRC),28,29 and participants underwent two 6MWTs. On the second day, measures were made for forced expiratory volume in 1 second, forced vital capacity and single-breath diffusing capacity for carbon monoxide (The Elite-Series-DX-plethysmograph, Medical Graphics Corporation, St Paul, Minnesota, USA)30–34 and participants completed a CPET.
Exercise capacity measures
Cardiopulmonary exercise test
A symptom-limited ramp cycle ergometry exercise test was performed on an electronically braked bicycle ergometer (ER-900 Jaeger, Viasys-Healthcare-GmbH, Germany) in accordance with published guidelines.14 The magnitude of work rate increments was individualized using a published equation,35 with the goal of achieving a test duration of 10 minutes.14,35,36 Increments varied from 1 watt (W) every 12 seconds to 1 W every 5 seconds. Participants rested on the bike for 3 minutes, then completed unloaded cycling (between 50 r min−1 and 60 r min−1) for 3 minutes, after which time the resistance on the bike progressively increased until symptom limitation. Breath-by-breath measurements were collected (Medgraphics-CardiO2; Medical Graphics Corporation). Blood pressure was measured every 2 minutes by automated sphygmomanometry, HR was measured via 12-lead electrocardiography, and peripheral capillary oxygen saturation (SpO2) was continuously monitored via pulse oximetry (Rad4; Masimo Corporation, Irvine, California, USA). The modified Borg scale (0–10)37 was used to quantify the level of dyspnoea (BORGd) and leg fatigue (BORGf) prior to starting the test, each minute during the test and on test completion.
Measures of oxygen uptake and minute ventilation collected over the final 20 seconds previous to their highest value were averaged to calculate VO2peak and maximum minute ventilation (VEmax).14,38,39 Measures of maximum work rate (W max) and VO2peak were expressed in absolute values and as a percentage of the predicted value.40 A single CPET was completed as studies in healthy people and in people with COPD have shown no improvement when a second test was performed.14
Six-minute walk test
The 6MWT was undertaken according to the ERS/ATS recommendations,26 with encouragement to walk as far and as fast as possible. The test was performed over a 45-metre straight course within an enclosed corridor, which is consistent with the course used to develop the reference equations for the Australian population.41 Standardized encouragement was given at the end of every minute. Also, at the end of every minute, measures were collected of HR (Polar-a1; Polar Electro Oy, Finland) and SpO2 (Rad-57; Masimo Corporation). The modified BORG scale37 was used to quantify level of dyspnoea and leg fatigue prior to starting the test and on test completion.
Two tests, separated by a 30-minute rest period, were conducted and the best 6MWD was recorded as the test result. Further, the six-minute walk work (6MWW) was calculated as the product of 6MWD (expressed in kilometre) and weight (expressed in kilogram). This variable represents the work of walking.42 For each participant, the 6MWD was expressed in absolute values and as a percentage of their predicted value.41
Statistical analysis
Statistical analyses were performed using SPSS® (Statistical Package for Social Sciences, version 22.0) and SigmaPlot (version 12.0). The distribution of data was examined using the Shapiro–Wilk test. Exercise capacity, peak HR, nadir SpO2, end-test BORGd and BORGf elicited during the two tests were compared using paired t-tests. Differences between the exercise tests are expressed as mean difference (95% confidence interval) (MD [95% CI]). Clinically important oxygen desaturation during the 6MWT and CPET was defined as a >4% fall in SpO2 from resting measures to a value <90%.43 A subgroup analysis comparing exercise responses during the two tests in people diagnosed with COPD44 versus people not diagnosed with COPD was undertaken using an independent t-test. In order to compare the patterns of changes in measures of HR and SpO2 during each test, data were grouped into deciles (i.e. epochs equivalent to 10% of the total test duration) using a two-dimensional data transformation. Comparison of both HR and SpO2 at each decile throughout the 6MWT and the CPET was performed using a one-way repeated-measures analysis of variance with Tukey’s post hoc test. Correlations between variables from the 6MWT (i.e. 6MWD and 6MWW) and variables from the CPET (i.e. VO2peak and W max) were calculated utilizing Pearson’s correlation coefficient (see online supplementary material). The effect of test repetition on 6MWD was examined using a paired t-test. For all analyses, the value of p ≤ 0.05 was considered significant. Data are expressed as mean ± standard deviation (SD).
This study represents a secondary analysis of data collected as part of a larger study that compared several outcomes in people following curative intent treatment for NSCLC with those in age and gender-matched healthy controls.45 For the larger study,45 the sample size calculation indicated that 18 participants with NSCLC and 18 healthy controls were needed to detect a between-group difference in VO2peak of 0.55 L min−1 with an SD of 0.57 L min−1 (α = 0.05, 1; β = 0.8). Data collected in the participants with NSCLC who completed this larger study were used in the analyses presented in the current study.
Results
Ninety-six people following lobectomy were screened to participate in this study, of whom 71% (n = 68) were eligible and approached. Of these, 22 (32%) consented to participate. The reasons for declining participation were (i) difficulty with travelling to the hospital (n = 16), (ii) no free time to attend assessments (n = 11), (iii) lack of interest (n = 11), (iv) personal issues (n = 6) and (v) travelling overseas for the next couple of weeks (n = 2). Two of the 22 were withdrawn due to health issues not related to lung cancer. Complete CPET and 6MWT data were available in 20 participants. The first assessment day occurred 56 ± 18 days following lobectomy. One participant received chemotherapy and their first assessment day occurred 28 days following their last cycle of chemotherapy. Characteristics of the participants are presented in Table 1. Their median (interquartile range) MMRC grade was 1 (1 to 1.75). Ten participants (50%) had a diagnosis of COPD. Their characteristics are presented in the online supplementary material.
Table 1.
Participant characteristics (n = 20).
| Variable | Mean ± SD | |
|---|---|---|
| Age (year) | 67 ± 10 | |
| BMI (kg m−2) | 26 ± 6 | |
| Smoking (pack/year) | 36 ± 26 | |
| FEV1 (L) | 1.65 ± 0.49 | |
| FEV1 (%pred) | 67 ± 16 | |
| FVC (L) | 2.69 ± 0.72 | |
| FVC (%pred) | 81 ± 11 | |
| FEV1/FVC | 0.63 ± 0.12 | |
| DLCO (ml mmHg−1 min−1) | 13.6 ± 3.4 | |
| DLCO (%pred) | 53 ± 11 | |
| n | % | |
| Gender, male/female | 6/14 | 30/70 |
| Smoking status | ||
| Ex-smoker | 17 | 85 |
| Never smoked | 3 | 15 |
| Type of NSCLC | ||
| Adenocarcinoma | 13 | 65 |
| Squamous cell carcinoma | 6 | 30 |
| Large cell carcinoma | 1 | 5 |
| NSCLC stage | ||
| I | 16 | 80 |
| II | 3 | 15 |
| IIIA | 1 | 5 |
| Type of surgery (lobectomy) | ||
| Open | 9 | 45 |
| VATS | 11 | 55 |
| Adjuvant chemotherapy | 1 | 5 |
BMI: body mass index; DLCO: single-breath diffusing capacity for carbon monoxide; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; NSCLC: non-small cell lung cancer; SD: standard deviation; VATS: video-assisted thoracoscopic surgery.
Cardiopulmonary exercise test
Results of the CPET are shown in Tables 2 and 3. One participant (5%) desaturated >4% to an SpO2 of 87% during the test. There was no difference between end-test BORGd (6.9 ± 2.6) and end-test BORGf (6.8 ± 2.4; p = 0.87). A total of 4 (20%) participants demonstrated evidence of a ventilatory limitation to exercise, defined as a ratio of VEmax to maximum voluntary ventilation of >85%.14 These participants had spirometric evidence of COPD.
Table 2.
Results of the exercise tests.
| Variable (N = 20) | Mean ± SD |
|---|---|
| CPET | |
| Duration (minutes) | 9.3 ± 1.6 |
| VO2peak(L min−1) | 1.03 ± 0.31 |
| VO2peak(mL kg−1 min−1) | 15 ± 3 |
| VO2peak(%pred) | 63 ± 15 |
| W max (W) | 76 ± 26 |
| W max (%pred) | 72 ± 20 |
| VEmax (L min−1) | 43 ± 15 |
| VEmax/MVV (%) | 70 ± 20 |
| AT (%V02peak) | 61 ± 10 |
| O2 pulse (mL beat−1) | 8 ± 3 |
| f R (breaths min−1) | 36 ± 4 |
| 6MWT | |
| 6MWD (metre) | 503 ± 71 |
| 6MWD (%pred) | 81 ± 10 |
| 6MWW (km kg−1) | 35 ± 11 |
6MWD: six-minute walk distance; VO2peak: peak rate of oxygen uptake; AT: anaerobic threshold as a percentage of the VO2peak; 6MWT: six-minute walk test; 6MWW: six-minute walk work; CPET: cardiopulmonary exercise test; f R: respiratory frequency; MVV: maximum voluntary ventilation; O2 pulse: oxygen pulse; VEmax: maximum minute; W max: maximum work rate.
Table 3.
Comparison of HR, SpO2 and symptoms during the 6MWT and CPET.
| Variable (N = 20) | CPET (mean ± SD) | 6MWT (mean ± SD) |
|---|---|---|
| Resting HR (beats minute−1) | 85 ± 13 | 83 ± 10 |
| Peak HR (beats minute−1) | 128 ± 18 | 119 ± 15b |
| Peak HR (%pred HRmax)a | 84 ± 11 | 79 ± 10b |
| ΔHR (beats minute−1) | 44 ± 16 | 37 ± 14 |
| Resting SpO2 (%) | 97 ± 2 | 97 ± 1 |
| Nadir SpO2 (%) | 95 ± 3 | 93 ± 2b |
| ΔSpO2 (%) | −1 ± 3 | −4 ± 2b |
| End-test BORGd | 6.9 ± 2.6 | 3.1 ± 1.6c |
| End-test BORGf | 6.8 ± 2.4 | 2.0 ± 1.9c |
6MWT: six-minute walk test; CPET: cardiopulmonary exercise test; BORGd: dyspnoea; BORGf: leg fatigue; HR: heart rate; SpO2: peripheral capillary oxygen saturation measured via pulse oximetry; ΔSpO2: nadir SpO2 − initial SpO2); HRmax: maximum heart rate.
aHRmax was calculated from the equation HRmax = 220 − age.
b p < 0.05: Statistically significant differences between tests.
c p < 0.01: Statistically significant differences between tests.
Six-minute walk test
Results of the best 6MWT are shown in Tables 2 and 3. Two participants (10%) desaturated >4% to an SpO2 <90% during the test. There was no difference between end-test BORGd (3.1 ± 1.6) and end-test BORGf (2.0 ± 1.9; p = 0.06).
Comparison of exercise responses during the two exercise tests
The impairment in exercise capacity expressed in terms of VO2peak was greater than the impairment expressed in terms of 6MWD (63 ± 15 vs. 81 ± 10%predicted; p < 0.001). Table 3 presents a comparison of HR, SpO2 and symptoms between the two tests. Compared with responses measured during the CPET, the 6MWT elicited a lower peak HR (MD [95% CI] = −9[−16, −1] beats minute−1; p = 0.02) and greater oxygen desaturation (MD [95% CI] nadir SpO2 = −2 [−4, −1]%; p = 0.01). Both the BORGd and BORGf were also lower on completion of the 6MWT (MD [95% CI] = −4 [−5, −3] and −5 [−6, −3], respectively; p < 0.001 for both).
The pattern of change in HR and SpO2 during the two tests is presented in Figure 1. During the CPET, HR increased linearly with time, whereas during the 6MWT, HR increased from the beginning of the test until the end of the third minute (ΔHR; 32 ± 12 beats minute−1; p < 0.001), after which there was a plateau until test completion (ΔHR between the end of the third minute and test completion; 4 ± 4 beats minute−1; p = 0.34). During the CPET, there was a linear decrease in SpO2, whereas during the 6MWT, there was a reduction in SpO2 from the beginning of the test until the end of the second minute (ΔSpO2 = −3 ± 2%; p = 0.01), after which there was a plateau until test completion (ΔSpO2 between the end of the second minute and test completion; −1 ± 1%; p = 0.67).
Figure 1.
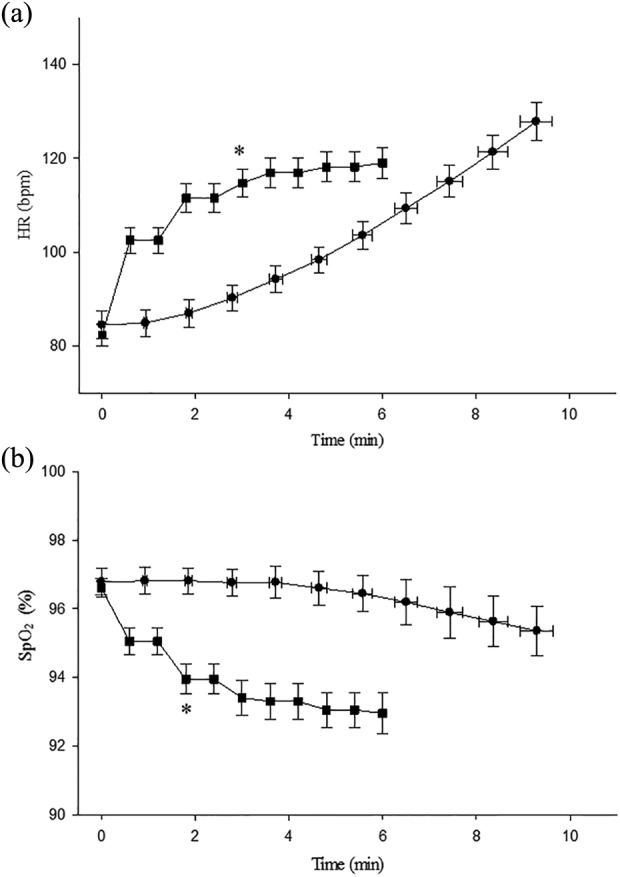
Patterns of responses for: (a) HR and (b) peripheral SpO2 for each test. Data are represented as mean and standard error. All participants contributed to each decile (data point). •: CPET; ▪:6MWT. For the CPET, time 0 (zero) is the start of the ramp. *Plateau from this data point to test completion. HR: heart rae; SpO2: capillary oxygen saturation; CPET: cardiopulmonary exercise test; 6MWT: six-minute walk test.
Effect of test repetition on 6MWD
The 6MWD increased with test repetition from 484 ± 72 to 503 ± 71 metre; p < 0.001. The MD was 19 ± 19 metre (range: −8 to 57m) or 4 ± 4% (range: −2% to 20%). Eighteen of the 20 participants (90%) walked further on the second 6MWT. None of the participants required a rest during the tests.
Subgroup analyses comparing responses of people with COPD to people without COPD
No difference between those with and without COPD were found in any of the exercise responses during the two tests (see online supplementary material).
Discussion
The important findings of this study are that in people following curative intent treatment for NSCLC (i) the impairment in exercise capacity measured during the CPET, expressed as VO2peak (% predicted) was considerably greater than the impairment expressed as 6MWD (% predicted); (ii) the causes of exercise limitation were variable with a small proportion of participants demonstrating evidence of ventilatory limitation; (iii) compared with the CPET, the 6MWT elicited a lower peak HR, less symptoms of dyspnoea and leg fatigue, but greater oxygen desaturation; and (iv) the 6MWD increased with test repetition. Differences in the pattern of response in HR and SpO2 were observed during the CPET and 6MWT.
During the CPET, people following curative intent treatment for NSCLC achieved a VO2peak of 15 ± 3 mL kg−1 min−1 or 63 ± 15% predicted. The impairment in 6MWD was less, being 503 ± 71 metre or 81 ± 10% predicted. Therefore, it seems that people following curative intent treatment for NSCLC have considerable impairment in peak exercise capacity, but relatively modest impairment in the capacity to walk as far as possible in 6 minutes. Regarding the mechanisms of exercise limitation, four (20%) participants, all of whom had evidence of COPD, demonstrated evidence of a ventilatory limitation. Although the other six participants with spirometric evidence of COPD did not demonstrate a ventilatory limitation, we cannot be certain that they did not develop lung hyperinflation during exercise as we did not perform flow-volume loops or monitor changes in inspiratory capacity. Earlier work has shown that people with mild COPD progressively develop lung hyperinflation during exercise and this contributes to their sensation of dyspnoea.46 In those without COPD, the most likely mechanism of limitation was peripheral muscle deconditioning.
Our data show that compared with the CPET, the 6MWT elicited a lower peak HR and less symptoms. This is different to earlier work in people with COPD, which shows that the CPET and 6MWT elicit a similar peak HR as their ventilatory limitation to exercise precludes them from attaining a higher peak HR during the CPET.18,19 The implications of these findings are threefold. First, for clinicians who are unable to access CPET, people following curative intent treatment for NSCLC who present with modest impairments in 6MWD may have considerable impairment in peak exercise capacity and therefore may still benefit from participating in exercise training. Second, given that the mean 6MWD was greater than 500 metre, or 80%predicted, it is possible that this test is not as responsive as it is in people with moderate-to-severe COPD to change following exercise training.47 Although earlier work in people with bronchiectasis, who were characterized by a high baseline 6MWD, demonstrated an increase in 6MWD of 41 metre (95%CI (metre) = 19, 63) following 8 weeks of exercise training,48 alternative field-based tests, such as the modified incremental shuttle walk test,49 may be more responsive to change following interventions. Third, the way in which the 6MWD has been used to prescribe the intensity of walking-based training in people with COPD may require some reconsideration in people following curative intent treatment for NSCLC. That is, in people with COPD, although the 6MWT has been shown to elicit similar peak responses as the CPET, walking-based exercise training is often prescribed at a high percentage (e.g. 80%) of the average speed achieved during the 6MWT.24 In contrast, our data show that in people following curative intent treatment for NSCLC, the 6MWT elicited sub-maximal responses. As the 6MWT is a sub-maximal test in this population, it is likely that they will tolerate an initial prescription of walking-based training at any intensity greater than that used for COPD.25
The magnitude of impairment in VO2peak and 6MWD between those with and without COPD was very similar. Although the lack of statistical difference is likely to reflect the small sample size available for this comparison, the data suggest that any difference is very small and unlikely to be clinically meaningful. The similarity in measures between those with versus without COPD may also relate to the fact that our sample included individuals with mild-to-moderate, rather than severe airflow obstruction.
Although the extent of oxygen desaturation observed during both tests was modest, the SpO2 of people following curative intent treatment for NSCLC decreased to a greater extent during the 6MWT. Greater desaturation during walking compared with cycle-based exercise corroborates the findings of previous studies in people with moderate-to-severe COPD.18–21,50 Had the CPET been performed on a treadmill, it is unlikely that the decrease in SpO2 would have been different between the two tests.51 The finding of greater oxygen desaturation during the 6MWT in this study may relate to both the larger muscle mass involved in walking compared to cycling and the specific load imposed on the quadriceps during cycle-based exercise, which results in a significant concentration of blood lactate on test completion, heightening the ventilatory response.52 The specific load on the quadriceps is likely to explain the greater severity of symptoms seen during the CPET and the higher level of ventilation previously reported during cycle-based exercise serves to minimize the extent of desaturation.51,53
This study revealed clear differences in patterns of HR and SpO2 responses during the 6MWT compared with the CPET. Whilst HR and SpO2 during the CPET changed gradually and linearly with time, during the 6MWT, these two variables changed exponentially over the first 2 to 3 minutes of the test, followed by a relative plateau until test completion. These patterns of response are consistent with data reported in people with moderate-to-severe COPD18,19 and reflect the way in which these tests are conducted. That is, compared to the CPET in which work rate increases in a linear fashion, work rate during the 6MWT is almost constant after the initial acceleration.19,54 Consistent with earlier work in COPD, both W max and VO2peak were associated with 6MWD. The strength of this association increased when work done during the 6MWT was considered using the variable 6MWW.42,55
Our data demonstrated that there was a significant effect of test repetition. The 6MWD improved by an average of 19 metre (or 4%) on the second test. This is consistent with the findings from studies in people with COPD (mean increase ranges from 13 to 37 metre or 4% to 11%)22,56,57 and idiopathic pulmonary fibrosis (mean increase reported to be 11 metre or 4%).22 This finding supports that a minimum of two 6MWT should be used when assessing people following curative intent treatment for NSCLC.
Limitations
Although our sample size was somewhat modest, it was similar to that of previous studies that have compared responses during the CPET to those during field-based walk tests in people with COPD (sample size ranged from 20 to 24 participants)18–20 and greater than a previous study in people with heart failure (n = 15).58 We acknowledge that the sample may not be large enough to detect differences between the subgroup of people with COPD and the subgroup of people who did not have COPD. We also acknowledge that the use of reference equations to quantify impairment in exercise capacity has its limitations. That is, differences in sample characteristics, equipment and assessment protocols between the study that developed the regression equations and the current study may have introduced error to our estimate of impairment, expressed as a percentage of the predicted value in a healthy population. As measures of exercise capacity were not collected prior to and following a specific intervention, we were not able to investigate the responsiveness of the two tests. The need for a third 6MWT was not assessed, however, data from previous study in people with COPD demonstrated no significant increase in distance on a third test.20 Another limitation is that ventilatory and metabolic responses during the 6MWT were not measured using a portable gas analysis system. Additionally, people who underwent wedge resection or pneumonectomy for NSCLC were not included in the study, thus the results cannot be extended to all people undergoing lung resection for NSCLC.
In conclusion, this study demonstrated that people following curative intent treatment for NSCLC presented with greater impairment in exercise capacity expressed as VO2peak than as 6MWD. This suggests that those who present with modest impairments in 6MWD may have considerable impairment in peak exercise capacity and may still benefit from participating in exercise training. The SpO2 of people following curative intent treatment for NSCLC decreased to a greater extent during the 6MWT, and therefore this test may assist in identifying those who desaturate during exercise. In contrast to people with COPD, in whom the 6MWT and CPET elicit similar peak VO2peak and HR response, in people following curative intent treatment for NSCLC, the 6MWT elicited sub-maximal responses. As the 6MWT is a sub-maximal test in this population, it is likely that they will tolerate an initial prescription of walking-based training at an intensity greater than that used for COPD, which is often 80% of the average walking speed achieved during the 6MWT. A minimum of two 6MWTs need to be performed as the 6MWD increased with test repetition. Future studies should investigate (i) the optimal initial speed for the walking training prescription based on the average walking speed achieved during the 6MWT, (ii) the responsiveness of the 6MWT to interventions such as exercise training and (iii) properties of other common field-based walking tests such as the incremental and endurance shuttle walk test in people following curative intent treatment for NSCLC.
Supplementary Material
Acknowledgements
VC is supported by the Curtin Strategic International Research Scholarship (CSIRS) and Lung Institute of Western Australia (LIWA) PhD Top-up Scholarship.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study received funding from Sir Charles Gairdner Hospital Research Advisory Committee (grant number: 2011/12/013).
Supplemental material: The online [appendices/data supplements/etc] are available at http://crd.sagepub.com/supplemental.
References
- 1. Alberg AJ, Brock MV, Ford JG, et al. Epidemiology of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143: e1S–e29S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143: e278S–e313S. [DOI] [PubMed] [Google Scholar]
- 3. AIHW and Cancer Australia. Lung cancer in Australia: an overview. Cat. no. CAN 58 Canberra: AIHW, 2011. [Google Scholar]
- 4. Jones LW. Physical activity and lung cancer survivorship. Recent Results Canc Res 2011; 186: 255–274. [DOI] [PubMed] [Google Scholar]
- 5. Pelletier C, Lapointe L, LeBlanc P. Effects of lung resection on pulmonary function and exercise capacity. Thorax 1990; 45: 497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagamatsu Y, Maeshiro K, Kimura NY, et al. Long-term recovery of exercise capacity and pulmonary function after lobectomy. J Thorac Cardiovasc Surg 2007; 134: 1273–1278. [DOI] [PubMed] [Google Scholar]
- 7. Win T, Groves AM, Ritchie AJ, et al. The effect of lung resection on pulmonary function and exercise capacity in lung cancer patients. Respir Care 2007; 52: 720–726. [PubMed] [Google Scholar]
- 8. Wang JS, Abboud RT, Wang LM. Effect of lung resection on exercise capacity and on carbon monoxide diffusing capacity during exercise. Chest 2006; 129: 863–872. [DOI] [PubMed] [Google Scholar]
- 9. Nezu K, Kushibe K, Tojo T, et al. Recovery and limitation of exercise capacity after lung resection for lung cancer. Chest 1998; 113: 1511–1516. [DOI] [PubMed] [Google Scholar]
- 10. Bolliger CT, Jordan P, Soler M, et al. Pulmonary function and exercise capacity after lung resection. Eur Respir J 1996; 9: 415–421. [DOI] [PubMed] [Google Scholar]
- 11. Edvardsen E, Skjonsberg OH, Holme I, et al. High-intensity training following lung cancer surgery: a randomised controlled trial. Thorax 2015; 70: 244–250. [DOI] [PubMed] [Google Scholar]
- 12. Cavalheri V, Tahirah F, Nonoyama M, et al. Exercise training for people following lung resection for non-small cell lung cancer – a Cochrane systematic review. Canc Treat Rev 2014; 40: 585–594. [DOI] [PubMed] [Google Scholar]
- 13. Cavalheri V, Tahirah F, Nonoyama M, et al. Exercise training undertaken by people within 12 months of lung resection for non-small cell lung cancer. Cochrane Database Syst Rev 2013; 7: CD009955. [DOI] [PubMed] [Google Scholar]
- 14. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003; 167: 211–277. [DOI] [PubMed] [Google Scholar]
- 15. Johnston CL, Maxwell LJ, Alison JA. Pulmonary rehabilitation in Australia: a national survey. Physiotherapy 2011; 97: 284–290. [DOI] [PubMed] [Google Scholar]
- 16. Spruit MA, Pitta F, Garvey C, et al. Differences in content and organisational aspects of pulmonary rehabilitation programmes. Eur Respir J 2014; 43: 1326–1337. [DOI] [PubMed] [Google Scholar]
- 17. Desveaux L, Janaudis-Ferreira T, Goldstein R, et al. An international comparison of pulmonary rehabilitation: a systematic review. COPD 2015; 12: 144–153. [DOI] [PubMed] [Google Scholar]
- 18. Hill K, Dolmage TE, Woon L, et al. Comparing peak and submaximal cardiorespiratory responses during field walking tests with incremental cycle ergometry in COPD. Respirology 2012; 17: 278–284. [DOI] [PubMed] [Google Scholar]
- 19. Troosters T, Vilaro J, Rabinovich R, et al. Physiological responses to the 6-min walk test in patients with chronic obstructive pulmonary disease. Eur Respir J 2002; 20: 564–569. [DOI] [PubMed] [Google Scholar]
- 20. Turner SE, Eastwood PR, Cecins NM, et al. Physiologic responses to incremental and self-paced exercise in COPD: a comparison of three tests. Chest 2004; 126: 766–773. [DOI] [PubMed] [Google Scholar]
- 21. Luxton N, Alison JA, Wu J, et al. Relationship between field walking tests and incremental cycle ergometry in COPD. Respirology 2008; 13: 856–862. [DOI] [PubMed] [Google Scholar]
- 22. Kozu R, Jenkins S, Senjyu H, et al. Peak power estimated from 6-minute walk distance in Asian patients with idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease. Respirology 2010; 15: 706–713. [DOI] [PubMed] [Google Scholar]
- 23. Fowler RM, Jenkins SC, Maiorana AJ, et al. Measurement properties of the 6-min walk test in individuals with exercise-induced pulmonary arterial hypertension. Intern Med J 2011; 41: 679–687. [DOI] [PubMed] [Google Scholar]
- 24. Jenkins S, Hill K, Cecins NM. State of the art: how to set up a pulmonary rehabilitation program. Respirology 2010; 15: 1157–1173. [DOI] [PubMed] [Google Scholar]
- 25. Zainuldin R, Mackey MG, Alison JA. Prescription of walking exercise intensity from the 6-minute walk test in people with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev 2015; 35: 65–69. [DOI] [PubMed] [Google Scholar]
- 26. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014; 44: 1428–1446. [DOI] [PubMed] [Google Scholar]
- 27. Singh SJ, Puhan MA, Andrianopoulos V, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J 2014; 44: 1447–1478. [DOI] [PubMed] [Google Scholar]
- 28. Fletcher CM, Elmes PC, Fairbairn AS, et al. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J 1959; 2: 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350: 1005–1012. [DOI] [PubMed] [Google Scholar]
- 30. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 31. Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005; 26: 720–735. [DOI] [PubMed] [Google Scholar]
- 32. American Thoracic Society/European Respiratory Society. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med 2002; 166: 518–624. [DOI] [PubMed] [Google Scholar]
- 33. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999; 159: 179–187. [DOI] [PubMed] [Google Scholar]
- 34. Crapo RO, Morris AH. Standardized single breath normal values for carbon monoxide diffusing capacity. Am Rev Respir Dis 1981; 123: 185–189. [DOI] [PubMed] [Google Scholar]
- 35. Pretto JJ, Braun GW, Guy PA. Using baseline respiratory function data to optimize cycle exercise test duration. Respirology 2001; 6: 287–291. [DOI] [PubMed] [Google Scholar]
- 36. Buchfuhrer MJ, Hansen JE, Robinson TE, et al. Optimizing the exercise protocol for cardiopulmonary assessment. J Appl Physiol: Respir Environ Exerc Physiol 1983; 55: 1558–1564. [DOI] [PubMed] [Google Scholar]
- 37. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14: 377–381. [PubMed] [Google Scholar]
- 38. Nanas S, Sakellariou D, Kapsimalakou S, et al. Heart rate recovery and oxygen kinetics after exercise in obstructive sleep apnea syndrome. Clin Cardiol 2010; 33: 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Win T, Jackson A, Groves AM, et al. Comparison of shuttle walk with measured peak oxygen consumption in patients with operable lung cancer. Thorax 2006; 61: 57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blackie SP, Fairbarn MS, McElvaney GN, et al. Prediction of maximal oxygen uptake and power during cycle ergometry in subjects older than 55 years of age. Am Rev Respir Dis 1989; 139: 1424–1429. [DOI] [PubMed] [Google Scholar]
- 41. Jenkins S, Cecins N, Camarri B, et al. Regression equations to predict 6-minute walk distance in middle-aged and elderly adults. Physiother Theory Pract 2009; 25: 516–522. [DOI] [PubMed] [Google Scholar]
- 42. Chuang ML, Lin IF, Wasserman K. The body weight-walking distance product as related to lung function, anaerobic threshold and peak VO2 in COPD patients. Respir Med 2001; 95: 618–626. [DOI] [PubMed] [Google Scholar]
- 43. British Thoracic Society. Clinical component for the home oxygen service in England and Wales. London: British Thoracic Society, 2006. [Google Scholar]
- 44. Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD), http://www.goldcopd.org/ (2015, accessed 10 June 2015).
- 45. Cavalheri V, Jenkins S, Cecins N, et al. Impairments after curative intent treatment for non-small cell lung cancer: a comparison with age and gender-matched healthy controls. Respir Med 2015; 109: 1332–1339. [DOI] [PubMed] [Google Scholar]
- 46. Ofir D, Laveneziana P, Webb KA, et al. Mechanisms of dyspnea during cycle exercise in symptomatic patients with GOLD stage I chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008; 177: 622–629. [DOI] [PubMed] [Google Scholar]
- 47. Frost AE, Langleben D, Oudiz R, et al. The 6-min walk test (6MW) as an efficacy endpoint in pulmonary arterial hypertension clinical trials: demonstration of a ceiling effect. Vasc Pharmacol 2005; 43: 36–39. [DOI] [PubMed] [Google Scholar]
- 48. Lee AL, Hill CJ, Cecins N, et al. Minimal important difference in field walking tests in non-cystic fibrosis bronchiectasis following exercise training. Respir Med 2014; 108: 1303–1309. [DOI] [PubMed] [Google Scholar]
- 49. Campo LA, Chilingaryan G, Berg K, et al. Validity and reliability of the modified shuttle walk test in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2006; 87: 918–922. [DOI] [PubMed] [Google Scholar]
- 50. Poulain M, Durand F, Palomba B, et al. 6-minute walk testing is more sensitive than maximal incremental cycle testing for detecting oxygen desaturation in patients with COPD. Chest 2003; 123: 1401–1407. [DOI] [PubMed] [Google Scholar]
- 51. Hsia D, Casaburi R, Pradhan A, et al. Physiological responses to linear treadmill and cycle ergometer exercise in COPD. Eur Respir J 2009; 34: 605–615. [DOI] [PubMed] [Google Scholar]
- 52. Man WD, Soliman MG, Gearing J, et al. Symptoms and quadriceps fatigability after walking and cycling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003; 168: 562–567. [DOI] [PubMed] [Google Scholar]
- 53. Mahler DA, Gifford AH, Waterman LA, et al. Mechanism of greater oxygen desaturation during walking compared with cycling in patients with COPD. Chest 2011; 140: 351–358. [DOI] [PubMed] [Google Scholar]
- 54. Butland RJ, Pang J, Gross ER, et al. Two-, six-, and 12-minute walking tests in respiratory disease. BMJ 1982; 284: 1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Carter R, Holiday DB, Nwasuruba C, et al. 6-minute walk work for assessment of functional capacity in patients with COPD. Chest 2003; 123: 1408–1415. [DOI] [PubMed] [Google Scholar]
- 56. Hernandes NA, Wouters EF, Meijer K, et al. Reproducibility of 6-minute walking test in patients with COPD. Eur Respir J 2011; 38: 261–267. [DOI] [PubMed] [Google Scholar]
- 57. Jenkins S, Cecins NM. Six-minute walk test in pulmonary rehabilitation: do all patients need a practice test? Respirology 2010; 15: 1192–1196. [DOI] [PubMed] [Google Scholar]
- 58. Deboeck G, Muylem AV, Vachiery JL, et al. Physiological response to the 6-minute walk test in chronic heart failure patients versus healthy control subjects. Eur J Prev Cardiol 2014; 21: 997–1003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


