Abstract
Background
The administration of endocrine therapy for 5 years substantially reduces recurrence rates during and after treatment in women with early-stage, estrogen-receptor (ER)–positive breast cancer. Extending such therapy beyond 5 years offers further protection but has additional side effects. Obtaining data on the absolute risk of subsequent distant recurrence if therapy stops at 5 years could help determine whether to extend treatment.
Methods
In this meta-analysis of the results of 88 trials involving 62,923 women with ER-positive breast cancer who were disease-free after 5 years of scheduled endocrine therapy, we used Kaplan–Meier and Cox regression analyses, stratified according to trial and treatment, to assess the associations of tumor diameter and nodal status (TN), tumor grade, and other factors with patients’ outcomes during the period from 5 to 20 years.
Results
Breast-cancer recurrences occurred at a steady rate throughout the study period from 5 to 20 years. The risk of distant recurrence was strongly correlated with the original TN status. Among the patients with stage T1 disease, the risk of distant recurrence was 13% with no nodal involvement (T1N0), 20% with one to three nodes involved (T1N1–3), and 34% with four to nine nodes involved (T1N4–9); among those with stage T2 disease, the risks were 19% with T2N0, 26% with T2N1–3, and 41% with T2N4–9. The risk of death from breast cancer was similarly dependent on TN status, but the risk of contralateral breast cancer was not. Given the TN status, the factors of tumor grade (available in 43,590 patients) and Ki-67 status (available in 7692 patients), which are strongly correlated with each other, were of only moderate independent predictive value for distant recurrence, but the status regarding the progesterone receptor (in 54,115 patients) and human epidermal growth factor receptor type 2 (HER2) (in 15,418 patients in trials with no use of trastuzumab) was not predictive. During the study period from 5 to 20 years, the absolute risk of distant recurrence among patients with T1N0 breast cancer was 10% for low-grade disease, 13% for moderate-grade disease, and 17% for high-grade disease; the corresponding risks of any recurrence or a contralateral breast cancer were 17%, 22%, and 26%, respectively.
Conclusions
After 5 years of adjuvant endocrine therapy, breast-cancer recurrences continued to occur steadily throughout the study period from 5 to 20 years. The risk of distant recurrence was strongly correlated with the original TN status, with risks ranging from 10 to 41%, depending on TN status and tumor grade. (Funded by Cancer Research UK and others.)
IN ESTROGEN-RECEPTOR (ER)–POSITIVE early-stage breast cancer, 5 years of adjuvant endocrine therapy substantially reduces the risks of locoregional and distant recurrence, contralateral breast cancer, death from breast cancer, and hence death from any cause.1–3 In trials of 5 years of tamoxifen therapy versus no endocrine therapy, the recurrence rate in the tamoxifen group was approximately 50% lower than that in the control group during the first 5 years (the treatment period) and approximately 30% lower during the next 5 years. The rate of death from breast cancer in the tamoxifen group was approximately 30% lower than that in the control group during the first 15 years (i.e., including 10 years after the cessation of therapy).2 In trials comparing an aromatase inhibitor versus tamoxifen in postmenopausal women, the aromatase inhibitor was even more effective than tamoxifen, with about one third fewer recurrences during the treatment period (but with little further gain thereafter) and with approximately 15% fewer deaths from breast cancer during the first decade.3
Extending adjuvant endocrine therapy (with either tamoxifen or an aromatase inhibitor) beyond 5 years can further reduce recurrence.4–9 For some women, however, receiving endocrine therapy for 5 years causes appreciable side effects, such as menopausal symptoms and arthropathy, which could continue with extended treatment.9–11 Much less common, but potentially life-threatening, side effects of adjuvant endocrine therapy include pulmonary embolus and endometrial cancer associated with tamoxifen and osteoporotic fracture associated with aromatase inhibitors, and the cumulative risk of these toxic events increases with longer treatment.2,3,5,6,9
Thus, decisions about extending adjuvant endocrine therapy after 5 years without any recurrence need to balance additional benefits against additional side effects. Since the proportional reduction in recurrence from a given regimen appears to be approximately similar in different risk groups,2,3,6 the absolute benefits of continuing endocrine therapy after 5 years depend on the absolute risk of later recurrence if no further therapy is given. Here, we report the influence of various characteristics of the original tumor on the 20-year incidence of breast-cancer outcomes in women with ER-positive, early-stage breast cancer who were scheduled to receive adjuvant endocrine therapy for 5 years and then to stop therapy. Our meta-analysis combines individual patient data from 88 trials in the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) database of randomized trials.
Methods
Patients
We analyzed data from women who had ER-positive breast cancer that had been diagnosed before the age of 75 years and who had T1 disease (tumor diameter, ≤2.0 cm) or T2 disease (tumor diameter, >2.0 to 5.0 cm), fewer than 10 involved nodes (stratified according to a pathological nodal status of no nodes [N0], 1 to 3 nodes [N1–3], or 4 to 9 nodes [N4–9]), and no distant metastases. All the patients were scheduled to receive endocrine therapy for 5 years then stop, regardless of actual adherence. Most of the patients entered the study at the time of diagnosis, but some entered later, having already received 2 to 5 years of endocrine therapy, but were scheduled to stop therapy at 5 years.
Main Outcomes
The main outcomes were the rate of distant recurrence (as defined by each trial), regardless of any local or contralateral events; the rate of any breast-cancer event (distant recurrence, locoregional recurrence, or contralateral new primary tumor, regardless of unrelated deaths); and the rate of death from breast cancer, as estimated by means of log-rank subtraction (i.e., subtracting the log-rank statistics from analyses of death without recurrence from causes other than breast cancer from the log-rank statistics for any death), as in previous EBCTCG reports.1–3
Study Oversight
All the authors participated in designing the study, writing the manuscript, and making the decision to submit the manuscript for publication. The members of the EBCTCG secretariat collected and analyzed the data and vouch for the accuracy and completeness of the data and analyses. No commercial sponsors or confidentiality agreements were involved.
Statistical Analysis
We used Kaplan–Meier analyses to assess the associations of breast-cancer outcomes with tumor diameter and nodal status. To smooth single-year irregularities in Kaplan–Meier analyses, for some subgroups we estimated the event rate in a particular time period as the rate (first event per patient-year) for that whole period. We used Cox regression analyses, stratified according to tumor and nodal (TN) status, to assess the associations of event rate ratios with age (<35, 35 to 44, 45 to 54, 55 to 64, and 65 to 74 years at diagnosis) and, when available, with tumor grade (low, moderate, or high), Ki-67 antibody expression (a marker of proliferation in early breast cancer: 0 to 9%, 10 to 19%, or ≥20%), and status with respect to progesterone receptor and human epidermal growth factor receptor type 2 (HER2). All the analyses were stratified according to trial and study group, if more than one study group was assigned to receive 5-year endocrine therapy. For multigroup comparisons, the variance of the log risk in each group was calculated12 and used to obtain group-specific confidence intervals for rate ratios.
Results
Characteristics of the Patients
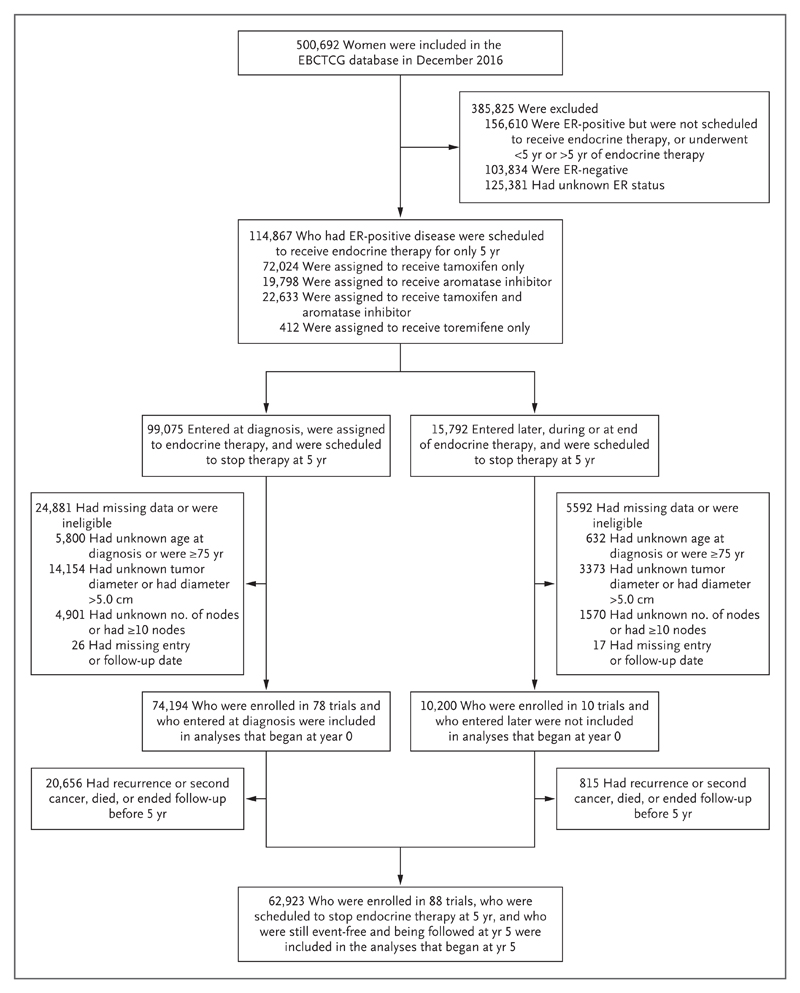
Of the 500,692 women in the EBCTCG trials database, 114,867 had ER-positive, early-stage breast cancer and had been scheduled to receive endocrine therapy for 5 years and then stop. Of these women, 63% had been assigned to receive tamoxifen, 17% to receive an aromatase inhibitor, and 20% to receive some sequence of tamoxifen and an aromatase inhibitor (Fig. 1). After the exclusion of patients who were 75 years of age or older at the time of diagnosis, those who had a tumor diameter of more than 5.0 cm, those who had more than 9 involved lymph nodes, and those with missing data with respect to age or TN status, a total of 74,194 women (in 78 trials) had entered a study at the time of diagnosis and were included in analyses that began at year 0. We included an additional 10,200 women who had entered the study later (at 2, 3, or 5 years) only in analyses that began at year 5, for a total of 62,923 women in 88 trials who were still being followed at 5 years without recurrence or a second cancer.
Figure 1. Selection of Women with Breast Cancer from 88 Trials of Adjuvant Breast-Cancer Therapy.
The analyses combine individual patient data from 88 trials in the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) database of randomized trials. All the women who were included in the study were scheduled to receive adjuvant endocrine therapy for only 5 years, regardless of actual adherence, after a diagnosis of estrogen-receptor (ER)–positive breast cancer before the age of 75 years. A total of 74,194 women who were enrolled in 78 trials at the time of diagnosis were included in the analyses that began at year 0. A total of 62,923 women who were enrolled in 88 trials either at the time of diagnosis or later (having already received 2 to 5 years of endocrine therapy) were scheduled to stop therapy at 5 years and were included in the analyses that began at year 5.
The characteristics of the women in the study are provided in Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org. Approximately 50% of the women had received a diagnosis of breast cancer before 2000 (range, 1976 to 2011). All the women had data on age and TN status. Data were available regarding tumor grade in 43,590 of the patients (69%), progesterone-receptor status in 54,115 (86%), HER2 status (regardless of the use of trastuzumab) in 24,145 (38%), and Ki-67 status in 7692 (12%). No gene-expression profiles were available. Similar numbers of women had undergone breast-conserving therapy and mastectomy; all the women had undergone lymph-node dissection. Approximately three quarters of those with node-positive disease had undergone chemotherapy. The receipt of chemotherapy was strongly correlated with nodal status and, in N0 disease, with tumor diameter and grade. Only a quarter of those with known HER2-positive disease had been scheduled to receive trastuzumab (Table S2 in the Supplementary Appendix).
Analyses of Data Starting at Year 0
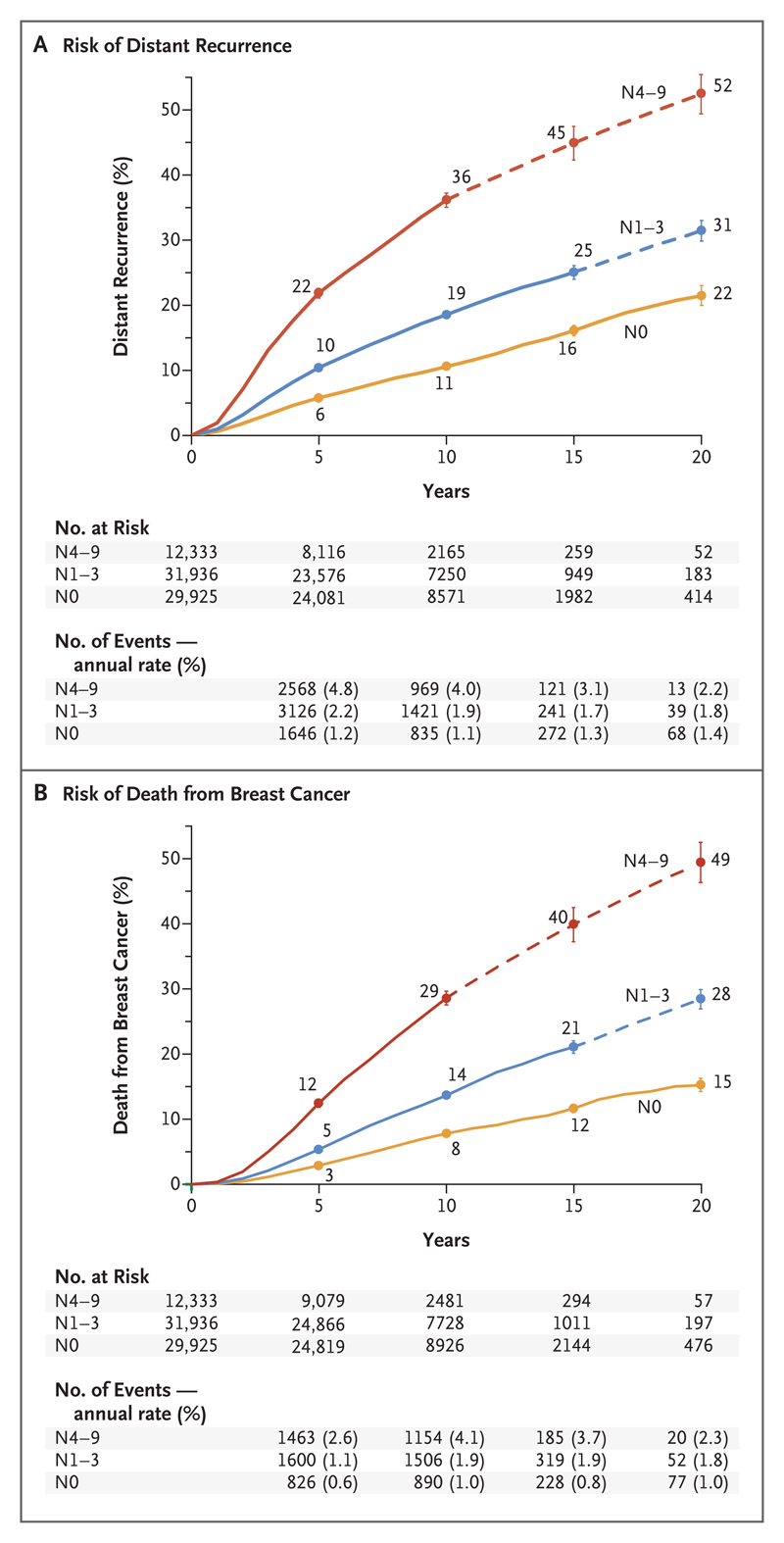
The cumulative risk and annual rates of distant recurrence and death from breast cancer in each 5-year period from year 0 to year 20, subdivided according to nodal status at the time of diagnosis, are shown in Figure 2. Within each category of nodal status, distant recurrences occurred steadily throughout the 20-year period. The annual risk was strongly related (P<0.001) to nodal status, with a 20-year risk of distant recurrence of 22% in women with no positive nodes, 31% in those with one to three positive nodes, and 52% in those with four to nine positive nodes.
Figure 2. Association between Pathological Nodal Status and the Risk of Distant Recurrence or Death from Breast Cancer during the 20-Year Study Period.
Shown are data regarding the risk of distant recurrence (Panel A) and death from breast cancer (Panel B) among 74,194 women with ER-positive T1 or T2 disease who were enrolled in 78 trials at year 0 and were scheduled to receive 5 years of endocrine therapy. (Data for another 10,200 women who enrolled in 10 trials after year 0 are not shown here.) The risk was calculated according to the patients’ pathological nodal status at the time of diagnosis: N0, N1–3, or N4–9. The number of events and annual rate are shown for the preceding period (e.g., data for years 0 to 4 are shown at 5 years). The I bars indicate 95% confidence intervals. The dashed lines indicate that the event rate is for the whole 5-year period, rather than for individual years, as is otherwise shown. The annual rate of death from breast cancer was estimated by subtracting the death rate in women without recurrence from the rate in all women.
As expected in a population that was initially free of recurrence, the annual rates of death from breast cancer were low during the first 5 years (only about half the rates of distant recurrence). However, starting at year 5, the annual rates of death and distant recurrence were similar and the cumulative risk of death from breast cancer lagged behind that of distant recurrence by only a few years. Hence, the 20-year risks of death from breast cancer were 15% with N0 disease, 28% with N1–3 disease, and 49% with N4–9 disease — risks that were not much lower than the 20-year risks of distant recurrence.
Analyses Starting at Year 5
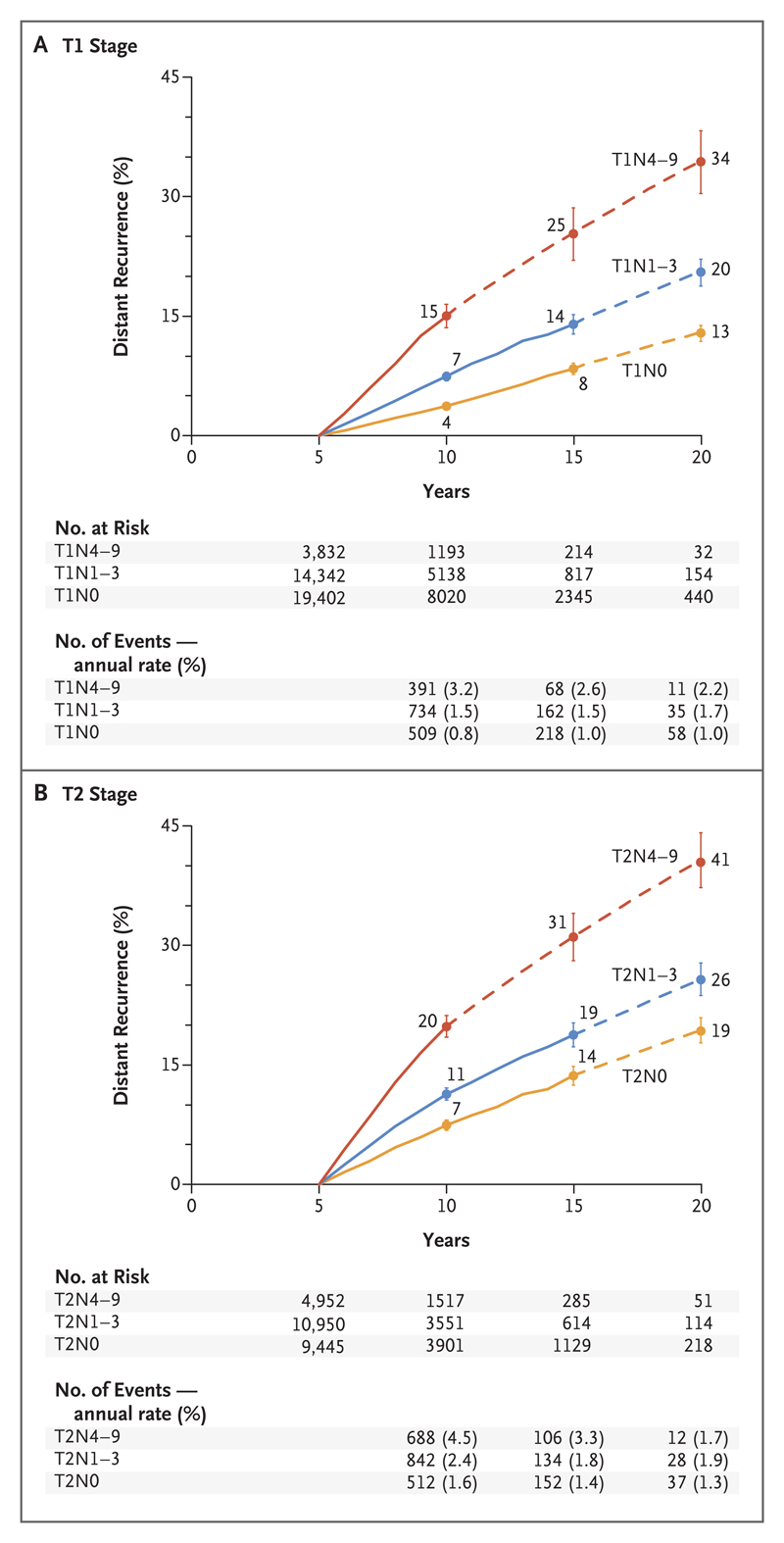
Cumulative risks and annual rates of distant recurrence in each 5-year period during the period from 5 to 20 years for the 62,923 women who reached year 5 without breast-cancer recurrence or any second cancer and who were scheduled to discontinue endocrine therapy are shown in Figure 3. The results are presented separately for T1 and T2 tumors and are subdivided according to nodal status at diagnosis. Although all the women had been clinically disease-free for many years, the original tumor diameter and especially the original nodal status remained powerful determinants of late distant recurrence, even during the second decade after diagnosis. Within each TN-status category, distant recurrences continued to occur steadily throughout the period from 5 to 20 years.
Figure 3. Association between Pathological Nodal Status and the Risk of Distant Recurrence during Years 5 to 20 of the Study, According to Tumor Stage.
Shown are the data for 62,923 women with ER-positive disease who were enrolled in 88 trials of breast-cancer therapy and who initiated therapy either at year 0 or within the first 5 years, according to whether they had T1 disease (Panel A) or T2 disease (Panel B). All the women were scheduled to receive 5 years of endocrine therapy and were event-free and still being followed at year 5. The I bars indicate 95% confidence intervals. The dashed lines indicate that the event rate is for the whole 5-year period, rather than for individual years, as is otherwise shown. Data for the number of events and annual rate begin at 5 years, since the analysis of the risk of distant recurrence starts at year 5.
Even for women with the best prognosis, the risks were appreciable. For those with T1N0 disease, the annual rate of distant recurrence remained approximately 1% throughout the period from 5 to 20 years, resulting in a cumulative risk of distant recurrence of 13% (Fig. 3A). The associations of tumor diameter and nodal status with the risk of distant recurrence during the period from 5 to 20 years were approximately additive, with a progressive increase from 13% for T1N0 to 41% for T2N4–9 disease (Fig. 3B). Similar results were observed for rates of death from breast cancer (Figs. S13 through S16 in the Supplementary Appendix).
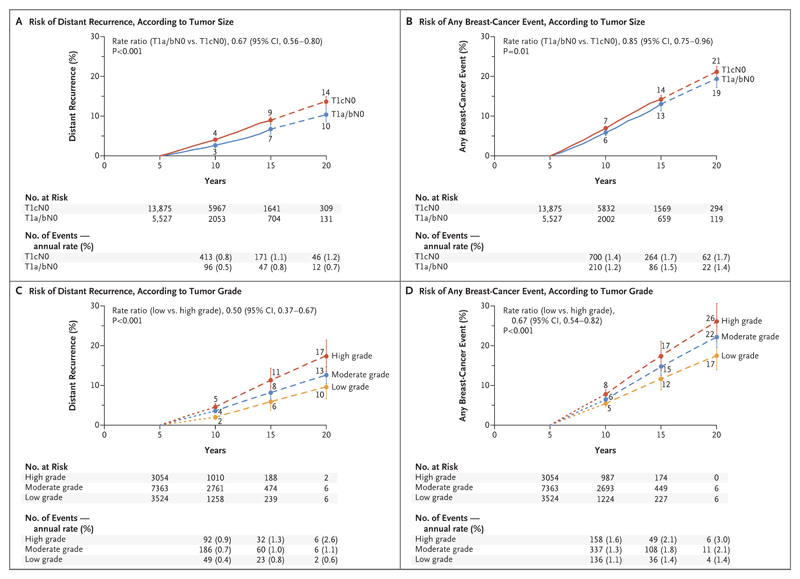
To further explore the possibility of identifying groups at very low risk, we subdivided the rates of distant recurrence and of any breast-cancer event (distant, local, or contralateral) among women with T1N0 disease according to tumor grade and size (T1a or T1b [≤1.0 cm] vs. T1c [>1.0 to 2.0 cm]) (Fig. 4). Although both tumor grade and tumor size significantly affected prognosis in T1N0 disease, even women with T1N0 tumors that were well differentiated or measured 1.0 cm or less in diameter (i.e., T1a or T1b) had appreciable recurrence rates throughout the period from 5 to 20 years. For low-grade T1N0 disease, the absolute risk during the study period was 10% for distant recurrence and 17% for any breast-cancer event (distant, local, or contralateral); the risks were similar for the combined T1a and T1b N0 group.
Figure 4. Association of Tumor Diameter and Tumor Grade with the Risk of Distant Recurrence or Any Breast-Cancer Event during Years 5 to 20 of the Study.
Shown are data for 19,402 women (13,941 of whom had a known tumor grade) with ER-positive breast cancer with a tumor size of 2 cm or less with no spread to lymph nodes (T1N0). All the women were scheduled to receive 5 years of adjuvant endocrine therapy and then discontinue therapy and were event-free and being followed at year 5. The findings were categorized according to tumor diameter (T1a or T1b [≤1.0 cm] vs. T1c [>1.0–2.0 cm]) and tumor grade. Panels A and C show the risk of distance recurrence during years 5 to 20 according to tumor diameter and tumor grade, respectively; Panels B and D show the risk of any breast-cancer event (distant or local recurrence or contralateral onset) during years 5 to 20 according to tumor diameter and tumor grade, respectively. The I bars indicate 95% confidence intervals. The dashed lines indicate that the event rate is for the whole 5-year period, rather than for individual years, as is otherwise shown.
Other Prognostic Factors
The importance of TN status as a determinant of the distant-recurrence rate, and the additional relevance of age, tumor grade, and various tumor markers, are described separately for recurrences during the first 5 years and for those during the subsequent 15 years (to year 20) (Fig. S5 in the Supplementary Appendix). The importance of TN status was similarly strong during both time periods, as was the importance of a young age at the time of diagnosis. In contrast, other factors that were of some additional relevance during the first 5 years were of less, or no, additional relevance thereafter, given the TN status. Tumor grade and the presence of Ki-67 antibody (which were strongly correlated with each other) were important independent factors of prognostic value during the first 5 years but were of only moderate relevance thereafter. Progesterone-receptor status was independently prognostic during years 0 to 5 but not thereafter. Only 2% of all the women in the study were scheduled to receive trastuzumab (Table S2 in the Supplementary Appendix), but in trials with no scheduled trastuzumab, 20,648 women had known HER2 status. Among these women, those with HER2-positive tumors who did not receive trastuzumab had a worse prognosis than those with HER2-negative tumors during years 0 to 5 but not thereafter.
Other Outcomes
Graphs showing the influence of age and tumor characteristics on absolute and relative risks of any breast-cancer event, death from breast cancer, locoregional recurrence, and contralateral breast cancer are provided in Figures S6 though S22 in the Supplementary Appendix. Locoregional recurrence and death from breast cancer showed much the same dependence on risk factors as distant recurrence. However, these risk factors were of little relevance to the risk of contralateral breast cancer, which continued at a rate of approximately 0.3% per year after the cessation of endocrine therapy, independent of age or TN status (Figs. S11 and S12 in the Supplementary Appendix). The risk of death without known recurrence depended chiefly on age but was also somewhat dependent on TN status, which suggests that some of these deaths were in fact from breast cancer and that approximately 5 to 10% of distant recurrences had been missed in trial records (Fig. S23 in the Supplementary Appendix).
Discussion
Among women with ER-positive, early-stage breast cancer who were scheduled to receive only 5 years of adjuvant endocrine therapy, distant recurrences occurred at a steady rate for at least another 15 years after the end of the 5-year treatment period. Throughout this time, the original nodal status and tumor diameter remained remarkably strong determinants of the annual recurrence rate (Table 1). However, even among women with small, node-negative (T1N0), low-grade tumors, there was a risk of distant recurrence of approximately 10% during years 5 to 20. TN status was also a strong determinant of locoregional recurrence, although not of contralateral disease.
Table 1. Association of Tumor Size and Nodal Status and Grade with the Risk of Distant Recurrence in Years 5 to <10 and in Years 10 to 20.*.
| Variable | Women Who Were Event-free at 5 Yr | Annual Rate of Distant Recurrence | Cumulative Risk from 5 Yr to 20 Yr | ||
|---|---|---|---|---|---|
| Total | Chemotherapy Scheduled | 5 to <10 Yr | 10 to 20 Yr | ||
| no. | no. (%) | percent | percent | ||
| Nodal involvement | |||||
| N0 | 28,847 | 9,136 (32) | 1.0 | 1.1 | 15 |
| N1–3 | 25,292 | 17,280 (68) | 1.9 | 1.7 | 23 |
| N4–9 | 8,784 | 6,664 (76) | 3.9 | 2.8 | 38 |
| Tumor diameter in N0 only | |||||
| T1a or T1b: ≤1.0 cm | 5,527 | 910 (16) | 0.5 | 0.8 | 10 |
| T1c: 1.1–2.0 cm | 13,875 | 4,034 (29) | 0.8 | 1.1 | 14 |
| T2: 2.1–3.0 cm | 6,700 | 2,859 (43) | 1.5 | 1.4 | 19 |
| T2: 3.1–5.0 cm | 2,745 | 1,333 (49) | 1.7 | 1.4 | 20 |
| Tumor grade in T1N0 only | |||||
| Low | 3,524 | 401 (11) | 0.4 | 0.8 | 10 |
| Moderate | 7,363 | 1,861 (25) | 0.7 | 1.0 | 13 |
| High | 3,054 | 1,414 (46) | 0.9 | 1.5 | 17 |
Data are for 62,923 women with T1 or T2 estrogen-receptor–positive disease with 0 to 9 positive nodes who were scheduled to receive 5 years of adjuvant endocrine therapy and were disease-free at year 5. Most of the women entered the study at the time of diagnosis, but some entered later, having already received 2 to 5 years of endocrine therapy, and were randomly assigned to stop therapy at 5 years. P<0.001 for all subgroup comparisons.
Given the TN status, the other risk factors were of limited additional prognostic relevance after the cessation of 5-year endocrine therapy. There was a strong association of tumor grade and Ki-67 status with the risk of distant recurrence during years 0 to 5 but only a moderate association during years 5 to 20. Data regarding tumor grade and Ki-67 status were sometimes from local institutions, so accuracy could be variable, but both factors were strongly predictive of 5-year risk, which suggests that these values were assessed with reasonable accuracy. Better assessments should better predict outcome but would probably not alter the observed pattern of substantially weaker associations with distant recurrence after 5 years than before 5 years. Likewise, tumors that were negative for progesterone receptor had a worse prognosis during years 0 to 5 but not thereafter, given the TN status. The data set did not include gene-expression assays,13–17 which may be shown to predict a very low risk of distant recurrence even without further therapy for some women when long-term follow-up data become available.18 However, decisions with respect to extending endocrine therapy will probably still depend on disease stage, so these findings will remain relevant.
Our main aim was to determine whether we could identify subgroups of women who stop endocrine therapy after 5 years in whom long-term risks are so small that any additional benefits from extended therapy would be unlikely to outweigh the additional side effects. However, even among women with T1N0 disease, the cumulative risk of distant recurrence was 13% during years 5 to 20. Although reliable trial evidence is not yet available on the long-term effects of extending endocrine therapy for 5 additional years on mortality,5–9,19–21 an absolute reduction of a few percentage points in the risk of distant metastases over the next 15 years might well be possible even for such low-risk women, with correspondingly greater absolute benefits for women with larger tumors or node-positive disease. Except for women who are older than 70 years of age or those in poor health, these probabilities will not be much attenuated by the risk of death from another cause, since in the absence of breast cancer, more than 80% of the women in the United States in reasonable health at the age of 65 years would be expected to survive beyond the age of 80 years.
Meta-analyses of data from individual patients will eventually reconcile any apparently conflicting findings from the trials of extending endocrine therapy beyond 5 years, especially after 5 years of aromatase inhibitor therapy.5–9,19–21 When these findings are available, the likely benefits of extending therapy will have to be weighed against uncommon but potentially life-threatening side effects, such as bone fracture for aromatase inhibitors3 and pulmonary embolus and (for women with a uterus) endometrial cancer for tamoxifen.2,5,6 The risks of such side effects increase with longer treatment, although the absolute risk of death from them is low (<0.5%).5–9
In addition, tamoxifen and aromatase inhibitors can cause bothersome but non–life-threatening side effects, including menopausal symptoms, arthropathy, and carpal tunnel syndrome, which adversely affect the quality of life. However, such symptoms are quite common even without endocrine therapy. In placebo-controlled trials7–10,22–24 that have systematically sought patient-reported outcomes, women in the placebo group have reported more than half as many episodes as those in the endocrine-therapy group, which highlights the importance of placebo control to assess many pharmacologic side effects. Trials have also shown little difference in discontinuation rates between endocrine therapy and placebo, although in clinical practice substantial numbers of women discontinue adjuvant endocrine therapy prematurely because of symptoms.25,26 However, women who have already completed 5 years of endocrine therapy are less at risk for unexpected symptoms than those who are initiating therapy.
These findings underline the need to help women who are receiving endocrine therapy to discover whether any symptoms are actually caused or exacerbated by therapy. Such patients could try stopping treatment for short periods or switching from one agent to another.26 Switching between an aromatase inhibitor and tamoxifen, or among various aromatase inhibitors, is safer than discontinuing therapy prematurely.3 Adherence may also be helped by interventions that can mitigate some of the symptoms attributed to endocrine therapy,27 including nonhormonal interventions that reduce menopausal symptoms,28,29 musculoskeletal symptoms,30 sexual dysfunction,31,32 and osteoporosis.33
Our study has several limitations. First, the recurrence rates reported here are in women who were scheduled to receive 5 years of endocrine therapy, not in those who completed treatment. Only a few of the trials in our study provided detailed data with respect to adherence, but a substantial minority of women in trials of 5-year endocrine therapy did not complete their treatment.2 Because 5 years of therapy is more effective than only 2 years,1,2 the risks among women who actually completed 5 years of therapy would be somewhat lower than those in our study. However, an expected lower risk with greater adherence is to some extent counterbalanced by unreported breast-cancer events: the association between TN status and the rate of death without reported recurrence suggests that some of these deaths were from unreported breast cancer and that the rate of distant recurrence would have been higher by 5 to 10% with complete ascertainment. Although a persisting recurrence risk among women with ER-positive breast cancer is well recognized,13–17,34 our study quantifies the 20-year risk more reliably than previous studies, since it is large and has long follow-up. However, long follow-up means that most of the patients received the breast-cancer diagnosis well before 2000. Since then, the prognosis for women in particular TN categories has somewhat improved owing to earlier diagnosis, more accurate tumor staging, and better surgical, radiation, and systemic therapies. Gene-expression arrays have also been adopted to guide the use of chemotherapy in ER-positive disease, a factor that has somewhat affected the mix of patients receiving chemotherapy.35 However, it seems unlikely that such factors would have a substantive effect on the generalizability of our findings to current patients, since the use of chemotherapy in our cohort was similar to that in current practice (Table S2 in the Supplementary Appendix).36
Furthermore, we could not reliably assess the relevance of chemotherapy to prognosis after year 5, since the women who received chemotherapy and those who did not receive chemotherapy differed in the extent of nodal involvement, tumor size, tumor grade, and perhaps unrecorded selection factors. The relevance of chemotherapy to prognosis after year 5 is best assessed in meta-analyses of the trials of chemotherapy, since randomization balances known and unknown risk factors between treatment groups. Previous EBCTCG meta-analyses have shown that most of the reduction in the recurrence rate with chemotherapy occurred in the first 5 years, with similar proportional reductions among women with ER-positive and ER-negative disease, but also indicated further benefit in years 5 to 9 with more effective regimens.37 Thus, the risk of recurrence after 5 years may well be somewhat lower in women who receive contemporary chemotherapy than among those in our study.
In addition, only 2% of the women in our study received trastuzumab. Wider use of trastuzumab in women with HER2-positive disease might have improved prognosis after 5 years,38 although again such a hypothesis would be best evaluated by meta-analyses of trastuzumab trials. In trials without trastuzumab, the recurrence risk in years 0 to 4 was higher for HER2-positive tumors than for HER2-negative tumors, but HER2 status was of little relevance to prognosis thereafter. Hence, although HER2 status was unknown for many tumors, the overall findings are applicable to women with ER-positive, HER2-negative disease (i.e., to most women with ER-positive disease).
In conclusion, even after 5 years of adjuvant endocrine therapy, women with ER-positive, early-stage breast cancer still had a persistent risk of recurrence and death from breast cancer for at least 20 years after the original diagnosis. This finding has implications for long-term follow-up strategies and highlights the need for new approaches to reduce late recurrence. The risk could be somewhat reduced by extending the duration of endocrine therapy,5–11 with greater absolute benefits for those at highest risk for recurrence. A major predictor of risk is TN status, although even among women with large, strongly node-positive tumors, most who complete 5 years of endocrine therapy will remain free of distant recurrence 20 years after diagnosis. However, even low-grade T1N0 disease carries an appreciable risk of distant recurrence and contralateral breast cancer, a risk that is sufficient for at least the consideration of extended endocrine therapy. Recognition of the magnitude of the long-term risks of ER-positive disease can help women and their health care professionals decide whether to extend therapy beyond 5 years and whether to persist if adverse events occur.
Supplementary Material
Acknowledgments
Supported by core funding from Cancer Research UK, the British Heart Foundation, and the Medical Research Council (MRC) to the Clinical Trial Service Unit and MRC Population Health Research Unit, Nuffield Department of Population Health, University of Oxford. Drs. Taylor and McGale are supported by a grant (C8225/A21133) from Cancer Research UK.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the women who entered these trials and their caregivers, the practitioners who conducted the studies and shared their data through the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), and the past and present members of the EBCTCG secretariat.
Contributor Information
Kathleen I. Pritchard, Sunnybrook Health Sciences Centre and the University of Toronto, Toronto
Jonas Bergh, Karolinska Institutet and Karolinska University Hospital, Stockholm
Mitch Dowsett, Royal Marsden Hospital and Institute of Cancer Research, London, United Kingdom
Daniel F. Hayes, University of Michigan Comprehensive Cancer Center, Ann Arbor
References
- 1.Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.The Early Breast Cancer Trialists’ Collaborative Group. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patientlevel meta-analysis of randomised trials. Lancet. 2011;378:771–84. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Early Breast Cancer Trialists’ Collaborative Group. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–52. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 4.Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2014;32:2255–69. doi: 10.1200/JCO.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray RG, Rea DW, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early-stage breast cancer. J Clin Oncol. 2013;31(suppl 5) abstract. [Google Scholar]
- 6.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–16. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005;97:1262–71. doi: 10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 8.Mamounas EP, Jeong JH, Wickerham DL, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast and Bowel Project-33 trial. J Clin Oncol. 2008;26:1965–71. doi: 10.1200/JCO.2007.14.0228. [DOI] [PubMed] [Google Scholar]
- 9.Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med. 2016;375:209–19. doi: 10.1056/NEJMoa1604700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher B, Dignam J, Bryant J, et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptorpositive tumors. J Natl Cancer Inst. 1996;88:1529–42. doi: 10.1093/jnci/88.21.1529. [DOI] [PubMed] [Google Scholar]
- 11.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 12.Plummer M. Improved estimates of floating absolute risk. Stat Med. 2004;23:93–104. doi: 10.1002/sim.1485. [DOI] [PubMed] [Google Scholar]
- 13.Sestak I, Dowsett M, Zabaglo L, et al. Factors predicting late recurrence for estrogen receptor-positive breast cancer. J Natl Cancer Inst. 2013;105:1504–11. doi: 10.1093/jnci/djt244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolmark N, Mamounas E, Baehner F, et al. Prognostic impact of the combination of recurrence score and quantitative ER expression (ESR1) on predicting late distant recurrence risk in ER+ breast cancer after 5 years of tamoxifen: results from NRG Oncology/National Surgical Adjuvant Breast and Bowel Project B-28 and B-14. J Clin Oncol. 2016;34:2350–8. doi: 10.1200/JCO.2015.62.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sestak I, Cuzick J, Dowsett M, et al. Prediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian Breast and Colorectal Cancer Study Group 8 and Arimidex, Tamoxifen Alone or in Combination randomized trials using the PAM50 risk of recurrence score. J Clin Oncol. 2015;33:916–22. doi: 10.1200/JCO.2014.55.6894. [DOI] [PubMed] [Google Scholar]
- 16.Dubsky P, Brase JC, Jakesz R, et al. The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer. 2013;109:2959–64. doi: 10.1038/bjc.2013.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sgroi DC, Sestak I, Cuzick J, et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the Trans-ATAC study population. Lancet Oncol. 2013;14:1067–76. doi: 10.1016/S1470-2045(13)70387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris LN, Ismaila N, McShane LM, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34:1134–50. doi: 10.1200/JCO.2015.65.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mamounas EP, Bandos H, Lembersky BC, et al. A randomized, double-blinded, placebo-controlled clinical trial of extended adjuvant endocrine therapy with letrozole in postmenopausal women with hormone-receptor-positive breast cancer who have completed previous adjuvant treatment with an aromatase inhibitor. In: Proceedings of the 2016 San Antonio Breast Cancer Symposium, San Antonio, TX. Cancer Res. 2017;77(Suppl 4):S1–05. abstract. [Google Scholar]
- 20.Tjan-Heijnen VCG, Van Hellemond IEG, Peer PGM, et al. Extended adjuvant aromatase inhibition after sequential endocrine therapy (DATA): a randomised, phase 3 trial. Lancet Oncol. 2017 Oct 11; doi: 10.1016/S1470-2045(17)30600-9. ( http://www.thelancet.com/pdfs/journals/lanonc/PIIS1470-2045(17)30600-9.pdf) [DOI] [PubMed] [Google Scholar]
- 21.Blok EJ, Kroep JR, Meershoek-Klein Kranenbarg E, et al. Optimal duration of extended adjuvant endocrine therapy for early breast cancer; results of the IDEAL trial (BOOG 2006-05) J Natl Cancer Inst. 2018 Jan 1; doi: 10.1093/jnci/djx134. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 22.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 23.Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–91. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 24.Sestak I, Harvie M, Howell A, Forbes JF, Dowsett M, Cuzick J. Weight change associated with anastrozole and tamoxifen treatment in postmenopausal women with or at high risk of developing breast cancer. Breast Cancer Res Treat. 2012;134:727–34. doi: 10.1007/s10549-012-2085-6. [DOI] [PubMed] [Google Scholar]
- 25.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–8. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henry NL, Azzouz F, Desta Z, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol. 2012;30:936–42. doi: 10.1200/JCO.2011.38.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayes DF. Follow-up of patients with early breast cancer. N Engl J Med. 2007;356:2505–13. doi: 10.1056/NEJMcp067260. [DOI] [PubMed] [Google Scholar]
- 28.Loprinzi CL, Barton DL, Rhodes D. Management of hot flashes in breast-cancer survivors. Lancet Oncol. 2001;2:199–204. doi: 10.1016/S1470-2045(00)00289-8. [DOI] [PubMed] [Google Scholar]
- 29.Stearns V, Ullmer L, López JF, Smith Y, Isaacs C, Hayes D. Hot flushes. Lancet. 2002;360:1851–61. doi: 10.1016/s0140-6736(02)11774-0. [DOI] [PubMed] [Google Scholar]
- 30.Henry NL, Banerjee M, Wicha M, et al. Pilot study of duloxetine for treatment of aromatase inhibitor-associated musculoskeletal symptoms. Cancer. 2011;117:5469–75. doi: 10.1002/cncr.26230. [DOI] [PubMed] [Google Scholar]
- 31.Loprinzi CL, Abu-Ghazaleh S, Sloan JA, et al. Phase III randomized double-blind study to evaluate the efficacy of a polycarbophil-based vaginal moisturizer in women with breast cancer. J Clin Oncol. 1997;15:969–73. doi: 10.1200/JCO.1997.15.3.969. [DOI] [PubMed] [Google Scholar]
- 32.Goetsch MF, Lim JY, Caughey AB. A practical solution for dyspareunia in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2015;33:3394–400. doi: 10.1200/JCO.2014.60.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brufsky A, Harker WG, Beck JT, et al. Zoledronic acid inhibits adjuvant letrozole-induced bone loss in postmenopausal women with early breast cancer. J Clin Oncol. 2007;25:829–36. doi: 10.1200/JCO.2005.05.3744. [DOI] [PubMed] [Google Scholar]
- 34.Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738–46. doi: 10.1200/JCO.1996.14.10.2738. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Kurian AW, Bondarenko I, et al. The influence of 21-gene recurrence score assay on chemotherapy use in a populationbased sample of breast cancer patients. Breast Cancer Res Treat. 2017;161:587–95. doi: 10.1007/s10549-016-4086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Cancer Institute. (SEER) Program. Research Data (1973-2014), DCCPS, Surveillance Research Program, Surveillance Systems Branch. released April 2017 ( https://www.seer.cancer.gov/)
- 37.The Early Breast Cancer Trialists’ Collaborative Group. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of longterm outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–44. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32:3744–52. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.