Abstract
Purpose
The comparative effectiveness of continuing or discontinuing long-term alendronate (ALN) on fractures is unknown. A large pragmatic ALN discontinuation study has potential to answer this question.
Methods
We conducted a 6-month pilot study of the planned The Effectiveness of DiscontinuinG bisphosphonatEs (EDGE) study among current long-term ALN users (women aged ≥65 with ≥3 years of ALN use) to determine study work flow and feasibility including: evaluating the administrative aspects of trial conduct (e.g. time to contract, IRB approval); assessing rates of site and participant recruitment; and evaluating post-randomization outcomes, including adherence, bisphosphonate-associated adverse events, and participant and site satisfaction. We assessed outcomes 1 and 6 months after randomization.
Results
Nine sites participated, including 7 community-based medical practices, and 2 academic medical centers. On average (SD) contract execution took 3.4 (2.3) months and IRB approval took 13.9 (4.1) days. Sites recruited 27 participants (13 to continue ALN, and 14 to discontinue ALN). Over follow-up, 22% of participants did not adhere to their randomization assignment: 30.8% in the continuation arm and 14.3% in the discontinuation arm. No fractures or adverse events were reported. Sites reported no issues regarding work flow, and participants were highly satisfied with the study.
Conclusions
Administrative procedures of the EDGE study were generally feasible, with minimal disruption to clinic flow. In this convenience sample, participant recruitment was suboptimal across most practice sites. Accounting for low treatment arm adherence, a comprehensive recruitment approach will be needed to effectively achieve the scientific goals of the EDGE study.
Keywords: Alendronate, Osteoporosis, Discontinuation, Drug Holiday
INTRODUCTION
Bisphosphonates, the most commonly used anti-osteoporosis medication, significantly reduce clinical fracture risk.[1, 2] However, bisphosphonates have prolonged skeletal retention and have been associated with long-term safety concerns such as atypical fractures and osteonecrosis of the jaw (ONJ). The optimal duration of bisphosphonate therapy and the need for possible “drug holidays” is a topic of considerable international controversy.[3–7] The decision to consider “drug holidays” is further complicated by the inconsistency between bone turnover markers and fracture risk.[8, 9] A delicate equipoise surrounding the decision to consider “drug holidays” exists for both patients and physicians.
Although there have been a few head-to-head randomized controlled trials (RCTs) of bisphosphonates in postmenopausal osteoporosis, these studies have focused on surrogate outcomes like bone mineral density (BMD) [10–13], but not fractures or optimal duration of bisphosphonate use.[3, 14] Safety of longer-term bisphosphonates [15–29] and drug holidays have been incompletely studied in RCTs due to inadequate sample size and sufficient follow-up time needed to provide optimal clinical decision-making information. For example, the Fracture Intervention Trial Long-term Extension (FLEX) study had limited power to detect modest differences in fracture rates.[30] Despite the lack of evidence, many patients have stopped taking or have been instructed to discontinue bisphosphonates. Roughly half as many persons are using bisphosphonates annually in the United States (US) compared to peak use in the mid-2000s.[7] This reduction in the most commonly used osteoporosis therapy has created concern about a crisis in osteoporosis management.[31]
Additionally, traditional RCTs, such as the FLEX trial, have limited generalizability to ‘real world’ patients and heterogeneous community medical practice settings. Pragmatic clinical trials (PCTs) were designed to include randomization procedures with the use of minimal inclusion/exclusion criteria and allow for the study of ‘real world’ patient populations who are often excluded from traditional RCTs. Osteoporosis, PCTs could provide larger subject numbers and longer-term surveillance data of fractures and mortality, the clinically relevant endpoints.[32, 33] To achieve maximal generalizability, patient recruitment should occur in community-based medical practices. However, the ability of sites, particularly community sites, to recruit and retain long-term bisphosphonate users in such a study is largely unknown.
We designed the Effectiveness of DiscontinuinG bisphosphonatEs (EDGE) study as a PCT to determine the effectiveness of randomizing alendronate (ALN) users to continue or discontinue ALN after at least three years of past therapy. ALN, generic since 2008, is the most widely prescribed bisphosphonate, and women aged 65+ constitute the vast majority (~90%) of alendronate users.[7] The key study question was to define the optimal duration of bisphosphonate therapy. Here we report the results of the 6-month pilot trial conducted to assess the feasibility and operational aspects of a future full scale study. The pilot focused on: 1) evaluating administrative aspects (e.g. time to contract, institutional review board (IRB) approval) of conducting such a PCT, 2) assessing site and participant recruitment rates, and 3) evaluating post-randomization: adherence to the study protocol, feasibility of data collection, and participant and site satisfaction.
METHODS
EDGE Pilot Overview
To test study procedures in different clinical settings, we sought to enroll 36 participants from community-based practices affiliated with the American Academy of Family Physicians (AAFP) National Research Network (NRN) (http://www.aafp.org), community-based osteoporosis specialty practices affiliated with Osteoporosis Net (OsNET) (http://www.osnet.org/), a clinical research network, and a convenience sample of academic medical centers focused on osteoporosis care delivery. Once sites were selected, long-term ALN users at each site were identified and enrolled using an interactive electronic informed consent tool.[34] Following consent, participants were randomized to either continue or discontinue ALN. We followed participants for up to 6-months post-randomization for outcome ascertainment. We used a computer assisted telephone (CATI) interface (SurveyMonkey, San Mateo, CA) to administer the surveys at 1- and 6- months.
Study Site Recruitment and Enrollment
Participating sites were identified and selected that might qualify for the future EDGE study based upon the site self-reporting a large number of female patients ≥ 65 years of age. We also selected sites that had heterogeneous characteristics with respect to urban/rural variation, racial and ethnic minority representativeness, and socioeconomic diversity. We selected a convenience sample of academic medical centers with specialists focused on osteoporosis, based on a known large proportion of ALN users estimated from a review of 2011-2013 Medicare data.
In order to streamline administrative processes we developed a standardized “fee-for-service” agreement and “scope of work” template that was used by sites. The University of Alabama at Birmingham’s (UAB) Institutional Review Board (IRB) served as central IRB for eight of the nine sites, meaning that UAB was the IRB of record. Following execution of agreements and IRB approval, we provided each site with participant recruitment materials that included: detailed instructions for (1) participant recruitment, (2) use of tablet computers (iPads) for participant data capture, and (3) procedures for the consenting processes. We hosted webinars with each site, to review the study aims, protocol, and visit procedures. Before any participants were recruited, we verified each site’s principal investigator and associated staff were certified to conduct human subjects’ research.
Study Participant Recruitment
Our pilot study’s minimal eligibility criteria mirrored the anticipated criteria for the future EDGE study: women ≥65 years of age with Medicare coverage who had a current ALN prescription, a history of >3 consecutive years of ALN use with a maximum cumulative allowable treatment gaps <90 days, currently under the care of the enrolling physician, and no history of any other metabolic bone disease (e.g. Paget’s disease of bone). Participants were identified, consented, and randomized at their health care provider’s office during a single visit. The provider and staff answered questions and provided assistance if needed, but allowed participants to self-navigate the consent process on the study provided tablet computer.
Since we specifically designed EDGE as a PCT to diminish barriers to participant recruitment, and given the high degree of heterogeneity among recruitment sites (e.g., organizational structure, practice size, type and level of clinical trials support staff involvement), we left the specifics of participant recruitment to each site’s discretion.[34] In general, two approaches for participant recruitment were used: “Real-time” and “Pre-selection” recruitment. Using the “pre-selection” approach, eligible participants were identified by searching the electronic health record or database systems at each site. Participants meeting inclusion criteria were scheduled (individually or as a group) for a study visit and offered participation. For sites that lacked the capacity to readily query electronic medical records or databases ahead of routine care visits, the “real-time” recruitment approach was used. With this more traditional clinical trials approach, a staff member identified all potential eligible patients at check-in. Patients deemed eligible for the study were offered participation. All interested patients, from both approaches, participated in the informed consent process.
Informed Consent
Informed consent was delivered through an interactive tablet based system developed in collaboration with Mytrus, Inc. (San Francisco, CA), which was approved by the UAB and Cleveland Clinic IRBs, the only site not relying on the central IRB. Development of this platform has previously been described,[34] but in brief, this integrated system delivered an electronic informed consent (e-consent) and provided real time randomization. The study-supplied tablet delivered the e-consent and included an introductory study video, visual and audio informed consent material, and multiple choice quiz questions on key elements of the informed consent. Prior to this study, we pilot tested this process and found it to be acceptable to both patients and study site staff.[34] To finalize the informed consent, each participant provided their electronic signature, social security number (needed for linkage to Medicare data, another source to capture exposure and outcome data), and completed demographic questions. The site staff or physician reviewed the informed consent and certified the participant’s qualifications. We compensated participants $50 at enrollment and $25 for each completed follow-up survey.
Randomization Process
Participants were randomized to either continue or discontinue ALN, using a blocked scheme, dynamically provided to the healthcare provider in real-time, during the office visit, through the study-provided EDGE tablet computer. In keeping with the pragmatic nature of the study, notification of the randomization assignment to the participant was left to the discretion of the provider.[34]
Study Outcomes
We collected information on three types of outcomes: 1) administrative procedures such as start-up time, 2) participant outcomes including adherence to study arms, safety, and satisfaction, and 3) site outcomes including participant recruitment and personnel satisfaction.
Administrative procedure outcomes included length of time for each site to complete contracting and IRB agreements. The first participant outcome included study arm adherence. Adherent participants included those who were assigned to continue ALN and reported maintaining ALN use, as well as those assigned to discontinue ALN who were not using ALN after 6-months. Additionally, participants were asked if they had begun taking any other prescribed medicine for their osteoporosis. Participants who withdrew, were lost-to-follow-up, or crossed over from their assigned arm were considered non-adherent.
Our post-enrollment participant safety outcomes in the pilot and considered for the full-scaled study included: fractures, ONJ, healthcare facility stays (e.g. hospital, nursing home), length of time to complete the follow-up surveys, and participant satisfaction with: compensation, the informed consent process, and the time required to enroll in the study. We planned to adjudicate all presumed fracture and ONJ cases using the criteria defined by the American Society for Bone and Mineral Research (ASBMR) for atypical femur fractures and ONJ.[6, 35] We also queried about anxiety around physical activity and falling to determine if individuals were altering their activity/behavior post-randomization based on potential fears of being on/off a bisphosphonate. We ascertained anxiety using questions adapted from the Patient Reported Outcome Measurement System (PROMIS) surveys [36, 37] including: “During this study, did you have any anxiety about any physical activities you might do during a typical day, such as going to a gym, taking a walk, gardening, doing laundry or housework, or walking your dog?” and “During this study, did you have any anxiety about falling?”
To assess participant satisfaction, we asked the following questions with a Likert-like response set (1-low to 7- high): 1) rank your ability to complete the informed consent process without any staff assistance; 2) how satisfied were you with the time required to enroll in the study?; 3) how satisfied were you with the level of compensation for your time to enroll in the study?; 4) rank your ability to complete the 1-month follow-up survey; 5) rank your ability to complete the 6-month follow-up survey; 6) how satisfied were you with level of compensation for your time to complete these surveys.
Site outcomes included participant recruitment and personnel satisfaction. For participant recruitment, we were interested in the proportion of participants enrolled and the length of time to enroll participants by site, specifically, the time to the first and last participant enrolled. Personnel satisfaction was measured at the end of the study through a series of questions containing Likert response sets ranging from 1 (strongly disagree) to 7 (strongly agree) including: 1) Completion of contracting with UAB was complicated, 2) obtaining IRB approval with UAB was difficult, 3) the remote training session for the EDGE pilot study was clear and easy to follow, 4) the study procedures binder with the tablet guide was clear and easy to use, 5) participant enrollment for the EDGE pilot study disrupted clinic flow, 6) validating participant data on the iPad was a time-consuming task for me/my staff, 7) participants liked the EDGE study questions and concept. Additionally, we asked the time it took to recruit and enroll participants in the pilot study (1: very long – 7: very short).
Covariates
Surveys were performed post-randomization to streamline enrollment process and limit the amount of data collected by sites at baseline. We collected information on self-reported: height, weight, cigarette use, alcohol consumption, use of a walking aid, falls in the last year, personal and family fracture history at one month post-randomization. We assessed current, past use and duration of osteoporosis medications, estrogen, glucocorticoids, calcium, vitamin D, and strontium supplementation, as well as general health rating, and activities of daily living at both the one and six month surveys.
Statistical Analysis
We descriptively evaluated the administrative outcomes. We calculated mean and standard deviations (SD) for contract execution and IRB approval time. Secondly, we assessed the proportion of participants enrolled and the amount of time (mean, SD) to enroll participants. We compared demographic and comorbidity variables collected by randomization arm, using t-tests for continuous variables and chi-square tests for categorical variables. Using a per-protocol approach, we calculated the proportion of participants adhering to randomized arm after 6-months. Lastly, we calculated the median scores (interquartile ranges) for participant and site satisfaction. All analyses were performed in SAS v.9.3 (Cary, NC) and Stata v.12 (College Station, TX).
RESULTS
Nine sites participated in the EDGE pilot study, including seven community physicians and two academic medical centers in six states (AL, CO, NM, CT, CA, and PA). Of the community sites, three were affiliated with the AAFP NRN, two were affiliated OsNET, and two were rheumatology research specialty clinics. All sites had various degrees of research experience, with two having limited research experience. On average (SD) it took 3.4 (2.3) months to execute contracts and 13.9 (4.1) days for IRB approval (Figure 1).
Figure 1.
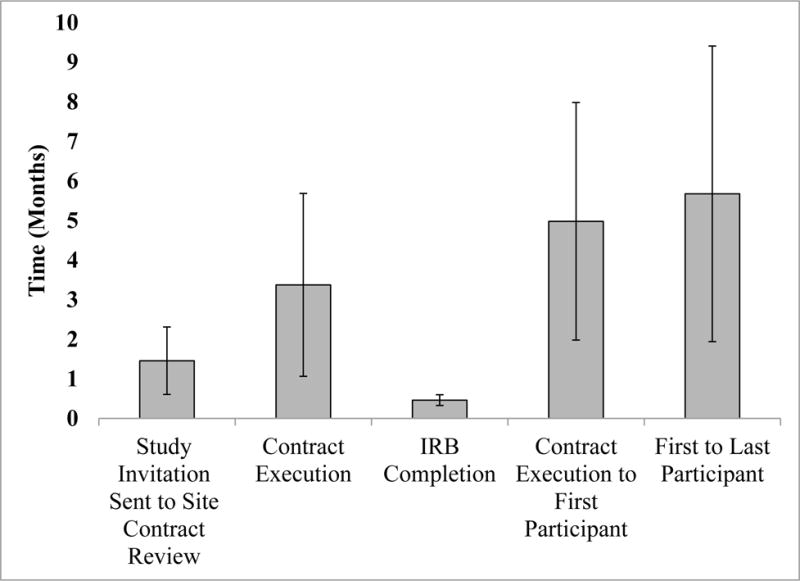
Time in Months of Administrative and Participant Recruitment Outcomes
Participant recruitment occurred over 22 months from April of 2014 through December of 2015. The majority of practices in our pilot study (n = 8) employed the “pre-selection” method or variation thereof, to target eligible patients for participation. We enrolled 27 (75%) of the targeted 36 participants. Two sites, a community practice and a rheumatology research specialty clinic, did not enroll any participants. For sites enrolling at least one participant, the average (SD) time from study initiation to the first participant recruited was 5.0 (3.0) months (Figure 1). The average (SD) time from the first participant to the last participant enrolled was 6.0 (3.7) months.
Of the 27 participants enrolled, 13 were randomized to continue ALN and 14 were randomized to discontinue ALN. Participants were on average (SD) 74.5 (6.1) years old, had used ALN for 5.1 (2.5) years and 14.8% were non-Caucasian (Table 1). Nearly 40% had history of previous fracture, 63% had history of other osteoporosis medication use, 85% were current vitamin D supplementation users, and 67% reported calcium supplementation (Table 1). The 1-month surveys took an average (SD) of 12.9 (2.9) minutes to complete, and the 6-month surveys took 5.7 (2.3) minutes to complete.
Table 1.
EDGE Pilot Study Participant Demographics by Study Arm
| Continue (N=13) |
Discontinue (N=14) |
Population (N=27) |
|
|---|---|---|---|
| Age, Mean (SD) | 74.4 (6.7) | 74.5 (5.9) | 74.5 (6.1) |
| Race, N (%) | |||
| White | 11 (84.6) | 12 (85.7) | 23 (85.2) |
| Black | 1 (7.7) | 2 (14.3) | 3 (11.1) |
| Other | 1 (7.7) | 0 (0.0) | 1 (3.7) |
| Education, N (%)* | |||
| Less than High School | 0 (0.0) | 1 (7.1) | 1 (3.7) |
| High School or G.E.D. | 5 (38.5) | 4 (28.6) | 9 (33.3) |
| Some College | 3 (23.1) | 3 (21.4) | 6 (22.2) |
| 4-year college degree or higher | 5 (38.5) | 5 (35.7) | 10 (37.0) |
| Body Mass Index, N (%)* | |||
| Underweight | 0 (0.0) | 1 (7.7) | 1 (3.7) |
| Normal | 9 (69.2) | 6 (46.2) | 15 (56.0) |
| Overweight | 4 (30.8) | 4 (30.8) | 8 (29.6) |
| Obese | 0 (0.0) | 2 (15.4) | 2 (7.4) |
| Previous History of Fracture, N (%) | 5 (38.5) | 5 (35.7) | 10 (37.0) |
| ALN Duration years, Mean (SD) | 4.8 (2.1) | 5.3 (3.1) | 5.1 (2.5) |
| History of Other Osteoporosis Medication Use, N (%) | 8 (61.5) | 9 (64.3) | 17 (63.0) |
| Vitamin D Supplementation, N (%) | 11 (84.6) | 12 (85.7) | 23 (85.2) |
| Calcium Supplementation, N (%) | 10 (76.9) | 8 (57.1) | 18 (66.7) |
| General Health, N (%) | |||
| Excellent | 6 (46.5) | 3 (21.4) | 9 (33.3) |
| Very Good | 2 (15.4) | 4 (28.6) | 6 (22.2) |
| Good | 4 (30.8) | 5 (35.7) | 9 (33.3) |
| Fair | 1 (7.7) | 2 (14.3) | 3 (11.1) |
| Poor | |||
| SF-36 Physical Function Score, Mean (SD) | 2.39 (0.54) | 2.43 (0.55) | (0.54) |
Numbers do not add to total number of participants randomized due to 1 participant skipping the education status question, and 1 declining to provide height/weight
Over follow-up, 30.8% (n= 4) of the participants in the continuation and 14.3% (n=2) of the participants in the discontinuation arms were not adherent (Figure 2). Two of the continuation arm participants stopped using ALN over the 6 months (one discontinued based on medication costs, and the other switched to a different osteoporosis medication), and two were lost-to-follow up. In the discontinuation arm, one participant restarted ALN and one was lost-to-follow up. The difference in non-adherence to the randomized treatment arm was not significantly different (p = 0.303). No fractures or other adverse events (ONJ or hospital stays) were reported over follow-up. We did not observe any differences in anxiety around performing physical activities or falling between the two groups. The mean (SD) anxiety around physical activity was 1.09 (0.09) in the continuation and 1.23 (0.23) in the discontinuation arm (p = 0.603), and the mean (SD) anxiety around falling was 1.64 (0.39) in the continuation and 1.23 (0.12) in the discontinuation arm (p = 0.296).
Figure 2. Study Arm Adherence at 6-month Follow-up.
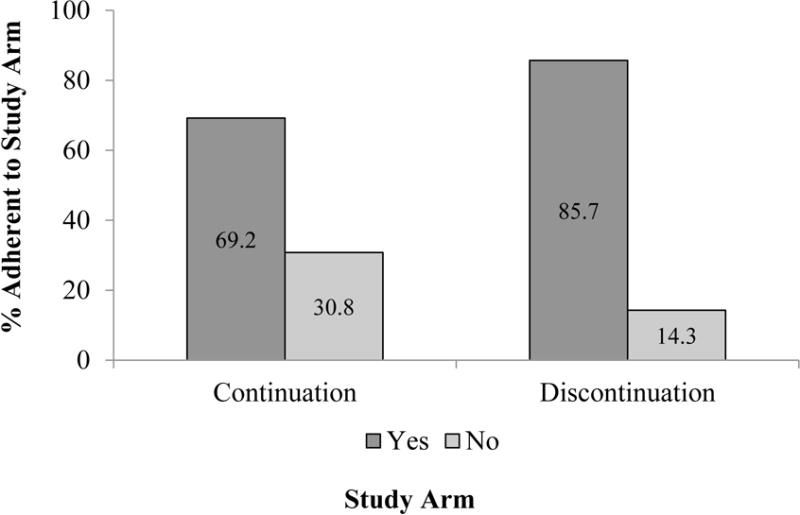
X2 P = 0.303
Among participants remaining in the study through 6-months, we observed high satisfaction with the study. Median satisfaction score with the ability to complete the one month survey was 7 (scale 1-7, higher value denotes higher satisfaction), with no difference between the two groups. The median satisfaction on the ability to complete the six month survey was 7.0, and the median satisfaction with compensation for completing surveys was 7.0, again with no differences observed between the groups.
We received responses on the site satisfaction surveys from 24 staff at the nine sites. Respondents included executive directors, nurse practitioners, and research managers. With respect to the completion of contracting necessary for the study (scale 1-7), sites gave the contracting procedures a median score of 3.3, a neutral rating (Figure 3). Sites did not rank obtaining IRB approval as a difficult task (median score: 1.5). The remote training session and the study binder were each given a median score of a 7 on being clear and easy to follow/use. Sites strongly disagreed that the EDGE study disrupted clinic workflow (median score: 1), and did not think that validating patient data was time consuming (median score: 2.7) (Figure 3). Providers perceived that patients were interested in the EDGE study questions (median score: 5.8) Sites estimated that it took an average (SD) of 35 (13.5) minutes to randomize and enroll participants in the study (range: 10-50 minutes).
Figure 3.
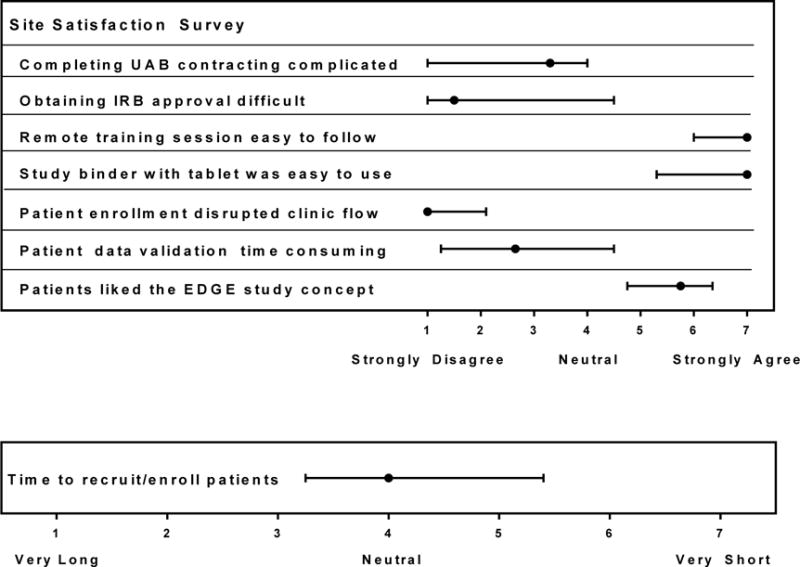
Median (range) Values of Site Satisfaction
DISCUSSION
The goal of the future EDGE trial is to determine the optimal duration of bisphosphonate use. In this pilot for EDGE, we found that the study administrative procedures were generally feasible, with minimal disruption to clinic flow. However, in our convenience sample of sites, participant recruitment was suboptimal; we were only able to recruit 75% of our participant goal, and over 20% of participants either crossed over from their randomized study arm to the alternate arm or dropped out. Reasons for suboptimal recruitment included a surprisingly limited pool of eligible patients on ALN, even among osteoporosis specialists, limited resources to support robust recruitment efforts, and prolonged time to study start-up.
The limited pool of eligible users could be due in part to our choice of the osteoporosis medication for this study. We chose ALN since it historically dominated the bisphosphonate therapy market. ALN represented 60% of prescriptions among new users of anti-osteoporosis medications in 2007-2009 national US Medicare data[38], and in our examination of 2009-2011 Medicare data, we also found that among long-term osteoporosis medications users, 78% were using ALN compared to only 13.9% for risedronate and 8.1% ibandronate.[39] While ALN still constitutes one of the primary first line osteoporosis medications, many women have already discontinued bisphosphonate therapy either on their own or under their physician’s recommendation, thus reducing the pool of available women.[31, 40]
Although a strength of PCT design allows one to include community sites and achieve greater generalizability of the findings, very low numbers of women meeting our rather basic inclusion criteria were seen in our community sites in a given day. We have previously estimated that there are over 500,000 long-term ALN users in the US[39], but it became evident that these women are not seen at practices at the frequency needed to meet recruitment goals. Most sites recognized this early, and when possible, chose to screen their databases for potential participants instead of utilizing the real-time recruitment method. Recruitment could be improved in the future EDGE study if we require sites to pre-screen participants.
In addition, to our recruitment challenges, we also observed that a moderate proportion of participants crossed-over from their randomization assignment or dropped out; 30.8% randomized to continue ALN and 14.3% randomized to discontinue ALN were not adherent to their assigned arm over the 6-month study. Half of our non-adherent participants were lost to follow-up, thus we do not know if they continued with their assigned study arm. However, in the future study, participants could be linked to Medicare or other administrative data claims, thus making treatment and some outcome information accessible. Medicare linkage was not established in this pilot given the length of time to ascertain and acquire Medicare claims data.
Although the cross-over proportions are based on a small number of participants and may not be generalizable to all women taking ALN, the results from this pilot have further informed our statistical assumptions for powering the future EDGE study. Based on the fracture probabilities observed in the Fracture Intervention Trial [30, 41], we powered the future EDGE study to ensure that we would maintain 80% power even if up to 50% of patients were lost to follow-up. Given the number of participants censored and lost-to-follow-up that we observed in the pilot, we should be able to maintain sufficient power as originally proposed with a sample size of 9,500 patients (4,750 per study arm). However, this pilot highlights the difficulty we may face conducting EDGE as originally designed, and has caused us to critically reassess our planned recruitment methods. Further pilot testing focused on different recruitment/enrollment methods including a direct-to-patient approach is needed.
In the 6-month follow-up we did not observe any fractures or other adverse events; however, we did not obtain information on changes in BMD. Although a limitation, this was done to keep with the pragmatic nature of the design and not force busy community-based practices to retrieve information that may not be readily available. Previous formative work [42] and clinical experiences revealed there might be heightened concern or fear around physical activity and falling, particularly in those women randomized to discontinue medication. We found that anxiety around changes to physical activity or falling was low, and although the discontinuation arm had slightly higher anxiety scores, there was no significant difference between groups.
Although recruitment and adherence to the study protocol were significant challenges, the participant and site experience was overall positive. Participants were very satisfied with the amount of time for the study procedures and level of compensation. Procedures associated with the EDGE study did not disrupt overall clinic workflow and there was modest to strong agreement around the usefulness of the tools provided and the overall EDGE project itself. While this study had a relatively light “foot print” and demand of staff resources, one lesson we learned was that significant attention must be paid to keeping these busy physicians and their staff engaged. This was particularly challenging with one of our community practice sites that failed to enroll a participant. We attempted to supplement our recruitment efforts by including osteoporosis specialty clinics in this pilot study. We found that the number of long-term users of ALN was also relatively low at these specialty clinics, as was the case with our second site that failed to enroll any participants. Given the recruitment challenges identified in this pilot it is clear we will need to develop a more comprehensive/multi-faceted approach to readily identify and target eligible participants for the future large-scale EDGE study. Our team is critically evaluating the planned patient recruitment methods and is considering a direct-to-patient approach. This may require supplementation from other administrative databases and large health maintenance organizations.
Efficacy of bisphosphonates is well established, but with the reports of the rare yet serious adverse events particularly associated with long-term use, patients and providers have more questions surrounding duration of therapy. Recent scientific [43] and lay press [40] articles have highlighted a crisis associated with the decreasing use of osteoporosis medications, and have made the observation that previously declining hip fracture rates in the US have begun to plateau.[44] While the FLEX study found a reduction in clinical vertebral fracture risk in those who continued ALN, it found no differences in overall rates of non-vertebral fractures and hip fractures between women randomized to continue ALN for 10-years compared to 5-years of ALN followed by 5-years of placebo.[30] FLEX was underpowered to assess and provide definitive information on fracture risk and did not investigate the optimal duration of treatment, and therefore does not provide information patient and providers need to make an informed decision of whether to continue or stop ALN. The future EDGE study aims to provide this evidence and assess the cumulative incidence of clinical fractures and determine the optimal duration of bisphosphonate use. Although in our convenience sample, participant recruitment was suboptimal across most practice sites, we found the administrative procedures of the EDGE study were generally feasible, with minimal disruption to clinic flow. A comprehensive/multi-faceted recruitment approach will be needed to effectively achieve the scientific goals of the EDGE study, which would provide rigorous and timely information on the effectiveness of long term bisphosphonate use.
Acknowledgments
The authors would like to extend their most sincere appreciation to the physicians and staffs of the participating community practices for their diligent efforts and support of this project. The research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases under award number: 1R21AR062300. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Footnotes
Financial Conflicts of Interest
NCW – Research Contracts: Amgen; Consultant: Pfizer
PJF – None
ASM – None
JAM – None
EML – Research Contracts: Amgen, Merck, Lilly; Scientific Advisory Boards: Amgen, Merck, Lilly, Radius Health, Shire, Alexion, AbbVie; Speakers’ Bureau: Shire, Alexion.
WJS – None
JRC – Research Contracts: Amgen; Consultant: Amgen
GRC – None
MID – None
MLK – Research Contracts: Amgen
CEL – None
SLM – None
DTR – None
AHW – None
KGS – Research Contracts: Amgen
References
- 1.Ensrud KE, et al. Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007;62(7):744–51. doi: 10.1093/gerona/62.7.744. [DOI] [PubMed] [Google Scholar]
- 2.Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006;35(Suppl 2):ii37–ii41. doi: 10.1093/ageing/afl084. [DOI] [PubMed] [Google Scholar]
- 3.Compston JE, Bilezikian JP. Bisphosphonate therapy for osteoporosis: the long and short of it. J Bone Miner Res. 2012;27(2):240–2. doi: 10.1002/jbmr.1542. [DOI] [PubMed] [Google Scholar]
- 4.Diab DL, Watts NB. Bisphosphonates in the treatment of osteoporosis. Endocrinol Metab Clin North Am. 2012;41(3):487–506. doi: 10.1016/j.ecl.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 5.McClung M, et al. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med. 2013;126(1):13–20. doi: 10.1016/j.amjmed.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Shane E, et al. Atypical Subtrochanteric and Diaphyseal Femoral Fractures: Second Report of a Task Force of the American Society for Bone and Mineral Research. Journal of Bone and Mineral Research. 2014;29(1):1–23. doi: 10.1002/jbmr.1998. [DOI] [PubMed] [Google Scholar]
- 7.Wysowski DK, Greene P. Trends in osteoporosis treatment with oral and intravenous bisphosphonates in the United States, 2002–2012. Bone. 2013;57(2):423–8. doi: 10.1016/j.bone.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Burch J, et al. Systematic review of the use of bone turnover markers for monitoring the response to osteoporosis treatment: the secondary prevention of fractures, and primary prevention of fractures in high-risk groups. Health Technol Assess. 2014;18(11):1–180. doi: 10.3310/hta18110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasikaran S, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22(2):391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 10.Miller PD, et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43(2):222–9. doi: 10.1016/j.bone.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Reid DM, et al. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2009;373(9671):1253–63. doi: 10.1016/S0140-6736(09)60250-6. [DOI] [PubMed] [Google Scholar]
- 12.Rosen CJ, et al. Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res. 2005;20(1):141–51. doi: 10.1359/JBMR.040920. [DOI] [PubMed] [Google Scholar]
- 13.Saag KG, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007;357(20):2028–39. doi: 10.1056/NEJMoa071408. [DOI] [PubMed] [Google Scholar]
- 14.Boonen S, et al. Postmenopausal osteoporosis treatment with antiresorptives: effects of discontinuation or long-term continuation on bone turnover and fracture risk–a perspective. J Bone Miner Res. 2012;27(5):963–74. doi: 10.1002/jbmr.1570. [DOI] [PubMed] [Google Scholar]
- 15.Abrahamsen B, Eiken P, Eastell R. Subtrochanteric and diaphyseal femur fractures in patients treated with alendronate: a register-based national cohort study. J Bone Miner Res. 2009;24(6):1095–102. doi: 10.1359/jbmr.081247. [DOI] [PubMed] [Google Scholar]
- 16.Brown JP, et al. Comparison of the Effect of Denosumab and Alendronate on Bone Mineral Density and Biochemical Markers of Bone Turnover in Postmenopausal Women With Low Bone Mass: A Randomized, Blinded, Phase 3 Trial. J Bone Miner Res. 2009:1–34. doi: 10.1359/jbmr.0809010. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Girgis CM, Sher D, Seibel MJ. Atypical femoral fractures and bisphosphonate use. N Engl J Med. 2010;362(19):1848–9. doi: 10.1056/NEJMc0910389. [DOI] [PubMed] [Google Scholar]
- 18.Green J, et al. Oral bisphosphonates and risk of cancer of oesophagus, stomach, and colorectum: case-control analysis within a UK primary care cohort. BMJ. 2010;341:c4444. doi: 10.1136/bmj.c4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kendler DL, et al. Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res. 2009;25(1):72–81. doi: 10.1359/jbmr.090716. [DOI] [PubMed] [Google Scholar]
- 20.Lenart BA, Lorich DG, Lane JM. Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med. 2008;358(12):1304–6. doi: 10.1056/NEJMc0707493. [DOI] [PubMed] [Google Scholar]
- 21.Lenart BA, et al. Association of low-energy femoral fractures with prolonged bisphosphonate use: a case control study. Osteoporos Int. 2009;20(8):1353–62. doi: 10.1007/s00198-008-0805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mammo Z, et al. Oral Bisphosphonates and Risk of Wet Age-Related Macular Degeneration. Am J Ophthalmol. 2016;168:62–7. doi: 10.1016/j.ajo.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 23.Mavrokokki T, et al. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. J Oral Maxillofac Surg. 2007;65(3):415–23. doi: 10.1016/j.joms.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 24.Odvina CV, et al. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90(3):1294–301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- 25.Ott SM. Fractures after long-term alendronate therapy. J Clin Endocrinol Metab. 2001;86(4):1835–6. doi: 10.1210/jcem.86.4.7436-1. [DOI] [PubMed] [Google Scholar]
- 26.Rizzoli R, et al. Osteonecrosis of the jaw and bisphosphonate treatment for osteoporosis. Bone. 2008;42(5):841–7. doi: 10.1016/j.bone.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Ruggiero SL, et al. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62(5):527–34. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Shane E, et al. Atypical subtrochanteric and diaphyseal femoral fractures: Report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010 doi: 10.1002/jbmr.253. [DOI] [PubMed] [Google Scholar]
- 29.Wysowski DK. Reports of esophageal cancer with oral bisphosphonate use. N Engl J Med. 2009;360(1):89–90. doi: 10.1056/NEJMc0808738. [DOI] [PubMed] [Google Scholar]
- 30.Black DM, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296(24):2927–38. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 31.Khosla S, Shane E. A Crisis in the Treatment of Osteoporosis. Journal of Bone and Mineral Research. 2016;31(8):1485–1487. doi: 10.1002/jbmr.2888. [DOI] [PubMed] [Google Scholar]
- 32.Berry DA. Statistical innovations in cancer research. In: Holland EFJ, Kufe DW, Pollock RE, Weichselbaum RR, Bast RC, Gansler TS, editors. Cancer Medicine. 6th. BC Decker; Hamilton: 2003. pp. 465–478. [Google Scholar]
- 33.Godwin M, et al. Pragmatic controlled clinical trials in primary care: the struggle between external and internal validity. BMC Med Res Methodol. 2003;3:28. doi: 10.1186/1471-2288-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warriner AH, et al. A pragmatic randomized trial comparing tablet computer informed consent to traditional paper-based methods for an osteoporosis study. Contemporary Clinical Trials Communications. 2016;3:32–38. doi: 10.1016/j.conctc.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khosla S, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22(10):1479–91. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 36.Kroenke K, et al. Operating Characteristics of PROMIS Four-Item Depression and Anxiety Scales in Primary Care Patients with Chronic Pain. Pain Med. 2014;15(11):1892–901. doi: 10.1111/pme.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pilkonis PA, et al. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS(R)): depression, anxiety, and anger. Assessment. 2011;18(3):263–83. doi: 10.1177/1073191111411667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yun H, et al. Generic alendronate use among Medicare beneficiaries: are Part D data complete? Pharmacoepidemiol Drug Saf. 2013;22(1):55–63. doi: 10.1002/pds.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright NCC, S M, Smith WK, Burden AM, Warriner AH, Foster PJ, Yun H, Melton ME, Curtis JR, Saag KG. Long-term Oral Bisphosphonate Use for Osteoporosis Among Older Women – US and Canadian Perspective. Arthritis & Rheumatism. 2014;66(10):S20. [Google Scholar]
- 40.Kolata G. New York Times. New York Times Company; New York: 2016. Osteoporosis Drugs Shunned for Fear of Rare Side Effects; p. A1. [Google Scholar]
- 41.Black DM, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348(9041):1535–41. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 42.Wright NC, F P, Fullman S, Randall S, Melton ME, Pace W, Calmbach W, Saag KG. Patient Perspectives on Participating in the Effectiveness of Discontinuing Bisphosphonates (EDGE) Study. Journal of Bone and Mineral Research. 2015;30(Suppl 1) [Google Scholar]
- 43.Jha S, et al. Trends in Media Reports, Oral Bisphosphonate Prescriptions, and Hip Fractures 1996-2012: An Ecological Analysis. J Bone Miner Res. 2015;30(12):2179–87. doi: 10.1002/jbmr.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewiecki EM, et al. Hip fractures and declining DXA testing: At a breaking point? J Bone Miner Res. 2016;31(Suppl 1) [Google Scholar]


