Abstract
BACKGROUND
The efficacy of percutaneous stellate ganglion block (SGB) for managing electrical storm (ES) is not well understood.
OBJECTIVE
To characterize the efficacy of SGB as a treatment for ES.
METHODS
We conducted literature searches using PubMed/Medline and Google Scholar, for mixed combinations of terms including “stellate ganglion block”, *ganglion block (ade)”, “sympathetic block (ade)” and “arrhythmia”, “ventricular arrhythmia (VA)” or “tachycardia" (VT), "ventricular fibrillation" (VF), "electrical storm”. Inclusion criteria were presentation with guideline-defined ES and treatment with SGB. Exclusion criteria: presentation with any supraventricular arrhythmia, VA without ES, or surgical sympathectomy. Studies lacking basic demographic data, arrhythmia description, and outcomes were excluded.
RESULTS
Of 3,374 publications reviewed, 38 patients from 23 studies met study criteria (52 ± 19.1 years, 11 F, 17 with ischemic cardiomyopathy). Anti-arrhythmics were used in all patients. Mean Left ventricular ejection fraction was 31 ± 10%. ES was triggered by acute myocardial infarction in 15 patients and QT prolongation in 7 patients. The most common local anesthetic used for SGB was bupivacaine (0.25–0.5%). SGB resulted in a significant decrease in VA burden (12.4±8.8 vs. 1.04±2.12 episodes/day, p< 0.001) and number of external and ICD shocks (10.0±9.1 vs. 0.05±0.22 shocks/day, p< 0.01). Following SGB, 80.6% of patients survived to discharge.
CONCLUSION
SGB is an effective acute treatment for ES. However, larger prospective randomized studies are needed to better understand the role of SGB in ES and other VAs.
Keywords: Stellate ganglion block, electrical storm, neuromodulation, autonomic nervous system, sympathetic nerves
INTRODUCTION
Electrical storm (ES) is commonly defined as the occurrence of three or more episodes of sustained ventricular arrhythmia (VA) over 24 hours (1,2). Antiarrhythmic medications and catheter ablation remain the standard of care in patients with ES or other refractory VA (3). The role of the autonomic nervous system in ventricular arrhythmogenesis is well recognized (4), and neural modulation via a number of avenues is increasingly gaining traction (5). Cardiac sympathetic denervation (CSD), the surgical resection of the lower half of the stellate (cervicothoracic) ganglion and T2–4 sympathetic ganglia, has been shown to be effective in the setting of ES or other refractory VA (6–14). Other forms of neuromodulation include pharmacologic beta-adrenergic receptor blockade, thoracic epidural anesthesia (TEA) spinal cord stimulation (SCS), and stellate ganglion block (SGB) (5). Stellate ganglion block (SGB) is performed by injecting local anesthetic agents percutaneously to stellate ganglion, which is less invasive than CSD and can be performed at bedside in emergent setting in patients with hemodynamic instability Currently, besides case reports and small series, limited evidence is available regarding the role of SGB in ES (15–37). To better understand the role of SGB in ES (particularly to address patient characteristics, techniques, and overall efficacy of SGB), we performed dedicated literature searches and performed this systemic review.
METHODS
Literature search, and criteria for inclusion or exclusion
Using PubMed/Medline and Google Scholar, we performed varying combinations of searches using the following terms, “left stellate ganglion block”, “ganglion block (ade)”, “sympathetic block (ade)”, “arrhythmia”, “ventricular arrhythmia (VA)” "ventricular tachycardia" (VT), "ventricular fibrillation" (VF), "electrical storm (ES)”. ES was defined as three or more episodes of sustained VT or VF within a 24-hour period (1). The search was limited to human subjects only. The search strategy was neither restricted by the language, nor the date of publication. Inclusion criteria were patients presenting with ES who underwent SGB. Bilateral SGB was also included. Exclusion criteria included patients presenting with any supraventricular tachycardia, ventricular arrhythmia without ES (for example, premature ventricular contractions), or patients treated only with surgical sympathectomy. The articles were carefully reviewed for inclusion and exclusion criteria. A lack of basic patient demographics, arrhythmia description, or outcomes, which are critical for this study, was also a reason for exclusion.
All clinical variables were extracted from the selected studies including number of patients, age, gender, type of VA, episodes of VA and shocks before and immediately after SGB, presence of underlying cardiomyopathy, left ventricular ejection fraction (LVEF), trigger of ES, antiarrhythmic medications used, other procedures or treatment received prior to or after SGB, anesthetic administration techniques (i.e. laterality, bolus injection vs. continuous pump infusion, use of imaging guidance), type and volume of local anesthetic agent used, inpatient survival to discharge. Relative reduction of VA and defibrillator shocks are calculated as the difference in episodes per day pre-vs. post-SGB, divided by the number of episodes per day pre-SGB.
Statistical analysis
Continuous variables were summarized as mean ± SD. Comparison of VA episodes and number of external or ICD shocks before and after SGB was performed using Wilcoxon signed-rank test, given the non-normal distribution of the data. Change in VA burden or defibrillator shocks was expressed as relative reduction (i.e. post-SGB/pre-SGB). The relationship between arrhythmia reduction and LV EF was examined by linear regression. Comparison of cardiomyopathy subtypes was performed using Analysis of Variance (ANOVA), and arrhythmia subtypes were compared using the Kruskall-Wallis test, as these data were non-normally distributed. A p-value < 0.05 was considered significant.
RESULTS
Patient characteristics
A total of 3374 publications were reviewed and 38 patients from 23 studies published between 1976 and 2016 were included based on inclusion and exclusion criteria. The mean age of the patient population was 52 ± 19.1 years. Twenty-seven (71%) patients were male. Cardiomyopathy (CMY) was present in 24 (63.2%) patients (ischemic CMY (ICMY) in 17 patients, and non-ischemic CMY (NICMY) in 7 patients, Table 1). The mean LVEF was 31±10 %. Acute myocardial infarction (AMI) was the most common trigger of ES (15 patients), followed by prolonged QT (7 patients). Interestingly, intracranial hemorrhage was the etiology in 2 patients. In 37% patients, the trigger of ES was unspecified. Of the arrhythmia types, mixed VT/VF was the most common type encountered (n=15), followed by polymorphic VT (n=12). Four patients had monomorphic VT and 7 patients had primary VF without VT (Table 1). Almost all patients were treated with beta-blocker therapy (33/38). All patients except one received antiarrhythmic medications prior to SGB (Table 2) and the most common agents were amiodarone (82%) and lidocaine (68%). On average, 1.82±0.82 anti-arrhythmic drugs were used, along with beta-blockers and/or calcium channel blockers prior to SGB. Regarding other interventions prior to SGB, 36.8% patients were intubated and deeply sedated, while15.8% patients were treated with catheter-based ablation (Table 3).
Table 1.
Patient Characteristics.
| Mean age (years) | 52±19.1 |
| Male | 27 (71%) |
| Presence of Cardiomyopathy | 35 (92%) |
| Ischemic CMY | 17 |
| Non-ischemic CMY | 7 |
| Unspecified | 11 |
| Arrhythmia Type | |
| Mixed VT/VF | 15 |
| PMVT | 12 |
| MMVT | 4 |
| VF | 7 |
| Left Ventricular Ejection Fraction | 31±10% |
| Acute trigger of ES | |
| Acute MI | 15 |
| Prolonged QT | 7 |
| Intracranial hemorrhage | 2 |
| Unspecified | 14 |
CMY: cardiomyopathy, MI: myocardial infarction, MMVT: monomorphic ventricular tachycardia, PMVT: polymorphic ventricular tachycardia, VF: ventricular fibrillation, VT: ventricular tachycardia.
Table 2.
Medications used before institution of stellate ganglion block.
| Amiodarone | 23 (82%) |
| Beta blocker | 31 (82%) |
| Metoprolol | 7 (25%) |
| Propranolol | 6 (21%) |
| Esmolol | 4 (14%) |
| Carvedilol | 1 (4%) |
| Unspecified | 14 (50%) |
| Lidocaine | 19 (68%) |
| Sotalol | 1 (4%) |
| Procainamide | 8 (29%) |
| Mexiletine | 3 (11%) |
| Qunidine | 2 (7%) |
| Ajmaline | 1 (4%) |
| Bretylium | 1 (4%) |
| Phenytoin | 8 (29%) |
| Digitalis | 1 (4%) |
| Isoproterenol | 1 (4%) |
| Verapamil | 1 (4%) |
Table 3.
Procedures/Interventions performed prior to stellate ganglion block.
| Intubation and deep sedation | 14 (36.8%) |
| Cardiac catheter ablation | 6 (15.8%) |
| IABP | 6 (15.8%) |
| ECMO | 3 (7.9%) |
| TEA | 2 (5.3%) |
| Tandem heart | 1 (2.6%) |
ECMO: Extracorporeal membrane oxygenation, IABP: Intra-aortic balloon pump, TEA: thoracic epidural anesthesia.
Approach to SGB
SGB was achieved by administering local anesthetic percutaneously to the SG. We examined the delivery method, the type and amount of local anesthetic, as well as the utility of imaging guidance with SGB. 34 patients received only left SGB whereas in 4 patients, both left and right SGB were performed. As noted in Table 4, local anesthetic agents were administered as bolus injections in 28 patients (73.7%), whereas continuous infusion with pump system was used in nine (one patient had both bolus and continuous infusion). Among patients receiving bolus injections, bupivacaine was the most commonly used anesthetic in patients with SGB (n=16), while ropivacaine was the next most commonly used. The mean volume of bupivacaine used was 9 ± 5.6 ml with concentration ranging from 0.25% to 0.5%. We also examined the utility of imaging when performing SGB. Ultrasound guidance was utilized in 21 patients, and fluoroscopy in 4 patients (Table 4). In the remaining13 patients, SGB was performed with anatomic landmarks only without imaging guidance.
Table 4.
Approaches to anesthetic use for stellate ganglion block.
| Anesthetic Agent | Number of patients |
Dose (Concentration, %) |
Volume (ml) |
|---|---|---|---|
| Bupivacaine | 16 | 0.25–0.5 | 9 ± 5.6 |
| Ropivacaine | 11 | 0.2 | 6 ± 5.7 |
| Lidocaine | 9 | 1–4 | 8 ± 3.8 |
| Mepivacaine | 2 | 2 | 4 ± 0.0 |
| Type of administration of anesthetics | Number of patients | ||
| Bolus injections | 28 | ||
| Continuous infusion | 9 | ||
| Both bolus injections and continuous infusion | 1 | ||
| Utility of imaging guidance | Number of patients | ||
| Landmark only without imaging | 13 | ||
| Ultrasound | 21 | ||
| Fluoroscopy | 4 | ||
Efficacy of SGB in immediate reduction of VA burden and shocks
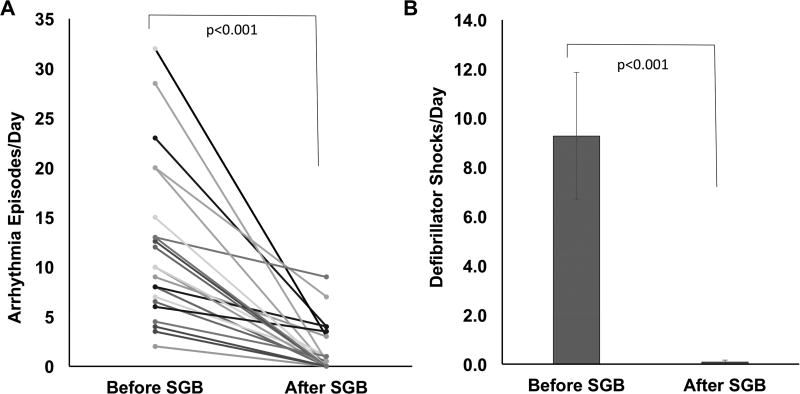
To determine the efficacy of SGB, we quantified ES burden as the episodes of VA per day and number of external or ICD shocks per day. As shown in Figure 1, there was a significant reduction in both the episodes of VA, and number of shocks in patients with ES after SGB. SGB decreased VA burden from 12.4±8.8 episodes/day to 1.04±2.12 episodes/day (p< 0.001). The number of external or ICD shocks was decreased from 10.0±9.1/day to 0.05±0.22/day (p< 0.01).
Figure 1. Impact of stellate ganglion block on ventricular arrhythmia episodes and defibrillator shocks.
Reduction in the number of ventricular arrhythmia episodes (A.) and number of internal or external defibrillator shocks (B.) before and after stellate ganglion block (SGB) are shown. Panel A (n=24) and panel B (n=11).
In this cohort, 24 of the 38 patients demonstrated complete arrhythmia suppression following SGB during the immediate follow up period, while the remaining 14 showed partial success. The impact of SGB did not depend on the subtype of VA causing ES. Relative reduction in VA episodes for the four subtypes studied, monomorphic VT, polymorphic VT, mixed VT/VF, and primary VF were 0.94±0.1; 0.76±0.25; 1±0; and 0.97±0.05, respectively (p=0.124). The duration of clinical suppressive effect after bolus injection was 6–24 hours for ropivacaine, 8–18 hours for lidocaine, 6 hours to 1 week for bupivacaine, 11 hours to 4 weeks for mepivacaine.
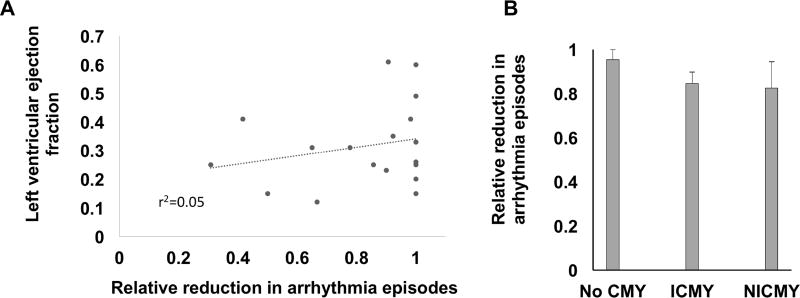
We examined whether the anti-arrhythmic benefit of SGB was influenced by the presence and degree of LV dysfunction. As shown in figure 2A, there was no correlation between the LV EF, and arrhythmia reduction (r2=0.05, p=0.384). Patients with normal LV function, mild, moderate, or severe LV dysfunction, equally benefitted from SGB. Similarly, the presence and etiology of CMY did not influence the ability of SGB to exert anti-arrhythmic benefits (figure 2B). Relative reduction in VA burden was 0.95±0.07, 0.85±0.19, and 0.83±0.29 respectively for no CMY, ICMY, and NICMY, respectively (p=0.78). Using relative VA reduction < 50% as a cut off for poor response to SGB, 10% of patients were labeled as poor responders to SGB. The rest of the patients were considered to have good response. Characteristics of good and poor responders were are summarized in Table 5. All poor responders were male and presented with PMVT. Mean LVEF in this group was 27 ± 13%.
Figure 2. Relationship between arrhythmia burden reduction and left ventricular dysfunction.
A. The relationship between left ventricular ejection fraction (LV EF) and the relative reduction in ventricular arrhythmia (VA) episodes (n=18), r2=0.05, p=0.384). B. Reduction in arrhythmia episodes was independent of the presence and etiology of cardiomyopathy (CMY), n=24, p=0.78).
Table 5.
Comparison of patients with poor response and good response to LSGB.
| Poor response | Good response | |
|---|---|---|
| Number of patients | 3 | 35 |
| Mean age (years) | 61±9 | 51±20 |
| Male | 3 (100%) | 24 (68.6%) |
| Presence of Cardiomyopathy | 3 (100%) | 24 (68.6%) |
| Ischemic CMY | 2 (66.7%) | 15 (42.9%) |
| Non-ischemic CMY | 1 (33.3%) | 6 (17.1%) |
| Unspecified CMY | 0 | 3 (8.6%) |
| Arrhythmia Type | ||
| Mixed VT/VF | 0 | 12 (34.3%) |
| PMVT | 3 (100%) | 12 (34.3%) |
| MMVT | 0 | 7 (20%) |
| VF | 0 | 4 (11.4%) |
| Left Ventricular Ejection Fraction | 27±13% | 32±14% |
| Acute trigger of ES | ||
| Acute MI | 2 (66.7%) | 14 (40%) |
| Prolonged QT | 1 (33.3%) | 7 (20%) |
| Intracranial events | 0 | 2 (5.7%) |
| Unspecified | 0 | 12 (34.3%) |
| Type of local anesthetics | ||
| Bupivacaine | 2 (66.7%) | 14 (40%) |
| Ropivacaine | 1 (33.3%) | 10 (28.6%) |
| Mepivacaine | 0 | 9 (25.7%) |
| Lidocaine | 0 | 2 (5.7%) |
| Method of administration | ||
| Bolus injection | 2 (66.7%) | 27 (77.1%) |
| Continuous infusion | 1 (33.3%) | 7 (20%) |
| Both bolus and continuous infusion | 0 | 1 (2.9%) |
| Type of imaging guidance | ||
| Ultrasound | 3 (100%) | 18 (51.4%) |
| Fluoroscopy | 0 | 4 (11.4%) |
| Inpatient survival | ||
| Discharged | 1 (33.3%) | 30 (85.7%) |
| Deceased | 2 (66.7%) | 5 (14.3%) |
Following SGB, 80.6% of patients survived to discharge (hospital day 6–28), and terminal sympathectomy via surgical CSD was performed in 11 patients. One patient underwent orthotopic heart transplantation.
DISCUSSION
The major findings of the present study on the efficacy of SGB for ES are 1) SGB is effective in reducing the number episodes and therapies for VA, and 2) this efficacy was independent of the subtype of ventricular arrhythmia, the presence or absence of cardiomyopathy, and the degree of LV dysfunction in the patients studied. To our knowledge, this is the first systemic review of the efficacy of the SGB in patients with ES, and strongly supports the use of SGB in patients with ES.
There is a strong link between cardiac sympathetic activity and ventricular arrhythmogenesis (38,39). In a rabbit myocardial infarction model, left stellate ganglion blockade (LSGB) prolonged the action potential duration (measured as MAPD90) in all layers of the myocardial wall, reducing transmural repolarization heterogeneity, increased the effective refractory period (ERP), and reduced ventricular fibrillation threshold (40). SGB, by mitigating catecholamine release, likely reverses the findings in a canine post-infarct model of shortened ERP in abnormal ventricular myocardium, and increased inducibility of arrhythmias in the presence of catecholamines (41). SGB may also be particularly useful for attenuating burst discharge activity in left stellate ganglion, which has been shown to precede most VT and sudden cardiac death in a canine model of ischemic cardiomyopathy (42–43). Sympathoexcitation increases repolarization heterogeneity (44–46), increases risk of delayed after depolarizations (47,48), and increases arrhythmia inducibility (49). It also modulates peri-infarct tissues harboring circuits capable of facilitating MMVT (50). The efficacy of beta-adrenergic receptor antagonism in patients with cardiomyopathy (51), is related to minimizing the adverse effects of adrenergic activation signaling. However, despite use of beta-adrenergic receptor blockers, anti-arrhtyhmic medications (some of which further antagonize beta-adrenergic receptors), and catheter ablation, some patients continue to have arrhythmias. In this study, we examine the efficacy of SGB in this population.
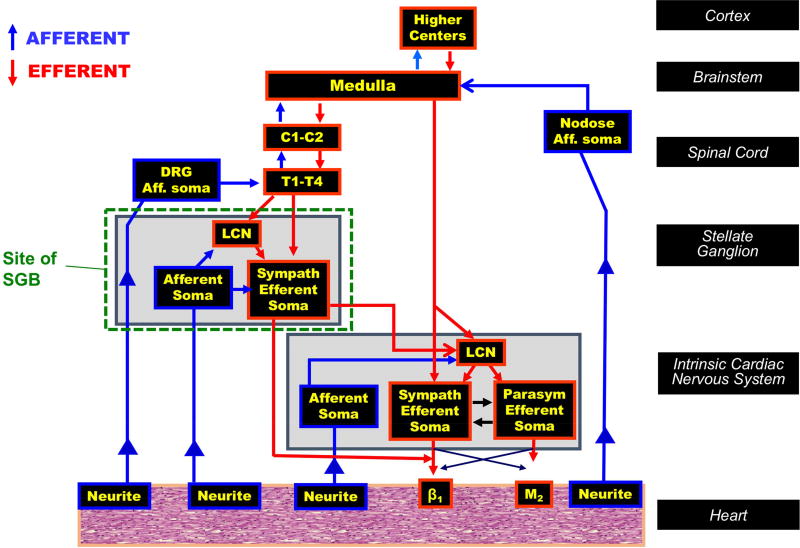
A number of mechanisms may explain the therapeutic benefits imparted by SGB. The bulk of efferent sympathetic outflow to the heart travels through the SG. Preganglionic fibers mediating neurotransmission to the heart synapse on postganglionic neurons within the SG (and adjacent ganglia within the sympathetic chain) (Figure 3). In addition, postganglionic fibers from other ganglia may also travel through the SG to middle cervical ganglia and cardiac nerves (52). This represents an effective site to target cardiac adrenergic activation while limiting systemic effects. Further, post-ganglionic axons release a variety of neurotransmitters, of which noradrenaline, targeted by beta blockers, is only one. Additional neurotransmitters such as neuropeptide Y and galanin modulate adrenergic signaling at the myocardial level (53,54. However, in some cases, as evidenced by the patients examined in this study, beta-adrenergic blockade, antiarrhythmic drugs, and catheter ablation do not mitigate these other mechanisms. Interventions targeting the SG however, would attenuate not only noradrenaline signaling, but these additional signaling pathways.
Figure 3. Schematic of cardiac innervation and site of stellate ganglion block.
Stellate ganglion block directly impacts both efferent and afferent neurotransmission to the heart. The site of action and components are shown. Aff, afferent; β, β-adrenergic receptor; C, cervical; DRG, dorsal root ganglion; LCN, local circuit neuron; M, muscarinic receptor, Sympath, sympathetic, and T, thoracic.
Sensory neurotransmission is also mitigated by SGB. Sympatho-excitatory reflexes that occur at multiple levels are triggered by sensory afferent nerves that relay information intrinsic cardiac, mediastinal, SG, spinal cord and brain stem (38). At these centers, the sensory information is processed, and reflex sympathetic activity is generated. The bulk of spinal sensory afferents, which have been implicated in the pathogenesis of heart failure (55,56), and along the same lines arrhythmogenesis pass through the SG, enroute to the dorsal horn of the spinal cord. Infusion of anesthetic agents to block the SG also targets these fibers, and therefore attenuates both afferent and efferent neurotransmission at the SG (Figure 3). Although SGB does not affect vagal afferents, the reduction of spinal afferents likely debulks overall cardiac afferent neurotransmission.
The burden of VAs and defibrillator shocks were significantly reduced after SGB in patients with ES. This occurred in patients independent of the etiology of the arrhythmia, triggering mechanisms, and LV function. VA suppression varied after bolus of injection of local anesthetics (LA), ranging from hours to weeks. The longer duration of efficacy relative to the anesthetic half-life is most likely related to short- and long-term adaptations in neurotransmission and neural processing. Due to the highly plastic nature of neurons, a short-term intervention can produce long-lasting effects, well beyond what is expected for the drug along. That said, there is likely to be some variation related to pharmacodynamics of the agents used, proximity of the injection of the ganglion, and thickness of the ganglion sheath.
We present the most commonly used approaches, anesthetic agents and doses to achieve SGB. The patient characteristics and technical data generated by the present study may be helpful as a reference for the institution of SGB. These data may also be useful in the design and patient selection for randomized prospective studies to improve SGB techniques in clinical practice.
LIMITATIONS
This study has a number of limitations, which include the number of studies in the literature meeting inclusion/exclusion criteria. As these were predominantly case reports and case series, data reported here are retrospective. Further the small sample size limits potentially instructive subgroup analyses, as well as the reliability of the analyses made. To improve the accuracy of the present study, reports in the literature lacking basic patient demographics were excluded, reducing the overall number of patients in the study (59).
CONCLUSION
The findings of the present study support the routine use of SGB as an effective adjunct to contemporary therapies in managing ES. SGB is efficacious for a variety of VA subtypes and patient demographics. Prospective randomized studies are needed to better understand the role of the SGB in ES and other VA.
Perspectives.
Competency in Medical Knowledge 1
The role of neuromodulation in managing electrical storm is gaining traction.
Competency in Medical Knowledge 2
Anesthetic blockade of the stellate ganglion achieved percutaneously represents an attractive approach that can be implemented in a variety of settings, including at the patient’s bedside.
Competency in Patient Care
The types of anesthetic agents, doses, and methods of dosing are important in applying stellate ganglion block. Characteristics of patients, and the arrhythmias controlled by stellate ganglion block would guide patient care.
Competency in Interpersonal & Communication Skills
It is important to discuss the available options with patients and the medical team.
Translational Outlook 1
Stellate ganglion block is effective in controlling electrical storm, and is not limited by the type of ventricular arrhythmia, or the presence/type of structural heart disease.
Translational Outlook 2
Additional research is needed to understand the safety and efficacy of stellate ganglion block in prospective randomized trials to improve is use.
Acknowledgments
Funding: The authors acknowledge the National Heart Lung and Blood Institutes of the National Institutes of Health for HL084261 to KS and HL125730 to OAA.
Abbreviations
- CMY
cardiomyopathy
- ES
electrical storm
- LA
local anesthetics
- LQT
long QT syndrome)
- LVEF
left ventricular ejection fraction
- SGB
stellate ganglion block
- VA
ventricular arrhythmias
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Aliot EM, Stevenson WG, Almendral-Garrote JM, et al. EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA) Heart Rhythm. 2009;6:886–933. doi: 10.1016/j.hrthm.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 2.Gao D, Sapp JL. Electrical storm: definitions, clinical importance, and treatment. Curr Opin Cardiol. 2013;28:72–9. doi: 10.1097/HCO.0b013e32835b59db. [DOI] [PubMed] [Google Scholar]
- 3.Zipes DP, Camm AJ, Borggrefe M, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death,Äîexecutive summaryA report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. European Heart Journal. 2006;27:2099–2140. doi: 10.1093/eurheartj/ehl199. [DOI] [PubMed] [Google Scholar]
- 4.Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circulation research. 2014;114:1004–21. doi: 10.1161/CIRCRESAHA.113.302549. [DOI] [PubMed] [Google Scholar]
- 5.Shivkumar K, Ajijola OA, Anand I, et al. Clinical neurocardiology defining the value of neuroscience-based cardiovascular therapeutics. J Physiol. 2016;594:3911–54. doi: 10.1113/JP271870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ajijola OA, Lellouche N, Bourke T, et al. Bilateral Cardiac Sympathetic Denervation for the Management of Electrical Storm. Journal of the American College of Cardiology. 2012;59:91–92. doi: 10.1016/j.jacc.2011.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ajijola OA, Vaseghi M, Mahajan A, Shivkumar K. Bilateral cardiac sympathetic denervation: why, who and when? Expert review of cardiovascular therapy. 2012;10:947–9. doi: 10.1586/erc.12.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaseghi M, Gima J, Kanaan C, et al. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: intermediate and long-term follow-up. Heart rhythm. 2014;11:360–6. doi: 10.1016/j.hrthm.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collura CA, Johnson JN, Moir C, Ackerman MJ. Left cardiac sympathetic denervation for the treatment of long QT syndrome and catecholaminergic polymorphic ventricular tachycardia using video-assisted thoracic surgery. Heart Rhythm. 2009;6:752–9. doi: 10.1016/j.hrthm.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Liu Y, Yang F, Jiang G, Li C, Hu D, et al. Video-assisted thoracoscopic left cardiac sympathetic denervation: a reliable minimally invasive approach for congenital long-QT syndrome. Ann Thorac Surg. 2008;6:1955–8. doi: 10.1016/j.athoracsur.2008.07.100. [DOI] [PubMed] [Google Scholar]
- 11.Moss AJ, McDonald J. Unilateral cervicothoracic sympathetic ganglionectomy for the treatment of long QT interval syndrome. N Engl J Med. 1971;16:903–4. doi: 10.1056/NEJM197110142851607. [DOI] [PubMed] [Google Scholar]
- 12.Nitter-Hauge S, Storstein O. Surgical treatment of recurrent ventricular tachycardia. Br Heart J. 1973;11:1132–5. doi: 10.1136/hrt.35.11.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz PJ, Priori SG, Cerrone M, Spazzolini C, Odero A, Napolitano C, et al. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation. 2004;15:1826–33. doi: 10.1161/01.CIR.0000125523.14403.1E. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz PJ, Zaza A, Locati E, Moss AJ. Stress and sudden death. The case of the long QT syndrome. Circulation. 1991;83:II71–80. [PubMed] [Google Scholar]
- 15.Biagini A, Sabino F, Paladini G, et al. Treatment of perinfarction recurrent ventricular fibrillation by percutaneous pharmacological block of left stellate ganglion. Clin Cardiol. 1985;8:111–3. doi: 10.1002/clc.4960080209. [DOI] [PubMed] [Google Scholar]
- 16.Boe BA, Webster G, Asher Y, Tsao S, Suresh S, Steinhorn DM. Percutaneous, ultrasound-guided stellate ganglion nerve block suppresses recurrent ventricular fibrillation in an infant awaiting heart transplant. Circulation Arrhythmia and electrophysiology. 2012;5:e93–4. doi: 10.1161/CIRCEP.112.974329. [DOI] [PubMed] [Google Scholar]
- 17.Gadhinglajkar S, Sreedhar R, Unnikrishnan M, Namboodiri N. Electrical storm: Role of stellate ganglion blockade and anesthetic implications of left cardiac sympathetic denervation. Indian J Anaesth. 2013;57:397–400. doi: 10.4103/0019-5049.118568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Moran E, Sandin-Fuentes MG, Alvarez Lopez JC, Duro-Aguado I, Uruena-Martinez N, Hernandez-Luis C. Electrical storm secondary to acute myocardial infarction and heart failure treated with left stellate ganglion block. Rev Esp Cardiol. 2013;66:595–7. doi: 10.1016/j.rec.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Moran E, Sliwinski-Herrera F, Cortes-Villar C, Sandin-Fuentes M, Pastor Baez G, San Roman A. Refractory Electrical Storm: A Role for Transient Sympathetic Blockade. Rev Esp Cardiol. 2016;69:76–8. doi: 10.1016/j.rec.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 20.Grossman MA. Cardiac arrhythmias in acute central nervous system disease. Successful management with stellate ganglion block. Arch Intern Med. 1976;136:203–7. [PubMed] [Google Scholar]
- 21.Hayase J, Patel J, Narayan SM, Krummen DE. Percutaneous stellate ganglion block suppressing VT and VF in a patient refractory to VT ablation. Journal of cardiovascular electrophysiology. 2013;24:926–8. doi: 10.1111/jce.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoepp HW, Eggeling T, Hombach V. Pharmacologic blockade of the left stellate ganglion using a drug-reservoir-pump system. Chest. 1990;97:250–1. doi: 10.1378/chest.97.1.250. [DOI] [PubMed] [Google Scholar]
- 23.Hulata DF, Le-Wendling L, Boezaart AP, Hurley RW. Stellate ganglion local anesthetic blockade and neurolysis for the treatment of refractory ventricular fibrillation. A A Case Rep. 2015;4:49–51. doi: 10.1213/XAA.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 24.Loyalka P, Hariharan R, Gholkar G, et al. Left stellate ganglion block for continuous ventricular arrhythmias during percutaneous left ventricular assist device support. Tex Heart Inst J. 2011;38:409–11. [PMC free article] [PubMed] [Google Scholar]
- 25.Malik AA, Khan AA, Dingmann K, et al. Percutaneous inferior cervical sympathetic ganglion blockade for the treatment of ventricular tachycardia storm: case report and review of the literature. J Vasc Interv Neurol. 2014;7:48–51. [PMC free article] [PubMed] [Google Scholar]
- 26.Mesa A, Kaplan RF. Dysrhythmias controlled with stellate ganglion block in a child with diabetes and a variant of long QT syndrome. Reg Anesth. 1993;18:60–2. [PubMed] [Google Scholar]
- 27.Nair L, Tseng PS, Manninen PH, Teo WS. Anaesthetic management of idiopathic long QT syndrome--a case report. Ann Acad Med Singapore. 1994;23:582–5. [PubMed] [Google Scholar]
- 28.Nielsen H, Badskjaer J. Blockade of the left stellate ganglion. Treatment of ventricular arrhythmias in secondary QT prolongation. Ugeskr Laeger. 1986;148:3221–3. [PubMed] [Google Scholar]
- 29.Parris WC, Reddy BC, White HW, McGrath DM. Stellate ganglion blocks in pediatric patients. Anesth Analg. 1991;72:552–6. doi: 10.1213/00000539-199104000-00024. [DOI] [PubMed] [Google Scholar]
- 30.Patel RA, Priore DL, Szeto WY, Slevin KA. Left stellate ganglion blockade for the management of drug-resistant electrical storm. Pain Med. 2011;12:1196–8. doi: 10.1111/j.1526-4637.2011.01167.x. [DOI] [PubMed] [Google Scholar]
- 31.Platia EV, Griffith LS, Watkins L, Mirowski M, Mower MM, Reid PR. Management of the prolonged QT syndrome and recurrent ventricular fibrillation with an implantable automatic cardioverter-defibrillator. Clin Cardiol. 1985;8:490–3. doi: 10.1002/clc.4960080907. [DOI] [PubMed] [Google Scholar]
- 32.Prabhu MA, Prasad SB, Abhilash SP, Thajudeen A, R BK, Namboodiri N. Left sympathetic cardiac denervation in managing electrical storm: acute outcome and long term follow up. J Interv Card Electrophysiol. 2016;47:285–292. doi: 10.1007/s10840-016-0153-2. [DOI] [PubMed] [Google Scholar]
- 33.Scanlon MM, Gillespie SM, Schaff HV, Cha YM, Wittwer ED. Urgent Ultrasound-Guided Bilateral Stellate Ganglion Blocks in a Patient With Medically Refractory Ventricular Arrhythmias. Crit Care Med. 2015;43:e316–8. doi: 10.1097/CCM.0000000000001086. [DOI] [PubMed] [Google Scholar]
- 34.Smith DI, Jones C, Morris GK, Kralovic S, Massey HT, Sifain A. Trial ultrasound-guided continuous left stellate ganglion blockade before surgical gangliolysis in a patient with a left ventricular assist device and intractable ventricular tachycardia: a pain control application to a complex hemodynamic condition. Asaio J. 2015;61:104–6. doi: 10.1097/MAT.0000000000000163. [DOI] [PubMed] [Google Scholar]
- 35.Tan AY, Abdi S, Buxton AE, Anter E. Percutaneous stellate ganglia block for acute control of refractory ventricular tachycardia. Heart rhythm. 2012;9:2063–7. doi: 10.1016/j.hrthm.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 36.Fudim M, Boortz-Marx R, Patel CB, Sun AY, Piccini JP. Autonomic Modulation for the Treatment of Ventricular Arrhythmias: Therapeutic Use of Percutaneous Stellate Ganglion Blocks. J Cardiovasc Electrophysiol. 2016 doi: 10.1111/jce.13152. [DOI] [PubMed] [Google Scholar]
- 37.Fudim M, Boortz-Marx R, Patel CB, Sun AY, Piccini JP. Autonomic Modulation for the Treatment of Ventricular Arrhythmias: Therapeutic Use of Percutaneous Stellate Ganglion Blocks. Journal of cardiovascular electrophysiology. 2016 doi: 10.1111/jce.13152. [DOI] [PubMed] [Google Scholar]
- 38.Shivkumar K, Ajijola OA, Anand I, et al. Clinical neurocardiology-defining the value of neuroscience-based cardiovascular therapeutics. J Physiol. 2016 doi: 10.1113/JP271870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ardell JL, Andresen MC, Armour JA, et al. Translational Neurocardiology: preclinical models and cardioneural integrative aspects. J Physiol. 2016 doi: 10.1113/JP271869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu Y, Wang L, Wang X, Tang Y, Cao F, Fang Y. Assessment of ventricular electrophysiological characteristics at periinfarct zone of postmyocardial infarction in rabbits following stellate ganglion block. J Cardiovasc Electrophysiol. 2012;23:S29–35. doi: 10.1111/j.1540-8167.2012.02437.x. [DOI] [PubMed] [Google Scholar]
- 41.Zipes DP, Rubart M. Neural modulation of cardiac arrhythmias and sudden cardiac death. Heart Rhythm. 2006;3:108–13. doi: 10.1016/j.hrthm.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou S, Jung BC, Tan AY, Trang VQ, Gholmieh G, Han SW, et al. Spontaneous stellate ganglion nerve activity and ventricular arrhythmia in a canine model of sudden death. Heart Rhythm. 2008;5:131–9. doi: 10.1016/j.hrthm.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Doytchinova A, Patel J, Zhou S, Chen LS, Lin H, Shen C, et al. Subcutaneous nerve activity and spontaneous ventricular arrhythmias in ambulatory dogs. Heart Rhythm. 2015;12:612–20. doi: 10.1016/j.hrthm.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ajijola OA, Yagishita D, Patel KJ, et al. Focal myocardial infarction induces global remodeling of cardiac sympathetic innervation: neural remodeling in a spatial context. American journal of physiology Heart and circulatory physiology. 2013;305:H1031–40. doi: 10.1152/ajpheart.00434.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaseghi M, Yamakawa K, Sinha A, et al. Modulation of regional dispersion of repolarization and T-peak to T-end interval by the right and left stellate ganglia. American journal of physiology Heart and circulatory physiology. 2013;305:H1020–30. doi: 10.1152/ajpheart.00056.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yagishita D, Chui RW, Yamakawa K, et al. Sympathetic Nerve Stimulation, Not Circulating Norepinephrine, Modulates T-Peak to T-End Interval by Increasing Global Dispersion of Repolarization. Circulation Arrhythmia and electrophysiology. 2014 doi: 10.1161/CIRCEP.114.002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Priori SG, Corr PB. Mechanisms underlying early and delayed afterdepolarizations induced by catecholamines. The American journal of physiology. 1990;258:H1796–805. doi: 10.1152/ajpheart.1990.258.6.H1796. [DOI] [PubMed] [Google Scholar]
- 48.Priori SG, Mantica M, Schwartz PJ. Delayed afterdepolarizations elicited in vivo by left stellate ganglion stimulation. Circulation. 1988;78:178–85. doi: 10.1161/01.cir.78.1.178. [DOI] [PubMed] [Google Scholar]
- 49.Irie T, Yamakawa K, Hamon D, Nakamura K, Shivkumar K, Vaseghi M. Cardiac Sympathetic Innervation Via the Middle Cervical and Stellate Ganglia and Anti-Arrhythmic Mechanism of Bilateral Stellectomy. Am J Physiol Heart Circ Physiol. 2016 doi: 10.1152/ajpheart.00644.2016. ajpheart 00644 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ajijola OA, Lux RL, Khahera A, et al. Sympathetic Modulation of Electrical Activation In Normal and Infarcted Myocardium: Implications for Arrhythmogenesis. Am J Physiol Heart Circ Physiol. 2017 doi: 10.1152/ajpheart.00575.2016. ajpheart 00575 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016;68:1476–88. doi: 10.1016/j.jacc.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 52.Janes RD, Brandys JC, Hopkins DA, Johnstone DE, Murphy DA, Armour JA. Anatomy of human extrinsic cardiac nerves and ganglia. The American journal of cardiology. 1986;57:299–309. doi: 10.1016/0002-9149(86)90908-2. [DOI] [PubMed] [Google Scholar]
- 53.Herring N, Cranley J, Lokale MN, et al. The cardiac sympathetic co-transmitter galanin reduces acetylcholine release and vagal bradycardia: implications for neural control of cardiac excitability. Journal of molecular and cellular cardiology. 2012;52:667–76. doi: 10.1016/j.yjmcc.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herring N, Lokale MN, Danson EJ, Heaton DA, Paterson DJ. Neuropeptide Y reduces acetylcholine release and vagal bradycardia via a Y2 receptor-mediated, protein kinase C-dependent pathway. Journal of molecular and cellular cardiology. 2008;44:477–85. doi: 10.1016/j.yjmcc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Zucker IH, Patel KP, Schultz HD. Neurohumoral stimulation. Heart failure clinics. 2012;8:87–99. doi: 10.1016/j.hfc.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang HJ, Wang W, Cornish KG, Rozanski GJ, Zucker IH. Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure. Hypertension. 2014;64:745–55. doi: 10.1161/HYPERTENSIONAHA.114.03699. [DOI] [PMC free article] [PubMed] [Google Scholar]