Abstract
Hydrogen sulfide (H2S) is a highly neurotoxic gas. Acute exposure can lead to neurological sequelae among survivors. A drug for treating neurological sequelae in survivors of acute H2S intoxication is needed. Using a novel mouse model we evaluated the efficacy of cobinamide (Cob) for this purpose. There were two objectives: (1) to determine the dose–response efficacy of Cob and (2) to determine the effective therapeutic time window of Cob. To explore objective 1, mice were injected intramuscularly with Cob at 0, 50 or 100 mg/kg at 2 min after H2S exposure. For objective 2, mice were injected intramuscularly with 100 mg/kg Cob at 2, 15, and 30 min after H2S exposure. For both objectives, mice were exposed to 765 ppm of H2S gas. Cob significantly reduced H2S-induced lethality in a dose-dependent manner (P < 0.05). Cob-treated mice exhibited significantly fewer seizures and knockdowns compared with the H2S-exposed group. Cob also reversed H2S-induced weight loss, behavioral deficits, neurochemical changes, cytochrome c oxidase enzyme inhibition, and neurodegeneration in a dose- and time-dependent manner (P < 0.01). Overall, these findings show that Cob increases survival and is neuroprotective in a mouse model of H2S-induced neurological sequelae.
Keywords: cobinamide, hydrogen sulfide, neurological sequelae, neuroprotection
Introduction
Hydrogen sulfide (H2S) is a highly toxic gas. Following exposure, at physiological pH, H2S almost instantaneously disassociates into its anion form (HS–), while less than 50% stays in its parent form.1–3 For the purposes of this paper, the term H2S will be used to refer to both species of hydrogen sulfide in vivo. Among toxic gases, H2S is the second most common cause of human death after carbon monoxide poisoning.2,4 High acute exposure to H2S leads to severe toxic effects, including death, with most deaths occurring at the scene of exposure.5 Whereas most H2S-induced deaths are occupational-related,5–8 suicide in confined spaces is a growing cause of death by H2S exposure.9,10
There are many sources of H2S in the environment, including natural discharges from volcanic activities and hot springs.11,12 Hydrogen sulfide is a contaminant of oil and natural gas and is a hazard to oil and petroleum industry workers;4,13 in addition, sour crude oil pipeline explosion accidents have led to mass civilian casualties.14 Rotting animal and plant matter (as in sewer systems), animal manure from intensive animal agriculture, and waste products of food processing plants have also been sources of acute H2S poisoning.15,16 In the Midwest, deaths have been reported due to acute H2S exposure following livestock manure pit agitation and pumping.17,18 Because H2S is relatively easy to make at home from ingredients commonly available in retail stores, concerns exist about potential misuse of H2S as a chemical weapon, particularly in confined spaces such as underground train stations and high-rise buildings.19,20 Mass civilian exposure to H2S has occurred following industrial accidents, as was the case in Poza Rica, Mexico14 and following a sour oil pipeline explosion in Kaixian County, China; the Poza Rica accident led to 22 deaths and hundreds affected, and the Kaixian accident led to hundreds of civilian deaths and affected several thousands.21
Acute inhalation exposure to high H2S concentrations affects the nervous, cardiovascular, and respiratory systems.22,23 Death from acute H2S exposure arises from cessation of breathing owing to inhibition of the respiratory center in the brain stem.24,25 During the Poza Rica and Kaixian industrial accidents, as well as during other occupational accidents, most deaths occurred at the scene, but a number of others died within 12 h after exposure.14,21 Of survivors discharged from the hospital with apparent recovery, many return 3–7 days postexposure complaining of neurological complications.6,26,27 These neurological sequelae include movement disorders, persistent migraines, memory loss, cognitive dysfunction and learning impairment, fatigue, hearing impairment, blindness, and seizures.6,22,27 Typically, neurological sequelae occur in victims who have suffered a knockdown and have been unconscious for at least 5 minutes.4
Currently, no specific treatment exists for treatment of neurological sequelae in survivors of acute H2S poisoning. Treatments, such as sodium nitrite, hydroxocobalamin, oxygen supplementation, hyperbaric oxygen, and hypothermia are directed toward treatment of the immediate effects of acute H2S poisoning.1,17,28,29 Cobinamide (Cob), a vitamin B12 precursor, is currently being investigated for treatment of acute cyanide and H2S intoxication.
Our study below has two specific objectives. One was to conduct a dose–response study in a mouse model (which we have recently reported on) to identify a therapeutic dose of Cob for treatment of neurological sequelae in mice that survive a specified exposure of H2S. The second objective was to conduct a time-course study to determine the therapeutic window of Cob for treatment of neurological sequelae using a dosage chosen from objective one above. We found that Cob markedly reduced H2S-induced mortality and neurological sequelae in mice exposed to H2S.
Materials and methods
Chemicals and reagents
Methanol (HPLC grade), acetonitrile (HPLC grade), MD-TM mobile phase, and formic acid were obtained from Fisher Scientific (Waltham, MA). D- and L-Glutamic acid, γ-aminobutyric acid (GABA), dopamine (DA), 3,4 dihydroxyphenlyacetic acid (DOPAC), homovanillic acid (HVA), norepinephrine (NE), 5-hydroxtryptaamine (5-HT), 5-hydroxyindoleacetic acid (5-HIAA), paraformaldehyde, and 60% perchloric acid were purchased from Sigma Aldrich (St. Louis, MO). All aqueous solutions were prepared using 18.2 MΩ·cm water (Aries Filter Network, West Berlin, New Jersey). Cobinamide was produced from hydroxocobalamin as described previously.30
Animals
All animal studies were approved by the Iowa State University Institutional Animal Care and Use Committee (IACUC). Only male mice were used in this study, because previous studies showed that male are more sensitive to H2S-induced neurotoxicity than female mice.31 The 7- to 8-week-old C57/BL6 male mice were purchased from the Jackson Laboratories (Bar Harbor, ME), and weighed 20–25 g at the beginning of the experiment. Mice were housed five per cage in the Laboratory Animal Resource (LAR) Facility, Iowa State University College of Veterinary Medicine (ISU CVM, Ames, IA). They were maintained at a controlled room temperature of 68–70 °F, relative humidity of 35–50%, and a 12-h light/dark cycle. Mice were provided 14% Protein Rodent maintenance diet (Teklad HSD Inc., WI) and drinking water ad libitum. These mice were acclimated to these environmental conditions for at least 1 week before the start of the experiments.
Experiment 1
Determining an ideal efficacious cobinamide dosage for treatment of neurological sequelae
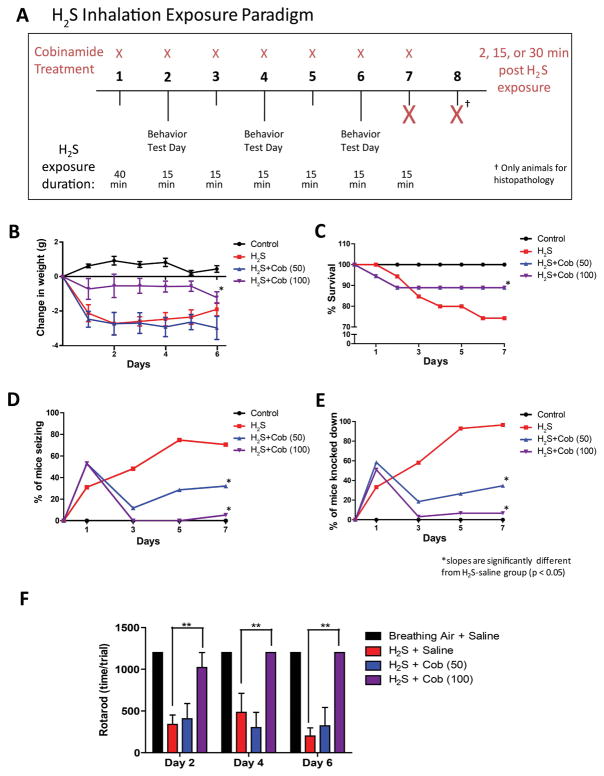
In this dose–response experiment, we tested the hypothesis that Cob manifests dose-dependent efficacy for treatment of neurological sequelae in a mouse model of H2S-induced neurological sequelae. To achieve this objective, we used a novel mouse model that was recently published.31 Briefly, fully conscious and freely moving mice were placed in a whole-body exposure chamber designed to hold up to 10 mice at a time. Mice were exposed by whole-body inhalation exposure, a route most relevant to human exposure scenarios. Gas access to the chamber was from two lines: one for breathing air from a tank under pressure and the other from a tank containing H2S under pressure. The two lines connected to the chamber via a control panel that allowed regulation of both breathing air and H2S flow (L/min). The real-time concentration of H2S in the exposure chamber was constantly monitored using a H2S monitor (Environmental Equipment and Supply, Harrisburg, PA) that was custom designed to measure concentrations of up to 1000 ppm of H2S. The H2S exposure paradigm used in this experiment is summarized in Figure 1A. This repeated acute exposure paradigm results in mice with behavioral, biochemical, and neurochemical changes and morphologic lesions recapitulating those of the human condition using a reasonably small number of mice.31 Detailed justification of this model was provided in an earlier publication in which results of neurological sequelae arising both from a single acute exposure (mimicking a typical human exposure scenario) and from the repeated short-term acute exposures were reported.31 Briefly, a single high-dose H2S exposure induced neurological sequelae in surviving mice, as did the acute short-term repeated exposure, but it caused very high mortality because of the steep dose–response effects of H2S.31 Using the repeated short-term, acute high-dose exposure paradigm, we were able to induce the same neurological sequelae humanely using a reasonable number of mice, allowing rigorous statistical evaluation of efficacy of the test article.
Figure 1.
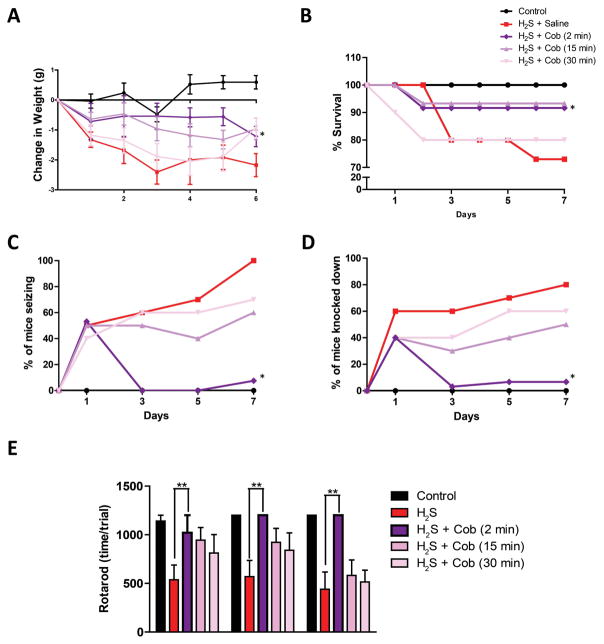
Cobinamide dose-dependently prevents H2S intoxication. (A) H2S exposure paradigm in a whole-body inhalation chamber for 7 days. (B) Weight loss, (C) mortality, (D) seizure activity, and (E) knockdown following H2S exposure. * P < 0.05; slopes are significantly different from H2S saline group. (F) Rotarod behavior testing post 2, 4, and 6 H2S exposures. ** P < 0.01 versus H2S/saline group.
Mice were randomly divided into four different groups as follows: group 1 mice were exposed to normal breathing air and injected with 0.9% saline; group 2 mice were exposed to 765 ppm H2S and injected with 0.9% normal saline (Cob 0 mg/kg bw); group 3 mice were exposed to 765 ppm H2S and injected with Cob at 50 mg/kg body weight; and group 4 mice were exposed to 765 ppm H2S and injected with Cob 100 mg/kg body weight. Cob or 0.9% saline was injected into the rear leg muscle in 50 μL of solution. Normal breathing air or H2S was delivered from gas cylinders. On the first day, mice in groups of 10 were exposed to 765 ppm H2S or breathing air for 40 minutes. On subsequent days, the same groups of mice were exposed either to 765 ppm H2S or to normal breathing air for 15 min only, each day. Following each exposure, the chamber was flushed out with breathing air for 2 min and injected with Cob or saline. All mice were euthanized 1 h after the last saline or Cob injection after the seventh exposure, except for mice designated for histopathological analysis, which were euthanized 24 h after the seventh H2S exposure. The reason for the latter was to allow a full 24 h for the test drug to work after the last Cob injection. End points monitored included daily clinical assessment during and after H2S exposure using a modified functional observational battery (FOB).32 Other tests included behavior tests and neurochemical, biochemical, molecular, and histopathology end points. The number of animals used for each of these tests is summarized in Table S1 (online only).
Clinical assessment
To obtain baseline data, animals were clinically evaluated and weighed daily starting 3 days before H2S exposure. Following exposure, mice were weighed daily until euthanasia. A modified FOB was used for clinical evaluation during exposure in the chamber.32 A clear advantage of this model employing freely moving un-anaesthetized mice is that it allowed clinical assessment of mice during exposure. To assess the clinical efficacy of Cob, clinical parameters evaluated included seizure activity and knockdown, which typically presented as lateral recumbency following seizure activity. Time to seizure and knockdown was also recorded. Other assessed parameters included lacrimation, salivation, piloerection, dyspnea, body posture, and gait. For consistency, the same trained observer assessed the mice throughout the entire experiment.
Behavior tests
Movement disorders are a common neurological sequela of acute H2S exposure in human beings.27 To test the efficacy of Cob on coordination and balance, mice were subjected to the AccuRotor 4-channel rotarod test (rod diameter = 30 mm, height = 38 cm). Mice were trained on the rotating rod for 2 consecutive days before H2S exposure at 24 rpm for 20 minutes. Following H2S exposure, mice were given five trials for 20 min at 24 rpm on test days 2, 4, and 6.
Histopathology and immunohistochemistry
In humans, neurodegeneration is a common sequela of acute H2S poisoning. To evaluate this and the efficacy of Cob, designated mice were anesthetized deeply with a cocktail of 100 mg/kg bw ketamine and 10 mg/kg bw xylazine IM. Once in a surgical plane of anesthesia, the thoracic cavity was surgically opened to expose the heart. Fresh 4% paraformaldehyde solution (PFA, pH 7.4) was then injected through the left ventricle to perfuse the animal. After perfusion, brains were post-fixed in 4% PFA for 24 h, paraffin embedded, sectioned at 5 microns, and stained with hematoxylin and eosin for routine histopathology. Additional brain sections were stained using an indirect immunostaining protocol (Vectastain Elite ABC kit, PK-6101, Vector Laboratories, Inc., Burlingame, CA) that employed primary antibodies directed at glial fibrillary acidic protein (GFAP, ab72600, Abcam), ionized calcium–binding adaptor molecule 1 (Iba1, ab153696, Abcam), and 4-hydroxynonenal (4HNE; ab46545, Abcam). Diaminobenzidine (DAB, SK-4100, Vector Laboratories, Inc.) was the chromogen used. Stained sections were examined microscopically using a Nikon Eclipse Ci-L microscope with a DS-Fi2 camera or EVOS FL fluorescence microscope. Routine histopathology, as well as immunohistopathology, was conducted by a board-certified veterinary pathologist blinded to the study design. Lesion severity was assessed using semi-quantitative scale, summarized in Table S2.
Biochemical and neurochemical analysis
H2S inhibits cytochrome c oxidase activity and induces neurochemical changes by inhibiting the monoamine oxidase enzyme. These were used to assess the efficacy of Cob. Mice were euthanized by decapitation 1 h after the last Cob injection. Brains were immediately removed from the skull, held on ice, and microdissected into different brain regions. Brain tissue samples were stored at –80 °C until analysis. Cytochrome c oxidase enzyme was extracted from microdissected brain regions (inferior colliculus, thalamus, and cortex) and enzyme activity determined using an assay kit (ab109909) from Abcam (Cambridge, MA) according to the manufacturer’s protocol.
To determine H2S-induced neurochemical changes, the striatum was analyzed for changes in dopamine (DA) and its metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA). The striatum was also analyzed for serotonin (5-HT), its metabolite 5-hydroxyindoleacetic acid (5-HIAA), and norepinephrine (NE). Samples were prepared and quantified as described previously.31 Briefly, neurotransmitters were extracted from different brain regions in 0.2 M perchloric acid solution containing 0.05% Na2EDTA, 0.1% Na2S2O5, and isoproterenol (internal standard). DA, 5-HT, NE, and their respective metabolites were analyzed by HPLC electrochemical detection consisting of a CoulArray model 5600A coupled with an analytical cell (microdialysis cell 5014B) and a guard cell (model 5020). Neurochemicals and metabolites were separated isocratically by a reverse-phase column with a flow rate of 0.6 mL/min mobile phase (10% acetonitrile, 1% sodium phosphate, 89% water) using a Dionex Ultimate 3000 HPLC system (pump ISO-3100SD, Thermo Scientific, Bannockburn, IL) equipped with a refrigerated automatic sampler (model WPS-3000TSL). Data acquisition and analysis were performed using Chromeleon 7 and ESA Coularray 3.10 HPLC Software.
Molecular targets
In order to elucidate the molecular effects of H2S-induced neurotoxicity and to evaluate efficacy of Cob as a therapeutic agent, the inferior colliculi from mice in the breathing air control group, H2S saline control, and H2S Cob (100 mg/kg) mice were analyzed for gene and protein changes. First, a total RNA isolation protocol was adapted from Seo et al.33 Briefly, brain tissue was placed in TRIzol Reagent, homogenized, and incubated for 5 minutes. Then, 200 μL of chloroform was added and shaken vigorously for 15 seconds. This mixture was incubated for 3 min and then centrifuged at 12,000 × g for 15 min at 4 °C. The upper, aqueous layer was transferred to a separate tube, and 500 μL of isopropanol was added. This suspension was incubated at RT for 10 min before being centrifuged for 10 min at 4 °C at 12,000× g.33 First-strand cDNA synthesis was performed using a High Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems, #4368814) following the manufacturer’s protocol. Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) was performed following previous protocols.34 The following validated mouse primers were purchased from Qiagen and used for performing q RT-PCR: TNF-α, IL-1β, IL-6, IL-12, and IL-18, TLR9, and MyD88. For normalization of each sample, the 18S rRNA gene (purchased from Qiagen) was used as the housekeeping gene. No-template controls (NTCs) and dissociation curves were run for all experiments to exclude cross-contamination.34
With the aim of studying protein changes via western blot, the inferior colliculus samples were lysed in modified RIPA lysis buffer (1% Triton X-100, 1 mM EDTA, 100 mM NaCl, 1 mM EGTA, 1 mM NaF, 20 mM Na4P2O7, 2 mM Na3VO4, 10% glycerol, 0.1% SDS, 0.5% deoxycholate, 50 mM Tris-Cl, pH7.4) via sonication. Brain homogenates were prepared as described previously.35 Protein concentration of samples was measured using the Bradford assay. Western blotting was performed as described previously.35,36 Briefly, the samples, containing equal amounts of proteins, were loaded and fractionated in a 10–12% SDS-PAGE gel and transferred onto a nitrocellulose membrane. Membranes were blocked with 5% milk in TBS supplemented with 0.1 % Tween-20 or TBS blocking buffer purchased from LI-COR (Lincoln, NE). Primary antibodies against specific proteins were incubated with the membrane overnight at 4 °C. These included TNF-α receptor 2 (TNF-αR2, ab15563, Abcam), BCL2-associated death promoter (Bad, sc-943, Santa Cruz Biotechnology), and TNF-α (R&D Biosystems). After rinsing thoroughly in PBS supplemented with 0.1% Tween-20, the membrane was incubated with Alexa Fluor 680 goat anti-mouse or IRDye 800 donkey anti-rabbit secondary antibodies. For the loading control, β-actin antibody was used. Immunoblot imaging was performed with an Odyssey Infrared Imaging system (LI-COR, Lincoln, NE). ImageJ software (National Institutes of Health, Bethesda, MD) was used to quantify Western blot bands of targeted proteins were normalized to β-actin or α-tubulin.
Experiment 2
Determining the time window of efficacy of cobinamide for treatment of neurological sequelae
Using an effective Cob dosage chosen from experiment 1 above, we tested the hypothesis that Cob at 100 mg/kg bw is efficacious for treatment of H2S-induced neurological sequelae given up to 30 mins after H2S exposure. The same H2S exposure paradigm as in experiment 1 was used for this study. We chose 100 mg/kg bw Cob because the dose–response study (experiment 1 above) revealed this to be the most effective dose. We selected the time window of 2–30 min as the study period because, in typical rescue scenarios, first responders arrive within this time period; treatment should be given as soon as possible to minimize toxic injury.
Mice were randomly divided into five groups as follows: group 1 mice were exposed to normal breathing air and injected with 0.9% normal saline 30 min postexposure; group 2 mice were exposed to H2S and injected with 0.9% normal saline 30 min postexposure; group 3 mice were exposed to H2S and injected with Cob (100 mg/kg) 2 min after H2S exposure; group 4 mice were exposed to H2S and injected with Cob (100 mg/kg) 15 min after H2S exposure; and group 5 mice were exposed to H2S and injected with Cob (100 mg/kg) 30 min after H2S exposure. Measured end points included clinical assessments using a modified FOB, behavioral tests, and neurochemical, biochemical, and histopathological end points as described for experiment 1. All mice were euthanized 1 h after saline or Cob injection after the seventh exposure.
Data analysis
Data are presented as mean and standard error of the mean. Clinical toxicity during exposures was analyzed using linear regression. Biochemical and neurochemical endpoints were analyzed using a Student’s t-test comparing the H2S/saline–treated group to the H2S/Cob treated group. Behavioral data was analyzed using 2-way analysis of variance with a Bonferroni post hoc test. Statistical tests were performed on Prism 6 (GraphPad Prism Software). Data was considered statistically significant at P < 0.05.
Results
Experiment 1: dose-response cobinamide study
Clinical assessment results
Results of clinical assessment are summarized in Table 1. Mice exposed to normal breathing air were normal and healthy throughout the duration of the study. Mice in the group exposed to H2S and treated with Cob scored better clinically compared with mice exposed to H2S and treated with saline (Table 1). For example, mice exposed to H2S and injected with saline exhibited typical clinical signs of H2S poisoning, such as lacrimation, salivation, ataxia, impaired righting reflex, and convulsions, which were absent in mice exposed to H2S and treated with Cob. However, all H2S-dosed mice exhibited dyspnea characterized by open-mouth breathing and hunched body posture, regardless of whether they were treated with Cob. Mice exposed to H2S and injected with Cob at 100 mg/kg lost significantly less weight than those injected with saline. The lower Cob dosage at 50 mg/kg dose did not prevent the H2S-induced weight loss (Fig. 1B). However, Cob at 50 mg/kg and 100 mg/kg both increased survival in this model (Fig. 1C).
Table 1.
Modified functional observational battery for evaluation of clinical signs during H2S exposure in the inhalation chamber.
| Measure | Breathing air + saline | H2S + saline | H2S + cobinamide (100 mg/kg) |
|---|---|---|---|
|
| |||
| Autonomic | |||
| Lacrimation | No | Present | No |
| Salivation | No | Present | No |
| Urination/defecation | Normal | Increased | No |
| Convulsions | |||
| Tonic movements | No | Mild | No |
| Clonic movements | No | Severe | Mild |
| Paddling feet | No | Severe | Mild |
| General measures | |||
| Piloerection | No | Present | No |
| Body posture | Normal | Hunched | Hunched |
| Dyspnea | No | Present | Present |
| Gait | |||
| Hindlimbs splayed | No | Present | No |
| Ataxia | No | Severe | Mild |
| Unable to support weight | No | Present | No |
| Righting reflex | No | Affected | Mild |
| Knockdown | No | Severe | Mild |
Over the course of the study, seizures were observed in about 80% of the mice exposed to H2S treated with saline, whereas only 5% of the Cob-treated mice exhibited seizure activity (Fig. 1D). Mice treated with the high dose of Cob (100 mg/kg) experienced significantly fewer knockdowns compared to the H2S-saline treated group, while the lower dosage Cob (50 mg/kg bw) did not protect against seizures as efficiently (Fig. 1E). Overall, Cob at the 100 mg/kg dosage was more effective in countering clinical signs induced by H2S.
Behavior tests
Results of the rotarod test are summarized in Figure 1F. Cob at the higher dose (100 mg/kg bw), but not at the lower dose (50 mg/kg bw), significantly protected mice against H2S-induced motor deficits.
Histopathology and immunohistochemistry
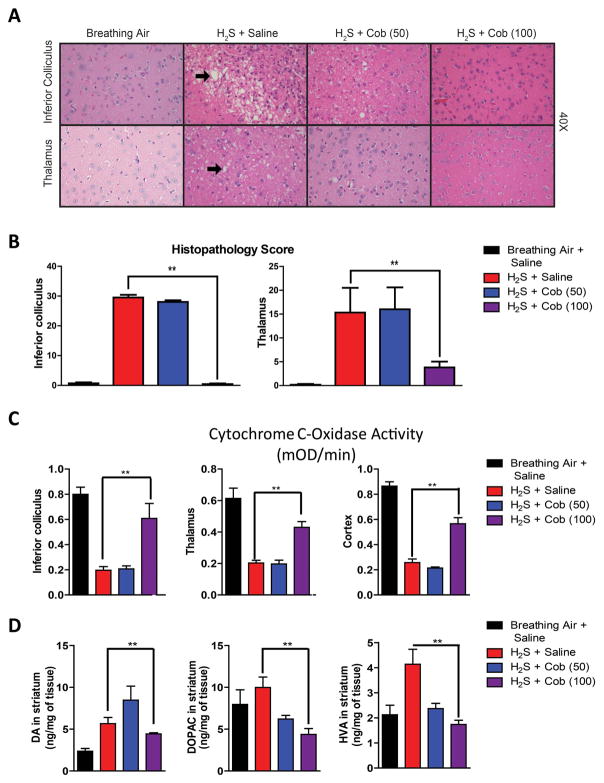
The inferior colliculus and the thalamus were the most consistently affected brain regions in this model. H2S-induced microscopic lesions consisted of vacuolar degeneration with formation of coalescing vacuoles in the neuropil, neuronal degeneration and cell death, and infiltration by activated neuroglia (Fig. 2A). In severely affected animals, there was complete loss of neurons in affected regions at 7 days, with increased numbers of astrocytes, microglia, and the presence of microglial phagocytic (Gitter) cells. Capillaries within injured regions had prominent endothelial cells and were mildly tortuous, consistent with glial scar formation. Cob at the highest dosage (100 mg/kg) significantly reduced the severity of lesions in the inferior colliculus and thalamus, resembling control animals, while several of the animals treated with the lower dosage (50 mg/kg) developed degenerative lesions. These results showed that Cob at the higher dose was more effective in reducing lesion severity and incidence than the lower dosage (Fig. 2A and 2B).
Figure 2.
Cobinamide is dose-dependently effective in preventing H2S neurotoxicity. (A and B) Lesion severity following treatment with Cob (50 mg/kg and 100 mg/kg). H2S-induced lesions are indicated by black arrows. (C) Cytochrome c oxidase activity inhibition and (D) neurochemical alterations induced by H2S after Cob treatment (100 mg/kg). ** P < 0.01 versus H2S/saline group. Representative photomicrographs of the inferior colliculus and thalamus (hematoxylin and eosin staining).
Biochemical and neurochemical analysis
Previous work in our lab found that H2S inhibits cytochrome c oxidase in multiple brain regions.31 We investigated cytochrome c oxidase activity in H2S-poisoned mice treated with saline versus those treated with Cob. Cytochrome c oxidase activity was significantly diminished in mice exposed to H2S and injected with saline but was rescued in mice injected with Cob at 100 mg/kg. However, Cob at 50 mg/kg bw failed to rescue H2S-induced suppression of H2S-induced cytochrome c oxidase activity. This was observed in the inferior colliculus, thalamus, and cortex (Fig. 2C).
Mice exposed to H2S and injected with saline showed a statistically significant increase in DA and its respective metabolites DOPAC and HVA. Cob at the highest dose (100 mg/kg bw) prevented the H2S-induced increase in DA, DOPAC, and HVA (Fig. 2D).
Molecular targets
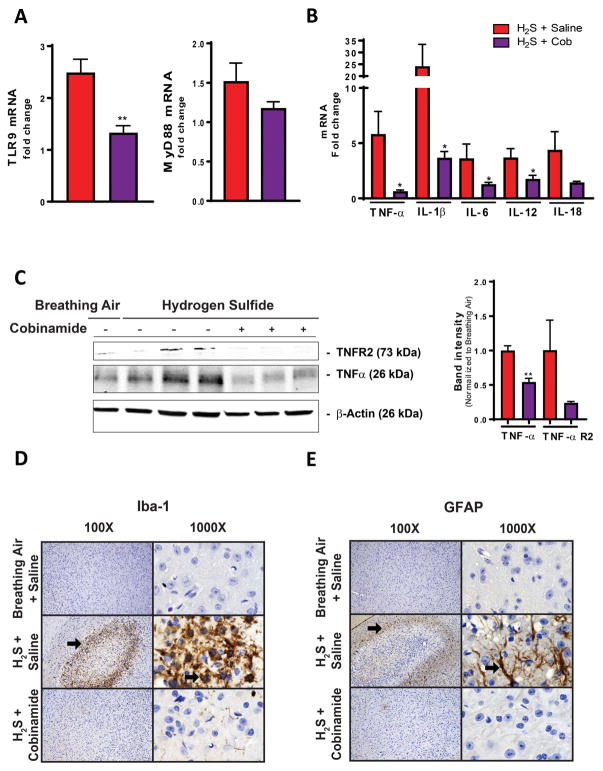
We previously reported that neuroinflammation is one of the mechanisms that we hypothesize to be involved in H2S-induced neurodegeneration.22 Since Cob was most effective at 100 mg/kg bw, we only investigated this dosage in this portion of the study. Also, we focused on the inferior colliculus, the most consistently affected brain region in this model. We found that TLR9 and MyD88 mRNA expression were significantly increased in the inferior colliculus of the H2S-exposed mice treated with saline (Fig. 3A). Cob at 100 mg/kg bw prevented increased expression of TLR9 and MyD88 mRNAs. Activation of MyD88 by TLR9 can lead to NF-κB activation, causing increased expression of proinflammatory cytokines. Therefore, we investigated the effect of H2S on cytokines and the impact of Cob. Mice exposed to H2S and injected with saline showed a significant increase in mRNA expression of TNF-α, IL-1β, IL-6, and IL-12. Cob at 100 mg/kg significantly lowered the expressions of these proinflammatory cytokines (Fig. 3B). We also found upregulation of TNF-α and TNF-α receptor in mice exposed to H2S, but these TNF-α–related H2S-induced changes were prevented in mice exposed to H2S and treated with 100 mg/kg Cob (Fig. 3C). Increases in GFAP and Iba-1 protein typically reflect activated astrocytes and microglia, indicating a neuroinflammatory response. H2S significantly increased the protein expression of GFAP and Iba-1 in the inferior colliculus, surrounding the lesion. This GFAP and Iba-1 H2S-induced increase was reduced by Cob (Fig. 3D and 3E).
Figure 3.
Cobinamide (100 mg/kg) prevents H2S-induced neuroinflammation in the inferior colliculus. (A and B) TLR9, MyD88, and proinflammatory cytokine mRNA expression with treatment of Cob after H2S exposure. (C) TNF-αR2 and TNF-α protein by Cob treatment after H2S exposure. (D) Activation status of microglia and (E) astrocytes observed following treatment with Cob in H2S-exposed mice. Arrows in D and E highlight increased expression of Iba1 and GFAP, respectively, in animals exposed to H2S and untreated by Cob. Graphs are represented as mean values. * P < 0.05, ** P < 0.01 versus the H2S/saline group. Representative photomicrographs of the inferior colliculus (Iba1 and GFAP immunohistochemical staining).
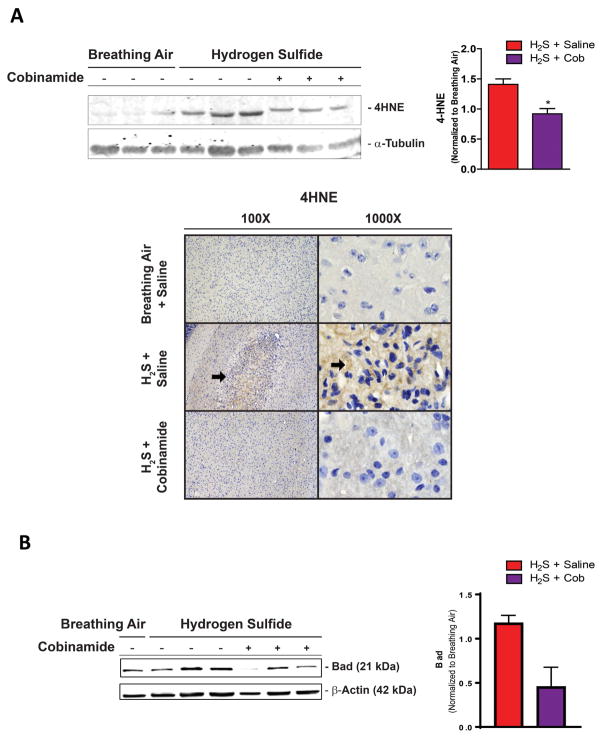
Oxidative stress is another common mechanism of toxin-induced neurodegeneration and is often coupled to inflammation. In this study, using western blot and immunohistochemistry analysis, we observed increased 4-HNE, a biomarker of lipid peroxidation, in mice exposed to H2S and injected with normal saline (Fig. 4A). Cob at 100 mg/kg prevented the generation of 4-HNE, implying reduced oxidative stress. H2S also induced an increase of Bad, a proapoptotic protein, in the inferior colliculus; while Cob (100 mg/kg) reduced Bad expression (Fig. 5B).
Figure 4.
Cobinamide (100 mg/kg) prevents oxidative stress and mitochondrial dysfunction induced by H2S exposure. Animals exposed to H2S and treated with Cob have less 4-HNE production in the inferior colliculus than untreated animals. (A) Representative photomicrographs of the inferior colliculus demonstrate similar amounts of 4-HNE in controls and in animals exposed to H2S and given Cob treatment, compared with animals exposed to H2S alone (arrows). (B) Bad expression induced by H2S with and without Cob treatment. * P < 0.05 versus H2S/saline group. Representative photomicrographs of the inferior colliculus (4HNE immunohistochemical staining).
Figure 5.
Cobinamide prevents clinical intoxication when administered 2 min after H2S exposure. (A) Weight loss, (B) survival, (C) seizure activity, and (D) knockdown during H2S exposure. * P < 0.05; slopes are significantly different from H2S/saline group. (E) Rotarod behavior testing post 2, 4, and 6 H2S exposures. ** P < 0.01 versus H2S/saline group.
Collectively, results of this mechanistic data show that Cob is neuroprotective against H2S-induced inflammation, oxidative stress, and mitochondrial dysfunction. Overall, results of the dose–response Cob study showed that Cob at 100 mg/kg bw was effective in treatment of H2S-induced neurotoxicity and neurodegeneration.
Experiment 2: therapeutic time window of cobinamide
Clinical assessment results
In this portion of study, we used only the high dose of Cob (100 mg/kg bw) because the dose–response study had revealed this to be the most effective dose. As observed in experiment 1, mice treated with 100 mg/kg Cob injected 2 min postexposure lost significantly less weight than the groups of mice injected with saline after H2S exposure throughout the 7 days of exposure. Body weights of the group of mice injected with Cob 15 or 30 min after H2S exposure were not significantly different from the group of mice exposed to H2S and injected with saline (Fig. 5A). However, H2S-exposed mice injected with Cob at 2 and 15 min postexposure had a significantly greater cumulative survival rate compared with the mice injected with saline (Fig 5B). All mice that died during the experiment died during H2S exposure or within 5 min of termination of H2S exposure.
Cob was most protective against seizure activity and knockdown induced by H2S when given 2 min postexposure compared with at 15 or 30 min postexposure. Cumulatively, by the seventh exposure, only about 10% of mice given Cob 2 min postexposure had seizures and knockdowns, which were significantly lower than that of H2S-exposed mice injected with saline (60%). With respect to seizure activity, H2S-exposed mice given Cob 15 or 30 min after H2S exposure were not protected and were not different than the H2S-saline treated group (Fig. 5C and 5D).
Behavior tests
Behavioral studies showed that motor deficits induced by H2S were significantly prevented by injecting Cob 2 min postexposure compared with the H2S group of mice injected with saline. Mice injected with Cob 15 and 30 min after H2S exposure did not perform significantly better than H2S-dosed mice injected with saline on the rotarod test (Fig. 5E). Overall, these behavioral results show that Cob is most efficacious against H2S-induced toxicity if given 2 min after H2S exposure rather than 15 or 30 min after H2S exposure.
Histopathology
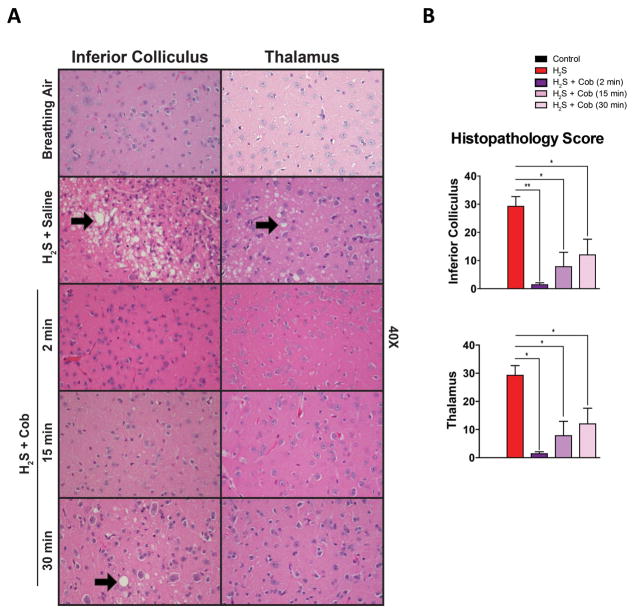
Control animals had no lesions. Animals exposed to H2S consistently developed severe lesions in the inferior colliculus and mild lesions in the thalamus. None of the mice given Cob 2 min after H2S exposure developed moderate or severe lesions. Twenty percent of mice treated with Cob at 15 min after H2S exposure developed inferior colliculus injury with a histopathology score of >15, while 40 % of mice treated with Cob at 30 min after H2S developed inferior colliculus damage. Cob treatment at 15 or 30 min resulted in protection from neuronal degeneration and death and reduced glial responses, although the best results were observed at 2 minutes. Treatment with Cob at either 15 or 30 min after H2S exposure strongly protected against thalamic injury, as did treating at 2 min after H2S exposure. Results of the histopathology study suggest that injecting Cob 30 min postexposure was still beneficial in some mice, but the best neuroprotection was achieved when treatment was given 2 min after H2S exposure (Fig. 6A and 6B).
Figure 6.
Cobinamide is efficacious in treating neurotoxicity when administered up to 30 min after H2S exposure. (A and B) H2S-induced lesion severity with Cob treatment was observed up to 30 min after H2S exposure. Vacuolization, neurodegeneration, and gliosis are present in the inferior colliculus and, to a lesser extent, in the thalamus of animals exposed to H2S (arrows). * P < 0.05, ** P < 0.01 versus H2S/saline group. Representative photomicrographs of the inferior colliculus and thalamus (hematoxylin and eosin staining).
Biochemical and neurochemical analysis
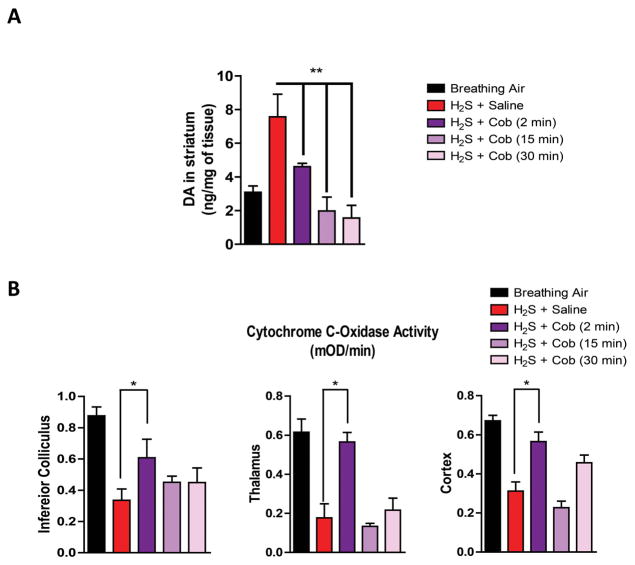
As observed in experiment 1, H2S induced an increase in dopamine in the striatum. Injecting Cob (100 mg/kg) 2, 15, and 30 min after H2S exposure prevented an increase in dopamine levels (Fig. 7A). H2S-induced changes were most responsive to Cob therapy at 2 min; and giving Cob even 30 min after was still beneficial (Fig. 7).
Figure 7.
Cobinamide is effective in preventing H2S-induced neurotoxicity when given up to 30 min after H2S exposure. (A) DA levels induced by H2S up to 30 min postexposure with and without Cob treatment. (B) Inhibition of cytochrome c oxidase activity after H2S exposure with and without Cob treatment. * P < 0.05, ** P < 0.01 versus H2S/saline group.
We also investigated the effects of Cob on the H2S-induced inhibition of cytochrome c oxidase when the drug is administered at 2, 15, or 30 min after H2S exposure. The results indicated that Cob was most effective in preventing cytochrome c oxidase inhibition when given 2 min postexposure. Cob injected 15 or 30 min postexposure did not prevent H2S-induced inhibition of cytochrome c oxidase enzyme in any of the brain regions examined, including the inferior colliculus, thalamus, and cortex. (Fig 7B).
Discussion
Currently, there is no drug for treatment of neurological sequelae in human survivors of acute H2S poisoning. Our data are the first evaluate the efficacy of any drug, including Cob, for treatment of neurological sequelae in survivors (as generated in the mouse model) of acute H2S poisoning. The study demonstrated that Cob is effective for treatment of H2S-induced neurodegeneration and other neurological sequelae in surviving mice and extends the work of others, which focused on testing the efficacy of Cob on reducing acute mortality.2 In our mouse model we found that at 100 mg/kg Cob (human equivalent dose = 8.1 mg/kg) was most effective when given immediately after rescue from H2S exposure. At 50 mg/kg, Cob was not consistently effective across the suite of end points monitored in this study. This observation is borne out by the percent survival; behavioral changes, including prevention of body weight changes, rotarod test results; protection against neurochemical changes; protection against cytochrome c oxidase inhibition; prevention of neural inflammation and oxidative stress; and reduction in neural lesion frequency and severity. As might be expected, delaying Cob injection after H2S exposure reduced its efficacy.
H2S dissociates into different sulfide species within a few minutes in vivo. Excellent studies by Haouzi et al., Jiang et al., Salnikov et al., and Cronican et al. suggest that following H2S exposure <50% of H2S exists in the parent form.1–3,29 Considering this observation, it makes sense that Cob therapy, if extrapolated to the case of human exposure, would be most beneficial when given during or a few minutes after termination of H2S exposure, as it is during this time that Cob would combine with H2S before it transforms into other sulfide species. It is interesting that, in our mouse model, the time-course study (experiment 2) showed that Cob had some efficacy at 15 and even at 30 min after H2S exposure. This suggests that Cob may harbor other mechanisms that collectively contribute to its efficacy in this mouse model.
While the role of H2S in oxidative stress is controversial, Jiang et al. demonstrated H2S-induced oxidative stress via generation of superoxide anions, but, more importantly, Cob neutralized these free radicals.2,37 Free radicals beget free radicals, triggering a chain reaction of oxidative stress, and giving Cob as late as 15 min postexposure may be beneficial in interrupting this process. We hypothesize that, by binding H2S and neutralizing free radicals, Cob protects critical molecular targets, reducing injury and associated inflammation.
Seizure activity in surviving mice was significantly reduced or prevented by Cob. although the mechanisms of H2S-induced seizures are unknown, changes in concentrations of neurotransmitters such as GABA and glutamate and neural inflammation have been associated with seizures.39 We did not measure GABA and glutamate in the above study, but we observed neuroinflammation. Cob at 100 mg/kg reduced neuroinflammation, and this may have reduced seizure activity. Seizure severity has been linked to neural injury in other models of chemical-induced neurotoxicity, such as nerve agents;40 reducing seizure activity could be one way in which Cob reduced lesion severity. Regardless of the mechanisms involved, the data above show that Cob reduced H2S-induced neurodegeneration.
In the broader context of effects in humans, the role of H2S in inflammation is controversial both in vitro and in vivo.41–44 H2S has been shown to increase proinflammatory cytokine expression in human monocytes via the ERK–NF-κB pathway.45 The data above show that Cob ameliorates the neuroinflammatory effects of H2S in the inferior colliculus of mice. Furthermore, we found that H2S exposure was associated with an increase in TLR9 expression and a subsequent increase in MyD88 expression, leading to transcription of proinflammatory cytokines via the NF-κB pathway. We also found an increase in IL-6 expression; increased IL-6 is an important factor in vascular inflammation,46 which is caused by monocyte infiltration into the injured vessel wall. In our mouse model, H2S exposure was associated with hemorrhage in areas of the lesion, indicative of vascular damage; the damage was prevented by pretreatment of the mice with Cob.
TNF-α is frequently implicated in inflammation. Under toxic conditions, microglia can release large amounts of TNF-α.47 Increased TNF-α has been implicated in excitotoxicity by inhibiting glutamate transporters on astrocytes and increasing AMPA and NMDA receptors, while also decreasing GABA-A receptors on neurons.39,47 These mechanisms could be involved in the seizure-like activity that has been reported in human H2S exposure cases.48 We found that both mRNA and protein expression of TNF-α were increased in mice exposed to H2S plus saline. The increased expression was significantly reduced by pretreatment of the mice with Cob.
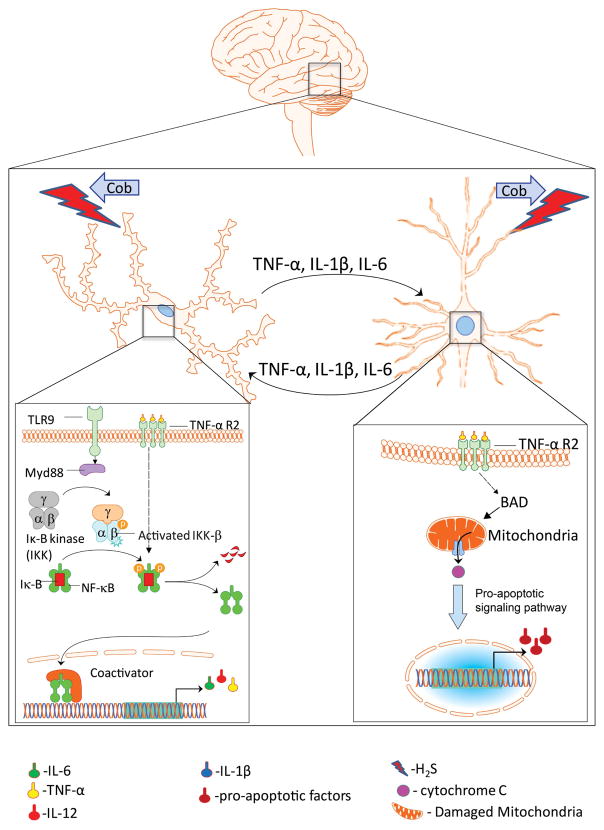
Release of proinflammatory cytokines (IL-1β, IL-6, IL-18, TNF-α, and IL-12) from microglia can be toxic to neurons as they are rapidly upregulated when reacting to a toxic insult. Proinflammatory cytokines then propagate the inflammatory response and can cause neuronal and synaptic dysfunction.50 Neurons themselves can produce cytokines in response to the peripheral cytokines,51 which then sustain neuroinflammatory responses (Fig. 8). Damaged neurons can also lead to the transformation of microglia into phagocytic cells that remove cellular debris.52 We note that Cob (100 mg/kg) injected into mice 2 min after H2S exposure prevented similar neuroinflammatory changes from occurring.
Figure 8.
A proposed mechanism. Cobinamide prevents the downstream effects of H2S-induced neurodegeneration by binding to H2S to attenuate neuroinflammation, oxidative stress, and mitochondrial dysfunction.
Oxidative stress has been implicated in neuronal injury. In our model of H2S-induced neurodegeneration oxidative stress can be caused by activated microglia,52 as well as directly from H2S. 4-Hydroxynonenal (4-HNE), a biomarker of oxidative stress, has been implicated as a factor in mitochondrial dysfunction.53 We found that H2S increased the production of 4-HNE and Cob prevented the increase.
A hallmark of H2S-induced toxicity is inhibition of cytochrome c oxidase activity.54,55 Low cytochrome c oxidase activity can result in a loss of membrane potential, leading to production of oxidative stress56,57 and mitochondrial dysfunction. Bad is a proapoptotic protein in the Bcl-2 family, a group of proteins that mediate pro- and antiapoptotic signaling in the mitochondria.57,58 Mitochondrial dysfunction has been shown to lead to neurodegeneration.59 We found that Cob (100 mg/kg) prevented the inhibition of cytochrome c oxidase by H2S as well as upregulation of H2S-induced expression of Bad. These results show that Cob likely mitigates H2S-induced mitochondrial dysfunction.
Collectively, the data above show potential novel mechanisms of action for H2S-induced neurotoxicity: H2S induced neuroinflammation results in production of proinflammatory cytokines that leads to neuronal dysfunction, including excitotoxicity and oxidative stress induced mitochondrial dysfunction, which then propagates inflammation and neuronal cell death (Fig. 8). Cob (100 mg/kg) injected 2 min postexposure prevented these physiological changes. We speculate that Cob binds directly to H2S preventing it from interacting with key molecular targets, thus preventing the pathological, neurochemical, and biochemical changes induced by H2S.
Conclusions
Together, the results of this study show that Cob is protective against H2S-induced neurotoxicity and neurodegeneration in an mouse model. Among the data are indications of the mechanistic effects of H2S, further identifying other potential targets for therapeutic strategies. The advantage of the mouse model used in this study is that the route of exposure––inhalation––is representative of the most common route of human H2S exposure. The model also recapitulates lesions found in human survivors of acute H2S intoxication.6,8,22,26,27,60–62 However, we acknowledge that the inhalation mouse model of H2S poisoning used here is quite different from human exposure to H2S. In particular, the short-term acute repeated H2S exposure used above is atypical of human exposure scenarios. Also, in humans neurological sequelae develop in survivors of single, acute high-dose exposures.6,26 Although it would have been ideal to use a single, acute high-dose H2S exposure, mice suffer high mortality, with only a few of the survivors developing neurodegeneration. Using a single, high-dose exposure to mimic the typical human single high-dose exposure scenario would have required an unreasonably large number of mice to test the efficacy of Cob. The approach used above induced H2S-induced neurological sequelae seen in humans but with low mortality, which enabled statistical assessment of drug efficacy with a reasonable number of mice. A similar approach was taken by Lund et al. in a monkey study because a single high-dose exposure caused mortality in monkeys; instead, two exposures 3 days apart were used, which resulted in brain lesions resembling the lesions found in humans.63
The half-life of Cob is 6–7 h, and by 24 h only about 2% of the maximal plasma concentration remains. Whereas some Cob was likely present at the subsequent injection, the remaining Cob was not enough to provide prophylactic coverage in subsequent Cob injections. This point is supported by results of the time-course study, where repeated administration of Cob at the same dosage (100 mg/kg) at 2, 15, and 30 mins after H2S exposure produced different results, with treatment at 2 mins postexposure yielding the best efficacy results. While this model was useful for the evaluation of the efficacy of Cob, it may not be suitable for evaluation of cumulative drugs, for which a modification of the model would be recommended. The study period of 1 week used above was sufficient to answer the questions posed; in future studies, it will be of interest to extend the observation period to about 4 weeks to allow assessment of reversibility of lesions.
In summary, Cob at 100 mg/kg bw was effective in treating H2S-induced neurodegeneration and neurological sequelae in this mouse model. Cob was most efficacious when given within 2 min of rescue of mice from H2S exposure, although the drug still showed some efficacy up to 30 min after H2S exposure. More work is recommended to further evaluate the efficacy of Cob for survivors of H2S in other contexts, including extending the observation period to assess whether the lesions are reversed and also to test the efficacy of Cob in other animal models. More generally, whether these results can be extrapolated to cases of human survival of acute H2S exposure remains an open question.
Supplementary Material
Summary of mice used per end point in experiments.
Grading scheme for evaluation of histopathological lesions in the brain.
Acknowledgments
This research is supported by the CounterACT Program, National Institutes of Health Office of the Director (NIH OD), and the National Institute of Neurological Disorder and Stroke (NINDS), Grant Number [NS089487]. The experiments were conceived and designed by W.K.R., P.A., A.G.K., A.K., and G.B. Cobinamide was prepared by A.C. The experiments were performed by P.A., B.M., C.S., S.S., and D.S.K. Analyses were done by P.A., S.S., D.S.K., and E.M.W., and they take responsibility for the integrity of the data analyzed. The manuscript was prepared by P.A., W.R.K., A.K., and E.M.W. We thank Dr. Jacek Koziel for setting up the inhalation chamber, Moriah Jenkins and Aubree Beenken for aiding with the animal work, and Patricia Lewis for histology.
Footnotes
Competing interests
The authors declare no competing interests.
Additional supporting information may be found in the online version of this article.
References
- 1.Haouzi P, Chenuel B, Sonobe T. High-dose hydroxocobalamin administered after H2S exposure counteracts sulfide-poisoning-induced cardiac depression in sheep. Clin Toxicol (Phila) 53:28–36. doi: 10.3109/15563650.2014.990976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang J, et al. Hydrogen Sulfide-Mechanisms of Toxicity and Development of an Antidote. Sci Rep. 2016;6:20831. doi: 10.1038/srep20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salnikov DS, et al. Kinetics and mechanism of the reaction of hydrogen sulfide with diaquacobinamide in aqueous solution. Eur J Inorg Chem. 2014;2014:4123–4133. doi: 10.1002/ejic.201402082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guidotti TL. Hydrogen sulfide intoxication. Handb Clin Neurol. 2015;131:111–133. doi: 10.1016/B978-0-444-62627-1.00008-1. [DOI] [PubMed] [Google Scholar]
- 5.Hendrickson RG, Chang A, Hamilton RJ. Co-worker fatalities from hydrogen sulfide. Am J Ind Med. 2004;45:346–350. doi: 10.1002/ajim.10355. [DOI] [PubMed] [Google Scholar]
- 6.Arnold IM, et al. Health implication of occupational exposures to hydrogen sulfide. J Occup Med. 1985;27:373–376. doi: 10.1097/00043764-198505000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Guidotti TL. Occupational exposure to hydrogen sulfide in the sour gas industry: some unresolved issues. Int Arch Occup Environ Health. 1994;66:153–160. doi: 10.1007/BF00380773. [DOI] [PubMed] [Google Scholar]
- 8.Hoidal CR, et al. Hydrogen sulfide poisoning from toxic inhalations of roofing asphalt fumes. Ann Emerg Med. 1986;15:826–830. doi: 10.1016/s0196-0644(86)80383-3. [DOI] [PubMed] [Google Scholar]
- 9.Nabeshima Y, et al. Analysis of Japanese Articles about Suicides Involving Charcoal Burning or Hydrogen Sulfide Gas. Int J Environ Res Public Health. 2016;13 doi: 10.3390/ijerph13101013. pii: E1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sams RN, et al. Suicide with hydrogen sulfide. Am J Forensic Med Pathol. 34:81–82. doi: 10.1097/PAF.0b013e3182886d35. [DOI] [PubMed] [Google Scholar]
- 11.Smith RP, Gosselin RE. Hydrogen sulfide poisoning. J Occup Med. 1979;21:93–97. doi: 10.1097/00043764-197902000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Daldal H, et al. Hydrogen sulfide toxicity in a thermal spring: a fatal outcome. Clin Toxicol (Phila) 2010;48:755–756. doi: 10.3109/15563650.2010.508044. [DOI] [PubMed] [Google Scholar]
- 13.Parra O, et al. Inhalation of hydrogen sulphide: a case of subacute manifestations and long term sequelae. Br J Ind Med. 1991;48:286–287. doi: 10.1136/oem.48.4.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mc CL, Clayton GD. Air pollution by hydrogen sulfide in Poza Rica, Mexico; an evaluation of the incident of Nov. 24, 1950. AMA Arch Ind Hyg Occup Med. 1952;6:199–213. [PubMed] [Google Scholar]
- 15.Guarrasi J, Trask C, Kirychuk S. A systematic review of occupational exposure to hydrogen sulfide in livestock operations. J Agromedicine. 20:225–236. doi: 10.1080/1059924X.2015.1009667. [DOI] [PubMed] [Google Scholar]
- 16.Gerasimon G, et al. Acute hydrogen sulfide poisoning in a dairy farmer. Clin Toxicol (Phila) 2007;45:420–423. doi: 10.1080/15563650601118010. [DOI] [PubMed] [Google Scholar]
- 17.Belley R, et al. Hyperbaric oxygen therapy in the management of two cases of hydrogen sulfide toxicity from liquid manure. Cjem. 2005;7:257–261. doi: 10.1017/s1481803500014408. [DOI] [PubMed] [Google Scholar]
- 18.Borst GH. Acute poisoning of pigs with hydrogen sulfide as a result of acidification of slurry on a pig farm. Tijdschr Diergeneeskd. 2001;126:104–105. [PubMed] [Google Scholar]
- 19.DHS. Federal Register. 1997;72:65423. [Google Scholar]
- 20.DHS. Federal Register. Vol. 2017. Department of Homeland Security; 2007. Appendix A: Chemicals of Interest List. [Google Scholar]
- 21.Yang D, Chen G, Zhang R. Estimated Public Health Exposure to H2S Emissions from a Sour Gas Well Blowout in Kaixian County, China. Aerosol and Air Quality Research. 2006;6:430–443. [Google Scholar]
- 22.Rumbeiha W, et al. Acute hydrogen sulfide-induced neuropathology and neurological sequelae: challenges for translational neuroprotective research. Ann N Y Acad Sci. 2016;1378:5–16. doi: 10.1111/nyas.13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vicas IMO. Hydrogen Sulfide. In: Haddad LM, Shannon MW, Winchester JF, editors. Clinical Management of Poisoning and Drug Overdose. W.B. Saunders Company; Philadelphia: 1990. pp. 906–912. [Google Scholar]
- 24.Reiffenstein RJ, Hulbert WC, Roth SH. Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol. 1992;32:109–134. doi: 10.1146/annurev.pa.32.040192.000545. [DOI] [PubMed] [Google Scholar]
- 25.Beauchamp RO, Jr, et al. A critical review of the literature on hydrogen sulfide toxicity. Crit Rev Toxicol. 1984;13:25–97. doi: 10.3109/10408448409029321. [DOI] [PubMed] [Google Scholar]
- 26.Tvedt B, et al. Brain damage caused by hydrogen sulfide: a follow-up study of six patients. Am J Ind Med. 1991;20:91–101. doi: 10.1002/ajim.4700200109. [DOI] [PubMed] [Google Scholar]
- 27.Matsuo M, Cummins JW, Anderson RE. Neurological sequelae of massive hydrogen sulfide inhalation. Arch Neurol. 1979;36:451–452. doi: 10.1001/archneur.1979.00500430081019. [DOI] [PubMed] [Google Scholar]
- 28.Hall AH, Rumack BH. Hydrogen sulfide poisoning: an antidotal role for sodium nitrite? Vet Hum Toxicol. 1997;39:152–154. [PubMed] [Google Scholar]
- 29.Cronican AA, et al. Antagonism of Acute Sulfide Poisoning in Mice by Nitrite Anion without Methemoglobinemia. Chem Res Toxicol. 2015;28:1398–1408. doi: 10.1021/acs.chemrestox.5b00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan A, et al. Nitrocobinamide, a new cyanide antidote that can be administered by intramuscular injection. J Med Chem. 2015;58:1750–1759. doi: 10.1021/jm501565k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anantharam P, et al. A mouse model for evaluating countermeasures against hydrogen sulfide-induced neurotoxicity and neurological sequelae. Ann N Y Acad Sci. 2017 doi: 10.1111/nyas.13419. XXXX: XX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDaniel KL, Moser VC. Utility of a neurobehavioral screening battery for differentiating the effects of two pyrethroids, permethrin and cypermethrin. Neurotoxicol Teratol. 1993;15:71–83. doi: 10.1016/0892-0362(93)90065-v. [DOI] [PubMed] [Google Scholar]
- 33.Seo J, Ottesen EW, Singh RN. Antisense methods to modulate pre-mRNA splicing. Methods Mol Biol. 2014;1126:271–283. doi: 10.1007/978-1-62703-980-2_20. [DOI] [PubMed] [Google Scholar]
- 34.Gordon R, et al. Prokineticin-2 upregulation during neuronal injury mediates a compensatory protective response against dopaminergic neuronal degeneration. Nat Commun. 2016;7:12932. doi: 10.1038/ncomms12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim DS, et al. p73 gene in dopaminergic neurons is highly susceptible to manganese neurotoxicity. Neurotoxicology. 2016 doi: 10.1016/j.neuro.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarkar S, et al. Manganese exposure induces neuroinflammation by impairing mitochondrial dynamics in astrocytes. Neurotoxicology. 2017;59:231–239. doi: 10.1016/j.neuro.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie ZZ, Liu Y, Bian JS. Hydrogen Sulfide and Cellular Redox Homeostasis. Oxid Med Cell Longev. 2016;2016:6043038. doi: 10.1155/2016/6043038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruder JB, et al. Hydrogen sulfide suicide: a new trend and threat to healthcare providers. J Burn Care Res. 2015;36:e23–25. doi: 10.1097/BCR.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 39.Vezzani A, et al. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hobson BA, et al. Magnetic Resonance Imaging Reveals Progressive Brain Injury in Rats Acutely Intoxicated with Diisopropylfluorophosphate. Toxicol Sci. 2017;157(2):342–353. doi: 10.1093/toxsci/kfx049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hegde A, Bhatia M. Hydrogen sulfide in inflammation: friend or foe? Inflamm Allergy Drug Targets. 2011;10:118–122. doi: 10.2174/187152811794776268. [DOI] [PubMed] [Google Scholar]
- 42.Whiteman M, Winyard PG. Hydrogen sulfide and inflammation: the good, the bad, the ugly and the promising. Expert Rev Clin Pharmacol. 2011;4:13–32. doi: 10.1586/ecp.10.134. [DOI] [PubMed] [Google Scholar]
- 43.Kida K, Ichinose F. Hydrogen Sulfide and Neuroinflammation. Handb Exp Pharmacol. 2015;230:181–189. doi: 10.1007/978-3-319-18144-8_9. [DOI] [PubMed] [Google Scholar]
- 44.Bhatia M. H2S and Inflammation: An Overview. Handb Exp Pharmacol. 2015;230:165–180. doi: 10.1007/978-3-319-18144-8_8. [DOI] [PubMed] [Google Scholar]
- 45.Zhi L, et al. Hydrogen sulfide induces the synthesis of proinflammatory cytokines in human monocyte cell line U937 via the ERK-NF-kappaB pathway. J Leukoc Biol. 2007;81:1322–1332. doi: 10.1189/jlb.1006599. [DOI] [PubMed] [Google Scholar]
- 46.Morales I, et al. Neuroinflammation and Neurodegeneration. In: Moretti DV, editor. Update on Dementia. Ch. 02. InTech; Rijeka: 2016. [Google Scholar]
- 47.Olmos G, Llado J. Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm. 2014;2014:861231. doi: 10.1155/2014/861231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo Y, et al. Aggravation of seizure-like events by hydrogen sulfide: involvement of multiple targets that control neuronal excitability. CNS Neurosci Ther. 2014;20:411–419. doi: 10.1111/cns.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Eldik LJ, et al. Glia proinflammatory cytokine upregulation as a therapeutic target for neurodegenerative diseases: function-based and target-based discovery approaches. Int Rev Neurobiol. 2007;82:277–296. doi: 10.1016/S0074-7742(07)82015-0. [DOI] [PubMed] [Google Scholar]
- 51.Carlson NG, et al. Inflammatory cytokines IL-1 alpha, IL-1 beta, IL-6, and TNF-alpha impart neuroprotection to an excitotoxin through distinct pathways. J Immunol. 1999;163:3963–3968. [PubMed] [Google Scholar]
- 52.Koutsilieri E, et al. Degeneration of neuronal cells due to oxidative stress--microglial contribution. Parkinsonism Relat Disord. 2002;8:401–406. doi: 10.1016/s1353-8020(02)00021-4. [DOI] [PubMed] [Google Scholar]
- 53.Dalleau S, et al. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ. 2013;20:1615–1630. doi: 10.1038/cdd.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicholls P, et al. Sulfide inhibition of and metabolism by cytochrome c oxidase. Biochem Soc Trans. 2013;41:1312–1316. doi: 10.1042/BST20130070. [DOI] [PubMed] [Google Scholar]
- 55.Truong DH, et al. Molecular mechanisms of hydrogen sulfide toxicity. Drug Metab Rev. 2006;38:733–744. doi: 10.1080/03602530600959607. [DOI] [PubMed] [Google Scholar]
- 56.Srinivasan S, Avadhani NG. Cytochrome c oxidase dysfunction in oxidative stress. Free Radic Biol Med. 2012;53:1252–1263. doi: 10.1016/j.freeradbiomed.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kadenbach B, et al. The possible role of cytochrome c oxidase in stress-induced apoptosis and degenerative diseases. Biochim Biophys Acta. 2004;1655:400–408. doi: 10.1016/j.bbabio.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 58.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johri A, Beal MF. Mitochondrial dysfunction in neurodegenerative diseases. J Pharmacol Exp Ther. 2012;342:619–630. doi: 10.1124/jpet.112.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lindenmann J, et al. Severe hydrogen sulphide poisoning treated with 4-dimethylaminophenol and hyperbaric oxygen. Diving Hyperb Med. 2010;40:213–217. [PubMed] [Google Scholar]
- 61.Fenga C, Cacciola A, Micali E. Cognitive sequelae of acute hydrogen sulphide poisoning. A case report. Med Lav. 2002;93:322–328. [PubMed] [Google Scholar]
- 62.Snyder JW, et al. Occupational fatality and persistent neurological sequelae after mass exposure to hydrogen sulfide. Am J Emerg Med. 1995;13:199–203. doi: 10.1016/0735-6757(95)90094-2. [DOI] [PubMed] [Google Scholar]
- 63.Lund OE, Wieland H. Pathologic-anatomic findings in experimental hydrogen sulfide poisoning (H2S). A study on rhesus monkeys. Int Arch Arbeitsmed. 1966;22:46–54. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of mice used per end point in experiments.
Grading scheme for evaluation of histopathological lesions in the brain.