Abstract
Background
Prescribing of medications with anticholinergic properties in older nursing home residents is relatively common, despite an association with increased risk for falls, delirium and other outcomes. Few studies have investigated what factors influence different levels of prescribing of these agents.
Objectives
The primary objective was to identify factors associated with low- and high-level anticholinergic burden in nursing home residents. A secondary objective was to examine in detail the contribution of different medications to low versus high burden, to aid in determining drugs to target in interventions.
Design
Retrospective, cross-sectional analysis.
Setting
National sample of 2009–2010 Medicare Part A and B claims, Part D prescription drug events, and Minimum Data Set (MDS) v2.0 assessments.
Participants
The cohort included 4730 Medicare beneficiaries age ≥65 with continuous Medicare Parts A, B, and D enrollment, admitted for non-skilled stays ≥14 days between 01/01/2010–09/30/2010.
Measurements
Anticholinergic burden was defined using the Anticholinergic Cognitive Burden(ACB) scale. Medication scores were summed at the patient-level and categorized as high (score ≥3), low (score 1–2), or none. Baseline predisposing (age, sex, race/ethnicity), enabling (prior year hospital, emergency department, primary care, specialist visits; region; Medicaid/low-income subsidy), and medical need factors (dementia severity, anti-dementia medication, Charlson co-morbidity index [CCI], select comorbidities) were evaluated for association with anticholinergic burden using multinomial logistic regression.
Results
Overall, 29.6% had high-level anticholinergic burden, and 35.2% had low-level burden. High-level burden was most often (72%) due to one highly anticholinergic medication rather than cumulative effect. In adjusted analyses, factors associated with increased risk of both low and high anticholinergic burden included: comorbidity, antidementia medication, depression, hypertension, and prior year hospitalization. Older age was associated with decreased odds of high anticholinergic burden. Urinary incontinence and prior year specialist visit were associated with increased odds of high anticholinergic burden. Severe and non-severe dementia were associated with decreased odds of low-level burden, but increased odds of high-level burden.
Conclusion
Almost two-thirds of nursing home patients have some degree of anticholinergic burden. Several medical need variables are significantly associated with increased risk for low-level and high-level anticholinergic burden. Interventions should be developed to optimize prescribing for residents at increased risk of receiving medications with anticholinergic properties. Future study is needed to evaluate the difference in the risk of adverse outcomes associated with various levels of anticholinergic burden.
1. Introduction
The use of medications with anticholinergic properties in older adults increases the risk for falls, delirium, and other negative outcomes.1–3 This risk is likely further increased among nursing home residents with advanced age, dementia, higher comorbidity level, and poorer functional status compared to community-dwelling older adults.4–6 Despite published evidence and clinical guidelines7 that highlight the negative implications of using these medications in older adults, they continue to be prescribed in practice. Previous studies have shown that over 50% of nursing home residents receive at least one medication with anticholinergic properties daily.8,9 The use of these medications is often seen as a necessary evil when prescribers have few efficacious options to use in their place. However, evidence suggests a possible dose-response relationship between anticholinergic exposure and negative outcomes.3,10,11 The degree to which these agents increase the risk for adverse events differs by medication and likely by the degree of cumulative exposure when taking into account a patient’s entire medication regimen, referred to as anticholinergic burden. Several validated scales exist that characterize the potential for adverse effects associated with individual medications and can be summed to generate an overall risk score.12–14 Therefore, a more realistic goal than avoiding these agents altogether may be to limit the overall burden of a patient’s medication regimen and/or avoid individual agents with strong anticholinergic properties.
Although previous investigations have described patient-level, prescriber-level, and facility-level characteristics associated with medications with anticholinergic properties,8,9 few studies have investigated whether different factors predict high versus low levels of anticholinergic burden. Having an improved understanding of differences in association across levels of anticholinergic burden will identify patients most at risk for high burden and associated negative outcomes that are most in need of intervention. Therefore, the objective of this investigation is to identify factors associated with varying levels of anticholinergic burden in a national sample of older Medicare beneficiaries in nursing homes. A secondary objective was to examine in detail the contribution of different medications to low versus high burden, to aid in determining drugs to target in future interventions.
2. Methods
2.1 Design and Data Sources
This retrospective, cross-sectional analysis merged Medicare enrollment, Part A and B claims, Part D prescription drug event data, and the Minimum Data Set (MDS) v2.0 from the years 2009–2010. The University of Pittsburgh Institutional Review Board (IRB) deemed this study to be exempt from IRB review and approval. Data originated from a 10% random sample of fee-for-service beneficiaries with continuous 2009 enrollment in Medicare Parts A and B medical benefits and Part D prescription drug benefits. Medicare Parts A and B provide payment for inpatient and outpatient services, respectively, whereas Medicare Part D provides coverage for prescription drugs. The MDS is a comprehensive database of health assessments conducted with nursing home residents upon admission and at least every 90 days thereafter, and considered one of the most reliable information sources in these patients.15 The MDS served as the primary source of variables to identify a cohort of newly admitted nursing home residents and create independent variables. The Medicare enrollment file was used to extract additional socio-demographic variables, while Part A and B claims were used to assess specific comorbidities not captured by MDS and inpatient and outpatient healthcare utilization prior to the admission. Medicare Part D prescription drug event data provided us with national drug code (NDC), drug name, date of dispensing, and estimated days’ supply for all prescriptions covered by Part D.
2.2 Cohort Construction
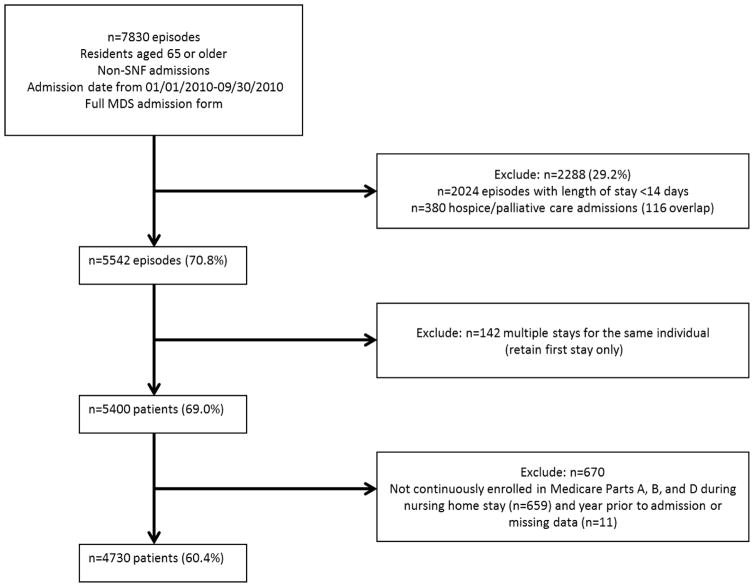
Derivation of the cohort is shown in Figure 1. We identified new nursing home admissions for beneficiaries aged 65 and older in 2009, who had non-skilled nursing home stays between January 1st, 2010 and September 30, 2010, and had a completed MDS admission assessment (n=7830). The September 30, 2010 end date corresponded to the last date on which the 2.0 version of the MDS assessments was used prior to switching to MDS version 3.0. MDS version 3.0 records were used only to identify discharge dates falling between the first date of use (October 1, 2010) and the end of data available (December 31, 2010). We did not incorporate assessment items from MDS v3.0 records because several items were either changed, removed, or replaced in the updated version and thus utilizing both sources would have affected the consistency of our analysis variables.
Figure 1. Cohort Construction.
(Abbreviations: SNF = Skilled Nursing Facility; MDS = Minimum Data Set)
We excluded patients admitted for skilled nursing stays because medications administered during these stays are covered by Part A rather than Part D and thus medication data were not available. We used the MDS reason for assessment fields (AA8A, AA8B) and MDS admission and discharge dates in combination with Skilled Nursing Facility (SNF) claims in the MedPAR file to define the duration of nursing home episodes16–18 and distinguish SNF and non-SNF stays. Specifically, we first identified all MDS forms with a primary reason for assessment (AA8A) equal to “admission assessment required by day 14” and an MDS admission date between 1/1/2010 and 9/30/2010 (n=41,674). We then excluded the subset of admissions with a non-blank value for AA8B, indicating the assessment was a Medicare Prospective Payment System (PPS) or state-required assessment (n=8,471). Medicare Part A skilled nursing facility (SNF) claims were then used to validate the accuracy of the MDS assessments in determining SNF versus non-SNF stays. We subsequently excluded any remaining stays associated with an overlapping SNF claim (~3%).
Patients were required to have a length of stay of at least 14 days, by which point the Centers for Medicare and Medicaid Services (CMS) mandates that an MDS assessment must be completed for every resident (n=2024 excluded). Patients admitted for hospice or palliative care services, identified on the MDS form, were excluded due to the different patterns of medication usage (n=380 excluded). For patients with multiple admissions during the specified time frame, we included only the first admission (n=142 excluded). We excluded patients who were not continuously enrolled in Medicare Parts A, B, and D for the entire year prior to their stay to ensure complete characterization of past medical history. In addition, we required patients to be enrolled for the entire duration of their stay, either until death or discharge, in order to capture all events and diagnoses occurring within the nursing home. (n=659). Finally, patients with missing data for covariates were excluded (<0.5%), resulting in a final cohort of 4,730 unique patient records.
2.3 Independent Variables
Selection of independent variables was guided by a literature search 8,9,19–28 and the Andersen Behavioral Model for Health Services Utilization (ABM)29. The ABM proposes that use of health services, including medications, is influenced by a combination of: predisposing factors, influencing need for healthcare based on their demographics; enabling factors, the environmental factors that facilitate or inhibit access to healthcare; and medical need factors or aspects of the individual’s health perceived by the individual or a medical professional as necessary for seeking healthcare.
Predisposing variables were extracted from the MDS and included age, sex, and race/ethnicity. Enabling factors were those that influence a patient’s access to health services and thus prescribers. These included: any hospitalizations and emergency department (ED) visits in the last year, outpatient primary care or specialist visits in the last year, geographic region, and low income subsidy (LIS) or Medicaid eligibility. Outpatient visits were identified using carrier claims for face-to-face outpatient encounters and were classified as primary versus specialty based on provider practice specialty codes per Pham et al.30 Geographic region was categorized using State identification codes and U.S. census region classifications (Northeast, Midwest, West, and South). Medicaid or LIS eligibility was included to serve as a measure of income, with individuals qualifying for Medicaid or LIS being eligible for reduced co-payments. This was coded as a three level categorical variable: enrolled neither, LIS only, or Medicaid.
Finally, medical need factors were those having the potential to lead to prescribing of medications with anticholinergic properties, by way of specific indications or need to utilize health services. We included all indications for medications with anticholinergic properties including: depression, anxiety, schizophrenia, Parkinson’s disease, incontinence, seizure disorder, hypertension and neuropathic pain. These were identified using indicator variables from within MDS assessments, with the exception of depression and neuropathic pain. Depression was identified using an International Classification of Diseases, ninth revision31 (ICD-9)-diagnosis based algorithm used by the Medicare Chronic Conditions Warehouse (CCW)32 or a depression rating scale33 score of greater than 3 in the MDS. Neuropathic pain was generated from Medicare claims using a validated set of ICD-9 codes34. Use of antidementia medications (donepezil, rivastigmine, galantamine, or memantine) was included due to cholinergic side effects that often lead to prescribing of medications with anticholinergic properties.35 Dementia severity was included because previous literature has shown that patients with severe dementia are less likely to be prescribed potentially inappropriate medications.21 Dementia was initially identified by the presence of a diagnosis code defined by the CCW32. Patients were further classified as having no dementia, non-severe dementia, or severe dementia based on a validated algorithm using a combination of the Cognitive Performance Scale (CPS) and Activities of Daily Living Scale, extracted from the MDS36. Overall comorbidity was measured by applying the Charlson Comorbidity Index (CCI)36 to claims and was categorized into a four-level variable (0–1; 2–3; 4–5; 6+) based on its distribution.
2.4 Anticholinergic Drug Burden
The dependent variable, anticholinergic drug burden, was defined using the Anticholinergic Cognitive Burden (ACB) scale 12,13 for all primary analyses. The ACB scale measures probable anticholinergic activity on a scale from 0–3, with 0 indicating no activity and 3 indicating high potential for inducing delirium. We defined medication usage based on medications received from days 8 to 14 after admission. To identify medications, NDCs were extracted from Medispan® Electronic Drug File v2 (Wolters Kluwer Health, Inc., Indianapolis, IN) by generic name and matched to NDCs in Part D. Medication classes were assigned based on the therapeutic class generic product identifier (TCGPI) level 2 classifications from Medi-Span. A total of 15,714 prescriptions for medications with anticholinergic properties were identified. Prescriptions for the cohort were reviewed to exclude dosage forms with low likelihood of systemic bioavailability and effect (topicals, ophthalmics, etc.).
ACB scores were derived for each individual by summing the score of each unique medication prescribed to generate an overall person-level score. We then categorized anticholinergic burden to three levels: no burden (score=0), low burden (sum score = 1–2), high burden (sum score = 3+). This categorical variable served as the primary outcome for all statistical analyses. To test the robustness of our results to alternative definitions of anticholinergic drug burden, we also constructed an alternative dependent variable using the Anticholinergic Drug Scale (ADS),14 for use in sensitivity analyses.
2.5 Statistical Analysis
Basic descriptive statistics including percentages, means, and standard deviations were generated for independent variables across entire sample. We described the most prevalent medication classes with anticholinergic properties and individual medications within each class for the entire sample and by level of anticholinergic burden (low or high). For patients with high level of anticholinergic burden, we created two subgroups. The first subgroup consists of those with no medications with an ACB score of 3, meaning these individuals only have high level anticholinergic burden due to the cumulative effect of multiple low burden medications. The second subgroup consists of those with at least one medication with an ACB score of 3, implying that high-level anticholinergic burden is attributable to one medication.
Bivariate and multivariate analyses were performed to assess the unadjusted and adjusted associations between individual predisposing, enabling, and medical need factors and anticholinergic drug burden. Specifically, we used multinomial logistic regression to generate odds ratios (ORs) and 95% confidence intervals (CIs) for three comparisons (low vs. no, high vs. no, and high vs. low). Analyses were conducted to determine the contribution of each set of predisposing, enabling, and medical need factors. The full model with all variables was compared to nested models with either predisposing, enabling, or medical need variables removed. Likelihood ratio tests were used to compare models and identify statistically significant changes in fit. Multicollinearity between independent variables was assessed using a regression model and examination of corresponding variance inflation factors.
Sensitivity analyses were conducted using ADS score as the dependent variable to determine whether results were dependent on the classification scale applied. In order to explore the stability of anticholinergic burden over time, sensitivity analyses were also conducted to measure how ACB scores changed among patients admitted for at least 37 days. We evaluated the change in ACB scores measured on admission (days 8–14) versus after 30 days (days 31–37) (n=3,665) to determine changes in the use of medications with anticholinergic properties over time in the same patients. We then re-evaluated multivariable associations, using ACB defined during days 31–37 to determine if there were any significant differences in factors associated with anticholinergic burden when compared to the original analysis.
3. Results
3.1 Patient Characteristics
The majority of individuals in the sample were age 80 or older (70.9%), female (74%) and white, non-Hispanic (85.96%) (Table 1). Approximately half were enrolled in Medicaid (41.9%) or LIS only (10.9%). In general, there was a high prevalence of comorbidities, with over 60% of individuals having a CCI greater than 3 and over 65% having dementia. Almost two-thirds of the sample had some degree of anticholinergic burden. Specifically, 35.2% had low-level anticholinergic burden (n=1663) and 29.6% had high-level anticholinergic burden (n=1399), with 35.3% having none (n=1399).
Table 1.
Overall Sample Characteristics
| Variable | n(%) N=4730 |
|---|---|
|
| |
| Predisposing Factors | |
|
| |
| Age (y) | |
| 65–70 | 303 (6.4) |
| 70–<80 | 1073 (22.7) |
| 80–<90 | 2124 (44.9) |
| 90+ | 1230 (26.0) |
|
| |
| Sex | |
| Male | 1230 (26.0) |
| Female | 3500 (74.0) |
|
| |
| Race/Ethnicity | |
| White(non-Hispanic) | 4066 (85.9) |
| Black(non-Hispanic) | 339 (7.2) |
| Hispanic | 208 (4.40) |
| Other | 117 (2.5) |
|
| |
| Medical Need Factors | |
|
| |
| Charlson Comorbidity Index | |
| 0 or 1 | 682 (14.4) |
| 2 or 3 | 1101 (23.3) |
| 4 or 5 | 1174 (24.8) |
| 6+ | 1773 (37.5) |
|
| |
| Dementia Severity (as defined previously by: CCW diagnosis, CPS and functional status) | |
| Non-Dementia | 1626 (34.4) |
| Dementia non-severe | 2744 (58.0) |
| Dementia severe | 360 (7.6) |
|
| |
| Use of Antidementia Medication | |
| AchEI only | 547 (11.6) |
| Memantine only | 165 (3.5) |
| AchEI + Memantine | 323 (6.8) |
| None | 3695 (78.1) |
|
| |
| Sensory Limitation | |
| Yes | 353 (7.5) |
| No | 4377 (92.5) |
|
| |
| Depression | |
| Yes | 1943 (41.1) |
| No | 2787 (58.9) |
|
| |
| Anxiety | |
| Yes | 1091 (23.1) |
| No | 3693 (76.9) |
|
| |
| Schizophrenia | |
| Yes | 114 (2.4) |
| No | 4616 (97.6) |
|
| |
| Parkinson’s Disease | |
| Yes | 301 (6.4) |
| No | 4429 (93.6) |
|
| |
| Incontinence | |
| Yes | 2067 (43.7) |
| No | 2663 (56.3) |
|
| |
| Seizure disorder | |
| Yes | 234 (4.9) |
| No | 4496 (95.1) |
|
| |
| Hypertension | |
| Yes | 3491 (73.8) |
| No | 1239 (26.2) |
|
| |
| Neuropathic Pain | |
| Yes | 2693 (56.9) |
| No | 2037 (43.1) |
|
| |
| Enabling Factors | |
|
| |
| Hospitalization in the last year | |
| Yes | 2399 (50.7) |
| No | 2331 (49.3) |
|
| |
| ED visit in the last year | |
| Yes | 3343 (70.7) |
| No | 1387 (29.3) |
|
| |
| Primary Care visit in the last year | |
| Yes | 3302 (69.8) |
| No | 1428 (30.2) |
|
| |
| Specialist visit in the last year | |
| Yes | 3348 (70.8) |
| No | 1382 (29.2) |
|
| |
| Geographic region | |
| Northeast | 813 (17.2) |
| Midwest | 1712 (36.2) |
| South | 1536 (32.5) |
| West | 669 (14.1) |
|
| |
| Low Income Subsidy/Medicaid Eligibility | |
| Neither | 2235 (47.3) |
| Low income subsidy only | 515 (10.9) |
| Medicaid | 1980 (41.8) |
CCW = Chronic Conditions Warehouse; CPS = Cognitive Performance Scale; AchEI = Acetylcholinesterase Inhibitor; ED = Emergency Department
3.2 Use of Medications with Anticholinergic Properties
The top ten prescribed medication classes with anticholinergic properties were diuretics (22.45%), beta-blockers (21.90%), antipsychotics/antimanic agents (16.45%), antidepressants (9.47%), anticoagulants (8.63%), antianginal agents (5.86%), cardiotonics (5.31%), urinary antispasmodics (5.10%), opioid analgesics (4.86%), and ulcer drugs (3.78%). (Table 2.)
Table 2.
Overall Medication Frequencies
| Drug Class | ACB* Score | % of all patients |
|---|---|---|
| Diuretics | 22.5 | |
| Furosemide | 1 | 20.8 |
| Triamterene/Hydrochlorothiazide | 1 | 1.6 |
| Beta Blockers | 21.9 | |
| Metoprolol Tartrate | 1 | 11.3 |
| Metoprolol Succinate | 1 | 6.0 |
| Atenolol | 1 | 4.8 |
| Antipsychotics/Antimanic Agents | 16.4 | |
| Quetiapine | 3 | 7.3 |
| Risperidone | 1 | 4.9 |
| Olanzapine | 3 | 2.2 |
| Antidepressants | 9.5 | |
| Trazodone | 1 | 3.9 |
| Paroxetine | 3 | 2.8 |
| Venlafaxine | 1 | 1.9 |
| Anticoagulants | 8.6 | |
| Warfarin | 1 | 8.6 |
| Antianginal Agents | 5.9 | |
| Isosorbide Mononitrate | 1 | 4.7 |
| Isosorbide Dinitrate | 1 | 1.2 |
| Cardiotonics | 5.3 | |
| Digoxin | 1 | 5.3 |
| Urinary Antispasmodics | 5.1 | |
| Oxybutinin Chloride | 3 | 3.3 |
| Solifenacin Succinate | 3 | 0.8 |
| Darifenacin Hydrobromide | 3 | 0.8 |
| Opioid Analgesics | 4.9 | |
| Fentanyl | 1 | 3.3 |
| Morphine Sulfate | 1 | 1.5 |
| Acetaminophen with Codeine | 1 | 0.6 |
| Ulcer Drugs | 3.8 | |
| Ranitidine | 1 | 3.3 |
| Dicyclomine | 3 | 0.4 |
ACB = Anticholinergic Cognitive Burden
Table 3 describes medication use by level of anticholinergic burden. Of the 1399 patients with high anticholinergic burden, 1013 (72%) had at least one medication with an ACB score of 3, indicating that high anticholinergic burden is most often attributable to one medication with high anticholinergic activity, rather than a cumulative effect. There is a great deal of overlap in the top prescribed medications between those patients with low burden and the subgroup with high burden due to multiple medications. The top prescribed medications for those prescribed medications with high-level anticholinergic activity was quite different from the other two groups. Low anticholinergic burden was most often attributable to use of multiple antihypertensives, whereas high anticholinergic burden was attributable to several classes including antispychotics, antihypertensives, urinary antispasmodics and antidepressants.
Table 3.
Medication Frequencies by Anticholinergic Burden Level
| Top Drugs among Patients with Low Burden (ACB* Score = 1–2) n=1663 | Top Drugs among Patients with High Burden (ACB* ≥3) n=1399 | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Patients with no medication with ACB* Score = 3 (n=386) | Patients with at least one medication with ACB* Score = 3 (n=1013) | |||||||
|
| ||||||||
| Drug Name | ACB* Score | % of group | Drug Name | ACB* Score | % of group | Drug Name | ACB* Score | % of group |
|
| ||||||||
| Furosemide | 1 | 28.9 | Furosemide | 1 | 62.7 | Quetiapine fumarate | 3 | 34.3 |
| Metoprolol Tartrate | 1 | 17 | Metoprolol Tartrate | 1 | 32.6 | Furosemide | 1 | 25.9 |
| Warfarin | 1 | 11.5 | Warfarin | 1 | 31.6 | Oxybutinin chloride | 3 | 15.3 |
| Metoprolol Succinate | 1 | 8.5 | Digoxin | 1 | 24.6 | Paroxetine HCl | 3 | 12.8 |
| Risperidone | 1 | 8.1 | Isosorbide mononitrate | 1 | 23.1 | Metoprolol tartrate | 1 | 12.5 |
| Atenolol | 1 | 7 | Metoprolol succinate | 1 | 18.7 | Olanzapine | 3 | 10.2 |
| Digoxin | 1 | 5.9 | Prednisone | 1 | 14.0 | Warfarin | 1 | 9.4 |
| Trazodone | 1 | 4.9 | Fentanyl | 1 | 11.7 | Promethazine HCL | 3 | 8.1 |
| Isosorbide Mononitrate | 1 | 4.7 | Trazodone | 1 | 11.4 | Metoprolol Succinate | 1 | 6.9 |
| Prednisone | 1 | 4.3 | Atenolol | 1 | 11.1 | Atenolol | 1 | 6.4 |
ACB = Anticholinergic Cognitive Burden
3.3 Factors Associated with Low and High Levels of Anticholinergic Burden
In unadjusted analyses, the following factors were significantly associated with increased odds of low or high anticholinergic burden at the p<0.05 level in unadjusted analyses: CCI >1, antidementia medication use, depression, hypertension, neuropathic pain, hospitalization in the last year, ED visit in the last year, specialist visit in the last year, Northeast region, LIS, and Medicaid eligibility (Table 4). No factors were associated solely with low levels of anticholinergic burden. Several variables were uniquely associated with high anticholinergic burden including anxiety, schizophrenia, and seizure disorder. In the third comparison, most factors that were associated with high anticholinergic burden individually were also significantly associated with high burden in the direct comparison to low burden, with the exception of hypertension, neuropathic pain, and geographic region. The only factor found to be significant solely within this comparison was urinary incontinence. The relationship between dementia severity and anticholinergic burden actually changed direction with increasing burden. The presence of any dementia (severe or non-severe) was associated with decreased odds of low anticholinergic burden, but increased odds of high anticholinergic burden, when compared to low anticholinergic burden.
Table 4.
Univariable Associations
| N=4730 | ACB Sum 1–2 vs. 0 | ACB Sum 3+ vs. 0 | ACB Sum 3+ vs. 1–2 |
|---|---|---|---|
|
| |||
| Variable | OR (95% CI) Unadjusted | OR (95% CI) Unadjusted | OR (95% CI) Unadjusted |
|
| |||
| Predisposing Factors | |||
|
|
|||
| Age (y) | overall ‡ | overall ‡ | |
| 65–70 | ref | ref | ref |
| 70–<80 | 0.72 (0.52, 1.00)* | 0.75 (0.55, 1.03) | 1.04 (0.77, 1.41) |
| 80–<90 | 0.82 (0.60, 1.11) | 0.55 (0.40, 0.74)‡ | 0.67 (0.50, 0.89)† |
| 90+ | 0.75 (0.54, 1.02) | 0.40 (0.29, 0.55)‡ | 0.54 (0.40, 0.73)‡ |
|
| |||
| Sex | |||
| Male | ref | ref | ref |
| Female | 0.94 (0.80, 1.09) | 1.08 (0.91, 1.27) | 1.15 (0.98, 1.35) |
|
| |||
| Race/Ethnicity | |||
| White(non-Hispanic) | ref | ref | ref |
| Black(non-Hispanic) | 1.21 (0.92, 1.57) | 1.14 (0.86, 1.51) | 0.95 (0.72, 1.24) |
| Hispanic | 1.04 (0.75, 1.45) | 0.94 (0.66, 1.33) | 0.90 (0.63, 1.27) |
| Other | 0.95 (0.62, 1.46) | 0.87 (0.55, 1.38) | 0.92 (0.57, 1.47) |
|
| |||
| Medical Need Factors | |||
|
|
|||
| Charlson Comorbidity Index | overall ‡ | overall ‡ | overall † |
| 0 or 1 | ref | ref | ref |
| 2 or 3 | 1.25 (1.00, 1.55)* | 1.60 (1.25, 2.05)‡ | 1.28 (0.99, 1.67) |
| 4 or 5 | 1.44 (1.16, 1.80)‡ | 2.15 (1.68, 2.75)‡ | 1.49 (1.15, 1.92)† |
| 6+ | 2.00 (1.63, 2.46)‡ | 2.84 (2.25, 3.59)‡ | 1.42 (1.12, 1.81)† |
|
| |||
| Dementia Severity (as defined previously by: CCW diagnosis, CPS and functional status) | overall † | overall † | overall ‡ |
| Non-Dementia | ref | ref | ref |
| Dementia non-severe | 0.85 (0.73, 0.98)* | 1.31 (1.12, 1.53)‡ | 1.54 (1.32, 1.80)‡ |
| Dementia severe | 0.64 (0.49, 0.84)† | 1.04 (0.79, 1.37) | 1.62 (1.21, 2.18)‡ |
|
| |||
| Use of Antidementia Medication | overall ‡ | overall ‡ | overall ‡ |
| AchEI only | 125 (0.99, 1.57) | 1.97 (1.58, 2.46)‡ | 1.58 (1.27, 1.96)‡ |
| Memantine only | 1.51 (1.02, 2.24)* | 1.90 (1.28, 2.84)† | 1.26 (0.87, 1.81) |
| AchEI + Memantine | 1.85 (1.36, 2.51)‡ | 2.88 (2.13, 3.89)‡ | 1.56 (1.20, 2.02)‡ |
| None | ref | ref | ref |
|
| |||
| Sensory Limitation | |||
| Yes | 0.93 (0.72, 1.20) | 0.93 (0.71, 1.22) | 1.00 (0.76. 1.32) |
| No | ref | ref | ref |
|
| |||
| Depression | |||
| Yes | 1.38 (1.20, 1.59)‡ | 2.03 (1.76, 2.35)‡ | 1.47 (1.28, 1.70)‡ |
| No | ref | ref | ref |
|
| |||
| Anxiety | |||
| Yes | 1.04 (0.88, 1.23) | 1.70 (1.44, 2.00)‡ | 1.63 (1.39, 1.93)‡ |
| No | ref | ref | ref |
|
| |||
| Schizophrenia | |||
| Yes | 1.10 (0.60, 2.03) | 4.47 (2.71, 7.38)‡ | 4.05 (2.50, 6.56))‡ |
| No | ref | ref | ref |
|
| |||
| Parkinson’s Disease | |||
| Yes | 0.86 (0.64, 1.14) | 1.08 (0.81, 1.43) | 1.26 (0.94, 1.68) |
| No | ref | ref | ref |
|
| |||
| Incontinence | |||
| Yes | 0.95 (0.83, 1.09) | 1.09 (0.95, 1.26) | 1.15 (0.99, 1.33) |
| No | ref | ref | ref |
|
| |||
| Seizure disorder | |||
| Yes | 1.05 (0.75, 1.46) | 1.53 (1.11, 2.11)† | 1.46 (1.06, 2.00)* |
| No | ref | ref | ref |
|
| |||
| Hypertension | |||
| Yes | 1.78 (1.52, 2.07)‡ | 1.86 (1.58, 2.19)‡ | 1.05 (0.88, 1.24) |
| No | ref | ref | ref |
|
| |||
| Neuropathic Pain | |||
| Yes | 1.29 (1.13, 1.48)‡ | 1.37 (1.19, 1.59)‡ | 1.06 (0.92. 1.23) |
| No | ref | ref | |
|
| |||
| Enabling Factors | |||
|
|
|||
| Hospitalization in the last year | |||
| Yes | 1.56 (1.36, 1.79)‡ | 1.89 (1.64, 2.19)‡ | 1.21 (1.05, 1.40)† |
| No | ref | ref | ref |
|
| |||
| ED visit in the last year | |||
| Yes | 1.40 (1.21, 1.62)‡ | 1.76 (1.50, 2.06)‡ | 1.26 (1.07, 1.48)† |
| No | ref | ref | ref |
|
| |||
| Primary Care visit in the last year | |||
| Yes | 1.02 (0.88, 1.18) | 1.10 (0.94, 1.28) | 1.08 (0.92 (1.26) |
| No | ref | ref | ref |
|
| |||
| Specialist visit in the last year | |||
| Yes | 1.19 (1.03, 1.38)* | 1.43 (1.22, 1.67)‡ | 1.20 (1.02, (1.41)* |
| No | ref | ref | ref |
|
| |||
| Geographic region | overall * | overall * | |
| Northeast | 1.35 (1.11, 1.65)† | 1.26 (1.02, 1.55)* | 0.93 (0.76, 1.14) |
| Midwest | ref | ref | ref |
| South | 1.06 (0.90, 1.25) | 1.16 (0.98, 1.38) | 1.10 (0.92, 1.31) |
| West | 1.12 (0.91, 1.38) | 0.92 (0.74, 1.16) | 0.82 (0.66, 1.04) |
|
| |||
| Low Income Subsidy/Medicaid Eligibility | overall * | overall ‡ | overall ‡ |
| Neither | ref | ref | ref |
| Low income subsidy only | 1.26 (1.00, 1.59)* | 1.47 (1.15, 1.87)† | 1.16 (0.92, 1.47) |
| Medicaid | 1.21 (1.05, 1.40)* | 1.61 (1.38, 1.87)‡ | 1.33 (1.14, 1.55)‡ |
p<0.05;
p<0.01;
<0.001
ACB = Anticholinergic Cognitive Burden; OR = Odds Ratio; CCW = Chronic Conditions Warehouse; CPS = Cognitive Performance Scale; AchEI = Acetylcholinesterase Inhibitor; ED = Emergency Department
Results of the multivariable logistic regression are displayed in Table 5. Several factors became non-significant in adjusted analyses including seizure disorder, neuropathic pain, ED visits, geographic region. Having at least one specialist visit in the last year remained significant, but was only associated with high anticholinergic burden. LIS was no longer significant, but Medicaid eligibility was uniquely associated with increased odds of high anticholinergic burden. Age was no longer a protective factor for low anticholinergic burden, but remained significant for high anticholinergic burden. Dementia status remained significantly associated with decreased odds of low anticholinergic burden, but was only associated with increased odds of high anticholinergic burden relative to low anticholinergic burden. All other variables remained significant as outlined in unadjusted analyses.
Table 5.
Multivariable Associations
| N=4730 | ACB Sum 1–2 vs. 0 | ACB Sum 3+ vs. 0 | ACB Sum 3+ vs. 1–2 |
|---|---|---|---|
|
| |||
| Variable | OR (95% CI) Adjusted | OR (95% CI) Adjusted | OR (95% CI) Adjusted |
|
| |||
| Predisposing Factors | |||
|
|
|||
| Age (y) | overall ‡ | ||
| 65–70 | ref | ref | ref |
| 70–<80 | 0.82 (0.58, 1.15) | 0.87 (0.62, 1.22) | 1.07 (0.78, 1.47) |
| 80–<90 | 1.03 (0.74, 1.43) | 0.75 (0.54, 1.05) | 0.73 (0.53, 0.99)* |
| 90+ | 1.04 (0.74, 1.47) | 0.67 (0.47, 0.96)* | 0.64 (0.46, 0.90)† |
|
| |||
| Sex | |||
| Male | ref | ref | ref |
| Female | 0.92 (0.78, 1.08) | 1.11 (0.92, 1.32) | 1.20 (1.01, 1.43)* |
|
| |||
| Race/Ethnicity | |||
| White(non-Hispanic) | ref | ref | ref |
| Black(non-Hispanic) | 1.04 (0.78, 1.39) | 0.89 (0.66, 1.22) | 0.86 (0.64, 1.15) |
| Hispanic | 0.92 (0.65, 1.30) | 0.69 (0.47, 1.02) | 0.76 (0.52, 1.10) |
| Other | 0.85 (0.54, 1.34) | 0.80 (0.49, 1.31) | 0.93 (0.57, 1.52) |
|
| |||
| Medical Need Factors | |||
|
|
|||
| Charlson Comorbidity Index | overall ‡ | overall ‡ | |
| 0 or 1 | ref | ref | ref |
| 2 or 3 | 1.15 (0.91, 1.44) | 1.43 (1.10, 1.86)† | 1.25 (0.95, 1.64) |
| 4 or 5 | 1.22 (0.96, 1.55) | 1.67 (1.27, 2.18)‡ | 1.36 (1.04, 1.80)* |
| 6+ | 1.59 (1.24, 2.03)‡ | 1.93 (1.46, 2.54)‡ | 1.21 (0.92, 1.60) |
|
| |||
| Dementia Severity (as defined previously by: CCW diagnosis, CPS and functional status) | overall ‡ | overall ‡ | |
| Non-Dementia | ref | ref | ref |
| Dementia non-severe | 0.71 (0.60, 0.83)‡ | 0.97 (0.81, 1.16) | 1.37 (1.15, 1.63)‡ |
| Dementia severe | 0.55 (0.41, 0.74)‡ | 0.88 (0.65, 1.21) | 1.62 (1.17, 2.22)‡ |
|
| |||
| Use of Antidementia Medication | overall ‡ | overall ‡ | overall ‡ |
| AchEI only | 1.48 (1.16, 1.89)‡ | 2.24 (1.76, 2.85)‡ | 1.51 (1.20, 1.90)‡ |
| Memantine only | 1.95 (1.30, 2.94)‡ | 2.09 (1.37, 3.19)‡ | 1.07 (0.73, 1.57) |
| AchEI + Memantine | 2.40 (1.73, 3.31)‡ | 3.26 (2.36 (4.49)‡ | 1.36 (1.03, 1.80)* |
| None | ref | ref | ref |
|
| |||
| Sensory Limitation | |||
| Yes | 1.01 (0.77, 1.33) | 1.04 (0.77, 1.39) | 1.02 (1.20, 1.90)‡ |
| No | ref | ref | ref |
|
| |||
| Depression | |||
| Yes | 1.29 (1.10, 1.50)‡ | 1.50 (1.28, 1.76)‡ | 1.17 (1.00, 1.37)* |
| No | ref | ref | ref |
|
| |||
| Anxiety | |||
| Yes | 1.00 (0.84, 1.19) | 1.51 (1.27, .181)‡ | 1.52 (1.27, 1.80)‡ |
| No | ref | ref | ref |
|
| |||
| Schizophrenia | |||
| Yes | 1.18 (0.63, 2.20) | 4.10 (2.41, 7.00)‡ | 3.50 (2.11, 5.79)‡ |
| No | ref | ref | ref |
|
| |||
| Parkinson’s Disease | |||
| Yes | 0.92 (0.69, 1.24) | 1.11 (0.82, 1.50) | 1.20 (0.89, 1.62) |
| No | ref | ref | ref |
|
| |||
| Incontinence | |||
| Yes | 0.97 (0.84, 1.12) | 1.14 (0.98. 1.33) | 1.17 (1.01, 1.36)* |
| No | ref | ref | ref |
|
| |||
| Seizure disorder | |||
| Yes | 0.95 (0.67, 1.35) | 1.14 (0.81, 1.61) | 1.19 (0.86, 1.67) |
| No | ref | ref | ref |
|
| |||
| Hypertension | |||
| Yes | 1.72 (1.47, 2.01)‡ | 1.80 (1.52, 2.14)‡ | 1.05 (0.88, 1.25) |
| No | ref | ref | ref |
|
| |||
| Neuropathic Pain | |||
| Yes | 1.12 (0.96, 1.30) | 1.09 (0.93, 1.28) | 0.97 (0.83, 1.14) |
| No | ref | ref | ref |
|
| |||
| Enabling Factors | |||
|
|
|||
| Hospitalization in the last year | |||
| Yes | 1.25 (1.05, 1.50)† | 1.32 (1.09, 1.59)† | 1.05 (0.87, 1.26) |
| No | ref | ref | ref |
|
| |||
| ED visit in the last year | |||
| Yes | 1.00 (0.83, 1.20) | 1.06 (0.87, 1.29) | 1.06 (0.87, 1.26) |
| No | ref | ref | ref |
|
| |||
| Primary Care visit in the last year | |||
| Yes | 0.95 (0.82, 1.11) | 1.03 (0.87, 1.22) | 1.08 (0.92, 1.27) |
| No | ref | ref | ref |
|
| |||
| Specialist visit in the last year | |||
| Yes | 0.99 (0.84, 1.16) | 1.19 (1.00. 1.42)* | 1.20 (1.01, 1.44)* |
| No | ref | ref | ref |
|
| |||
| Geographic region | |||
| Northeast | 1.31 (1.06, 1.61)† | 1.14 (0.91, 1.42) | 0.87 (0.70, 1.08) |
| Midwest | ref | ref | ref |
| South | 1.03 (0.86, 1.22) | 1.00 (0.73, 1.19) | 0.98 (0.81, 1.18) |
| West | 1.15 (0.92, 1.43) | 0.93 (0.73, 1.19) | 0.81 (0.64, 1.03) |
|
| |||
| Low Income Subsidy/Medicaid Eligibility | overall ‡ | ||
| Neither | ref | ref | ref |
| Low income subsidy only | 1.18 (0.93, 1.49) | 1.24 (0.96, 1.60) | 1.05 (0.83, 1.35) |
| Medicaid | 1.14 (0.97, 1.34) | 1.40 (1.17, 1.66)‡ | 1.22 (1.03, 1.45)* |
p<0.05;
p<0.01;
<0.001
ACB = Anticholinergic Cognitive Burden; OR = Odds Ratio; CCW = Chronic Conditions Warehouse; CPS = Cognitive Performance Scale; AchEI = Acetylcholinesterase Inhibitor; ED = Emergency Department
Likelihood ratio tests revealed that predisposing, enabling and medical need factors were all statistically significant in contributing to model fit and removal of any group resulted in a statistically significant decrease in model fit. Assessment of variance inflation factors revealed no problematic correlations between any of the independent variables included in our model that would have significantly impacted our findings.
3.4 Sensitivity Analyses
Using the ADS in place of the ACB to quantify anticholinergic burden in sensitivity analyses revealed substantively similar results (available from author upon request). Additional sensitivity analyses revealed that ACB is not stable over time. Among patients with a length of stay of at least 37 days, the prevalence of any anticholinergic burden during days 8–14 of stay was 65%, identical to the results presented above for the full cohort. Following these same patients over time, the prevalence of any anticholinergic burden increased to 72% during days 31–37 of stay. When examining the multivariable associations of predisposing, enabling, and medical need factors with anticholinergic burden in this group, there were no major changes to the pattern of associations, in that the directionality of associations remained stable. However, the magnitude of associations between certain factors were somewhat attenuated and as a result, some associations were no longer statistically significant. Detailed results may be found in the Electronic Supplementary Materials Tables S1–S3.
4. Discussion
This national analysis identifies several important associations in use of medications with anticholinergic properties among Medicare beneficiaries admitted to nursing homes for nonskilled stays. We found that exposure to these medications is common among nursing home residents, with nearly two thirds of nursing home residents having either low-level or high-level anticholinergic burden. Several medical need variables were consistently associated with increased risk for high or low-level anticholinergic burden, while few enabling or predisposing factors were associated. The relationship between dementia and anticholinergic burden was dependent on the level of anticholinergic burden, with dementia being associated with decreased risk for low-level burden, but increased risk for high-level burden. We also identified that high–level anticholinergic burden most often was due to exposure to one medication with high-level anticholinergic activity, rather than a cumulative effect of multiple medications. Sensitivity analyses revealed that anticholinergic burden is not stable and that it actually increases in the time from admission to 1 month post-admission. While this did not significantly impact the association between anticholinergic burden and patient factors, exploring changes in anticholinergic burden over time and its association with patient characteristics is a worthwhile direction for future study.
A number of medical need factors were consistently associated with increased likelihood of high or low levels of anticholinergic burden, including overall comorbidity, use of antidementia medications, depression, and hypertension. The association between use of antidementia medications and anticholinergic burden is concerning, given that this is a major drug interaction that essentially counteracts the cholinergic effects of acetylcholinesterase inhibitors (AchEIs), increasing risk for cognitive decline. This comes as no surprise, as this phenomenon, where urinary antispasmodics are used to treat the adverse effects of urinary incontinence caused by AchEIs, has previously been reported in the literature and is referred to as a “prescribing cascade”.35 Dementia status was the only factor to exhibit a significant protective effect, but only for low-level anticholinergic burden. This is reassuring because it suggests that prescribers are aware of the negative cognitive effects associated with use of these medications. However, the finding that dementia is a risk factor for having high anticholinergic burden is concerning, but can likely be explained by the fact that medications used to treat behavioral symptoms associated with dementia have high anticholinergic activity.
Few predisposing or enabling factors were significant. Older age exhibited a protective effect for high levels of anticholinergic burden, particularly among those over the age of 80 years. This implies that prescribers acknowledge the increased risk for adverse events associated with anticholinergic activity in advanced age. Prior hospitalization was associated with increased risk of low or high-level anticholinergic burden, while specialist visits and Medicaid eligibility were uniquely associated with increased risk for high-level anticholinergic burden. While this finding is interesting, it most likely speaks to the fact that these factors represent an enhanced ability to access services which puts patients with dual eligibility or more frequent access to the healthcare system at risk for prescribing of new drugs generally, and not specifically anticholinergic burden.
To our knowledge, only two analyses have investigated factors associated with prescribing of medications with anticholinergic properties in nursing home patients, both conducted in patients with dementia specifically. A retrospective analysis of Medicare data from 2007–2008 by Palmer et al.8 found that 77% of nursing home residents with dementia were prescribed at least one medication with any anticholinergic activity, with 33% prescribed medications with high-level anticholinergic activity. Women, African Americans, those over the age of 75 years old, and those with severe cognitive impairment were less likely to use these medications. Only high levels of aggression were associated with increased use of medications with anticholinergic properties. An analysis of National Nursing Home Survey Data from 2004 by Chatterjee et al.9 found that 73.6% of nursing home residents with dementia used at least one medication with anticholinergic properties, with 21.27% using highly anticholinergic medications. Older age, functional dependence, and behavioral symptoms were associated with decreased likelihood of receiving medications with high-level anticholinergic activity. In contrast, Medicaid eligibility, higher number of medications, depression, anxiety, and Parkinson’s disease were associated with increased likelihood of receiving medications with high-level anticholinergic activity. Our findings align relatively well with both analyses8,9 in terms of the frequency of prescribing of medications with anticholinergic properties, confirming the importance of anticholinergic burden as a medication safety issue. In terms of the factors associated with use of these medications, the findings of our analysis are more closely aligned with those identified in the analysis by Chatterjee et al.9 in which older age was associated with decreased use of medications with anticholinergic properties, while Medicaid eligibility and certain comorbid medical conditions were associated with increased use. In Palmer et al8, fewer factors were examined and thus there were fewer associations with prescribing of medications with anticholinergic properties. In addition, severe cognitive impairment was associated only with decreased likelihood of use. This variation may be explained by the fact that our sample included non-dementia patients, who served as the reference category for cognitive impairment. Thus the protective effect of severe cognitive impairment only holds true when comparing directly between non-severe and severe dementia. The most commonly prescribed medications with high-level anticholinergic activity in our analysis were a combination of antipsychotics, antihypertensives, urinary antispasmodics, and antidepressants. This is relatively consistent with previous analyses, with the exception of antipsychotics, which were not frequently prescribed in Chatterjee et al.9 A unique feature of our analysis is that we identified differences in medications that contribute to differing levels of anticholinergic burden, and identified that high anticholinergic burden is most often attributable to the effects of the use of one or more medications with high- anticholinergic activity, as opposed to the compounded effect of multiple medications with low anticholinergic activity. While we were not able to determine whether this equates to a substantial difference in the risk for adverse events, it does have some implications for clinical practice and policy development.
The findings of this analysis provide a means for identifying patients at the highest risk for having anticholinergic burden upon admission to the nursing home, as well as the mechanisms by which high-level anticholinergic burden is attained. Using this information, prescribers and pharmacists can identify and prioritize patients for specific interventions based on their risk. Among patients with high-level anticholinergic burden due to multiple medications, providers can conduct detailed medication therapy management reviews to monitor for polypharmacy and eliminate potentially unnecessary medications contributing to anticholinergic burden. For those patients with high-level anticholinergic burden primarily due to one medication (which our results suggest is the majority of high-burden patients), medications can be re-evaluated to determine appropriateness and identify potential alternatives. Modification may be fairly straightforward for medications such as tricyclic antidepressants, which may be substituted with selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs) to manage depression.38 Whereas, the use of antipsychotics such as quetiapine or olanzapine may require careful consideration of other factors before considering an alternative such as risperidone for managing behavioral complications of dementia.38 The same logic can also be applied to guide the initiation of new therapies, particularly among patients with existing anticholinergic burden, in an effort to reduce additional risk for adverse events. For example, a prescriber wishing to treat symptoms of nausea and vomiting may choose to initiate ondansetron, which has no anticholinergic activity, instead of an agent with high-level anticholinergic properties, such as promethazine. When selecting therapies for pain management, prescribers may choose to avoid anticholinergic skeletal muscle relaxants like methocarbamol and cyclobenzaprine. This kind of conscientious prescribing has the potential to limit additional anticholinergic exposure to the minimum extent when use of certain medications may not be medically necessary.
Our analysis has several important strengths compared to prior analyses, the most significant being that we were able to make the distinction between high-level anticholinergic burden due to one medication with high-level anticholinergic activity versus a cumulative effect of multiple medications. Also, this is a national analysis that used a nationally representative cohort, which makes our sample representative of all nonskilled nursing home patients. This analysis also included both dementia and non-dementia patients and thus we were able to compare differences in prescribing across these two groups. As a result, we were able to identify that dementia patients are at increased risk for receiving medication with high-level anticholinergic activity, compared to those without dementia. Our study also identifies enabling factors associated with baseline medication use on admission, external to the nursing home environment, such as hospitalizations and visits to specialists that have not been addressed in prior investigations. Taken together, these strengths provide an enhanced understanding of the factors that explain patterns of prescribing of potentially inappropriate medications with anticholinergic properties in this population.
There are some limitations of this analysis that should be taken into account in the interpretation of these findings. This study employed a cross-sectional design, which limits our abilities to establish causality and to evaluate temporal relationships between exposure factors and the outcome, prescribing of medications with anticholinergic properties. This also means that medications prescribed for short-term use were not captured since a short window was used to evaluate medication use. The data used in this analysis were from the years 2009–2010 and are not the most recent years of data available for use in research. Therefore, it is possible that patterns in prescribing of medications with anticholinergic properties may have changed in the years since these data were generated. Our sample was not very diverse with regards to race/ethnicity and while generalizability is a concern for all analyses, our cohort was derived from a national sample of Medicare beneficiaries. Therefore, we assume that this sample is fairly representative of the older adult nursing home population. We also were not able to capture any medications not covered by Medicare Part D. As a result, we may have not captured use of over the counter medications with anticholinergic properties or benzodiazepines which are also not covered by Medicare. There are also limitations to using an objective scale to classify inappropriate medication use, since this does not take into account inter-individual variability in terms of anticholinergic side effects. We did not assess duration of use or doses of individual medications, which may differentially affect the risk for adverse events on the patient level. Also, this objectivity classifies all medications with anticholinergic properties as “inappropriate” when in fact that may not be the case. Several disease states are appropriately managed with medications with anticholinergic properties. Therefore, there is also potential for collinearity between the medical need variables for specific comorbidities as well as overall comorbidity, although tests for multicollinearity indicated that there were no problematic correlations that would have affected our findings. Finally, the interpretability of the associations between specific diagnoses and anticholinergic burden is limited by the fact that most of the medications used to treat these conditions have at least some degree of anticholinergic activity. Thus there is limited clinical utility for the associations between diagnoses and anticholinergic burden. Even so, it was important to include these variables in our models to control for appropriate medication use in order to accurately measure the associations with other non-diagnosis related patient factors.
Our analysis points out several areas which require additional study, primarily focused on the relationship between anticholinergic burden and adverse events. The primary focus of this paper was to measure the prescribing of medications with anticholinergic properties on admission to nursing homes. Thus, this analysis only captures a short window of time in the patient’s stay. Despite the fact that the ACB was designed to assess the risk for delirium associated with these medications, we did not assess this risk as part of our analyses. One of the main reasons for this was that the MDS 2.0 does not contain a validated instrument for diagnosing delirium, but rather items that are related to delirium symptoms. The MDS 3.0, however, does contain a validated diagnostic instrument, the confusion assessment method (CAM) and thus newer years of data containing MDS 3.0 assessment records would be better suited for studying delirium in this population. A number of studies have established that there is a relationship between anticholinergic burden and outcomes such as cognitive decline39,40, delirium3,41, falls1,42, and mortality43–45. However, there is a substantial amount of heterogeneity in terms of the ways in which exposure to medications with anticholinergic properties is defined, and few studies actually address whether there is a difference in the risk for adverse events specifically between individuals with high-level versus low-level anticholinergic burden40,41,45. Interestingly, no studies have investigated whether there is a difference in the risk for adverse events for high-level anticholinergic burden due to a cumulative effect versus the effect of one medication with high-level anticholinergic activity. Therefore, future studies should assess differences in risk for adverse events across levels of anticholinergic burden to guide targeted prescribing strategies with the goal of reducing anticholinergic burden in a clinically meaningful way in this population.
5. Conclusion
This retrospective analysis revealed that almost two-thirds of nursing home patients are subject to some degree of anticholinergic burden. Logistic regression analyses revealed that several medical need variables are significantly associated with increased risk for having low-level and high-level anticholinergic burden. Interventions should be developed to optimize prescribing of medications for residents who are at increased risk of having anticholinergic burden. Future study is needed to evaluate differences in the risk of adverse outcomes associated with various levels of anticholinergic burden.
Supplementary Material
Electronic Supplementary Material Table S1. Overall Sample Characteristics from Sensitivity Analysis
Supplementary Material Table S2. Unadjusted Univariable Associations from Sensitivity Analysis
Electronic Supplementary Material Table S3. Multivariable Associations from Sensitivity Analysis
Key Points.
Approximately two thirds of older adult nursing home patients receive at least one medication with anticholinergic properties daily.
High anticholinergic burden is more often attributable to use of one medication with high-level anticholinergic activity, rather than a cumulative effect of multiple medications.
Certain subgroups of patients are at increased risk for receiving these medications based on their clinical characteristics, comorbid conditions, or access to healthcare.
Acknowledgments
Funding: Grants were received from the Donaghue Foundation, National Insitutes on Aging (T32AG021885, P30AG024827), and the Agency for Health Research and Quality (R18 HS023779). No other sources of funding were used to assist in the preparation of this article.
Previously presented as a poster at the AcademyHealth Annual Research Meeting, June 25, 2017, New Orleans, LA. The authors would like to acknowledge the University of Pittsburgh School of Pharmacy for providing resources and support necessary to conduct this project.
Footnotes
Compliance with Ethical Standards
Conflict of Interest: Joshua Niznik, Xinhua Zhao, Tao Jiang, Joseph Hanlon, Sherrie Aspinall, Joshua Thorpe, and Carolyn Thorpe declare that they have no conflicts of interest relevant to the content of this manuscript.
Contributor Information
Joshua Niznik, PhD Student, Department of Pharmaceutical Sciences, University of Pittsburgh School of Pharmacy, Research Health Science Specialist, VA Pittsburgh Healthcare System, Center for Health Equity Research and Promotion.
Xinhua Zhao, Biostatistician, University of Pittsburgh Schools of Pharmacy and Medicine, Senior Biostatistician, VA Pittsburgh Healthcare System, Center for Health Equity Research and Promotion.
Tao Jiang, University of Pittsburgh.
Joseph T. Hanlon, Professor of Medicine, Pharmacy and Epidemiology University of Pittsburgh; Research Health Scientist, VA, Pittsburgh Healthcare System, Center for Health Equity Research and Promotion/Geriatric Research Education and Clinical Center.
Sherrie L. Aspinall, Clinical Pharmacy Specialist, VA Center for Medication Safety, Core Investigator, VA Pittsburgh Healthcare System Center for Health Equity Research and Promotion, Associate Professor, Department of Pharmacy and Therapeutics, University of Pittsburgh School of Pharmacy.
Joshua Thorpe, Associate Professor, Department of Pharmacy and Therapeutics, University of Pittsburgh School of Pharmacy, Core Investigator, VA Pittsburgh Healthcare System Center for Health Equity Research and Promotion.
Carolyn Thorpe, Associate Professor, Department of Pharmacy and Therapeutics, University of Pittsburgh School of Pharmacy, Core Investigator, VA Pittsburgh Healthcare System Center for Health Equity Research and Promotion.
References
- 1.Marcum ZA, Perera S, Thorpe JM, et al. Anticholinergic use and recurrent falls in community-dwelling older adults: findings from the Health ABC Study. Ann Pharmacother. 2015;49(11):1214–1221. doi: 10.1177/1060028015596998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothberg MB, Herzig SJ, Pekow PS, et al. Association between sedating medications and delirium in older inpatients. J Am Geriatr Soc. 2013;61(6):923–930. doi: 10.1111/jgs.12253. [DOI] [PubMed] [Google Scholar]
- 3.Naja M, Zmudka J, Hannat S, et al. In geriatric patients, delirium symptoms are related to the anticholinergic burden. Geriatr Gerontol Int. 2016;16(4):424–431. doi: 10.1111/ggi.12485. [DOI] [PubMed] [Google Scholar]
- 4.Olsen C, Pedersen I, Bergland A, et al. Differences in quality of life in home-dwelling persons and nursing home residents with dementia - a cross-sectional study. BMC Geriatr. 2016;16:137. doi: 10.1186/s12877-016-0312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogaisky M, Dezieck L. Early hospital readmission of nursing home residents and community-dwelling elderly adults discharged from the geriatrics service of an urban teaching hospital: patterns and risk factors. J Am Geriatr Soc. 2015;63(3):548–552. doi: 10.1111/jgs.13317. [DOI] [PubMed] [Google Scholar]
- 6.Gassoumis ZD, Fike KT, Rahman AN, et al. Who transitions to the community from nursing homes? Comparing patterns and predictors for short-stay and long-stay residents. Home Health Care Serv Q. 2013;32(2):75–91. doi: 10.1080/01621424.2013.779353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.By the American Geriatrics Society Beers Criteria Update Expert P. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63(11):2227–2246. doi: 10.1111/jgs.13702. [DOI] [PubMed] [Google Scholar]
- 8.Palmer JB, Albrecht JS, Park Y, et al. Use of drugs with anticholinergic properties among nursing home residents with dementia: a national analysis of Medicare beneficiaries from 2007 to 2008. Drugs Aging. 2015;32(1):79–86. doi: 10.1007/s40266-014-0227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee S, Mehta S, Sherer JT, et al. Prevalence and predictors of anticholinergic medication use in elderly nursing home residents with dementia: analysis of data from the 2004 National Nursing Home Survey. Drugs Aging. 2010;27(12):987–997. doi: 10.2165/11584430-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Best O, Gnjidic D, Hilmer SN, et al. Investigating polypharmacy and drug burden index in hospitalised older people. Intern Med J. 2013;43(8):912–918. doi: 10.1111/imj.12203. [DOI] [PubMed] [Google Scholar]
- 11.Salahudeen MS, Nishtala PS, Duffull SB. The influence of patient characteristics on anticholinergic events in older people. Dement Geriatr Cogn Dis Extra. 2015;5(3):530–541. doi: 10.1159/000441718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boustani M, Campbell N, Munger S, et al. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4:311–320. [Google Scholar]
- 13.Campbell N, Boustani M, Limbil T, et al. The cognitive impact of anticholinergics: a clinical review. Clin Interv Aging. 2009;4:225–233. doi: 10.2147/cia.s5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46(12):1481–1486. doi: 10.1177/0091270006292126. [DOI] [PubMed] [Google Scholar]
- 15.Mor V. A comprehensive clinical assessment tool to inform policy and practice: applications of the minimum data set. Med Care. 2004;42(4 Suppl):III50–59. doi: 10.1097/01.mlr.0000120104.01232.5e. [DOI] [PubMed] [Google Scholar]
- 16.Intrator O, Hiris J, Berg K, et al. The residential history file: studying nursing home residents’ long-term care histories. Health Serv Res. 2011 Feb;46(1 Pt 1):120–37. doi: 10.1111/j.1475-6773.2010.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahman M, Tyler D, Acquah JK, et al. Sensitivity and specificity of the Minimum Data Set 3.0 discharge data relative to Medicare claims. J Am Med Dir Assoc. 2014;15(11):819–24. doi: 10.1016/j.jamda.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei YJ, Simoni-Wastila L, Zuckerman IH, et al. Algorithm for identifying nursing home days using Medicare claims and Minimum Data Set assessment data. Med Care. 2016 Nov;54(11):e73–e77. doi: 10.1097/MLR.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 19.Bhattacharya R, Chatterjee S, Carnahan RM, et al. Prevalence and predictors of anticholinergic agents in elderly outpatients with dementia. Am J Geriatr Pharmacother. 2011;9(6):434–441. doi: 10.1016/j.amjopharm.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Blazer DG, 2nd, Federspiel CF, Ray WA, et al. The risk of anticholinergic toxicity in the elderly: a study of prescribing practices in two populations. J Gerontol. 1983;38(1):31–35. doi: 10.1093/geronj/38.1.31. [DOI] [PubMed] [Google Scholar]
- 21.Cool C, Cestac P, Laborde C, et al. Potentially inappropriate drug prescribing and associated factors in nursing homes. J Am Med Dir Assoc. 2014;15(11):850.e851–859. doi: 10.1016/j.jamda.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Dhalla IA, Anderson GM, Mamdani MM, et al. Inappropriate prescribing before and after nursing home admission. J Am Geriatr Soc. 2002;50(6):995–1000. doi: 10.1046/j.1532-5415.2002.50252.x. [DOI] [PubMed] [Google Scholar]
- 23.Jiron M, Pate V, Hanson LC, et al. Trends in prevalence and determinants of potentially inappropriate prescribing in the United States: 2007 to 2012. J Am Geriatr Soc. 2016;64(4):788–797. doi: 10.1111/jgs.14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kachru N, Carnahan RM, Johnson ML, et al. Potentially inappropriate anticholinergic medication use in community-dwelling older adults: a national cross-sectional study. Drugs Aging. 2015;32(5):379–389. doi: 10.1007/s40266-015-0257-x. [DOI] [PubMed] [Google Scholar]
- 25.Lau DT, Kasper JD, Potter DE, et al. Potentially inappropriate medication prescriptions among elderly nursing home residents: their scope and associated resident and facility characteristics. Health Serv Res. 2004;39(5):1257–1276. doi: 10.1111/j.1475-6773.2004.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee EK, Lee YJ. Prescription patterns of anticholinergic agents and their associated factors in Korean elderly patients with dementia. Int J Clin Pharm. 2013;35(5):711–718. doi: 10.1007/s11096-013-9793-9. [DOI] [PubMed] [Google Scholar]
- 27.Morin L, Laroche ML, Texier G, et al. Prevalence of potentially inappropriate medication use in older adults living in nursing homes: a systematic review. J Am Med Dir Assoc. 2016;17(9):862.e861–869. doi: 10.1016/j.jamda.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Reppas-Rindlisbacher CE, Fischer HD, Fung K, et al. Anticholinergic drug burden in persons with dementia taking a cholinesterase inhibitor: the effect of multiple physicians. J Am Geriatr Soc. 2016;64(3):492–500. doi: 10.1111/jgs.14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav. 1995;36(1):1–10. [PubMed] [Google Scholar]
- 30.Pham HH, Schrag D, O’Malley AS, et al. Care patterns in Medicare and their implications for pay for performance. N Engl J Med. 2007;356(11):1130–1139. doi: 10.1056/NEJMsa063979. [DOI] [PubMed] [Google Scholar]
- 31.Moisio Marie A. A guide to health insurance billing. Cengage Learning. 2010 [Google Scholar]
- 32.Bucaneer Computer Systems & Service I. Chronic Condition Data Warehouse Medicare Administrative Data ser Guide. 2013. [Google Scholar]
- 33.Anderson RL, Buckwalter KC, Buchanan RJ, et al. Validity and reliability of the Minimum Data Set Depression Rating Scale (MDSDRS) for older adults in nursing homes. Age Ageing. 2003;32(4):435–438. doi: 10.1093/ageing/32.4.435. [DOI] [PubMed] [Google Scholar]
- 34.Pugh MJ, Fincke BG, Bierman AS, et al. Potentially inappropriate prescribing in elderly veterans: are we using the wrong drug, wrong dose, or wrong duration? J Am Geriatr Soc. 2005;53(8):1282–1289. doi: 10.1111/j.1532-5415.2005.53402.x. [DOI] [PubMed] [Google Scholar]
- 35.Gill SS, Mamdani M, Naglie G, et al. A prescribing cascade involving cholinesterase inhibitors and anticholinergic drugs. Arch Intern Med. 2005;165(7):808–813. doi: 10.1001/archinte.165.7.808. [DOI] [PubMed] [Google Scholar]
- 36.van der Steen JT, Volicer L, Gerritsen DL, et al. Defining severe dementia with the Minimum Data Set. Int J Geriatr Psychiatry. 2006;21(11):1099–1106. doi: 10.1002/gps.1618. [DOI] [PubMed] [Google Scholar]
- 37.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 38.Hanlon JT, Semla TP, Schmader KE. Alternative medications for medications in the use of high-risk medications in the elderly and potentially harmful drug-disease interactions in the elderly quality measures. J Am Geriatr Soc. 2015;63(12):e8–e18. doi: 10.1111/jgs.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carriere I, Fourrier-Reglat A, Dartigues JF, et al. Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-city study. Arch Intern Med. 2009;169(14):1317–1324. doi: 10.1001/archinternmed.2009.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell NL, Boustani MA, Lane KA, et al. Use of anticholinergics and the risk of cognitive impairment in an African American population. Neurology. 2010;75(2):152–159. doi: 10.1212/WNL.0b013e3181e7f2ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalisch Ellett LM, Pratt NL, Ramsay EN, et al. Multiple anticholinergic medication use and risk of hospital admission for confusion or dementia. J Am Geriatr Soc. 2014;62(10):1916–1922. doi: 10.1111/jgs.13054. [DOI] [PubMed] [Google Scholar]
- 42.Landi F, Dell’Aquila G, Collamati A, et al. Anticholinergic drug use and negative outcomes among the frail elderly population living in a nursing home. J Am Med Dir Assoc. 2014;15(11):825–829. doi: 10.1016/j.jamda.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Juola AL, Pylkkanen S, Kautiainen H, et al. Burden of potentially harmful medications and the association with quality of life and mortality among institutionalized older people. J Am Med Dir Assoc. 2016;17(3):276.e279–214. doi: 10.1016/j.jamda.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 44.Sarbacker GB, Espino DV, Wood RC, et al. Total anticholinergic burden and survival within a cohort of elderly Mexican Americans. Geriatr Gerontol Int. 2016 doi: 10.1111/ggi.12907. [DOI] [PubMed] [Google Scholar]
- 45.Chatterjee S, Bali V, Carnahan RM, et al. Risk of mortality associated with anticholinergic use in elderly nursing home residents with depression. Drugs Aging. 2017 doi: 10.1007/s40266-017-0475-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic Supplementary Material Table S1. Overall Sample Characteristics from Sensitivity Analysis
Supplementary Material Table S2. Unadjusted Univariable Associations from Sensitivity Analysis
Electronic Supplementary Material Table S3. Multivariable Associations from Sensitivity Analysis