Abstract
Bile acids are the amphipathic primary end-products of cholesterol metabolism that aid in digestion as well as participate in signal transduction in several hepatic and enteric pathways. Despite the reputation of bile acids as signaling molecules implicated in disease states such as cancer and diabetes, there remain numerous bile acid species that are weakly characterized in either physiological or pathological conditions. This review presents one such group: the flat or planar bile acids, a set of bile acids found in humans during infancy and occurring again during certain diseases. As their name implies, these molecules are structurally distinct from the typical human bile acids, retaining the planar structure of their cholesterol predecessor instead of bending or twisting at the A ring. This review defines these species of bile acids in detail and describes their presence in infancy, gestation, and in disease. The large gaps in research regarding the flat bile acids are highlighted and all available experimental knowledge collected as far as 60 years ago is summarized. Further, the potential for these molecules as endogenous biomarkers of liver disease and injury is discussed. Finally, the flat bile salts found in humans are compared to the ancestral and evolutionary older bile salts, which similarly have a flat steroidal structure, as mechanisms of flat bile acid biosynthesis are explored.
Keywords: Bile acid metabolism, bile acids and salts, cholesterol, bile acid biosynthesis
Graphical abstract
1. Introduction and background
Bile acids (BAs) are synthesized from cholesterol primarily in the hepatocytes, then transported to the gallbladder for storage [1, 2]. Ingestion of a meal causes the gallbladder or biliary tract to empty BAs into the duodenum of the small intestine, where they perform a critical role as digestive surfactants [1, 2]. 95% of those BAs excreted from the gallbladder are then reabsorbed throughout the intestinal tract and delivered back to the liver in a process known as enterohepatic circulation, whereas the remaining 5% are excreted into feces [1, 2]. Thus, most BAs in the body at any given time have been recycled and completed this circuit more than once [1, 2]. Accordingly, BA synthesis is tightly controlled by feedback mechanisms in health but may become dysregulated in pathological conditions [1, 2].
BAs have long been known for their ability to aid the solubilization and digestion of lipophilic xenobiotics, fat-soluble vitamins, fatty acids, and monoglycerides and have also been established to regulate their own synthesis via feedback mechanisms [1, 2]. Additionally, BAs feature many other physiological functions that are under active investigation, including: the binding of heavy metal cations such as zinc, iron, and copper excreted in the biliary tract; the binding of dietary proteins for easier cleavage by proteases; antimicrobial and osmosensitive activities within the intestinal tract; and signaling capabilities related to the activation of FXR (farnesoid X receptor), CAR (constitutive androstane receptor), PXR (pregnane X receptor), VDR (vitamin D receptor), PPAR-α (peroxisome proliferator-activated receptor, HNF-α (hepatocyte nuclear receptor factor 4), M-BAR (membrane-type receptor for bile acids; TGR5), and LXR (liver X receptor) [1, 2]. Moreover, BAs have been implicated in several disease states, such as cholestasis; hepatic and intestinal cancers; liver cirrhoses; and diabetes mellitus [1, 2]. Their multiple physiological, pathological, and pharmacological functions have reinstated BAs as a research focus within biology and pharmacology within the past few decades.
While BAs have been studied for nearly a century, human BAs encompass nearly 50 species synthesized by the human body alone, and modifications made by intestinal bacteria result in almost 400 derivatives of the C24 core structure [3]. Most of these species are still poorly characterized [3]. One of the least studied subspecies are the “flat” BAs, a group of C24 cholanoic acids that retain the planar structure of their cholesterol predecessor instead of the “bent” or “twisted” structure of the typical mammalian BAs [2, 4–11]. These so-called “flat” or “planar” BAs comprise several subspecies and will be the focus of this review.
Planar BAs are mostly found as highly abundant BAs in the healthy human fetus, newborn, and pregnant woman; however, they are normally very lowly abundant or undetectable in the healthy adult [12–14]. What makes these molecules relevant, however, is not necessarily their importance in early life, but instead their recurrence in adult patients suffering from various types of liver injury or disease, such as cancer [4, 11]. As discussed below, these typically fetal flat BAs become abundant at various stages of hepatocellular carcinoma, following liver ablation, and in other malignancies and subsequently disappear upon recovery. Thus, there has been renewed interest in studying these fetal flat bile acid species in recent years since their original discovery by Ohta in 1939 [15]. Common interests include using these molecules as biomarkers for liver proliferation and damage as well as discovering their role in disease states.
2. Chemical Characterization of ‘Flat’ BAs
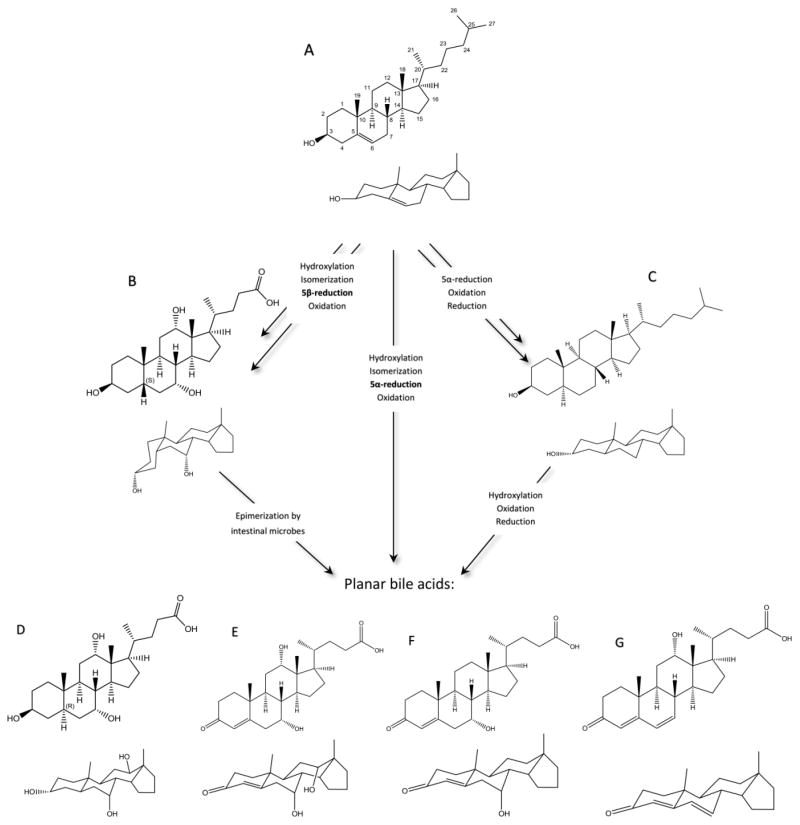
BAs are synthesized in the hepatocytes from cholesterol, a C27 steroid containing an alcohol group at C3, a double bond at C5, and an isooctane side chain (Fig. 1A) [1, 2]. During BA biosynthesis, the planar steroid backbone of the cholesterol molecule becomes “bent” via isomerization and subsequent saturation of the C5–C6 double bond by two enzymes: microsomal cholest-5-ene-3β,7α-diol-3β-dehydrogenase (3β-HSD; EC 1.1.1.181) and cytosolic Δ4-3-oxosteroid-5β-reductase. (AKR1D1; EC 1.3.1.3), respectively [1]. These two steps result in epimerization of the A/B ring system from the trans to the cis conformation, effectively bending the steroidal backbone of the molecule [1, 4]. The so-called “flat” BAs are thus molecules that retain the A/B trans structure of cholesterol, wherein the steroid backbone exists within the same spatial plane, instead of the A/B cis structure of typical human BAs (Fig. 1A, 1B). The A/B trans structure is relatively abnormal among the C24 BAs and is due to either the alpha arrangement of the C5 hydrogen atom – in contrast with the standard beta arrangement – or an unsaturation affecting the C5 bond [4]. Both of these configurations result in molecules that occupy the category of flat BAs and, as mentioned, are found in healthy humans only during gestation and shortly after birth.
Figure 1.
A simplified schematic of cholesterol (A) metabolism highlighting major enzymatic changes. Cholic acid (B) is used here to represent typical human BAs, which constitute most of cholesterol metabolism. Cholestanol (C) is another metabolic product of cholesterol found in small amounts in humans. All three of these are possible precursors to planar bile acids, the most common of which are shown here: (D) allo-cholic acid; (E) 7α,12α-dihydroxy-3-oxochol-4-en-24-oic acid; (F) 7α-hydroxy-3-oxochol-4-en-24-oic acid; (G) 12α-hydroxy-3-oxochol-4,6-dien-24-oic acid. The reaction mechanisms shown here summarize the most important enzymatic changes during the conversion of cholesterol to cholestanol or to typical 5β bile acids as well as summarize the likely enzymatic reactions taking place during conversion to the planar bile acids. As mentioned within the text, structures E, F, and G are also transiently formed within normal bile acid biosynthesis.
Historically, the C24 BAs with a 5α configuration are termed “allo” BAs, which are found as the major BAs in certain reptilian and marine species but not in mammals (Fig. 1D) [16]. An analogous allo – or 5α – epimer corresponding to every “typical” 5β bile acid molecule has been observed in nature and are usually found in less evolved species: for example, a version of allo-cholic acid is a primary BA and pheromone in sea lamprey [17]. The other type of flat BAs are those with a double bond between the C4 and C5 atoms, trivially called the Δ4-unsaturated BAs (Fig. 1E–G), and are regularly formed in humans as temporary BA intermediates during both hepatic biosynthesis and bacterial conjugation within the intestinal tract during enterohepatic circulation [2]. While these Δ4-unsaturated BAs encompass many transient species and intermediates, three species are encountered most abundantly both during early life and during disease: 7α,12α-dihydroxy-3-oxochol-4-en-24-oic acid (CAS 13587-11-6; LM ID LMST04010241), 12α-hydroxy-3-oxochola-4,6-dien-24-oic acid (CAS 13535-96-1; LM ID LMST04010245), and 7α-hydroxy-3-oxochol-4-en-24-oic acid (CAS 14772-95-3; LM ID LMST04010239) (Fig. 1E–G) [12, 13, 18]. The BAs in this subset are collectively termed the Δ4-unsaturated, 3-oxo-Δ4, oxo, or ketonic BAs and share a similarly planar structure with the allo-BAs; hence, both qualify as flat BAs. Neither the allo-BAs nor the Δ4-unsaturated BAs are normally found in high abundance in healthy adult mammals; however, they are among the most abundant of the human fetal BA species [12, 13, 18]. A final type of planar BA will be mentioned for the sake of completeness but not focused on within this review – the Δ5-unsaturated BAs, those with a double bond between the C5 and C6 atoms [19]. These molecules, unlike the other types of planar BAs described, do not resurface following liver injury and instead are found mostly at significant levels in patients with HSD3B7 (3β-hydroxy-Δ5-C27-steroid dehydrogenase) deficiency [19].
3. Nomenclature of Flat Bile Acids
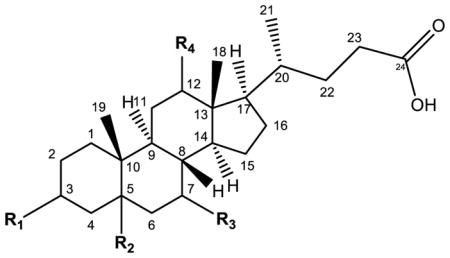
For allo and Δ4-unsaturated BA isomers as well as the more commonly found BAs, traditional and trivial nomenclature is more common than the technically correct terminology. Concerning the former, the prefix “allo” is defined as the more stable molecule of two geometric isomers and with regards to BAs describes the 5α analog of a typically 5β-cholanyl C24 bile acid [16]. This 5α configuration is common in the bile acid pool of certain reptilian and marine species and among the C27 bile alcohols, though the C27 bile alcohols are not historically referred to as “allo” [2, 16]. Thus, allo-cholic acid is technically termed 3α,7α,12α-trihydroxy-5α-cholanic acid instead of -5β-cholanic acid, the latter being termed cholic acid (Fig. 1B, 1D; Table 1); the other allo BAs follow the same pattern, swapping a 5β for a 5α in the traditional nomenclature (i.e. allo-taurocholate, allo-chenodeoxycholate).
Table 1.
Examples of bile acid nomenclature are shown for the typical human bile acids, allo bile acids, and the Δ4-unsaturated bile acids.
| ||||||
|---|---|---|---|---|---|---|
| Trivial name | Substituents | LIPIDMAPS name | ||||
|
|
||||||
| R1 (C3) | R2 (C5) | R3 (C7) | R4 (C12) | |||
|
|
||||||
| Primary human bile acids | Cholic acid (CA) | α-OH | β-H | α-OH | α-OH | 3α,7α,12α-trihydroxy-5β-cholan-24-oic acid |
| Chenodeoxycholic acid (CDCA) | α-OH | β-H | α-OH | — | 3α,7α-dihydroxy-5β-cholan-24-oic acid | |
| Deoxycholic acid (DCA) | α-OH | β-H | — | 12α-OH | 3α,12α-dihydroxy-5β-cholan-24-oic acid | |
| Lithocholic acid (LCA) | α-OH | β-H | — | — | 3α-hydroxy-5β-cholan-24-oic acid | |
| Allo (3α) bile acids | Allo-cholic acid (aCA) | α-OH | α-H | α-OH | α-OH | 3α,7α,12α-trihydroxy-5α-cholan-24-oic acid |
| Allo-chenodeoxycholic acid (aCDCA) | α-OH | α-H | α-OH | — | 3α,7α-dihydroxy-5α-cholan-24-oic acid | |
| Allo-deoxycholic acid (aDCA) | α-OH | α-H | — | α-OH | 3α,12α-dihydroxy-5α-cholan-24-oic acid | |
| Allo-lithocholic acid (aLCA) | α-OH | α-H | — | — | 3α-hydroxy-5α-chol-24-oic acid | |
| Δ4-unsaturated bile acids | CA-Δ4-3-one | oxo (=O) | — | α-OH | α-OH | 7α,12α-dihydroxy-3-oxo-4-cholen-24-oic acid |
| 3-oxo-Δ4,6-DCA | oxo (=O) | — | — | α-OH | 12α-hydroxy-3-oxo-chola-4,6-dien-24-oic acid | |
| CDCA-Δ4-3-one | oxo (=O) | — | α-OH | — | 7α-hydroxy-3-oxo-4-cholen-24-oic acid | |
The Δ4-unsaturated BAs do not individually have convenient trivial names and are referred to by the exhaustive names listed in the above section. Some authors, however, have referred to these molecules in the past as derivatives of more common BAs: for example, 7α,12α-dihydroxy-3-oxo-4-cholenoic acid has been referred to as CA-Δ4-3-one and 12α-hydroxy-3-oxo-chola-4,6-dien-24-oic acid as 3-oxo-Δ4,6-DCA [14]. These molecules share a ketone group at the C3 atom and a double bond connecting the C4 and C5 atoms, and each then has the corresponding hydroxyl groups seen commonly in typical human BAs and described within the name (table 1).
4. The Flat Bile Acids Are Typically Fetal BA Species
The Δ4-unsaturated ketonic bile acid species, especially those mentioned in the above sections – 7α,12α-dihydroxy-3-oxo-4-cholen-24-oic, 12α-hydroxy-3-oxo-4,6-cholandien-24-oic, and 7α-hydroxy-3-oxo-4-cholen-24-oic acids – are fetal BAs abundant in the amniotic fluid of healthy pregnant women, as well as in the meconium, feces, and urine of healthy newborns and infants [13, 18]. Allo-cholic and allo-chenodeoxycholic acid are also normally detectable in lower amounts both before and after delivery in the urine of the mother and child [13]. The Δ5-unsaturated BAs are also found in the meconium of healthy infants but more so in preterm than full-term infants [20]. As these molecules are intermediates of the acidic pathway of BA synthesis, it is thought that the acidic pathway is more prevalent before birth [20]. All of these planar bile acid species are thought to be synthesized by the fetal liver and transferred to the mother or excreted into the amniotic fluid and subsequently swallowed by the fetus, resulting in the presence of flat BAs in the meconium and early feces [13]. This theory is supported by the progression of elimination of flat BAs described herein: when the production of urine begins to increase in the fetus at 30 weeks of gestation, total maternal urinary BAs decrease as more fetal waste products are excreted into the amniotic fluid instead of via the umbilical cord and placenta; moreover, the levels of flat and fetal BA species such as Δ4-unsaturated and polyhydroxylated BAs are increased in the amniotic fluid and begin to decrease in maternal urine late in gestation [13, 18]. Upon birth, healthy newborns’ urinary and fecal BAs are made up of mainly polyhydroxylated and unsaturated ketonic BAs, but allo-cholic and -chenodeoxycholic acids are also abundant and are detectable up to three months of age [18]. Though this area has not been as extensively studied in animal models of pregnancy, high levels of allo-BAs are also detected in the perinatal period of developing rats, indicating that these animals provide a suitable model of human BA diversity [21]. Though it is normal for infants and very young (less than 3 months old) children to have higher levels of flat BAs, the Δ4-unsaturated BAs are found to be significantly increased in the urine of some children with severe liver disease, making up approximately 62–78% of total BAs excreted into the urine of cholestatic infants; for comparison, healthy infants excrete anywhere from 11 to 55% Δ4-unsaturated BAs [12–14, 16]. It is still unknown if the fetal liver is responsible for the synthesis of flat BAs, and, if so, how and why it produces them [12, 13, 18]. It may be a side effect of an immature liver, which may be incapable of expressing functional and/or mature hepatic enzymes. This and other theories of flat BA biosynthesis are discussed in the following section.
5. Biosynthesis
Despite the discovery of flat BAs being nearly eighty years ago, their biosynthesis in any organism remains undefined [22–24]. Several theories persist regarding the biosynthetic pathways of allo BAs, but current literature lends most support to a shift in the enzymatic expression and/or activity of 5β-reductase. The oxosteroid 5β-reductase enzyme, also called aldo-keto reductase 1D1 (AKR1D1), reduces the C4–C5 double bond in cholesterol early in the BA biosynthetic pathway, resulting in the formation of 5β-cholanoic acids [1, 25, 26]. A decrease in the activity or expression of this enzyme, then, would result in the build-up of Δ4-unsaturated pathway intermediates. In such cases, as occurs in the congenital disease CBAS2 (congenital bile acid synthesis defect type 2), BA biosynthesis may defer to another enzyme, 3-oxo-5α-steroid-4-dehydrogenase (5α-reductase, SRD5A), which can convert Δ4-BAs into the corresponding 5α-cholanoic acids [2, 4, 8, 27]. Indeed, the study of a patient deficient in active 5β-reductase due to a missense mutation lends some support to this theory. This patient’s deficiency required supplementation with prescribed exogenous BAs; however, she was found several years later to be healthy and thriving without replacement BA therapy. Upon examination, clinicians discovered that over sixty percent of the patient’s circulating BAs were in the 5α configuration, perhaps indicating that these flat or planar BAs are capable of filling the role of the missing typical 5β BAs [27]. Additional cases of 5β-reductase deficiency were discovered in twins with similar clinical presentations and BA profiles [28]. These individuals had high levels of oxo and allo BAs (approximately 75% and 30%, respectively) in serum and urine, whereas healthy infants had undetectable levels of these BAs [28]. This study also points to oxo and allo BA synthesis being liver-derived as opposed to being created by intestinal microbes, as the gut microbiome is still highly underdeveloped at this age [28]. As additional support, at least one type of 5α-reductase enzyme (EC 1.3.1.22) is proven to be capable of converting Δ4-unsaturated BAs to allo-cholic acid ex vivo [25], which may explain the predominance of allo-cholic acid to other allo BAs during disease [5]. Animal studies have provided supporting data: both after partial hepatectomy and during hepatocarcinogenesis in the rat, expression of 5β-reductase is decreased, while 5α-reductase expression remains stable, resulting in a significant enhancement of the 5α-reductase-to-5β-reductase ratio and simultaneous increased secretion of allo BAs into bile [8, 9]. This shift in enzymatic activity may also explain the differential proportions of flat BAs at different stages of hepatic cancer progression, as is discussed in more detail in section 7.1 [4].
Other pathways for the formation of flat BAs, specifically the allo BAs, have been demonstrated in animals and in in vitro models and cannot yet be ruled out as possible biosynthetic pathways in humans [10]. Rabbits and rats can reversibly convert the principal human BAs – cholic, chenodeoxycholic, deoxycholic, and lithocholic acids – into their 5α epimers, reactions that are mostly catalyzed by intestinal microorganisms [10, 29]. This pathway also results in the formation of Δ4-BAs. The transformative effects of human microflora on BAs are an active area of research. At least three bacterial species found in the human gut are already known to be involved in secondary BA production – Clostridium hylemonae, C. scindens, and C. hiranonis. These strains are also capable of forming 3-oxo-Δ4 BAs from primary BAs via flavoproteins encoded by the recently identified, conserved baiCD genes [30]. The oxo BAs formed by these bacteria are then reduced to form DCA or LCA through yet unknown reactions [30]. Interestingly, secondary allo BAs can be induced by the administration of primary allo BAs in gut bacterial strains; however, the formation of primary allo BAs by these species has yet to be shown [30]. Allo BAs may also be formed from Δ4-BAs themselves: oxo groups can be reduced to form hydroxyl groups, and the resonant 3-oxo-Δ4,6 intermediate that is created during dihydroxylation may be reduced further to the 5α conformation [2]. Thus, the biosynthesis and origin of the planar BAs is still a matter of speculation.
Early research into allo BAs found that the administration of cholestanol, a cholesterol metabolite, results in formation of allo BAs [10], specifically of allo-cholic and – deoxycholic acid, in the rat,gerbil, and rabbit [16, 31]. Cholestanol (Fig. 1C) is formed from cholesterol in small but significant amounts in the human body, a reaction shown in vitro to be catalyzed by 5α-reductase [16]. In vitro work has also demonstrated that this pathway is likely very analogous to that converting cholesterol to 5β BAs [10, 16]; however, as intermediates of this pathway are nearly exclusively converted to 5β BAs in vivo, early authors believed that the 5α-reductase enzyme must be inhibited somehow in healthy subjects despite its high activity ex vivo [16]. Although sufficient data do not exist to either support or refute this theory in humans, it is a possible mechanism of flat BA formation.
6. Physiological and Biochemical Properties of Flat BAs
Limited experimental data exist regarding the physiological and pharmacological properties of either the allo or Δ4-unsaturated BAs; moreover, the bulk of what has been studied has been performed in in vitro models. Still, these studies partially inform on the flat BAs.
6.1 Biochemical Properties of Flat BAs
The allo BAs are nearly entirely the same as their isomers in terms of their biochemical properties. The predicted logP values of allo-cholic and – chenodeoxycholic acid match perfectly those of cholic and chenodeoxycholic acids, respectively, meaning their lipophilicity and therefore membrane permeability is comparable [32]. Similarly, the critical micellar concentration (CMC) values of the allo BAs are approximately 2mM higher than the normal BAs, indicating that the allo BAs have a slightly lower detergent capability [33]. Despite the similarities of the allo BAs to their epimers, the Δ4-unsaturated BAs involve more variance. The lipophilicity of these species, inferred from software-predicted logP values, are distinct for each molecule: 7α,12α-dihydroxy-3-oxo-4-cholenoic acid is less membrane permeable than cholic acid, with a predicted logP value of about 1 unit less. The other Δ4-unsaturated BAs – 12α-hydroxy-3-oxo-chola-4,6-dien-24-oic acid and 7α-hydroxy-3-oxochol-4-en-24-oic acid – have similar permeability approximately between that of cholic acid and chenodeoxycholic acid [32]. The acidity of all of the bile acids discussed herein – as assessed by their calculated pKa values – is identical or nearly identical because each molecule shares a common carboxylic acid moiety.
6.2 Allo-BAs
Most of the data regarding the 5α BAs exists within a single study using allo-cholic acid (ACA) in comparison with its highly abundant epimer, cholic acid (CA) [6]. These experiments have elucidated some aspects of ACA transport, metabolism, and signaling capacity [6]. For instance, wild-type Chinese hamster ovary (CHO) cells displayed higher uptake of taurine-conjugated allo-cholic acid, or tauro-allo-cholic acid (TACA), than tauro-cholic acid (TCA), possibly due to inherent steroid uptake mechanisms that may exist in these steroidogenic cells coupled with the similar structure of allo BAs with steroid hormones [6]. Similarly, translocation into rat hepatocyte nuclei was higher for TACA than TCA, which may again be related to the ability of steroidal structures to enter cell nuclei [6]. Thus, based on structural similarity, it is possible that ACA can utilize some of the transport machinery used by steroidal hormones, such as the glucocorticoid receptor (GR) or mineralocorticoid receptor (MR) to penetrate the nucleus; however, this is so far an experimentally unsupported hypothesis. Transport studies of ACA and CA also elucidated some distinctions. Though uptake by transfected rat transporters – Oatp1a1 (organic anion transporting polypeptide 1a1) and Ntcp (sodium-taurocholate-cotransporting polypeptide) into CHO cells was comparable for both BAs, transport of TACA was not diminished when sodium was removed from the incubation medium but was still significantly higher than wild-type control. As TACA has also shown significantly elevated transport by transfected rabbit Ntcp, it is more likely that the transfection of the rat ortholog is similarly able to increase uptake of this BA through a mechanism independent of Ntcp [34]. TACA was also found to inhibit ATP-mediated transport of TCA in rat Bsep (bile salt export protein)-containing plasma membrane vesicles of Sf9 insect cells but was not itself transported by any ATP-dependent mechanisms in this system, suggesting that it is not transported by the rat Bsep protein [6]. TACA also induced a stronger choleretic effect, i.e. stimulated increased bile flow, than its 5β epimer [6]. Furthermore, although TACA was not found to affect the proliferation, differentiation, viability, or apoptosis of isolated rat hepatocytes [6], a separate group discovered that ACA could significantly upregulate BSEP, SHP, and OSTβ expression in FXR- and RXR-cotransfected human hepatoma Alexander cells to an extent similar to deoxycholic acid (DCA) and lithocholic acid (LCA), so it is possible that allo-BAs retain some signaling capacity, at least in the case of FXR [35]. Thus, more physiological data is needed to establish physiological and pharmacological properties of the allo BAs in human systems as opposed to in vitro and animal models. HepG2 cells, a human hepatoblastoma cell line, have been found to produce large amounts of the flat BA intermediates – i.e. the Δ4-unsaturated BAs – as well as allo BAs to an extent similar to CA and CDCA; though, it is unknown whether this is due to the cancerous traits of the cells or some abnormality that occurred during immortalization [36]. It would additionally be interesting to examine flat BAs in vitro using hepatic and fetal hepatic cells lines. The studies referenced here did not include the other allo-BAs, i.e. allo-chenodeoxycholic acid, allo-lithocholic acid, or allo-deoxycholic acid, so it is currently only known that these differences exist for one set of BA epimers. The known physiological properties of ACA based on current literature are illustrated in figure 2.
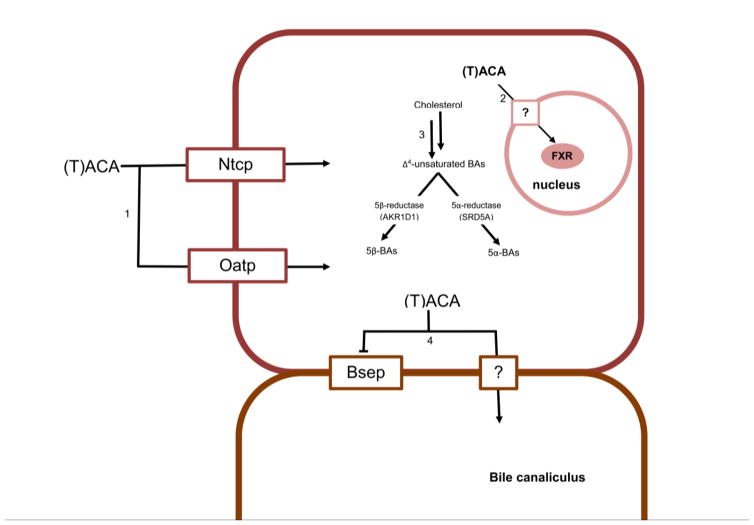
Figure 2.
An illustration of the known physiological pathways of one allo-BA, (taurine-conjugated)-allo-cholic acid, (T)ACA. The mechanisms shown below have been shown experimentally to occur in non-human systems as detailed in the text. In pathway 1, tauro-allo-cholic acid, (T)ACA. The mechanisms shown below have been shown experimentally to occur in non-human systems as detailed in the text. In pathway 1, tauro-allo-cholic acid is transported into hepatocytes by Ntcp and Oatp. In pathway 2, (T)ACA is transported efficiently into the hepatocyte nucleus and is capable of activating FXR, though the mechanism through which this happens is unknown. A question mark (?) indicates that the current mechanism of membrane penetration is unknown, such as in pathway 4, in which (T)ACA is transported into the bile canaliculus from hepatocytes through an unknown mechanism. In addition, (T)ACA inhibits but is not transported by Bsep. In the center simplified cholesterol metabolism schematic (3), the mechanism of planar BA formation is assumed to be via SRD5A-mediated cholesterol metabolism, though this has not been definitively proven as of yet.
6.3 Δ4-unsaturated BAs
The Δ4-unsaturated BAs are even less well studied than the 5α species, perhaps because they were long thought to be only intermediate steps in an elaborate biosynthetic pathway. The Meier group used a model compound to represent BAs with a hydroxylated 3-oxo-Δ4 structure in the interest of studying the transport of BAs synthesized by patients with oxosteroid 5β-reductase deficiency, that is, CBAS2 [37]. These patients accumulate an excess of BAs with this flat, Δ4-unsaturated structure due to their inability to reduce intermediates in the BA biosynthetic pathway. Taurine-conjugated 7α-hydroxy-3-oxo-4-cholenoic acid was synthesized as this experiment’s model bile acid and is a conjugated version of one of the three Δ4-unsaturated BAs that recurs most often in disease (Fig. 1F). Using plasma membrane vesicles isolated from rat liver, this Δ4-unsaturated BA was found to competitively inhibit canalicular ATP-dependent transport of TCA without itself being transported; in agreement with this early data, the Δ4-unsaturated BAs are now known to be potent cholestatic agents via their inhibition of BSEP [8, 37]. Additionally, the model Δ4-unsaturated BA used in this study exhibited competitive inhibition of sodium-dependent uptake mechanisms in vesicles isolated from basolateral liver; however, like TACA, sodium-dependent transport of 7α-hydroxy-3-oxo-4-cholyltaurine was comparable to that of taurocholic acid [37]. Thus, this Δ4-unsaturated BA competitively inhibits the hepatic sodium-dependent basolateral transporter(s), i.e. NTCP. At present, this represents the bulk of physiological data regarding the Δ4-unsaturated BAs. As these BAs represent perhaps more of a clinical concern than the allo BAs due to congenital defects in the 5β reductase enzyme, it is surprising that there is such a paucity of knowledge regarding their physio- and pharmacological properties.
7. Recurrence in Disease
7.1 Cancer
The renewed study of the flat BAs is motivated by their reappearance in adults during certain disease states, usually those which involve high hepatic proliferation. This phenomenon has been shown to occur in humans, rats, and mice in several hepatic diseases and disease models. One of the first reports of this was in patients with the most common type of liver cancer, hepatocellular carcinoma (HCC) [4]. Both categories of flat BAs – the allo BAs and Δ4-unsaturated BAs – reappear at significantly elevated levels above control in the serum and urine of these patients (table 2) [4]. Moreover, it would seem that the amount of flat BAs are positively correlated with hepatocyte proliferation or tumor size, as the concentration of Δ4-unsaturated BAs were found to be significantly lower in the urine of patients with small tumors (<3 cm) than in mid-size tumors (3–6 cm), and patients with large tumors (>6 cm) had an additional increase in Δ4-BAs compared with those of mid-size tumors [4]. This is one of the few studies investigating irregular BAs as prognostic or diagnostic indicators.
Table 2.
A summary of the data present regarding the occurrence of flat bile acids in humans. Values are given as a percentage (%) of total bile acids in the given biological tissue. Numbers in brackets represent the source from which this information was drawn, listed in the References of this work. If multiple allo or Δ4-unsaturated species were specified by a source manuscript, these values were summed in order to determine percent of total bile acids. Percentages could not be determined for the publication in the bottommost tale, but absolute concentrations of these bile acids were estimated from visual assessment of graphical results.
| Allo (5α) bile acids | Δ4-unsaturated bile acids | |||||
|---|---|---|---|---|---|---|
| Healthy individuals | ||||||
|
| ||||||
| Urine | Urine | Feces | ||||
|
|
||||||
| At birth (infant) | undetectable[12] | 48.4%[12] | 30.9%[19] | |||
| 0.29%[13] | 33.04%[13] | |||||
| 34.3%[19] | ||||||
| 3 days old | 0.06%[12] | 15.1%[12] | ||||
| 0.23%[13] | 8.96%[13] | |||||
| 15.4%[19] | ||||||
| 7 days old | 0.09%[13] | 14.7%[13] | 31.0%[19] | |||
| 16.3%[19] | ||||||
| 1 month old | undetectable[12] | 6.5%[12] | 0.79 [19] | |||
| 16.2%[19] | ||||||
| 2 months old | 3.1%[12] | 1.2%[12] | ||||
| 20.8%[19] | ||||||
| 3 months old | 3.1%[12] | 16.4%[19] | 15.2%[19] | |||
| 11–12 months old | 3.3%[12] | undetectable[12] | ||||
| 2–3 years old | 1.1%[12] | 3.4%[12] | ||||
| 9–14 years old | undetectable[12] | undetectable[12] | ||||
| Cholestatic infants | ||||||
|
| ||||||
| Urine | Serum | Urine | Serum | |||
|
|
||||||
| At birth | 0.5%[14] | 5.5%[14] | 70.6%[14] | 26.1%[14] | ||
| 1–2 months old | 0.5%[14] | 67.3%[14] | ||||
| Healthy pregnant women | ||||||
|
| ||||||
| Urine | Urine | |||||
|
|
||||||
| Nonpregnant | 1.89%[13] | 1.33%[13] | ||||
| 30–32 weeks | 0.74%[13] | 9.26%[13] | ||||
| 35–36 weeks | 1.3%[13] | 6.74%[13] | ||||
| 40 weeks | 0.34%[13] | 3.31%[13] | ||||
| 3–4 days following delivery | 2.86%[13] | 0.52%[13] | ||||
| 6–7 days following delivery | 1.78%[13] | 1.95%[13] | ||||
| 5β-Reductase deficiency | ||||||
|
| ||||||
| Plasma | Urine | Bile | Plasma | Urine | Bile | |
|
|
||||||
| 13 years old | 62%[27] | |||||
| Infants (identical twins) | 28.9%, 29.6%[28] | 2.6%, | 25.7%[28] | 17.1%, 12.0%[28] | 92.1%, | undetectable[28] |
| 9.5%[28] | 75.4%[28] | |||||
| Colon cancer | ||||||
|
| ||||||
| Serum | Feces | |||||
|
|
||||||
| undetectable[38] | 24.29%[38] | |||||
| Liver disease/injury | ||||||
|
| ||||||
| Urine (μmol/24 hours) | Serum (μM) | Urine (μmol/24 hours) | Serum (μM) | |||
|
|
||||||
| Cirrhosis | 0.1–0.15[4] | 0.1–0.2[4] | 0.6–0.7[4] | <0.1[4] | ||
| Metastasis | 0.35–0.40[4] | <0.1[4] | 0.5[4] | 0.25[4] | ||
| HCC | 0.25[4] | 0.25[4] | 2.5[4] | 0.6[4] | ||
| Partial hepatectomy | 0.13–1.04[11] | |||||
The presence of the flat BAs in hepatic cancer is mirrored in animal experiments. During the progression of chemically induced hepatocarcinogenesis in rats, flat BA species were markedly elevated in bile; though, the concentrations of each peaked at different time points of disease advancement, indicating a timeline progression of the specific flat BA species [5]. This study examined the BA content in liver and bile at three different time points and in different hepatic compartments during hepatocarcinogenesis development: the foci stage (12 weeks following chemical induction), hepatoma stage (20 weeks), and carcinoma stage (32 weeks) in rat hepatocyte nuclei, homogenate, and in bile [5]. The allo-BAs, predominantly allo-cholic acid, reached a peak concentration in bile at the hepatoma stage (20 weeks), whereas Δ4-unsaturated BAs were elevated at the carcinoma stage (32 weeks) [3, 5]. The authors noted that increased abundance of Δ4-unsaturated BAs approximately coincided with the maximal loss of hepatocyte differentiation at the carcinoma stage, whereas the allo-BAs’ increased secretion began much earlier in hepatocarcinogenesis and was maintained throughout disease progression [5]. The unsaturated BAs were absent from bile at the hepatoma stage of hepatocarcinogenesis when allo-cholic acid reached its maximum [5]. Conversely, the planar unsaturated BAs were not found in liver homogenate at the points examined during hepatocarcinogenesis, but allo-cholic acid was found at the hepatoma stage (20 weeks) as 0.3% of total BAs [5]. It has not been determined if humans also have differential levels of each subtype of flat BA at different points of hepatic cancer progression and if this could be used in HCC diagnosis and/or prognosis.
The flat BAs have been found in cases of colon cancer as well. Allo-BAs, both primary and secondary (i.e. allo-lithocholic and allo-deoxycholic), were found to be elevated in the feces but not serum of colon cancer patients; however, allo-cholic acid and – chenodeoxycholic acid levels were not significantly different from control [38]. Conversely, the secondary allo BAs, allo-lithocholic and -deoxycholic acids, were significantly increased in the feces of the colon cancer patients - up to 2.6-fold higher for allo-LCA and 40-fold higher for allo-DCA – the latter being especially high in males [38]. The higher ratio of secondary-to-primary allo BAs in this particular disease is most likely due to a combination of longer retention of feces in the large intestine and changes in colonic bacteria associated with colon cancer [38]. Though this study demonstrates the body’s capability of producing BAs in the 5α configuration during colon cancer, it is possible that the secondary allo BAs were modified by microflora from host-synthesized primary allo BAs.
7.2 Liver ablation
Following partial hepatectomy, transplantation, or liver injury, hepatocytes rapidly proliferate to regenerate the organ; moreover, BAs have recently been found to play important roles in this process via their activation of FXR and TGR5 [39]. Thus, it follows that the flat BAs found in the rapidly proliferating hepatocytes of HCC and other hepatic cancers would also be found in the regenerating liver, and indeed, they are. In the urine of patients who had undergone partial hepatectomy (PH), flat BAs – both the Δ4-unsaturated and allo-BAs – were significantly increased at day 3 following surgery and continued to rise for the next seven days (the remainder of the study), reaching an increase of up to 8-fold control values (table 2) [11]. Though total urinary BA output was similar in all patients examined, excretion of flat BAs was significantly higher for some patients that had undergone major PH – meaning a larger portion of the liver was removed – versus those in the minor PH group [11]. This data is corroborated by earlier animal studies, in which a transient increase of Δ4-unsaturated and allo-BAs of about 6 times control was found in the bile of rats that had also undergone PH [8]. These studies did not establish whether a differential proportion of flat BA subspecies occur at separate time points in liver recovery, nor did they examine if and when the flat BAs disappeared after recovery. Additional research in this area may also elucidate if reappearance of the flat BAs is correlated with the proliferative state of the liver.
7.3 Additional occurrence of planar BAs in various studies
The flat bile acid species have been found in hepatic disorders other than primary hepatic or colon cancer and mechanical ablation. Allo and Δ4-unsaturated BAs were found at low concentrations in the serum samples of patients with liver metastasis and liver cirrhosis; additionally, Δ4-unsaturated BAs were raised in patients with liver cirrhosis and chronic viral hepatitis (table 2) [4]. This study and a few others demonstrate that, while the flat BAs seem to resurface most consistently in cases of high hepatic proliferation such as cancer and organ regeneration, other hepatic conditions can result in production of these molecules as well. Because of the limited studies of uncommon BAs in human disease and liver injury, it is difficult to discern the origin of the flat BAs in humans.
Due to the inverse relationship between cellular proliferation and differentiation, it is possible that recurrence of flat BAs in disease states may be due to retro-differentiation of the liver, during which hepatocytes become less specialized and lose much of the capabilities of mature cells; thus, the hepatic environment may resemble that of the fetus and newborn during periods of high proliferative activity [40]. Other typically fetal bile acid molecules, such as BAs with unusual hydroxylation points – i.e. 1β, 3β, and 6α hydroxylations – were also found in the serum and urine of these patients, presenting additional evidence of a retro-differentiated hepatic state. However, flat BA species are noted to reappear at elevated levels in other cases of liver injury, such as cirrhosis, cholestasis, and metastasis, conditions that do not reflect high hepatic proliferation [4, 5, 11]. Thus, it is presently still uncertain what causes the reappearance of flat BAs in adult humans during disease, demonstrating a need for additional research in this field.
8. Evolutionary Considerations
BAs are produced by every class of vertebrate animals, with chemical variation within exceeding that of any other type of small molecule [23, 24]. The generically termed “bile salts” are made up of several subgroups of cholesterol end products across species: the C27 bile alcohols, C24 bile acids, and C24 bile acids, differing in the length of the carbon side chain and oxidation state [23, 24]. The C27 bile alcohols are considered the ancestral bile salts, as they are found as the bile salts of the earliest evolving extant species (jawless and lobe-finned fish such as the lamprey and hagfish), whereas the most recently evolved species utilize C24 BAs [23, 24]. The C27 bile alcohols are also in the 5α configuration, whereas the more evolutionarily furthered vertebrate species (nearly all mammals and humans, for example) have 5β C24 BAs, demonstrating two evolutionary shifts: the C5 hydrogen atom from an alpha to a beta configuration and the decrease in side chain length from 8 to 5 carbon atoms [23, 24]. The latter represents the addition of several enzymatic steps taking place within multiple cellular compartments, but little is known about the biosynthetic pathways that produce either the 5α C27 alcohols or 5α C24 acids.[23, 24] Moreover, there are several extant species, mostly lizards in the Iguanidae phylogenetic family, that utilize entirely 5α (allo) bile acids or bile alcohols [23, 24]. Thus, one hypothesis behind the presence of flat BAs is that the immature hepatic state in humans during liver proliferation is responsible for the formation of 5α BAs and is enzymatically and metabolically reflective of a less evolved BA biosynthesis pathway.
9. Conclusions and Implications
Although bile acids have received much scientific attention, the bulk of the molecules within this family are under-characterized. Of these are a subset of BAs that retain the flat or planar structure of cholesterol and ancestral BAs found in lower-order species. Although these typically fetal BAs are consistently elevated in several hepatic disease conditions, the origin and function of these molecules are unknown, opening the research floor to a number of opportunities. Current science has yet to elucidate how the planar BAs are biosynthesized either in primitive reptilian and marine species or in man. Research concerning these compounds could also continue to examine their physiological and pharmacological effects in more relevant systems, such as in primary human cells and in vivo, as the planar BAs have so far only been studied in in vitro nonhuman models. Additionally, only a few of the flat BAs have been investigated, so additional study into the remaining planar BAs, both the other oxo and allo species, should also be considered, as small changes in BA structure are associated with drastically different physiological effects. Due to the well-characterized signaling capabilities and toxicity associated with the primary human BAs, it is necessary to understand how these endogenous molecules can affect the hepatobiliary system in the disease states in which they are present, especially in those with poor prognoses, such as hepatocellular carcinoma. Several authors have expressed interest in using irregular or uncommon BAs such as these as endogenous biomarkers of disease, which would allow physicians to identify and begin treatment for conditions such as HCC earlier, but considerable research is necessary to reach clinical significance. Quantitative methods of characterizing BA profiles would be beneficial in determining if these BA species are present in other disease states in addition to the limited number that has been studied in order to further explore these molecules as possible biomarkers. Much further study is required to establish whether these molecules are capable of bioactive signaling, markers of injury, or relics of less specialized BA biosynthesis.
Highlights.
Planar bile acids are structurally and functionally different from common 5β bile acids
Planar bile acids are abundant in the fetus, newborn, and pregnant woman
They are undetectable in healthy adults, but recur during hepatic injury, cancer and ablation
They could serve as biomarkers for liver disease, but require further characterization in humans
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (5R01DK061425 to PWS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alnouti Y. Bile Acid Sulfation: A Pathway of Bile Acid Elimination and Detoxification. Tox Sci. 2009;108:225–246. doi: 10.1093/toxsci/kfn268. [DOI] [PubMed] [Google Scholar]
- 2.Hofmann AF, Hagey LR. Bile Acids: Chemistry, Pathochemistry, Biology, Pathobiology, and Therapeutics. Cell Mol Life Sci. 2008;65:2461–2483. doi: 10.1007/s00018-008-7568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lan K, Su M, Xie G, Ferslew BC, Brouwer KLR, Rajani C, Liu C, Jia W. Key Role for the 12-Hydroxy Group in the Negative Ion Fragmentation of Unconjugated C24 Bile Acids. Anal Chem. 2016;88:7041–7048. doi: 10.1021/acs.analchem.6b00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-mir MY, Badia MD, Luengo N, Monte MJ, Marin JJG. Increased levels of typically fetal bile acid species in patients with hepatocellular carcinoma. Clin Sci. 2001;100:499–208. [PubMed] [Google Scholar]
- 5.Mendoza ME, Monte MJ, El-mir MY, Badia MD, Marin JJG. Changes in the pattern of bile acids in the nuclei of rat liver cells during hepatocarcinogenesis. Clin Sci. 2002;102:143–150. [PubMed] [Google Scholar]
- 6.Mendoza ME, Monte MJ, Serrano MA, Pastor-Anglada M, Steiger B, Meier PJ, Medarde M, Marin JJG. Physiological characteristics of allo-cholic acid. J Lipid Res. 2003;44:84–92. doi: 10.1194/jlr.m200220-jlr200. [DOI] [PubMed] [Google Scholar]
- 7.Monte MJ, El-mir MY, Sainz GR, Bravo P, Marin JJG. Bile acid secretion during synchronized rat liver regeneration. Biochim Biophys Acta. 1997;1362:56–66. doi: 10.1016/s0925-4439(97)00063-x. [DOI] [PubMed] [Google Scholar]
- 8.Monte MJ, Fernandez-Tagarro M, Macias RIR, Jimenez F, Gonzalez-San Martin F, Marin JJG. Changes in the expression of genes related to bile acid synthesis and transport by the rat liver during hepatocarcinogenesis. Clin Sci. 2005;109:199–207. doi: 10.1042/CS20050035. [DOI] [PubMed] [Google Scholar]
- 9.Monte MJ, Fernandez-Tagarro M, Marin JJG. Transient changes in the expression pattern of key enzymes for bile acid synthesis during rat liver regeneration. Biochim Biophys Acta. 2005;1734:127–135. doi: 10.1016/j.bbalip.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Sjovall J, Meurant G. Sterols and bile acids. Elsevier Science; Amsterdam: 1985. [Google Scholar]
- 11.Stärkel P, Shindano T, Horsmans Y, Gigot JF, Fernandez-Tagarro M, Marin JJG, Monte MJ. Foetal 'flat' bile acids reappear during human liver regeneration after surgery. Eur J Clin Invest. 2009;39:58–64. doi: 10.1111/j.1365-2362.2008.02059.x. [DOI] [PubMed] [Google Scholar]
- 12.Kimura A, Mahara R, Inoue T, Nomura Y, Murai T, Kurosawa T, Tohma M, Noguchi K, Hoshima A, Fujisawa T, Kato H. Profile of Urinary Bile Acids in Infants and Children: Developmental Pattern of Excretion of Unsaturated Ketonic Bile Acids and 7β-Hydroxylated Bile Acids. Ped Res. 1999;45:603–609. doi: 10.1203/00006450-199904010-00022. [DOI] [PubMed] [Google Scholar]
- 13.Kimura A, Suzuki M, Murai T, Inoue T, Kato H, Hori D, Nomura Y, Yoshimura T, Kurosawa T, Tohma M. Perinatal bile acid metabolism: analysis of urinary bile acids in pregnant women and newborns. J Lipid Res. 1997;38:1954–1962. [PubMed] [Google Scholar]
- 14.Suzuki M, Murai T, Yoshimura T, Kimura A, Kurosawa T, Tohma M. Determination of 3-oxo-Δ4- and 3-oxo-Δ4,6-bile acids and related compounds in biological fluids of infants with cholestasis by gas chromatography-mass spectrometry. J Chrom B. 1997;693:11–21. doi: 10.1016/s0378-4347(97)00063-7. [DOI] [PubMed] [Google Scholar]
- 15.Ohta K. Tetraoxy-norsterocholansaure aus der Gigi“-Fischgalle (Pelteobagrus nudiceps) Hoppe-Seyl Z. 1939;259:53–61. [Google Scholar]
- 16.Elliott WH. The allo bile acids. NATO Adv Study Inst Ser, Ser A. 1976;A7:469–483. [Google Scholar]
- 17.Chung-Davidson YW, Wang H, Bryan MB, Wu H, Johnson NS, Li W. An anti-steroidogenic inhibitory primer pheromone in male sea lamprey (Petromyzon marinus) General and Comparative Endocrinology. 2013;189:24–31. doi: 10.1016/j.ygcen.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 18.Inoue T, Kimura A, Aoki K, Tohma M, Kato H. Developmental pattern of 3-oxo-Δ4 bile acids in neonatal bile acid metabolism. Arch Dis Child. 1997;77:F52–F56. doi: 10.1136/fn.77.1.f52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamato Y, Kimura A, Murai T, Yoshimura T, Kurosawa T, Terazawa S, Takao A, Maeda K, Yamashita Y, Kato H. 3β-Hydroxy-Δ5 -C27-steroid dehydrogenase deficiency: Diagnosis and treatment. Journal of Paediatrics and Child Health. 2001;37:516–519. doi: 10.1046/j.1440-1754.2001.00751.x. [DOI] [PubMed] [Google Scholar]
- 20.Kumagai M, Kimura A, Takei H, Kurosawa T, Aoki K, Inokuchi T, Matsuishi T. Perinatal bile acid metabolism: bile acid analysis of meconium of preterm and full-term infants. Journal of Gastroenterology. 2007;42:904–910. doi: 10.1007/s00535-007-2108-y. [DOI] [PubMed] [Google Scholar]
- 21.Juan SCd, Monte MJ, Macias RIR, Wauthier V, Calderon PB, Marin JJG. Ontogenic development-associated changes in the expression of genes involved in rat bile acid homeostasis. J Lipid Res. 2007;48:1362–1370. doi: 10.1194/jlr.M700034-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Anderson IG, Haslewood GAD. Comparative studies of ‘bile salts’. 15. The natural occurrence and preparation of allocholic acid. Biochemical Journal. 1962;85:236–242. doi: 10.1042/bj0850236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagey LR, Vidal N, Hofmann AF, Krasowski MD. Evolutionary diversity of bile salts in reptiles and mammals, including analysis of ancient human and extinct giant ground sloth coprolites. BMC Evolutionary Biology. 2010;10:133. doi: 10.1186/1471-2148-10-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hofmann AF, Hagey LR, Krasowski MD. Bile salts of vertebrates: structural variation and possible evolutionary significance. Journal of Lipid Research. 2010;51:226–246. doi: 10.1194/jlr.R000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zimniak P, Lester R. Bile Acid Metabolism in the Perinatal Period. In: Lebenthal E, editor. Human Gastrointestinal Development. Raven Press; 1989. pp. 561–580. [Google Scholar]
- 26.Chen M, Penning TM. 5β-Reduced steroids and human Δ4-3-ketosteroid 5β-reductase (AKR1D1) Steroids. 2015;83:17–26. doi: 10.1016/j.steroids.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palermo M, Marazzi MG, Hughes BA, Stewart PM, Clayton PT, Shackleton CHL. Human Δ4-3-oxosteroid 5β-reductase (AKR1D1) deficiency and steroid metabolism. Steroids. 2008;73:417–423. doi: 10.1016/j.steroids.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Setchell KD, Schwarz M, O'Connell NC, Lund EG, Davis DL, Lathe R, Thompson HR, Weslie Tyson R, Sokol RJ, Russell DW. Identification of a new inborn error in bile acid synthesis: mutation of the oxysterol 7alpha-hydroxylase gene causes severe neonatal liver disease. The Journal of Clinical Investigation. 1998;102:1690–1703. doi: 10.1172/JCI2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kallner A, Knutsson L, Larsen B, Dumanovic J. On the biosynthesis and metabolism of allodeoxycholic acid in the rat. Acta Chem Scand. 1967;21:315–321. doi: 10.3891/acta.chem.scand.21-0315. [DOI] [PubMed] [Google Scholar]
- 30.Ridlon JM, Kang DJ, Hylemon PB. Isolation and characterization of a bile acid inducible 7α-dehydroxylating operon in Chlostridium hylemonae TN271. Anaerobe. 2010;16:137–146. doi: 10.1016/j.anaerobe.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofmann AF, Mosbach EH. Identification of Allodeoxycholic Acid as the Major Component of Gallstones Induced in the Rabbit by 5α-Cholestan-3β-ol. Journal of Biological Chemistry. 1964;239:2813–2821. [PubMed] [Google Scholar]
- 32.Advanced Chemistry Development, Inc., Toronto, ON, Canada, 2017.
- 33.Poa M, Sebenji A. Chemometric and conformational approach to the analysis of the aggregation capabilities in a set of bile salts of the allo and normal series. J Pharm Biomed Anal. 2016;121:316–324. doi: 10.1016/j.jpba.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 34.Baringhaus KH, Matter H, Stengelin S, Kramer W. Substrate specificity of the ileal and the hepatic Na+/bile acid cotransporters of the rabbit. II. A reliable 3D QSAR pharmacophore model for the ileal Na+/bile acid cotransporter. Journal of Lipid Research. 1999;40:2158–2168. [PubMed] [Google Scholar]
- 35.Vaquero J, Monte MJ, Dominguez M, Muntané J, Marin JJG. Differential activation of the human farnesoid X receptor depends on the pattern of expressed isoforms and the bile acid pool composition. Biochem Pharmacol. 2013;86:926–939. doi: 10.1016/j.bcp.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 36.Axelson M, Mörk B, Everson GT. Bile acid synthesis in cultured human hepatoblastoma cells. Journal of Biological Chemistry. 1991;266:17770–17777. [PubMed] [Google Scholar]
- 37.Steiger B, Zhang J, O'Neill B, Sjovall J, Meier PJ. Differential interaction of bile acids from patients with inborn errors of bile acid synthesis with hepatocellular bile acid transporters. Eur J Biochem. 1997;244:39–44. doi: 10.1111/j.1432-1033.1997.00039.x. [DOI] [PubMed] [Google Scholar]
- 38.Tadano T, Kanoh M, Matsumoto M, Sakamoto K, Kamano T. Studies of Serum and Feces Bile Acids Determination by Gas Chromatogrphy-Mass Spectrometry. Rinsho Byori. 2006;54:103–110. [PubMed] [Google Scholar]
- 39.Fan M, Wang X, Xu G, Yan Q, Huang W. Bile acid signaling and liver regeneration. Biochim Biophys Acta. 2015;1849:196–200. doi: 10.1016/j.bbagrm.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuhlmann WD, Peschke P. Hepatic progenitor cells, stem cells, and AFP expression in models of liver injury. International Journal of Experimental Pathology. 2006;87:343–359. doi: 10.1111/j.1365-2613.2006.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]