Abstract
Parasitic nematodes infect hundreds of millions of people and farmed livestock. Further, plant parasitic nematodes result in major crop damage. The pipeline of therapeutic compounds is limited and parasite resistance to the existing anthelmintic compounds is a global threat. We have developed an INVertebrate Automated Phenotyping Platform (INVAPP) for high-throughput, plate-based chemical screening, and an algorithm (Paragon) which allows screening for compounds that have an effect on motility and development of parasitic worms. We have validated its utility by determining the efficacy of a panel of known anthelmintics against model and parasitic nematodes: Caenorhabditis elegans, Haemonchus contortus, Teladorsagia circumcincta, and Trichuris muris. We then applied the system to screen the Pathogen Box chemical library in a blinded fashion and identified compounds already known to have anthelmintic or anti-parasitic activity, including tolfenpyrad, auranofin, and mebendazole; and 14 compounds previously undescribed as anthelmintics, including benzoxaborole and isoxazole chemotypes. This system offers an effective, high-throughput system for the discovery of novel anthelmintics.
Keywords: Parasitic nematodes, C. elegans, Chemical library screening, Automated phenotyping, Anthelmintic, Benzoxaborole
Graphical abstract
Highlights
-
•
A rapid system for quantifying motility and growth of nematodes is described.
-
•
The system was validated by quantifying the activity of known anthelmintics.
-
•
The Pathogen Box was screened for molecules blocking C. elegans growth.
-
•
Compounds with known anthelmintic/anti-parasitic activity were identified.
-
•
Fourteen compounds previously undescribed as anthelmintics were discovered.
1. Introduction
Parasitic nematodes infect around one billion people, with the soil transmitted nematodes Ascaris, hookworm and whipworm (Trichuris trichiura) each afflicting hundreds of millions of people (Hotez, 2013). These diseases cause high morbidity and are closely linked to poverty in the developing world. The global impact of parasitic nematodes is worsened by their effect on livestock, equids and companion animals. Parasitic nematodes of livestock are thought to cost approximately $10 billion annually with anthelmintic products comprising a major segment of the veterinary pharmaceuticals market (Roeber et al., 2013).
There are concerns that mass drug administration (MDA) programs aimed at controlling or eradicating human parasitic nematodes will be made unsustainable by anthelmintic resistance, jeopardising attempts to eradicate these important tropical diseases (Prichard et al., 2012). In the case of Trichuris, modelling indicates that existing benzimidazole drugs are insufficiently effective for single dose MDA programs designed to break transmission; therefore eradication cannot be achieved (Keiser and Utzinger, 2008, Levecke et al., 2014, Turner et al., 2016). The current arsenal of veterinary anthelmintics is threatened by the emergence and spread of resistance in parasitic nematodes of domesticated animals, a recent example of which is the report of resistance to monepantel in sheep nematode species only four years after introduction of the compound (Sales and Love, 2016, Scott et al., 2013). Multi-class resistance is also common in parasitic nematodes of ruminants (Rose et al., 2015, Sutherland, 2015) and equids (Matthews, 2014). Thus, there is an urgent requirement for the identification of novel classes of anthelmintics.
Phenotypic screening is enjoying a resurgence in recent years and is playing an increasingly important role in the modern drug discovery paradigm (Al-Ali, 2016). Furthermore, employment of this approach using live nematodes ex vivo has been historically successful for target identification and lead optimisation. For example, a series of acetonitrile derivatives were identified using a Haemonchus contortus in vitro larval development assay, and this led to the discovery of monepantel (Kaminsky et al., 2008).
1.1. Automated systems for phenotypic screening of parasitic nematodes and models of parasites
Given the need for new anthelmintics, there has been growing interest both in phenotypic screens for identification of new classes of active small molecules and in systems for quantifying relevant readouts of nematode viability such as growth and motility (Buckingham et al., 2014, Buckingham and Sattelle, 2008). Manual scoring of motility, growth or viability has been effective and used to screen libraries of up to 67,000 compounds (Burns et al., 2015, Tritten et al., 2011, Tritten et al., 2012). Automated systems offer the potential of higher throughput and greater reliability. Such approaches include indirect assessment of viability by using the xCELLigence System; assessment of metabolic activity via colorimetric assays such as resazurin, MTT, and acid phosphatase activity; assessment of motor activity via isothermal microcalorimetry and quantification of movement-related light scattering (Nutting et al., 2015, Silbereisen et al., 2011, Smout et al., 2010, Wangchuk et al., 2016a, Wangchuk et al., 2016b).
Imaging-based systems for quantification of motility or growth have also been developed. The “WormAssay” system quantifies the motility of macroscopic parasites such as Brugia malayi adult worms (Marcellino et al., 2012). This system has been further developed into “The Worminator”, which quantifies smaller, microscopic nematode stages and has been validated by quantifying the activity of several anthelmintics (Storey et al., 2014). This system has a reported scan time of 30 s per well, hence a throughput of around one and a quarter 6-well plates per hour. A system based on single-well imaging and thresholding of motile pixels with a throughput of around five 96-well plates per hour has been reported (Preston et al., 2016a). Its utility has been demonstrated by the successful screening of a 522-compound kinase inhibitor library and the 400-compound Medicine for Malaria Venture Pathogen box on Haemonchus contortus larvae (Preston et al., 2015, Preston et al., 2016b). A notable recently-described screen of the effects of 26,000 compounds on Caenorhabditis elegans growth/survival used WormScan, a system that uses a conventional flat-bed scanner to capture two frames of images of whole plates and then uses an algorithm based on the difference image to assign a value to each well that reflects motility/growth (Mathew et al., 2012, Mathew et al., 2016). This led to the identification of several compounds with previously unreported anthelmintic activity, including compounds targeting PINK-1 and MEV-1. The authors reported a throughput of approximately 25–40 96-well plates per hour.
1.2. Developing a new robust motility/growth quantification system focussed on rapid, high-throughput chemical screening
It is clear that recent developments in phenotypic screening of parasitic and model nematodes have led to an acceleration of the discovery of potential novel anthelmintic compounds. Given the large sizes of drug-like compound libraries and the need to efficiently identify the hit compounds therein that have the potential to be developed into potent and selective anthelmintic lead molecules, it is desirable that nematode phenotypic screening be further accelerated. Here, we present the development of the INVAPP/Paragon system, which quantifies nematode motility/growth with a throughput of approximately 100 96-well plates per hour, with a robust and unbiased approach. We validate the system by quantifying the activity of a panel of known anthelmintics on a variety of nematode species, and then by screening, in a blinded fashion, the Medicines for Malaria Venture Pathogen Box for compounds that block or reduce nematode growth.
2. Materials and methods
2.1. Ethics statement
All mouse experiments were approved by the University of Manchester Animal Welfare and Ethical Review Board and performed under the regulation of the United Kingdom Home Office Scientific Procedures Act (1986) and the Home Office project licence 70/8127.
All ovine experiments were approved by the Moredun Research Institute Experiments and Ethics Committee and performed under the regulation of the United Kingdom Home Office Scientific Procedures Act (1986) and the Home Office project licence 60/04421.
2.2. INVAPP/Paragon system
The INVAPP/Paragon system consists of a fast high-resolution camera (Andor Neo, resolution 2560x2160, maximum frame rate 100 frames per second) with a line-scan lens (Pentax YF3528). Microtiter plates are placed in a holder built into the cabinet and imaged from below. Illumination is provided by an LED panel with acrylic diffuser. Movies were captured using μManager (Edelstein et al., 2014). The desirable movie frame length and duration of filming depends on the particular organism under study and is specified below. Movies were analysed using MATLAB scripts. Briefly, movies were analysed by calculating the variance through time for each pixel. The distribution of these pixel variances was then considered, and pixels whose variance was above the threshold (typically those greater than one standard deviation away from the mean variance) were considered ‘motile’. Motile pixels were assigned by well, by dividing the matrix evenly across the width and height of the image, with plateColumns and plateRows parameters used to specify the number of wells in the plate image. The ‘motile’ pixels were then counted, generating a movement score for each well. The source code for this software has been released under the open source MIT license and is available at https://github.com/fpartridge/invapp-paragon. A further MATLAB script has been provided for batch processing of movies.
2.3. Caenorhabditis elegans motility and growth assays
C. elegans strains were maintained at 20 °C on nematode growth medium agar seeded with the E. coli strain OP50. To obtain worms for screening, a mixed-stage liquid culture was prepared by washing well-fed worms from one small NGM plate into a medium of 50 ml S-complete buffer with a pellet of approximately 2–3 g E. coli HB101. Cultures were agitated at 200 rpm, 20 °C, until there were many adults present, then synchronised at the L1 stage by bleaching. Fifty millilitre cultures were pelleted and bleaching mix (1.5 ml 4M NaOH, 2.4 ml NaOCl, 2.1 ml water) added. Mixing for 4 min led to the release of embryos, which were washed three times with 50 ml S-basal medium. The cultures were incubated in 50 ml S-basal at 20 °C and agitated at 200 rpm overnight to allow eggs to hatch and arrest as a synchronous L1 population.
For the growth assay, C. elegans were cultured in a 96-well plate format. Synchronised L1 were diluted to approximately 20 worms per 50 μl in S complete medium with around 1% w/v HB101 E. coli. Assay plates were prepared with 49 μl of S-basal and 1 μl of DMSO or compound in DMSO solution per well. Next, 50 μl of the L1 suspension were added to each well. Final DMSO concentration was 1% v/v. Plates were incubated at 20 °C before imaging using the INVAPP/Paragon system 5 days later. Prior to imaging, worm motion was stimulated mechanically by inserting and removing a 96-well PCR plate into/from the wells of the assay plate. Whole-plate 200 frame movies were recorded at 30 frames per second (7 s total).
For the adult motility assay, synchronised L1 were refed as a bulk 50 ml culture and cultured at 20 °C until they developed into young adults. Worms were washed in S-basal and dispensed, approximately 20 worms per well, into 96-well plates with compound dissolved in DMSO, or DMSO alone and then incubated for 3 h. Final DMSO concentration was 1% v/v. Whole-plate 200 frame movies were recorded at 30 frames per second (7 s total).
For the anthelmintic panel screen using C. elegans in the acute treatment adult motility assay and the growth assay, we screened n = 5 wells for each concentration, with each replicate concentration on different plates. Where the compound was active in the assay, we fitted a dose-response curve using 3-parameter log-logistic model and the R package drc (Ritz and Streibig, 2005).
2.4. Trichuris muris motility assay
For the adult T. muris motility assay, male and female severe combined immune deficiency mice (bred in the Biological Services Facility at the University of Manchester) were infected with 200 embryonated T. muris eggs in water by oral gavage. After 35 days, mice were killed and their caecae and colons removed, opened longitudinally, and washed with pre-warmed Roswell Park Memorial Institute (RPMI) 1640 media supplemented with penicillin (500 U/ml) and streptomycin (500 μg/ml). Adult T. muris worms were then removed using fine forceps and maintained in RPMI-1640/penicillin/streptomycin media at approximately 37 °C and studied on the same day. Individual live worms were placed into 96 well plates containing 75 μl of RPMI-1640/penicillin/streptomycin medium plus 1% v/v final concentration of DMSO or compound dissolved in DMSO. Plates were incubated at 37 °C, 5% v/v CO2, and motility was analysed after 24 h. Whole plate 200 frame movies were recorded at 10 frames per second (20 s total).
2.5. Ovine parasitic nematode isolation
Six month-old, male Texel cross lambs that were raised under helminth-free conditions were infected per os either with 15,000 T. circumcincta (isolate MTci2) larvae (L3) or 5000 H. contortus (isolate MHco3) L3. Once a patent infection was detected by the observation of nematodes eggs in faeces (around 21 days post-infection), the lambs were fitted with a collection harness and bag to enable faecal collection. Pelleted faeces were collected from each bag 24 h later and placed in a covered seed tray, which was incubated at room temperature (>15 °C) for 10 days before larval recovery using the modified Baermann technique (Manual of Veterinary Parasitological Laboratory Techniques, 1986). The next day L3 were recovered in ∼250 ml H2O, number estimation performed then the larvae were stored in 100 ml tap water in 75 cm2 surface area vented cap, suspension culture flasks (Sarstedt Ltd UK) at ∼ 5 °C for MTci2 and 8 °C for MHco3, respectively.
2.6. T. circumcincta assay
Ensheathed L3 T. circumcincta were used in this assay, which are the infective form of the parasite that is ingested by the grazing host. Approximately 30 worms were added to wells containing compound + DMSO or DMSO alone (final concentration 1% v/v). Worms were incubated for 2 h in the dark at 25 °C. Movement was stimulated by illuminating the plate with bright white light for 3 min (Zeiss HAL100), before acquiring movies on the INVAPP/Paragon system (200 frames, 10 frames per second).
2.7. H. contortus assay
H. contortus exsheathed L3 (xL3) were prepared by treatment with sodium hypochlorite. A 1 ml solution containing around 1000 H. contortus L3 was placed in a 35 mm petri dish. 20 μl sodium hypochlorite solution (Fisher, S/5040/PC17) was added and incubated at room temperature for 4 min. Exsheathment was monitored using a dissecting microscope. The worms were filtered using a 10 μm cell strainer (pluriSelect), rinsed with 10 × 1 ml S-basal solution, and eluted with 1 ml S-basal solution. Around 30 xL3 worms (in S-basal solution) were added to wells containing compound + DMSO or DMSO alone (final DMSO concentration 1% v/v). Worms were incubated for 2 h in the dark at 25 °C. Movement was stimulated by illuminating the plate with bright white light for 3 min (Zeiss HAL100), before acquiring movies on the INVAPP/Paragon system (200 frames, 30 frames per second). The INVAPP/Paragon movementIndexThreshold parameter was set to 2 for analysis of H. contortus xL3, due to a lower prior expectation of worm movement in the movie for this nematode.
2.8. Pathogen Box screening
The Pathogen Box library was obtained from the Medicines for Malaria Venture as 10 mM solutions in DMSO, and then diluted in DMSO to 1 mM. It was then screened in the C. elegans growth assay as described (final concentration 10 μM, n = 5, 1% v/v final DMSO). Solid material for confirmatory screening of actives was obtained from Sigma-Aldrich (tolfenpyrad) and Santa Cruz Biotechnology (auranofin). Solid samples of MMV007920, MMV020152, MMV652003 and MMV688372 were obtained from the Medicines for Malaria Venture.
3. Results
3.1. INVAPP/Paragon: a high-throughput assay for quantifying nematode motility and growth
We wanted to develop an assay for screening small molecules for their effect on the motility and growth of diverse parasites. A high-throughput and automated system was particularly desirable, given the large size of available small molecule libraries. To this end, we designed the INVAPP/Paragon system. A schematic of the INVAPP hardware is shown in Fig. 1A. This allows recording of movies of entire microplates at high frame rate, reducing the per plate acquisition time to 10–30 s. Tens of thousands of compounds or conditions can therefore be readily screened per day.
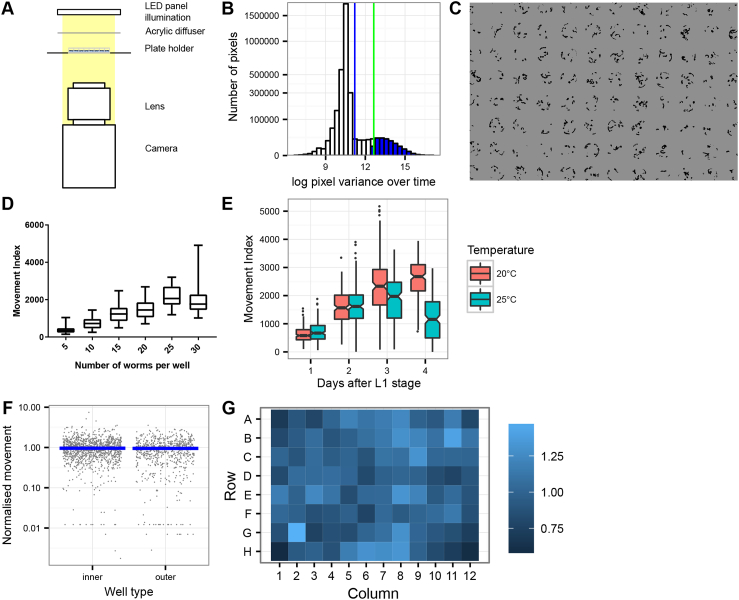
Fig. 1.
The INVAPP /Paragon movement index algorithm is a fully-automated high-throughput system able to determine motility and growth rate. (A) Schematic of the INVAPP setup (B) Principle of the algorithm: thresholding of moving pixels by statistical analysis of variance of each pixel through time. Histogram shows the distribution of pixel variance over time. Data was from obtained from a movie of all wells in a single 96-well plate containing wild-type C. elegans. The distribution is obtained by counting the number of pixels that fall into each bin of log pixel variance over time. Blue vertical line indicates mean pixel variance. The green vertical line indicates mean plus standard deviation of pixel variance; the blue shaded portion of the histogram indicates pixels that exceed this threshold so are deemed to be ‘motile’. (C) Image of 96-well plate containing C. elegans adults processed by the INVAPP/Paragon movement index system. Dark pixels are those categorized as moving by the algorithm. (D) Increasing the number of C. elegans worms per well leads to increase in reported movement index. Boxplot bars indicate 95% confidence interval. Dataset contains 16 wells per group from a single 96-well plate. (E) Movement index algorithm is able to quantify C. elegans growth in 96-well plates. Movement index increases with growth. Synchronised L1 population refed on day 0. Decrease in movement index in 25 °C group on Day 4 reflects completion of the C. elegans lifecycle and exhaustion of the bacterial food source. Boxplot notches indicate 95% confidence interval, n = 192 wells of worms for each of the 20 °C and 25 °C groups. (F) Absence of edge effects in this assay – analysis of a 1920-well C. elegans growth dataset shows no difference of the normalised movement score for 96-well plate outer edge wells (the wells found in columns 1 and 12 or rows A and H) compared to the score for inner wells (the other wells in the plate). Dataset is from a total of 20 96-well plate movies obtained from C. elegans grown at 20 °C, 25 °C and 26 °C to show that this assay is robust over a range of experimentally useful temperatures. Movement index for each well is normalised by dividing by the mean movement index for all wells of that plate. The blue bar indicates median. (G) No edge effects or other inhomogeneity across the plate – heat map shows average normalised movement index for each well location. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
We took a statistical approach to quantifying motility. The variance through time for each pixel in the plate was calculated and the distribution of the variances examined. Pixels whose variance is greater than a threshold of the mean plus typically one standard deviation are determined to be “motile” (Fig. 1B). For organisms that are particularly small or have limited motility, such as H. contortus L3s, this threshold was increased to the mean plus two standard deviations, reflecting a lower prior expectation of worm movement in the movie. A similar approach was recently reported for the determination of H. contortus motility (Preston et al., 2015). An example of this thresholding model is shown in Fig. 1C, which shows analysis of a 96-well plate containing uniformly-treated, adult, wild-type C. elegans. Dark pixels are those that have been determined to be motile. Once the motility threshold has been applied to the data, ‘motile’ pixels are assigned by well to their plate location and counted. All analysis is fully automated via a set of MATLAB scripts, available at https://github.com/fpartridge/invapp-paragon (Partridge et al., 2017a).
This approach was able to quantify motility. To illustrate this, we analysed plates containing a variable number of synchronised adult C. elegans worms. As expected, quantified movement increased with the number of worms per well, reflecting a larger number of ‘motile’ pixels in the recording (Fig. 1D).
The system was also able to quantify nematode growth. To test this, we synchronised C. elegans at the L1 stage, before refeeding them in plates at two temperatures commonly used in C. elegans culture (20 °C and 25 °C). Plates were then analysed using INVAPP/Paragon every 24 h. The results are shown in Fig. 1E. The quantified movement index increases as worms develop from L1 to adult stage. The drop in motility in the 25 °C group on day 5 reflects growth of L1 progeny leading to exhaustion of the bacterial food source and starvation.
When establishing a high-throughput assay it is important to consider the issue of edge effects (Malo et al., 2006). Systematic biases across the plate are particularly common around edges. Typical causes are evaporation, which is often worse at the edges, or temperature inhomogeneity. In our assay, given that it involves imaging of whole plates, we also wanted to exclude the possibility of systemic bias caused by optical distortion. To address these concerns, we analysed a 1920-well C. elegans growth dataset. This was chosen because the long four-day incubation time gave the maximum possibility of confounding evaporation differences. We classified wells on the outer rows and columns of the plate as being outer wells, and compared their quantified motility to the inner wells (Fig. 1F). There was no significant difference between these groups (Mann-Whitney-Wilcoxon test, P = 0.77), and therefore no evidence of problematic edge effects in this assay. To further exclude the possibility of assay inhomogeneity across the plate, we calculated a heat map showing average normalised motility for each well (Fig. 1G). Again, this showed no evidence of systemic bias by plate position.
3.2. Validation of the INVAPP/Paragon assay using existing commercial anthelmintic standards
Having set up this high-throughput motility and growth assay, we wanted to validate its utility by examining the effects of a panel of known anthelmintics. We selected nine anthelmintics with a variety of reported mechanisms of action. Piperazine is a GABA agonist that acts at the neuromuscular junction (Martin, 1985). Diethylcarbamazine has been proposed to have a similar mechanism, although other mechanisms including targeting host arachidonic acid metabolism are also thought to be important (Maizels and Denham, 1992). Levamisole, oxantel and pyrantel are nicotinic acetylcholine receptor agonists that induce spastic paralysis (Martin et al., 1997). Mebendazole is an inhibitor of beta-tubulin polymerisation (Driscoll et al., 1989). Ivermectin is a positive allosteric modulator of glutamate-gated chloride channels although other targets have also been suggested (Cully et al., 1994). Trichlorfon is a member of the organophosphate group of acetylcholine esterase inhibitors. Praziquantel is thought to act by disrupting calcium ion homeostasis but its target is unclear (Caffrey, 2007).
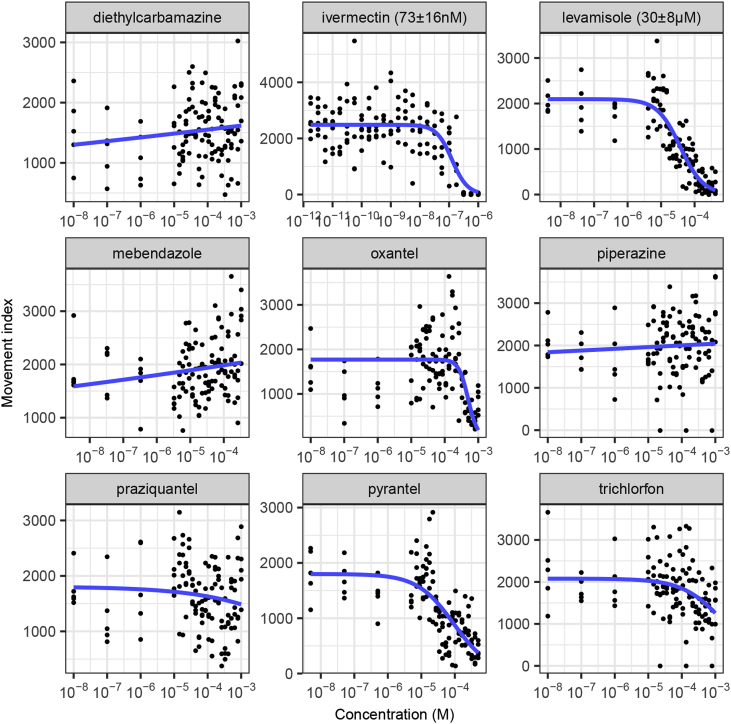
We first measured concentration-response curves for this panel of anthelmintics in an acute treatment (3 h) adult C. elegans assay. The results are shown in Fig. 2. As expected, the major ion channel-targeting drugs ivermectin, levamisole, oxantel and pyrantel were active in this assay, reflecting their direct effects on worm motility.
Fig. 2.
Determination of the effect of acute treatment (3 h) with important anthelmintics on C. elegans adult motility using the INVAPP/Paragon movement index algorithm. Blue line fitted using the 3-parameter log-logistic model. EC50 values are shown in parentheses, with standard error for this estimate calculated using drc (Ritz and Streibig, 2005). Each dot indicates movement score for one well. n = 5 wells for each concentration, with each replicate concentration on different plates. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
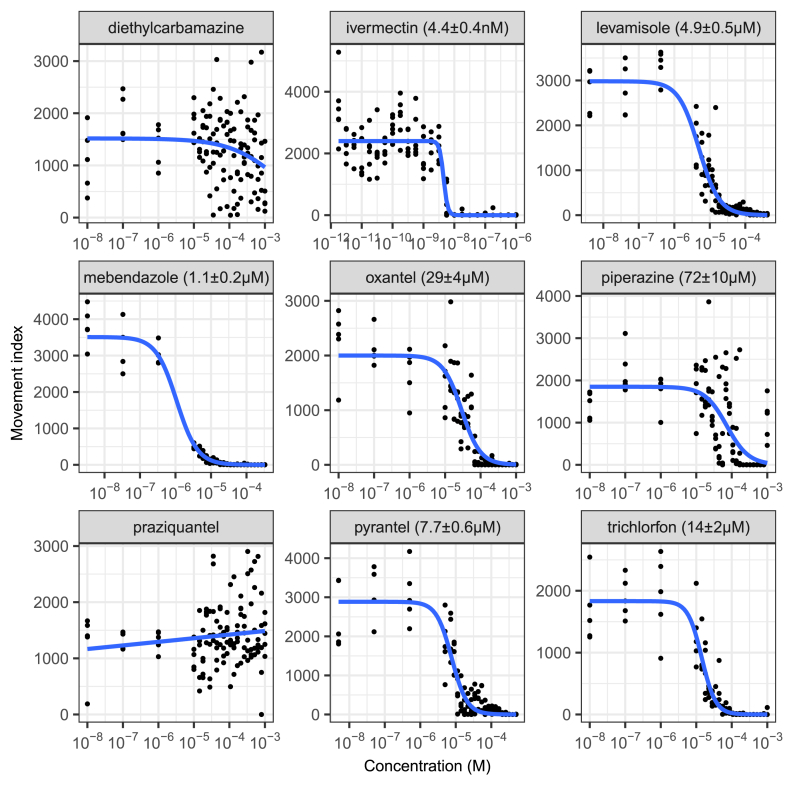
We then measured concentration-response curves for this panel of anthelmintics in a chronic treatment C. elegans growth assay (5 days, 20 °C, from L1 stage). The results are shown in Fig. 3. Activity was again found with ivermectin, levamisole, oxantel and pyrantel and, additionally, with mebendazole, piperazine and trichlorfon. This reflects that some anthelmintic modes of action may not be measured in purely acute motility assays (at least for shorter-lived nematodes such as C. elegans), supporting the use of assays that involve growth or development. Indeed, an action of macrocyclic lactones distinct from that on motility has been proposed (Wolstenholme et al., 2016). Diethylcarbamazine and praziquantel were not active in these assays as expected. This reflects previously reported low in vitro activity of diethylcarbamazine and its proposed mechanism of acting on host arachidonic acid metabolism (Maizels and Denham, 1992). Praziquantel, used primarily to treat flukes and tapeworms, is known to have limited efficacy against nematodes (Holden-Dye, 2007). Successfully demonstrating the ability of the INVAPP/Paragon system to determine the effects of known anthelmintics increased our confidence in this approach.
Fig. 3.
Determination of the effect of important anthelmintics on C. elegans growth using the INVAPP/Paragon movement index algorithm. Chronic treatment for 5 days from L1 stage. Blue line fitted using the 3-parameter log-logistic model. EC50 values are shown in parentheses, with standard error for this estimate calculated using drc (Ritz and Streibig, 2005). Each dot indicates movement score for one well. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. Adaptation of the INVAPP/Paragon assay to parasitic nematodes
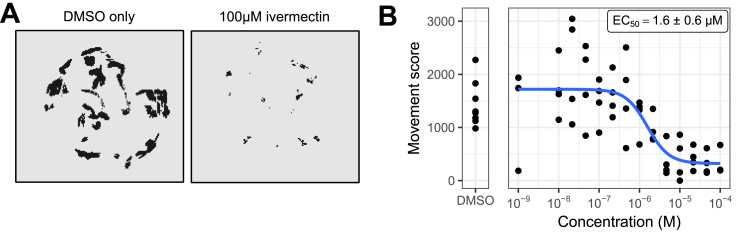
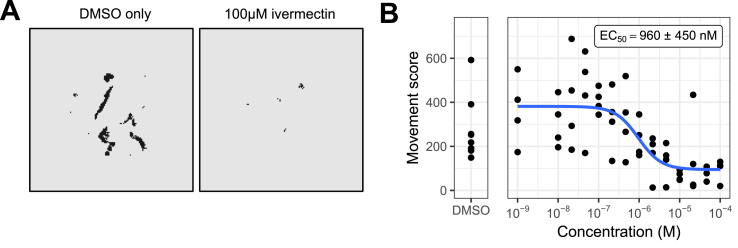
C. elegans is a useful model because of the ease of laboratory culture and access to powerful genetic tools. However, it is also valuable to screen parasitic nematodes directly. T. circumcincta is a globally important parasitic nematode that infects small ruminants. We tested the ability of the INVAPP/Paragon system to quantify the activity of the anthelmintic ivermectin on ensheathed T. circumcincta L3. Worms were incubated at 25 °C with the compounds for 2 h in the dark, after which movement was induced by bright light and movies recorded on the INVAPP system. Fig. 4A shows an example of the INVAPP/Paragon motility thresholding of single wells treated with DMSO or DMSO plus 100 μM ivermectin, showing much reduced, but not abolished movement, of these larvae in the assay. We obtained a concentration-response curve for ivermectin (Fig. 4B), demonstrating the ability of the system to quantify motility of this parasite.
Fig. 4.
Utilising the INVAPP/Paragon system with ensheathed L3 T. circumcincta. (A) INVAPP/Paragon motility thresholding of single wells treated with DMSO or a DMSO solution of ivermectin (assay concentration 100 μM). Black pixels show those scored as being ‘motile’ by the software. (B) Concentration-response curve for treatment with the anthelmintic ivermectin, n = 4. Each replicate was carried out in different experimental sessions, using one batch of L3 T. circumcincta. Curve fitted using the four-parameter log-logistic function. Error range on the EC50 estimate indicates standard error (delta method).
H. contortus, the barber's pole worm, is another economically important gastrointestinal parasite of small ruminants. We tested the ability of the INVAPP/Paragon system to quantify the activity of ivermectin on exsheathed L3 (xL3) H. contortus. Worms were incubated at 25 °C with the compounds for 2 h in the dark, after which movement was induced by bright light and movies recorded on the INVAPP system. An example of the INVAPP/Paragon motility thresholding of single wells treated with DMSO or a DMSO solution of ivermectin (assay concentration 100 μM) is shown in Fig. 5A. Movement of ivermectin-treated xL3s in the assay was considerably reduced. A concentration-response curve for ivermectin is shown in Fig. 5B, illustrating the ability of the system to quantify motility of this parasite. A caveat to these results, regarding their potential translation to in vivo potency, is that under physiological conditions H. contortus becomes exsheathed in the stomach at 38–41 °C. Therefore activity in this assay at 25 °C may not fully reflect the in vivo action of ivermectin.
Fig. 5.
Utilising the INVAPP/Paragon system with exsheathed L3 H. contortus. (A) INVAPP/Paragon motility thresholding of single wells treated with DMSO or a DMSO solution of ivermectin (assay concentration 100 μM). Black pixels show those scored as being ‘motile’ by the software. (B) Concentration-response curve for treatment with the anthelmintic ivermectin, n = 4. Each replicate drug treatment was carried out in different experimental sessions, each time freshly exsheathing the worms by bleach treatment, using one batch of ensheathed L3 H. contortus. Curve fitted using the four-parameter log-logistic function. Error range on the EC50 estimate indicates standard error (delta method).
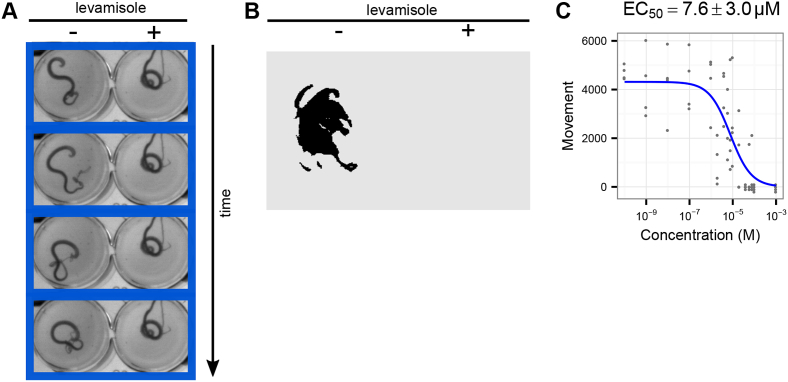
Trichuris muris, which infects mice, is a widely used laboratory model for investigating trichuriasis and has been used as a system for ex vivo and in vivo testing of anthelmintic candidates for activity against whipworm (Hurst et al., 2014, Tritten et al., 2011, Wimmersberger et al., 2013). We tested the ability of the INVAPP/Paragon system to quantify the activity of the anthelmintic levamisole on adult T. muris. Worms were incubated at 37 °C with the compounds for 24 h in the dark, after which movement was recorded on the INVAPP system. Fig. 6A shows control and levamisole treated wells. For illustration, selected frames of the movie are shown (3 second intervals). The readout of ‘motile’ pixels as determined by the INVAPP/Paragon algorithm is shown in Fig. 6B, showing the quantification of the loss of motility of the levamisole-treated worm. A concentration-response curve for levamisole was measured (Fig. 6C), which illustrates the ability of the system to quantify motility of this parasite and thus measure anthelmintic activity.
Fig. 6.
Utilising the INVAPP/Paragon system with adult T. muris. (A) Example of section of movie, showing control and levamisole-treated wells. Selected movie frames are shown at 3-s intervals (B) Pixels for these wells that are considered to be motile as determined by the algorithm are shown in red. (C) Concentration-response curves for treatment with the anthelmintic levamisole, n = 4 wells per concentration, each replicate well on different plates using independent batches of T. muris from different mice, over 2 experimental sessions. Curve fitted using the three-parameter log-logistic function. Error range on the EC50 estimate indicates standard error (delta method). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.4. Screening the Pathogen Box for compounds that affect C. elegans growth
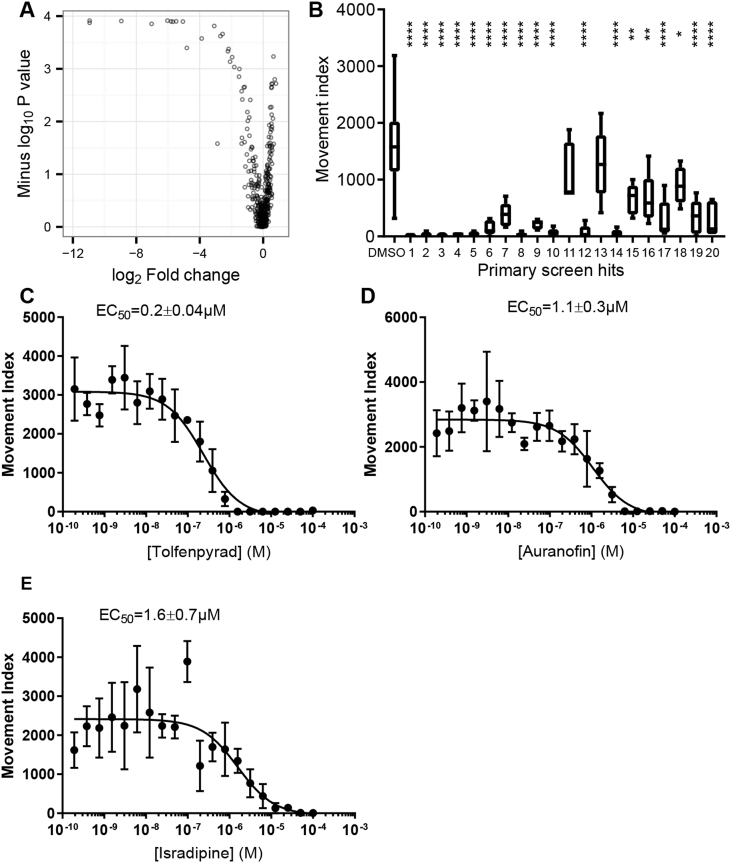
We then applied the INVAPP/Paragon assay system to the identification of novel anthelmintic small molecules. We used the Pathogen Box, a collection of 400 diverse drug-like molecules that are known to be active against various neglected disease pathogens (particularly, tuberculosis (or Mycobacterium spp.), malaria and kinetoplastid protozoa). This library is distributed as an open-science project by the Medicines for Malaria Venture. A screen of this library for compounds affecting motility of exsheathed L3 of H. contortus has been published recently (Preston et al., 2016b), which identified the insecticide, tolfenpyrad, as active against the larvae. To complement this approach, and with the aim of identifying compounds blocking growth of nematodes as opposed to solely immobilising them, we screened the library in a blinded fashion (5 separate library plate sets in total, using worms from 2 separately prepared batches of C. elegans L1 larvae, experiment conducted on 2 different occasions, final compound concentration 10 μM) using the INVAPP/Paragon C. elegans growth assay. For each compound, motility change and significance (Mann-Whitney-Wilcoxon test) were calculated relative to DMSO-only control wells. A volcano plot showing the results of this screen is shown in Fig. 7A. Compounds that reproducibly reduced growth are found towards the top left of this plot. To confirm identity of the hit molecules, we retested the top 20 putative hit compounds in a secondary screen with the same library material (n = 5, concentration 10 μM) in the same INVAPP/Paragon C. elegans growth assay (Fig. 7B). A total of 18 out of 20 compounds were active (P < 0.05, Dunnett's multiple comparison test).
Fig. 7.
Screening the Pathogen Box in the C. elegans growth screen. (A) Volcano plot showing the results of the primary screen (n = 5 replicates per compound, concentration = 10 μM, each replicate was on different plates, over two experimental sessions). Each point represents one compound. Effective size is shown on the x axis, as log2-fold change (ratio of the median movement for the repeats of the compound to the median movement of DMSO-only wells). Statistical significance is shown on the y axis as the -log10 P value in the Mann-Whitney-Wilcoxon test. A location at the top left of this plot indicates anthelmintic activity. (B) Secondary rescreen of hit compounds, using library material, in order of their activity in the primary screen. Statistical significance compared to DMSO-only control calculated by Dunnett's multiple comparison test (n = 5, * indicates P < 0.05, ** indicates P < 0.005, **** indicates P < 0.0005). (C,D,E) Concentration-response curves showing the activity of known anthelmintics – (C) tolfenpyrad, (D) auranofin, (E) isradipine – that were found in the Pathogen box screen retested using solid material (supplied by the Medicines for Malaria Venture) in the C. elegans growth assay. A concentration-response curve for mebendazole in the assay was presented in Fig. 3. Error bars indicate standard deviation, n = 4 wells per concentration per compound. Curve fitting was undertaken using three parameter log logistic model (Graphpad Prism).
The combined assay results from the primary and secondary screen are found in Supplementary File 1 and have been recorded in the PubChem database with Assay ID 1259336.
3.5. Identification of known anthelmintic compounds by screening the Pathogen Box in the C. elegans growth assay
We first considered known anthelmintic compounds that we found to be active in this screen (Table 1). Mebendazole, an anthelmintic from the benzimidazole group, acts by inhibiting microtubule synthesis (Laclette et al., 1980). Tolfenpyrad is a broad-spectrum acaricide and insecticide that acts as an inhibitor of complex I of the electron transport chain (Yu, 2014). It has been recently reported to reduce motility of H. contortus exsheathed L3 and to block L3 to L4 development of this parasite in vitro (Preston et al., 2016b). We confirmed activity of this compound using solid material. As shown in Fig. 7C, the EC50 was 200 ± 40 nM. Independently identifying these known anthelmintic compounds using a blinded screening approach further validates the INVAPP/Paragon system as a robust high throughput screening approach.
Table 1.
Named compounds that were active in the C. elegans growth screen. Log2-fold change in growth estimate compared to DMSO-only controls. Hit ID is as in Fig. 7B. EC50 confidence interval is the standard error. The EC50 estimate for mebendazole is from Fig. 3. Primary and secondary screens used solutions supplied as part of the Pathogen Box Library. EC50 estimates were determined using solid material.
| Compound | MMV ID | PubChem CID | Hit ID | Log2 fold change in growth |
EC50 (μM) ± standard error |
|
|---|---|---|---|---|---|---|
| (1° screen) | (2° screen) | (solid material) | ||||
| Tolfenpyrad | MMV688934 | 10,110,536 | 1 | −10.9 | −10.6 | 0.2 ± 0.04 |
| Auranofin | MMV688978 | 24,199,313 | 2 | −10.9 | −7.2 | 1.1 ± 0.3 |
| Mebendazole | MMV003152 | 4030 | 5 | −6.0 | −5.7 | 1.1 ± 0.2 |
| Isradipine | MMV001493 | 158,617 | 16 | −2.2 | −1.4 | 1.6 ± 0.7 |
| Tavaborole | n/a | 11,499,245 | n/a | n/a | 8.6 ± 1.9 | |
Auranofin is a gold(I) compound originally developed for the treatment of rheumatoid arthritis. It has received attention for repurposing as an anti-cancer agent, with a number of clinical trials under way. It has been shown that auranofin has in vitro and in vivo activity in several models of parasitic diseases, including schistosomiasis (Kuntz et al., 2007), amoebiasis (Debnath et al., 2012), leishmaniasis (Sharlow et al., 2014) and onchocerciasis (Bulman et al., 2015). A phase IIa trial of auranofin for gastrointestinal protozoal infection is ongoing. We confirmed activity of this compound in the C. elegans growth assay using solid material. As shown in Fig. 7D, the EC50 of this compound was 1.1 ± 0.3 μM.
Isradipine is an antihypertensive drug that belongs to the dihydropyridine family of L-type calcium channel blockers. A structurally related dihydropyridine, nemadipine-A, has been shown to cause growth and egg laying defects in C. elegans by antagonising the L-type calcium channel α1-subunit EGL-19 (Kwok et al., 2006). We confirmed activity of this compound in the C. elegans growth assay using solid material. As shown in Fig. 7E, the EC50 of this compound was 1.6 ± 0.7 μM.
3.6. Assessment of screen performance
The pathogen box screen was screened blindly without the addition of positive control compounds. However we wanted to assess the performance of the screen. We therefore treated wells containing mebendazole as positive controls for comparison against negative control DMSO-only wells. We then calculated the strictly standardized mean difference (SSMD) parameter, which is a principled statistical assessment of high-throughput screen performance (Birmingham et al., 2009, Zhang et al., 2007, Zhang, 2011). For the positive control mebendazole we calculated that SSMD β = −2.54. This indicates that screen performance was adequate to good (for a strong control −3 < β < −2 is considered good).
3.7. Novel anthelmintics that block C. elegans growth
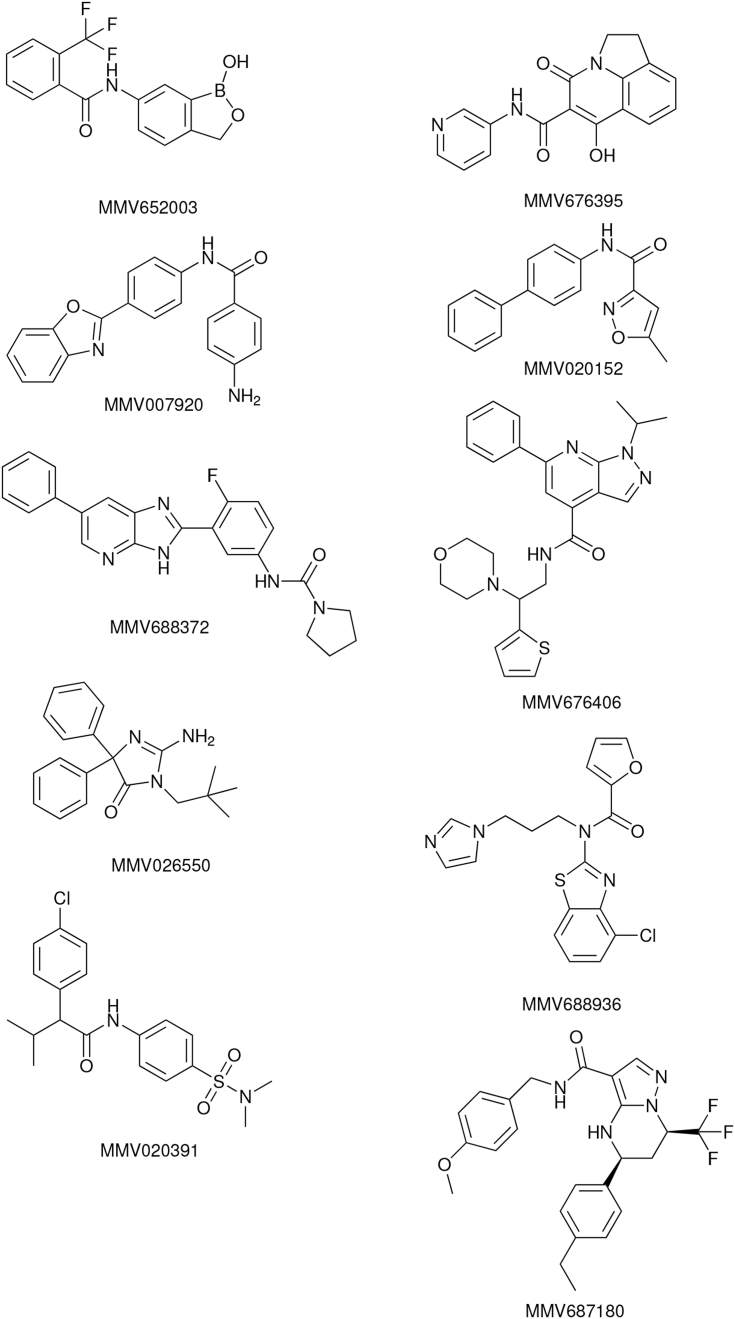
Fourteen compounds without previously-described anthelmintic activity were identified in the Pathogen Box screen (Table 2). The structures of the most active compounds are shown in Fig. 8. We examined four of these compounds more closely. First, we determined activity, using solid material, in the C. elegans growth assay (Fig. 9). The EC50 values for the confirmatory assay are shown in Table 2. These results have been recorded in the PubChem database with Assay ID 1259335.
Table 2.
Compounds with previously unreported anthelmintic activity that were active in the C. elegans growth screen. MMV ID is the compound identifier for the Medicines for Malaria Venture. PubChem CID is the compound identifier for the PubChem database. Hit ID is as in Fig. 7B. Log2 fold change in the growth estimate in the INVAPP/Paragon assay compared to DMSO-only controls. Primary and secondary screens used solutions supplied as part of the Pathogen Box Library. EC50 estimates were determined using solid material, supplied by the Medicines for Malaria Venture.
| MMV ID | PubChem CID | Hit ID | Log2 fold change in growth |
EC50 (μM) |
|
|---|---|---|---|---|---|
| (1° screen) | (2° screen) | (solid material) | |||
| MMV652003 | 46,196,110 | 3 | −8.9 | −7.6 | 4.3 ± 2.5 |
| MMV007920 | 721,133 | 4 | −7.0 | −5.4 | 6.2 ± 2.4 |
| MMV688372 | 72,710,598 | 6 | −5.8 | −4.0 | 3.3 ± 1.7 |
| MMV675994 | 44,222,802 | 7 | −5.7 | −2.0 | n.d. |
| MMV026550 | 44,530,521 | 8 | −5.5 | −5.9 | n.d. |
| MMV020391 | 7,918,647 | 9 | −5.0 | −2.7 | n.d. |
| MMV676395 | 54,678,166 | 10 | −4.8 | −4.8 | n.d. |
| MMV020152 | 8,880,740 | 12 | −3.1 | −6.9 | 2.5 ± 1.0 |
| MMV676406 | 30,238,526 | 14 | −2.7 | −8.0 | n.d. |
| MMV688417 | 58,346,931 | 15 | −2.6 | −1.1 | n.d. |
| MMV688936 | 18,589,797 | 17 | −2.2 | −3.7 | n.d. |
| MMV1028806 | 16,387,386 | 18 | −2.1 | −0.8 | n.d. |
| MMV688888 | 5,179,236 | 19 | −2.0 | −2.1 | n.d. |
| MMV687180 | 41,058,173 | 20 | −1.8 | −3.6 | n.d. |
Fig. 8.
Structures of the most active compounds, previously undescribed as anthelmintics, which were found to have activity in the C. elegans growth screen. Compound numbers from Medicines for Malaria Venture Pathogen Box.
Fig. 9.
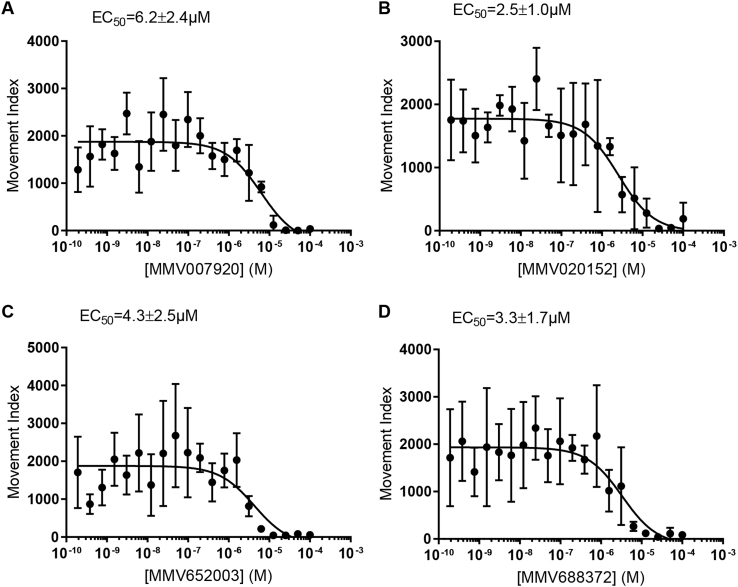
Concentration-response relationships for selected hit compounds in the C. elegans growth screen, using solid material supplied by Medicines for Malaria Venture. (A) MMV007920 (B) MMV020152 (C) MMV652003 (D) MMV688372. N = 4 wells per concentration per compound. Curve fitting was undertaken using three parameter log logistic model (Graphpad Prism).
MMV007920 is a benzoxazole-containing compound previously identified in a screen for agents that inhibit Plasmodium falciparum proliferation. The target of this compound is not known but it has been suggested that some benzoxazole compounds act on beta-tubulin (Satyendra et al., 2011). MMV020152 is an isoxazole-containing compound previously identified in a screen for compounds that inhibit P. falciparum growth. A number of other compounds also containing isoxazole motifs have been shown to have insecticidal activity (da Silva-Alves et al., 2013). MMV688372 is an imidazopyridine-containing compound that has been previously shown to have in vivo anti-trypanosomal activity (Tatipaka et al., 2014).
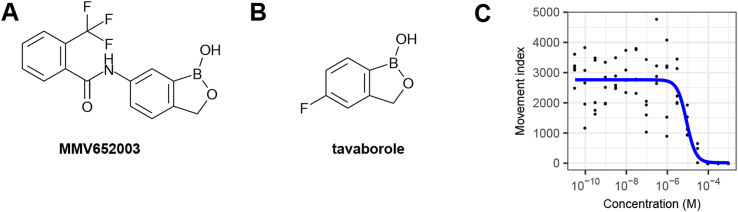
MMV652003 is a benzoxaborole-containing compound that has also been given the identifier AN3520 in the literature. This compound has potent activity against Trypanosoma sp., both in vitro, and in murine models of human African trypanosomiasis (Nare et al., 2010). In this context this compound has been iteratively improved leading to the identification of the close relative SCYX-7158 (Jacobs et al., 2011), which is currently in clinical trials. The anti-trypanosomal target of this benzoxaborole class is not known (Jones et al., 2015). A simpler benzoxaborole compound, tavaborole (Fig. 10B), has been approved as an anti-fungal (Elewski et al., 2015). This acts by inhibiting cytoplasmic leucyl-tRNA synthetase by forming an adduct with tRNALeu in the enzyme editing site (Rock et al., 2007). Benzoxaborole anthelmintic agents are being developed by Anacor/Eli Lilly (Akama et al., 2014, Zhang et al., 2011). Benzoxaborole compounds also show promise for other infectious diseases, including malaria, cryptosporidiosis, toxoplasmosis and tuberculosis, in each case acting via inhibition of leucyl-tRNA synthetase (Palencia et al., 2016a, Palencia et al., 2016b, Sonoiki et al., 2016).
Fig. 10.
Benzoxaborole compounds as anthelmintics. Structures of (A) the screen hit MMV652003 and (B) the related benzoxaborole tavaborole, which is approved as an anti-fungal. (C) Concentration-response curve for tavaborole in the C. elegans growth assay. N = 5 wells per concentration. Blue line fitted using three parameter log logistic model with drc (Ritz and Streibig, 2005). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Given the effect of MMV652003 and the similarity of this compound to the already approved drug tavaborole, we determined whether tavaborole was effective in the C. elegans growth assay. Tavaborole showed concentration-dependent growth inhibition (Fig. 10C) with an EC50 of 8.6 ± 1.9 μM (Table 1). This is similar to that of the benzoxaborole screen hit, MMV652003, which has an EC50 of 4.3 ± 2.5 μM (Table 1). These results support the development of a benzoxaborole anti-nematode agent.
4. Discussion
There is an urgent need for new anthelmintics. Despite encouraging progress with MDA programs, current strategies and therapies will not achieve eradication of, for example, T. trichiura (Keiser and Utzinger, 2008, Turner et al., 2016). Furthermore, MDA, particularly with drugs that do not fully clear infection, may lead to drug resistance (Vercruysse et al., 2011). The experience from veterinary parasitology is that resistance to new anthelmintics can develop relatively rapidly after registration, causing major economic impacts and risks to global food security (Sales and Love, 2016, Scott et al., 2013).
In this manuscript, we present the INVAPP/Paragon system which, based on imaging of whole microplates and thresholding pixel variance to determine motion, is able to quantify growth and/or motility of parasitic nematodes and the free-living nematode, C. elegans. A strength of this system is its high-throughput capability, typically imaging a whole plate for 5–20 s is sufficient to reliably quantify motion in all 96 wells. We demonstrated this effect by determining efficacy of a panel of anthelmintics in both acute motility and growth assays in C. elegans and of known anthelmintics in H. contortus, T. circumcincta, and T. muris assays. We further demonstrated utility of the system in a screen of small molecules for compounds that block or limit C. elegans growth. Current anthelmintic screens generally focus on motility reduction, as growth of parasitic nematodes can be difficult to model in vitro, with larvae failing to moult through their larval stages outside of the host. However anthelmintic activity in vivo can be much broader than inhibition of motility and thus screening compounds for their ability to inhibit C. elegans growth, rather than motility, represents a useful strategy to identify compounds which can subsequently be tested for growth inhibition activity in vivo. We used the Pathogen Box, a library of a collection of 400 diverse drug-like molecules known to be active against various neglected diseases, distributed as an open-science project by the Medicines for Malaria Venture.
Identifying the compounds with known anthelmintic or anti-parasitic activity mebendazole and tolfenpyrad (Preston et al., 2016b) using independent blinded screening approach serves as an important validation and supports the robustness of the screening platform. Repurposing of existing drugs for new indications is an established approach in drug discovery (Zheng et al., 2017) and is particularly valuable for neglected tropical diseases as it may reduce research and development costs and speed progress to clinical trials (Pollastri and Campbell, 2011). Auranofin has recently been shown to have activity against filarial nematode infection (Bulman et al., 2015). Our identification of auranofin as a compound that blocks C. elegans growth lends support to test repurposing of this compound for nematode infections. Isradipine, a safe and well-tolerated L-type calcium channel blocker, was also active in the screen. Assaying the activity of isradipine in in vivo models of parasitic infection is a priority and could lead to repurposing trials.
We also identified 14 compounds with previously undescribed anthelmintic activity in the C. elegans growth assay, belonging to a variety of chemical classes. These include benzoxazole and isoxazole compounds previously shown to have activity against P. falciparum (da Silva-Alves et al., 2013, Satyendra et al., 2011), and an imidazopyridine-containing compound previously shown to have in vivo anti-trypanosomal activity (Tatipaka et al., 2014). Another notable active compound was the benzoxaborole, MMV652003. Since the identification and successful progression into the clinic of the anti-fungal tavaborole, a number of benzoxaborole compounds have been reported to show potential for trypanosomiasis, malaria, cryptosporidiosis, toxoplasmosis and tuberculosis (Liu et al., 2014). These results support the idea that some drug chemotypes can have activity against a diversity of infectious agents. Given the costs of drug development and the limited resources available for the discovery of new drugs targeting neglected tropical diseases, it seems possible that an open source medicinal chemistry program could catalyse discovery for many different indications (Van Voorhis et al., 2016). Whilst preparing this manuscript we have applied the INVAPP/Paragon system to library-scale screening measuring motility of ex vivo T. muris adults, which led to identification of a new class of anthelmintics, the dihydrobenzoxazepinones (Partridge et al., 2017b). Taken together these results demonstrate the potential for anthelmintic discovery using this system, with new compounds with anthelmintic activity identified by screens using both the free-living nematode C. elegans as a parasite model, and the mouse whipworm T. muris.
The anthelmintic compounds we have identified in this study were active in a growth assay using C. elegans, which is typically considered a free-living nematode, although it undoubtedly has utility in anthelmintic drug discovery (Burns et al., 2015). A critical step to support the development of these compounds will be to determine their activity on important human and animal parasitic nematodes. These compounds were inactive in a recently reported screen that determined their effects on motility of H. contortus xL3 (Preston et al., 2016b). It is therefore possible that their activity requires active growth of the target nematode, perhaps reflecting a target distinct from the neuromuscular system. A recent set of criteria for hit-to-lead progression in neglected infective diseases provides a useful guide to the steps that need to be taken to progress these early-stage hit compounds (Nwaka et al., 2009). It will be particularly important to demonstrate activity against a range of nematode parasites and differential sensitivity between parasite and host to support the development of a new anthelmintic.
We focused our application of the INVAPP/Paragon system to investigating parasitic diseases. However, given its ability to determine growth and motility of C. elegans quickly and robustly, it could also be applied to the study of other human diseases modelled in C. elegans.
In conclusion, we have developed a high-throughput system for measuring the growth and/or motility of parasitic and free-living nematodes. Quantification of the activity of known anthelmintics and identification of novel chemotypes with anthelmintic activity was demonstrated, validating our approach. The system is well suited to library-scale screening of chemicals with many human and animal health applications.
Acknowledgements
The Pathogen Box was designed and supplied by the Medicines for Malaria Venture (www.mmv.org). Some C. elegans strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). F.A.P. is supported by Medical Research Council (www.mrc.ac.uk) grant MR/N024842/1 to D.A.L. and D.B.S. and prior to that was supported by CeBioscience Ltd and a University College London Fellowship. A.E.B. is supported by a studentship from the Rosetrees Trust. R.F. is supported by Medical Research Council (www.mrc.ac.uk) grant MR/N022661/1 to K.J.E. D.A.L. is supported by the NIHR UCLH Biomedical Research Centre. J.B.M. and A.A.D. are funded by the Scottish Government through the Rural and Environmental Science and Analytical Services (RESAS) Division.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ijpddr.2017.11.004.
Contributor Information
David A. Lomas, Email: d.lomas@ucl.ac.uk.
David B. Sattelle, Email: d.sattelle@ucl.ac.uk.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Results of primary and secondary screens of the Pathogen Box library using the C. elegans growth assay.
References
- Akama T., Jarnagin K., Plattner J.J., Pulley S.R., White W.H., Zhang Y.-K., Zhou Y. 2014. 1-hydroxy-benzooxaboroles as Antiparasitic Agents. WO2014149793 A1. [Google Scholar]
- Al-Ali H. The evolution of drug discovery: from phenotypes to targets, and back. MedChemComm. 2016;7:788–798. https://doi.org/10.1039/C6MD00129G [Google Scholar]
- Birmingham A., Selfors L.M., Forster T., Wrobel D., Kennedy C.J., Shanks E., Santoyo-Lopez J., Dunican D.J., Long A., Kelleher D., Smith Q., Beijersbergen R.L., Ghazal P., Shamu C.E. Statistical methods for analysis of high-throughput RNA interference screens. Nat. Methods. 2009;6:569–575. doi: 10.1038/nmeth.1351. https://doi.org/10.1038/nmeth.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham S.D., Partridge F.A., Sattelle D.B. Automated, high-throughput, motility analysis in Caenorhabditis elegans and parasitic nematodes: applications in the search for new anthelmintics. Int. J. Parasitol. Drugs Drug Resist. 2014;4:226–232. doi: 10.1016/j.ijpddr.2014.10.004. https://doi.org/10.1016/j.ijpddr.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham S.D., Sattelle D.B. Strategies for automated analysis of C. elegans locomotion. Invert. Neurosci. 2008;8:121. doi: 10.1007/s10158-008-0077-3. https://doi.org/10.1007/s10158-008-0077-3 [DOI] [PubMed] [Google Scholar]
- Bulman C.A., Bidlow C.M., Lustigman S., Cho-Ngwa F., Williams D., Rascón Alberto A., Jr., Tricoche N., Samje M., Bell A., Suzuki B., Lim K.C., Supakorndej N., Supakorndej P., Wolfe A.R., Knudsen G.M., Chen S., Wilson C., Ang K.-H., Arkin M., Gut J., Franklin C., Marcellino C., McKerrow J.H., Debnath A., Sakanari J.A. Repurposing auranofin as a lead candidate for treatment of lymphatic filariasis and onchocerciasis. PLoS Negl. Trop. Dis. 2015;9:e0003534. doi: 10.1371/journal.pntd.0003534. https://doi.org/10.1371/journal.pntd.0003534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns A.R., Luciani G.M., Musso G., Bagg R., Yeo M., Zhang Y., Rajendran L., Glavin J., Hunter R., Redman E., Stasiuk S., Schertzberg M., Angus McQuibban G., Caffrey C.R., Cutler S.R., Tyers M., Giaever G., Nislow C., Fraser A.G., MacRae C.A., Gilleard J., Roy P.J. Caenorhabditis elegans is a useful model for anthelmintic discovery. Nat. Commun. 2015;6:7485. doi: 10.1038/ncomms8485. https://doi.org/10.1038/ncomms8485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey C.R. Chemotherapy of schistosomiasis: present and future. Curr. Opin. Chem. Biol. 2007;11:433–439. doi: 10.1016/j.cbpa.2007.05.031. https://doi.org/10.1016/j.cbpa.2007.05.031 Next-generation therapeutics. [DOI] [PubMed] [Google Scholar]
- Cully D.F., Vassilatis D.K., Liu K.K., Paress P.S., Van der Ploeg L.H.T., Schaeffer J.M., Arena J.P. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature. 1994;371:707–711. doi: 10.1038/371707a0. https://doi.org/10.1038/371707a0 [DOI] [PubMed] [Google Scholar]
- da Silva-Alves D.C.B., dos Anjos J.V., Cavalcante N.N.M., Santos G.K.N., Navarro D.M. do A.F., Srivastava R.M. Larvicidal isoxazoles: synthesis and their effective susceptibility towards Aedes aegypti larvae. Bioorg. Med. Chem. 2013;21:940–947. doi: 10.1016/j.bmc.2012.12.006. https://doi.org/10.1016/j.bmc.2012.12.006 [DOI] [PubMed] [Google Scholar]
- Debnath A., Parsonage D., Andrade R.M., He C., Cobo E.R., Hirata K., Chen S., García-Rivera G., Orozco E., Martínez M.B., Gunatilleke S.S., Barrios A.M., Arkin M.R., Poole L.B., McKerrow J.H., Reed S.L. A high-throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat. Med. 2012;18:956–960. doi: 10.1038/nm.2758. https://doi.org/10.1038/nm.2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll M., Dean E., Reilly E., Bergholz E., Chalfie M. Genetic and molecular analysis of a Caenorhabditis elegans beta-tubulin that conveys benzimidazole sensitivity. J. Cell Biol. 1989;109:2993–3003. doi: 10.1083/jcb.109.6.2993. https://doi.org/10.1083/jcb.109.6.2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein A.D., Tsuchida M.A., Amodaj N., Pinkard H., Vale R.D., Stuurman N. Advanced methods of microscope control using μManager software. J. Biol. Methods. 2014;1:e10. doi: 10.14440/jbm.2014.36. https://doi.org/10.14440/jbm.2014.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elewski B.E., Aly R., Baldwin S.L., González Soto R.F., Rich P., Weisfeld M., Wiltz H., Zane L.T., Pollak R. Efficacy and safety of tavaborole topical solution, 5%, a novel boron-based antifungal agent, for the treatment of toenail onychomycosis: results from 2 randomized phase-III studies. J. Am. Acad. Dermatol. 2015;73:62–69. doi: 10.1016/j.jaad.2015.04.010. https://doi.org/10.1016/j.jaad.2015.04.010 [DOI] [PubMed] [Google Scholar]
- Holden-Dye L. 2007. Anthelmintic Drugs. WormBook; pp. 1–13.https://doi.org/10.1895/wormbook.1.143.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J. second ed. ASM Press; Washington, DC: 2013. Forgotten People, Forgotten Diseases: the Neglected Tropical Diseases and Their Impact on Global Health and Development. [Google Scholar]
- Hurst R.J., Hopwood T., Gallagher A.L., Partridge F.A., Burgis T., Sattelle D.B., Else K.J. An antagonist of the retinoid X receptor reduces the viability of Trichuris muris in vitro. BMC Infect. Dis. 2014;14(520) doi: 10.1186/1471-2334-14-520. https://doi.org/10.1186/1471-2334-14-520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs R.T., Nare B., Wring S.A., Orr M.D., Chen D., Sligar J.M., Jenks M.X., Noe R.A., Bowling T.S., Mercer L.T., Rewerts C., Gaukel E., Owens J., Parham R., Randolph R., Beaudet B., Bacchi C.J., Yarlett N., Plattner J.J., Freund Y., Ding C., Akama T., Zhang Y.-K., Brun R., Kaiser M., Scandale I., Don R. SCYX-7158, an orally-active benzoxaborole for the treatment of stage 2 human African trypanosomiasis. PLoS Negl. Trop. Dis. 2011;5:e1151. doi: 10.1371/journal.pntd.0001151. https://doi.org/10.1371/journal.pntd.0001151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.C., Foth B.J., Urbaniak M.D., Patterson S., Ong H.B., Berriman M., Fairlamb A.H. Genomic and proteomic studies on the mode of action of oxaboroles against the African trypanosome. PLoS Negl. Trop. Dis. 2015;9:e0004299. doi: 10.1371/journal.pntd.0004299. https://doi.org/10.1371/journal.pntd.0004299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky R., Ducray P., Jung M., Clover R., Rufener L., Bouvier J., Weber S.S., Wenger A., Wieland-Berghausen S., Goebel T., Gauvry N., Pautrat F., Skripsky T., Froelich O., Komoin-Oka C., Westlund B., Sluder A., Mäser P. A new class of anthelmintics effective against drug-resistant nematodes. Nature. 2008;452:176–180. doi: 10.1038/nature06722. https://doi.org/10.1038/nature06722 [DOI] [PubMed] [Google Scholar]
- Keiser J., Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937–1948. doi: 10.1001/jama.299.16.1937. https://doi.org/10.1001/jama.299.16.1937 [DOI] [PubMed] [Google Scholar]
- Kuntz A.N., Davioud-Charvet E., Sayed A.A., Califf L.L., Dessolin J., Arnér E.S.J., Williams D.L. Thioredoxin glutathione reductase from Schistosoma mansoni: an essential parasite enzyme and a key drug target. PLoS Med. 2007;4:e206. doi: 10.1371/journal.pmed.0040206. https://doi.org/10.1371/journal.pmed.0040206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok T.C.Y., Ricker N., Fraser R., Chan A.W., Burns A., Stanley E.F., McCourt P., Cutler S.R., Roy P.J. A small-molecule screen in C. elegans yields a new calcium channel antagonist. Nature. 2006;441:91–95. doi: 10.1038/nature04657. https://doi.org/10.1038/nature04657 [DOI] [PubMed] [Google Scholar]
- Laclette J.P., Guerra G., Zetina C. Inhibition of tubulin polymerization by mebendazole. Biochem. Biophys. Res. Commun. 1980;92:417–423. doi: 10.1016/0006-291x(80)90349-6. [DOI] [PubMed] [Google Scholar]
- Levecke B., Montresor A., Albonico M., Ame S.M., Behnke J.M., Bethony J.M., Noumedem C.D., Engels D., Guillard B., Kotze A.C., Krolewiecki A.J., McCarthy J.S., Mekonnen Z., Periago M.V., Sopheak H., Tchuem-Tchuenté L.-A., Duong T.T., Huong N.T., Zeynudin A., Vercruysse J. Assessment of anthelmintic efficacy of mebendazole in school children in six countries where soil-transmitted helminths are endemic. PLoS Negl. Trop. Dis. 2014;8:e3204. doi: 10.1371/journal.pntd.0003204. https://doi.org/10.1371/journal.pntd.0003204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.T., Tomsho J.W., Benkovic S.J. The unique chemistry of benzoxaboroles: current and emerging applications in biotechnology and therapeutic treatments. Bioorg. Med. Chem. 2014;22:4462–4473. doi: 10.1016/j.bmc.2014.04.065. https://doi.org/10.1016/j.bmc.2014.04.065 Unlocking Nature through Chemistry. [DOI] [PubMed] [Google Scholar]
- Maizels R.M., Denham D.A. Diethylcarbamazine (DEC): immunopharmacological interactions of an anti-filarial drug. Parasitology. 1992;105:S49–S60. doi: 10.1017/s0031182000075351. https://doi.org/10.1017/S0031182000075351 [DOI] [PubMed] [Google Scholar]
- Malo N., Hanley J.A., Cerquozzi S., Pelletier J., Nadon R. Statistical practice in high-throughput screening data analysis. Nat. Biotechnol. 2006;24:167–175. doi: 10.1038/nbt1186. https://doi.org/10.1038/nbt1186 [DOI] [PubMed] [Google Scholar]
- Manual of Veterinary Parasitological Laboratory Techniques, 3rd ed. ed, 1986. H.M.S.O., London.
- Marcellino C., Gut J., Lim K.C., Singh R., McKerrow J., Sakanari J. WormAssay: a novel computer application for Whole-plate motion-based screening of macroscopic parasites. PLoS Negl. Trop. Dis. 2012;6:e1494. doi: 10.1371/journal.pntd.0001494. https://doi.org/10.1371/journal.pntd.0001494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. j. γ-Aminobutyric acid- and piperazine-activated single-channel currents from Ascaris suum body muscle. Br. J. Pharmacol. 1985;84:445–461. doi: 10.1111/j.1476-5381.1985.tb12929.x. https://doi.org/10.1111/j.1476-5381.1985.tb12929.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R.J., Robertson A.P., Bjorn H. Target sites of anthelmintics. Parasitology. 1997;114:111–124. [PubMed] [Google Scholar]
- Mathew M.D., Mathew N.D., Ebert P.R. WormScan: a technique for high-throughput phenotypic analysis of Caenorhabditis elegans. PLoS One. 2012;7:e33483. doi: 10.1371/journal.pone.0033483. https://doi.org/10.1371/journal.pone.0033483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew M.D., Mathew N.D., Miller A., Simpson M., Au V., Garland S., Gestin M., Edgley M.L., Flibotte S., Balgi A., Chiang J., Giaever G., Dean P., Tung A., Roberge M., Roskelley C., Forge T., Nislow C., Moerman D. Using C. elegans forward and reverse genetics to identify new compounds with anthelmintic activity. PLoS Negl. Trop. Dis. 2016;10:e0005058. doi: 10.1371/journal.pntd.0005058. https://doi.org/10.1371/journal.pntd.0005058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J.B. Anthelmintic resistance in equine nematodes. Int. J. Parasitol. Drugs Drug Resist. 2014;4:310–315. doi: 10.1016/j.ijpddr.2014.10.003. https://doi.org/10.1016/j.ijpddr.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nare B., Wring S., Bacchi C., Beaudet B., Bowling T., Brun R., Chen D., Ding C., Freund Y., Gaukel E., Hussain A., Jarnagin K., Jenks M., Kaiser M., Mercer L., Mejia E., Noe A., Orr M., Parham R., Plattner J., Randolph R., Rattendi D., Rewerts C., Sligar J., Yarlett N., Don R., Jacobs R. Discovery of novel orally bioavailable oxaborole 6-carboxamides that demonstrate cure in a murine model of late-stage central nervous system African trypanosomiasis. Antimicrob. Agents Chemother. 2010;54:4379–4388. doi: 10.1128/AAC.00498-10. https://doi.org/10.1128/AAC.00498-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutting C.S., Eversole R.R., Blair K., Specht S., Nutman T.B., Klion A.D., Wanji S., Boussinesq M., Mackenzie C.D. Analysis of nematode motion using an improved light-scatter based system. PLoS Negl. Trop. Dis. 2015;9:e0003523. doi: 10.1371/journal.pntd.0003523. https://doi.org/10.1371/journal.pntd.0003523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwaka S., Ramirez B., Brun R., Maes L., Douglas F., Ridley R. Advancing Drug Innovation for Neglected Diseases—Criteria for Lead Progression. PLoS Negl. Trop. Dis. 2009;3 doi: 10.1371/journal.pntd.0000440. https://doi.org/10.1371/journal.pntd.0000440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palencia A., Li X., Bu W., Choi W., Ding C.Z., Easom E.E., Feng L., Hernandez V., Houston P., Liu L., Meewan M., Mohan M., Rock F.L., Sexton H., Zhang S., Zhou Y., Wan B., Wang Y., Franzblau S.G., Woolhiser L., Gruppo V., Lenaerts A.J., O'Malley T., Parish T., Cooper C.B., Waters M.G., Ma Z., Ioerger T.R., Sacchettini J.C., Rullas J., Angulo-Barturen I., Pérez-Herrán E., Mendoza A., Barros D., Cusack S., Plattner J.J., Alley M.R.K. Discovery of novel oral protein synthesis inhibitors of Mycobacterium tuberculosis that target Leucyl-tRNA synthetase. Antimicrob. Agents Chemother. 2016;60:6271–6280. doi: 10.1128/AAC.01339-16. https://doi.org/10.1128/AAC.01339-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palencia A., Liu R.-J., Lukarska M., Gut J., Bougdour A., Touquet B., Wang E.-D., Li X., Alley M.R.K., Freund Y.R., Rosenthal P.J., Hakimi M.-A., Cusack S. Cryptosporidium and toxoplasma parasites are inhibited by a benzoxaborole targeting Leucyl-tRNA synthetase. Antimicrob. Agents Chemother. 2016;60:5817–5827. doi: 10.1128/AAC.00873-16. https://doi.org/10.1128/AAC.00873-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge F.A., Brown A.E., Buckingham S.D., Sattelle D.B. 2017. INVAPP Paragon.https://doi.org/10.5281/zenodo.1064045 [Google Scholar]
- Partridge F.A., Murphy E.A., Willis N.J., Bataille C.J.R., Forman R., Heyer-Chauhan N., Marinič B., Sowood D.J.C., Wynne G.M., Else K.J., Russell A.J., Sattelle D.B. Dihydrobenz[e][1,4]oxazepin-2(3H)-ones, a new anthelmintic chemotype immobilising whipworm and reducing infectivity in vivo. PLoS Negl. Trop. Dis. 2017;11:e0005359. doi: 10.1371/journal.pntd.0005359. https://doi.org/10.1371/journal.pntd.0005359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollastri M.P., Campbell R.K. Target repurposing for neglected diseases. Future Med. Chem. 2011;3:1307–1315. doi: 10.4155/fmc.11.92. https://doi.org/10.4155/fmc.11.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston S., Jabbar A., Nowell C., Joachim A., Ruttkowski B., Baell J., Cardno T., Korhonen P.K., Piedrafita D., Ansell B.R.E., Jex A.R., Hofmann A., Gasser R.B. Low cost whole-organism screening of compounds for anthelmintic activity. Int. J. Parasitol. 2015;45:333–343. doi: 10.1016/j.ijpara.2015.01.007. https://doi.org/10.1016/j.ijpara.2015.01.007 [DOI] [PubMed] [Google Scholar]
- Preston S., Jabbar A., Nowell C., Joachim A., Ruttkowski B., Cardno T., Hofmann A., Gasser R.B. Practical and low cost whole-organism motility assay: a step-by-step protocol. Mol. Cell. Probes. 2016;30:13–17. doi: 10.1016/j.mcp.2015.08.005. https://doi.org/10.1016/j.mcp.2015.08.005 [DOI] [PubMed] [Google Scholar]
- Preston S., Jiao Y., Jabbar A., McGee S.L., Laleu B., Willis P., Wells T.N.C., Gasser R.B. Screening of the ‘Pathogen Box’ identifies an approved pesticide with major anthelmintic activity against the barber's pole worm. Int. J. Parasitol. Drugs Drug Resist. 2016 doi: 10.1016/j.ijpddr.2016.07.004. https://doi.org/10.1016/j.ijpddr.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard R.K., Basáñez M.-G., Boatin B.A., McCarthy J.S., García H.H., Yang G.-J., Sripa B., Lustigman S. A research agenda for helminth diseases of humans: intervention for control and elimination. PLoS Negl. Trop. Dis. 2012;6:e1549. doi: 10.1371/journal.pntd.0001549. https://doi.org/10.1371/journal.pntd.0001549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz C., Streibig J.C. Bioassay analysis using R. J. Stat. Softw. 2005;12:1–22. [Google Scholar]
- Rock F.L., Mao W., Yaremchuk A., Tukalo M., Crépin T., Zhou H., Zhang Y.-K., Hernandez V., Akama T., Baker S.J., Plattner J.J., Shapiro L., Martinis S.A., Benkovic S.J., Cusack S., Alley M.R.K. An antifungal agent inhibits an Aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science. 2007;316:1759–1761. doi: 10.1126/science.1142189. https://doi.org/10.1126/science.1142189 [DOI] [PubMed] [Google Scholar]
- Roeber F., Jex A.R., Gasser R.B. Impact of gastrointestinal parasitic nematodes of sheep, and the role of advanced molecular tools for exploring epidemiology and drug resistance - an Australian perspective. Parasit. Vectors. 2013;6(153) doi: 10.1186/1756-3305-6-153. https://doi.org/10.1186/1756-3305-6-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose H., Rinaldi L., Bosco A., Mavrot F., de Waal T., Skuce P., Charlier J., Torgerson P.R., Hertzberg H., Hendrickx G., Vercruysse J., Morgan E.R. Widespread anthelmintic resistance in European farmed ruminants: a systematic review. Vet. Rec. 2015;176(546) doi: 10.1136/vr.102982. https://doi.org/10.1136/vr.102982 [DOI] [PubMed] [Google Scholar]
- Sales N., Love S. Resistance of Haemonchus sp. to monepantel and reduced efficacy of a derquantel/abamectin combination confirmed in sheep in NSW, Australia. Vet. Parasitol. 2016;228:193–196. doi: 10.1016/j.vetpar.2016.08.016. https://doi.org/10.1016/j.vetpar.2016.08.016 [DOI] [PubMed] [Google Scholar]
- Satyendra R.V., Vishnumurthy K.A., Vagdevi H.M., Rajesh K.P., Manjunatha H., Shruthi A. Synthesis, in vitro antioxidant, anthelmintic and molecular docking studies of novel dichloro substituted benzoxazole-triazolo-thione derivatives. Eur. J. Med. Chem. 2011;46:3078–3084. doi: 10.1016/j.ejmech.2011.03.017. https://doi.org/10.1016/j.ejmech.2011.03.017 [DOI] [PubMed] [Google Scholar]
- Scott I., Pomroy W.E., Kenyon P.R., Smith G., Adlington B., Moss A. Lack of efficacy of monepantel against Teladorsagia circumcincta and Trichostrongylus colubriformis. Vet. Parasitol. 2013;198:166–171. doi: 10.1016/j.vetpar.2013.07.037. https://doi.org/10.1016/j.vetpar.2013.07.037 [DOI] [PubMed] [Google Scholar]
- Sharlow E.R., Leimgruber S., Murray S., Lira A., Sciotti R.J., Hickman M., Hudson T., Leed S., Caridha D., Barrios A.M., Close D., Grögl M., Lazo J.S. Auranofin is an apoptosis-simulating agent with in vitro and in vivo anti-leishmanial activity. ACS Chem. Biol. 2014;9:663–672. doi: 10.1021/cb400800q. https://doi.org/10.1021/cb400800q [DOI] [PubMed] [Google Scholar]
- Silbereisen A., Tritten L., Keiser J. Exploration of novel in vitro assays to study drugs against Trichuris spp. J. Microbiol. Methods. 2011;87:169–175. doi: 10.1016/j.mimet.2011.08.009. https://doi.org/10.1016/j.mimet.2011.08.009 [DOI] [PubMed] [Google Scholar]
- Smout M.J., Kotze A.C., McCarthy J.S., Loukas A. A novel high throughput assay for anthelmintic drug screening and resistance diagnosis by real-time monitoring of parasite motility. PLoS Negl. Trop. Dis. 2010;4:e885. doi: 10.1371/journal.pntd.0000885. https://doi.org/10.1371/journal.pntd.0000885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoiki E., Palencia A., Guo D., Ahyong V., Dong C., Li X., Hernandez V.S., Zhang Y.-K., Choi W., Gut J., Legac J., Cooper R., Alley M.R.K., Freund Y.R., DeRisi J., Cusack S., Rosenthal P.J. Antimalarial benzoxaboroles target Plasmodium falciparum Leucyl-tRNA synthetase. Antimicrob. Agents Chemother. 2016;60:4886–4895. doi: 10.1128/AAC.00820-16. https://doi.org/10.1128/AAC.00820-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B., Marcellino C., Miller M., Maclean M., Mostafa E., Howell S., Sakanari J., Wolstenholme A., Kaplan R. Utilization of computer processed high definition video imaging for measuring motility of microscopic nematode stages on a quantitative scale: “The Worminator.”. Int. J. Parasitol. Drugs Drug Resist. 2014;4:233–243. doi: 10.1016/j.ijpddr.2014.08.003. https://doi.org/10.1016/j.ijpddr.2014.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I.A. Recent developments in the management of anthelmintic resistance in small ruminants - an Australasian perspective. N. Z. Vet. J. 2015;63:183–187. doi: 10.1080/00480169.2015.1019947. https://doi.org/10.1080/00480169.2015.1019947 [DOI] [PubMed] [Google Scholar]
- Tatipaka H.B., Gillespie J.R., Chatterjee A.K., Norcross N.R., Hulverson M.A., Ranade R.M., Nagendar P., Creason S.A., McQueen J., Duster N.A., Nagle A., Supek F., Molteni V., Wenzler T., Brun R., Glynne R., Buckner F.S., Gelb M.H. Substituted 2-phenylimidazopyridines: a new class of drug leads for human African trypanosomiasis. J. Med. Chem. 2014;57:828–835. doi: 10.1021/jm401178t. https://doi.org/10.1021/jm401178t [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritten L., Braissant O., Keiser J. Comparison of novel and existing tools for studying drug sensitivity against the hookworm Ancylostoma ceylanicum in vitro. Parasitology. 2012;139:348–357. doi: 10.1017/S0031182011001934. https://doi.org/10.1017/S0031182011001934 [DOI] [PubMed] [Google Scholar]
- Tritten L., Silbereisen A., Keiser J. In vitro and in vivo efficacy of Monepantel (AAD 1566) against laboratory models of human intestinal nematode infections. PLoS Negl. Trop. Dis. 2011;5:e1457. doi: 10.1371/journal.pntd.0001457. https://doi.org/10.1371/journal.pntd.0001457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner H.C., Truscott J.E., Bettis A.A., Hollingsworth T.D., Brooker S.J., Anderson R.M. Analysis of the population-level impact of co-administering ivermectin with albendazole or mebendazole for the control and elimination of Trichuris trichiura. Parasite Epidemiol. Control. 2016;1:177–187. doi: 10.1016/j.parepi.2016.02.004. https://doi.org/10.1016/j.parepi.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W.C., Adams J.H., Adelfio R., Ahyong V., Akabas M.H., Alano P., Alday A., Resto Y.A., Alsibaee A., Alzualde A., Andrews K.T., Avery S.V., Avery V.M., Ayong L., Baker M., Baker S., Mamoun C.B., Bhatia S., Bickle Q., Bounaadja L., Bowling T., Bosch J., Boucher L.E., Boyom F.F., Brea J., Brennan M., Burton A., Caffrey C.R., Camarda G., Carrasquilla M., Carter D., Cassera M.B., Cheng K.C.-C., Chindaudomsate W., Chubb A., Colon B.L., Colón-López D.D., Corbett Y., Crowther G.J., Cowan N., D'Alessandro S., Dang N.L., Delves M., DeRisi J.L., Du A.Y., Duffy S., El-Sayed S.A.E.-S., Ferdig M.T., Robledo J.A.F., Fidock D.A., Florent I., Fokou P.V.T., Galstian A., Gamo F.J., Gokool S., Gold B., Golub T., Goldgof G.M., Guha R., Guiguemde W.A., Gural N., Guy R.K., Hansen M.A.E., Hanson K.K., Hemphill A., van Huijsduijnen R.H., Horii T., Horrocks P., Hughes T.B., Huston C., Igarashi I., Ingram-Sieber K., Itoe M.A., Jadhav A., Jensen A.N., Jensen L.T., Jiang R.H.Y., Kaiser A., Keiser J., Ketas T., Kicka S., Kim S., Kirk K., Kumar V.P., Kyle D.E., Lafuente M.J., Landfear S., Lee N., Lee S., Lehane A.M., Li F., Little D., Liu L., Llinás M., Loza M.I., Lubar A., Lucantoni L., Lucet I., Maes L., Mancama D., Mansour N.R., March S., McGowan S., Vera I.M., Meister S., Mercer L., Mestres J., Mfopa A.N., Misra R.N., Moon S., Moore J.P., da Costa F.M.R., Müller J., Muriana A., Hewitt S.N., Nare B., Nathan C., Narraidoo N., Nawaratna S., Ojo K.K., Ortiz D., Panic G., Papadatos G., Parapini S., Patra K., Pham N., Prats S., Plouffe D.M., Poulsen S.-A., Pradhan A., Quevedo C., Quinn R.J., Rice C.A., Rizk M.A., Ruecker A., Onge R.S., Ferreira R.S., Samra J., Robinett N.G., Schlecht U., Schmitt M., Villela F.S., Silvestrini F., Sinden R., Smith D.A., Soldati T., Spitzmüller A., Stamm S.M., Sullivan D.J., Sullivan W., Suresh S., Suzuki B.M., Suzuki Y., Swamidass S.J., Taramelli D., Tchokouaha L.R.Y., Theron A., Thomas D., Tonissen K.F., Townson S., Tripathi A.K., Trofimov V., Udenze K.O., Ullah I., Vallieres C., Vigil E., Vinetz J.M., Vinh P.V., Vu H., Watanabe N., Weatherby K., White P.M., Wilks A.F., Winzeler E.A., Wojcik E., Wree M., Wu W., Yokoyama N., Zollo P.H.A., Abla N., Blasco B., Burrows J., Laleu B., Leroy D., Spangenberg T., Wells T., Willis P.A. Open source drug discovery with the malaria box compound collection for neglected diseases and beyond. PLoS Pathog. 2016;12:e1005763. doi: 10.1371/journal.ppat.1005763. https://doi.org/10.1371/journal.ppat.1005763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercruysse J., Albonico M., Behnke J.M., Kotze A.C., Prichard R.K., McCarthy J.S., Montresor A., Levecke B. Is anthelmintic resistance a concern for the control of human soil-transmitted helminths? Int. J. Parasitol. Drugs Drug Resist. 2011;1:14–27. doi: 10.1016/j.ijpddr.2011.09.002. https://doi.org/10.1016/j.ijpddr.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangchuk P., Giacomin P.R., Pearson M.S., Smout M.J., Loukas A. Identification of lead chemotherapeutic agents from medicinal plants against blood flukes and whipworms. Sci. Rep. 2016;6(32101) doi: 10.1038/srep32101. https://doi.org/10.1038/srep32101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangchuk P., Pearson M.S., Giacomin P.R., Becker L., Sotillo J., Pickering D., Smout M.J., Loukas A. Compounds derived from the bhutanese daisy, ajania nubigena, demonstrate dual anthelmintic activity against Schistosoma mansoni and Trichuris muris. PLoS Negl. Trop. Dis. 2016;10:e0004908. doi: 10.1371/journal.pntd.0004908. https://doi.org/10.1371/journal.pntd.0004908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmersberger D., Tritten L., Keiser J. Development of an in vitro drug sensitivity assay for Trichuris muris first-stage larvae. Parasit. Vectors. 2013;6(42) doi: 10.1186/1756-3305-6-42. https://doi.org/10.1186/1756-3305-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme A.J., Maclean M.J., Coates R., McCoy C.J., Reaves B.J. How do the macrocyclic lactones kill filarial nematode larvae? Invertebr. Neurosci. IN. 2016;16(7) doi: 10.1007/s10158-016-0190-7. https://doi.org/10.1007/s10158-016-0190-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S.J. second ed. CRC Press; 2014. The Toxicology and Biochemistry of Insecticides. [Google Scholar]
- Zhang X.D. Cambridge University Press; 2011. Optimal High-throughput Screening: Practical Experimental Design and Data Analysis for Genome-scale RNAi Research.https://doi.org/10.1017/CBO9780511973888 [Google Scholar]
- Zhang X.D., Ferrer M., Espeseth A.S., Marine S.D., Stec E.M., Crackower M.A., Holder D.J., Heyse J.F., Strulovici B. The use of strictly standardized mean difference for hit selection in primary RNA interference high-throughput screening experiments. J. Biomol. Screen. 2007;12:497–509. doi: 10.1177/1087057107300646. https://doi.org/10.1177/1087057107300646 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhou H., Ding C., Plattner J., Freund Y. 2011. Boron-containing Small Molecules as Antihelminth Agents. WO/2011/063293. [Google Scholar]
- Zheng W., Sun W., Simeonov A. Drug repurposing screens and synergistic drug-combinations for infectious diseases. Br. J. Pharmacol. 2017 doi: 10.1111/bph.13895. https://doi.org/10.1111/bph.13895 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of primary and secondary screens of the Pathogen Box library using the C. elegans growth assay.