Abstract
Objectives
This study aimed to evaluate the association between sodium intake and metabolic syndrome (MetS) in Korean boys.
Methods
A total of 1,738 boys aged 10–18 years were included in this study from the Korea National Health and Nutrition Examination Survey (KNHANES) during the years 2010–2013. Sodium intake was assessed using the urinary sodium excretion to urinary specific gravity ratio (U-Na to U-SG ratio).
Results
The median U-Na to U-SG ratio was 133.27 mmol/L (interquartile range: 95.66–178.50 mmol/L). Significant positive associations were found between the U-Na to U-SG ratio and the TG (P = 0.001 for trend) and TG concentrations, and these concentrations were significantly higher in boys with a U-Na to U-SG ratio in the highest quartile compared with those with a ratio in the lowest (P = 0.001) and second (P = 0.033) quartiles, as demonstrated through analysis of covariance (ANCOVA) after adjustment for possible confounders, including age, BMI standard deviation score, ferritin, vitamin D, house income, smoking, alcohol intake, physical activity, season, total intake, total energy intake, protein intake, fat intake, carbohydrate intake, and water intake. Significant inverse associations were found for the U-Na to U-SG ratio with the HDL-C (P = 0.033 for trend) and HDL-C levels, and these values were significantly lower in boys with a ratio in the highest quartile compared with those with a ratio in the second quartile (P = 0.020), as demonstrated through an ANCOVA. Although the trends did not reach statistical significance, a higher U-Na to U-SG ratio tended to be associated with higher SBP (P = 0.086 for trend), DBP (P = 0.063 for trend), and glucose levels (P = 0.099 for trend), as illustrated through ANCOVA. Boys with a ratio in the highest quartile exhibited a 1.73-fold increased risk for elevated TG (95% CI, 1.19–2.51) and a 2.66-fold increased risk for MetS (95% CI, 1.11–6.35) compared with those with a ratio in the lowest quartile, as demonstrated through multivariate logistic regression analyses after adjusting for confounders.
Conclusions
Our results suggest that high sodium intake may be significantly independently associated with MetS in Korean boys aged 10–18 years.
Introduction
According to the 2013 Korea School Health Examination, the prevalence of obesity in Korean children and adolescents increased from 13.2% in 2009 to 15.3% in 2013 [1]. The high prevalence of childhood and adolescent obesity has been associated with increased adult obesity [2] and obesity-related complications, such as insulin resistance [3], hypertension [4], dyslipidemia [5], metabolic syndrome (MetS) [6], and type 2 diabetes mellitus (T2DM) [7]. According to the modified NCEP-ATP III for children and adolescents, a recent Korean study revealed that the prevalence of MetS is 5.8% and 5.5% in boys and girls aged with 10–18 years, respectively [8]. MetS is related to cardiovascular disease, cerebrovascular disease, kidney disease, and type 2 diabetes mellitus (T2DM) [9]. This constellation of cardiometabolic risk factors is considered to be modifiable, and the identification of children and adolescents with MetS is necessary because age-specific interventions can help improve their condition.
High sodium intake has become one of the major problems in the healthcare field throughout the world. High sodium intake is associated with increased blood pressure in children [10], adolescents [10] and adults [11], and high sodium intake is thought to be related to MetS because elevated blood pressure is a component of MetS [12]. However, recent studies have indicated that high sodium intake is associated with other components of MetS [13–15]. There are reports that high sodium intake is related to obesity [13.14], and it has also been suggested that high sodium intake is associated with cardiometabolic risk factors, such as dyslipidemia, insulin resistance, and metabolic syndrome, in adults [15]. However, no previous study has demonstrated a link between high sodium and insulin resistance-related diseases in children and adolescents. In addition, high salt intake is modifiable as part of a medical intervention.
In the current study, we aimed to evaluate the association between sodium intake, which was assessed using the urinary sodium excretion to urinary specific gravity ratio (U-Na to U-SG ratio), and MetS in Korean boys aged 10–18 years. Through a nationwide survey, we also assessed whether this relationship was independent or whether the association was mediated by confounders.
Materials and methods
Subjects
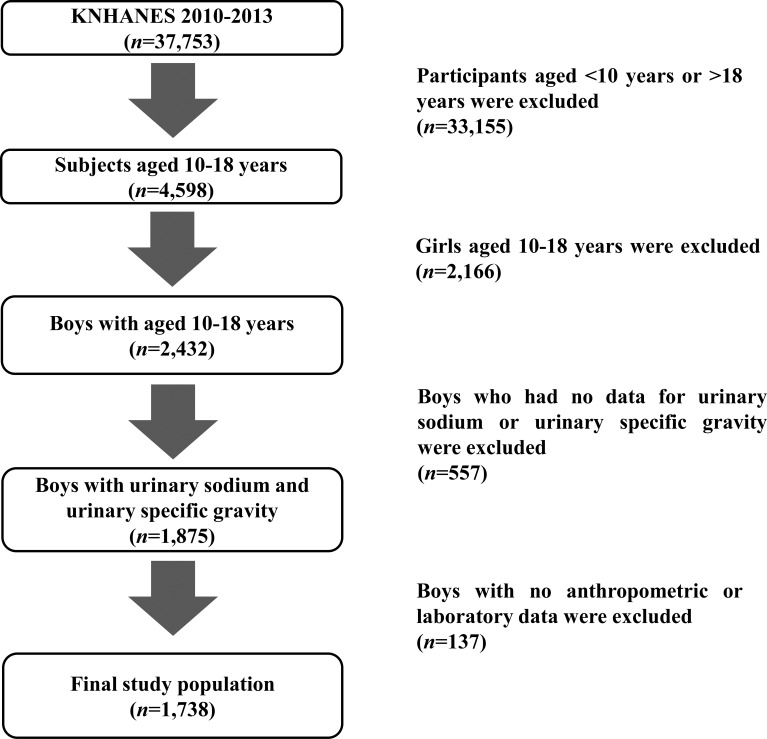
This study was conducted using data from the Korea National Health and Nutrition Examination Survey (KNHANES) during the period 2010–2013. The KNHANES is a cross-sectional, nationwide and representative survey that is conducted regularly by the Division of Chronic Disease Surveillance, Korean Centers for Disease Control and Prevention [15]. The survey, which consists of a health questionnaire, health examination, and nutritional assessment, uses a stratified, multistage probability sampling design for the selection of household units. Details of the KNHANES have been described previously [16]. The 37,753 subjects in the KNHANES during the years 2010–2013 included 4,598 children and adolescents aged 10 to 18 years (Fig 1). Boys exhibited higher levels of sodium intake and excretion than girls in preliminary analyses. Additionally, the overall prevalence of MetS in girls was significantly lower than that in boys in our analyses. A total of 2,432 boys were included in our study, of which 1,875 underwent laboratory examination, including measurements of urinary sodium (U-Na) and urinary specific gravity (U-SG); 137 boys who did not complete the anthropometrical examination were excluded. As a result, 1,738 participants were included in this study. The database is available to the public at the KNHANES website (http://knhanes.cdc.go.kr) [17]. Because the dataset did not provide any personal information and informed consent was provided by all of the KNHANES participants, this study was exempted by the institutional review board from needing to obtain participant consent.
Fig 1. Flow chart of the study population.
In total, 1,738 Korean boys were included in the present study.
Measurements
Anthropometric assessments using standard methods were conducted by a trained expert. In brief, the height and body weight were assessed to the nearest 0.1 cm using Seca 225 (Seca, Hamburg, Germany) and 0.1 kg using GL-6000-20 (G-tech, Seoul, Korea), respectively. The body mass index (BMI) was determined as the weight (kg)/square of height (m2). The waist circumference (WC) was measured at the midline between the lower rib margin and iliac crest to the nearest 0.1 cm. The standard deviation scores (SDSs) for the height, weight, BMI and WC were used because they were not evenly distributed between various age groups. The height SDS, weight SDS, BMI SDS and WC SDS were determined through LMS methods using 2007 Korean reference data. The systolic blood pressure (SBP, mmHg) and diastolic blood pressure (DBP, mmHg) were determined three times from the right upper arm using a calibrated sphygmomanometer (Baumanometer Desk model 0320, Baum, NY, USA) and an appropriately sized cuff. The measurements were taken at 2-minute intervals. The mean of the last two values was then used for the analysis.
Random samples from blood and urine were collected year-round after the participants had fasted for at least 8 hours, and these were immediately processed, refrigerated, and transported to a central laboratory (NeoDin Medical Institute, Seoul, Korea) for analysis within 24 hours. Routine biochemistry tests, including analyses of the levels of total cholesterol (T-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), glucose, U-Na and U-SG, were measured enzymatically using a Hitachi 7600 automatic analyzer (Hitachi, Tokyo, Japan). LDL cholesterol was determined with Friedewald’s equation [18]. Serum ferritin and vitamin D were determined through an immunoradiometric assay using a 1470 Wizard Gamma Counter (Perkin-Elmer, Turku, Finland).
Collection of data for lifestyle-related and metabolic parameters
Smoking, alcohol intake, physical activity, and household income were included in this study as lifestyle-related parameters. Smokers were defined as individuals who smoked more than a total of five packs worth of cigarettes throughout their life and were divided into the following two groups: smoker and non-smoker. Alcohol intake was defined as drinking at least two alcoholic beverages/month during the previous year and was divided into the following two groups: drinker and non-drinker. Physical activity was defined as meeting at least one of the following three criteria: (i) intense physical activity for 20 minutes at least three days/week, (ii) moderate physical activity for 30 minutes at least five days/week, or (iii) walking for 30 minutes at least five days/week. Physical activity was also divided into the following two groups: exercise or no exercise. Household income was reported in quartiles and was categorized into the following two groups: lowest quartile and at least second quartile. The Republic of Korea is located in a temperate region with four seasons, which are categorized as spring (March to May), summer (June to August), fall (September to November), and winter (February and December).
The dietary intake of nutrients, including total intake, total energy intake, total protein, total fat, total carbohydrates, and water intake, was assessed by a trained nutritionist using 24-hour recall. The data regarding the general participant characteristics were obtained from the KNHANES.
Definition
MetS and its components were defined according to modified criteria of the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) [19]. Elevated WC was defined as a WC greater than or equal to the 90th percentile for age and gender. Elevated BP was defined by an SBP or DBP greater than or equal to the 90th percentile for age, gender, and height according to 2007 Korean growth charts [20] or current administration of antihypertensive drugs. Elevated glucose was defined as fasting glucose concentrations greater than or equal to 110 mg/dL or a previous diagnosis of T2DM. T2DM was diagnosed in children and adolescents who met at least one of the following three categories: (i) subjects who self-reported their disease using a questionnaire comprising questions with yes or no answers, (ii) children and adolescents currently using medications or receiving insulin to manage T2DM, or (iii) participants with a fasting glucose level of at least 126 mg/dL during the national survey. Elevated TG was defined as serum TG concentrations greater than or equal to 110 mg/dL or current administration of drugs for dyslipidemia, whereas reduced HDL-C was defined as levels of serum HDL-C less than 40 mg/dL. MetS was defined as having at least three of the following five criteria: (i) elevated WC, (ii) elevated BP, (iii) elevated glucose, (iv) elevated TG, and (v) reduced HDL-C.
Statistical analyses
All analyses were conducted using SPSS software for Windows (SPSS version 23.0, IBM SPSS Inc., Chicago, IL, USA). The participants were divided into four groups according to their U-Na to U-SG ratio: (i) lowest quartile group (Q1), (ii) second quartile group (Q2), (iii) third quartile group (Q3), and (iv) highest quartile group (Q4). Normally distributed variables are presented as the means ± standard errors (SEs), whereas categorical variables are presented as percentages (%). Differences in categorical variables and normally distributed variables were analyzed using chi-square tests and analysis of variance (ANOVA) according to the U-Na to U-SG ratio. Adjusted means for MetS components, including WC SDS, SBP, DBP, glucose, HDL-C, and TG, were calculated through an analysis of covariance (ANCOVA) with Bonferroni’s post hoc test after adjustment for possible confounding factors, such as age, BMI SDS, ferritin, vitamin D, house income, smoking, alcohol intake, physical activity, season, total intake, total energy intake, protein intake, fat intake, carbohydrate intake, and water intake according to the U-Na to U-SG ratio, which was assessed as a surrogate for sodium intake and divided into quartiles. To investigate the associations of the U-Na to U-SG ratio with MetS and its components, a multivariate logistic regression analysis was conducted after adjusting for the previously described confounders, and the corresponding odds ratios (ORs) and 95% confidence intervals (95% CIs) were determined. The ORs for MetS components and MetS were determined according to the U-Na to U-SG ratio (divided into quartiles), with the lowest quartile serving as a reference. Trends across quartiles were assessed for each serum ferritin quartile as continuous variables in the multivariate logistic regression models. All significances were analyzed using a two-tailed method, and a P value <0.05 was considered to indicate statistical significance.
Results
Clinical characteristic of study participants according to the U-Na to U-SG ratio
The mean age of the study participants was 13.79 ± 0.06 years, and the median U-Na to U-SG ratio was 133.27 mmol/L, with an interquartile range (IQR) of 95.66–178.50 mmol/L. The median U-Na to U-SG ratios in the lowest, second, third and highest quartiles were 71.99 mmol/L (13.92–95.66 mmol/L), 115.76 ng/mL (95.66–133.27 mmol/L), 155.64 mmol/L (133.27–178.50 mmol/L), and 212.33 mmol/L (178.50–463.80 mmol/L), respectively. The clinical characteristics of the study population are shown in Table 1. The subjects with a U-Na to U-SG ratio in the highest quartile tended to have increased mean values for the weight SDS (P<0.001), BMI SDS (P<0.001), WC SDS(P<0.001), SBP (P = 0.011), DBP(P = 0.013), glucose levels (P = 0.003), T-C levels (P = 0.034), TG levels (P<0.001), ferritin levels (P = 0.010), U-SG (P<0.001), and U-Na (P<0.001), respectively. Boys with a U-Na to U-SG ratio in the higher quartiles were more likely to have a lower house income (P = 0.004) and a lower mean HDL-C level (P = 0.003).
Table 1. Clinical characteristic of the study participants according to the urinary sodium to urinary specific gravity ratio in Korean boys aged 10–18 years (n = 1,738).
| Urinary sodium to urinary specific gravity ratio (mmol/L) | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| n = 434 | n = 435 | n = 435 | n = 434 | ||
| [<95.66] | [95.66–133.27] | [133.27–178.50] | [≥178.50] | P | |
| Age (years) | 13.90 ± 0.12 | 13.73 ± 0.12 | 13.72 ± 0.12 | 13.82 ± 0.12 | 0.697 |
| Height SDS | 0.38 ± 0.05 | 0.52 ± 0.05 | 0.53 ± 0.05 | 0.47 ± 0.05 | 0.147 |
| Weight SDS | 0.02 ± 0.05 | 0.16 ± 0.05 | 0.28 ± 0.05 | 0.38 ± 0.06 | <0.001 |
| BMI SDS | -0.19 ± 0.05 | -0.08 ± 0.05 | 0.04 ± 0.05 | 0.21 ± 0.05 | <0.001 |
| WC SDS | -0.44 ± 0.05 | -0.31 ± 0.05 | -0.19 ± 0.05 | -0.01 ± 0.06 | <0.001 |
| SBP (mmHg) | 106.93 ± 0.51 | 107.57 ± 0.59 | 107.49 ± 0.52 | 109.33 ± 0.54 | 0.011 |
| DBP (mmHg) | 66.77 ± 0.47 | 66.83 ± 0.47 | 66.32 ± 0.46 | 67.87 ± 0.46 | 0.013 |
| Glucose (mg/dL) | 89.16 ± 0.30 | 89.45 ± 0.31 | 90.04 ± 0.27 | 90.64 ± 0.31 | 0.003 |
| T-C (mg/dL) | 151.83 ± 1.23 | 157.11 ± 1.36 | 154.24 ± 1.24 | 155.93 ± 1.53 | 0.034 |
| HDL-C (mg/dL) | 53.26 ± 0.50 | 54.24 ± 0.52 | 52.98 ± 0.51 | 51.58 ± 0.49 | 0.003 |
| TG (mg/dL) | 75.87 ± 1.96 | 80.60 ± 2.21 | 85.31 ± 2.54 | 93.46 ± 3.20 | <0.001 |
| LDL-C (mg/dL) | 83.40 ± 1.03 | 86.75 ± 1.16 | 84.39 ± 1.07 | 86.23 ± 1.37 | 0.145 |
| Ferritin (ng/mL) | 53.44 ± 1.91 | 51.00 ± 1.64 | 48.84 ± 1.40 | 45.94 ± 1.56 | 0.010 |
| Vitamin D (ng/mL) | 17.88 ± 0.29 | 17.93 ± 0.27 | 18.30 ± 0.28 | 17.92 ± 0.26 | 0.672 |
| Urinary specific gravity (mmol/L) | 1.02 ± 0.00 | 1.02 ± 0.00 | 1.02 ± 0.00 | 1.02 ± 0.00 | <0.001 |
| Urinary sodium (mmol/L) | 70.94 ± 0.93 | 117.92 ± 0.55 | 159.18 ± 0.64 | 224.43 ± 1.87 | <0.001 |
| Urinary sodium to urinary specific gravity ratio | 69.50 ± 0.90 | 115.27 ± 0.54 | 155.44 ± 0.62 | 219.26 ± 1.83 | <0.001 |
| Season | 0.576 | ||||
| Spring | 84 (19.4%) | 96 (22.1%) | 78 (17.9%) | 72 (16.6%) | |
| Summer | 118 (27.2%) | 115 (26.4%) | 109 (25.1%) | 121 (27.9%) | |
| Autumn | 110 (25.3%) | 105 (24.1%) | 120 (27.6%) | 126 (29.0%) | |
| Winter | 122 (28.1%) | 119 (27.4%) | 128 (29.4%) | 115 (26.5%) | |
| House income ≤lowest quartile (%) | 39 (9.0%) | 54 (12.4%) | 45 (10.3%) | 72 (16.6%) | 0.004 |
| Smoking (%) | 41 (9.4%) | 34 (7.8%) | 38 (8.7%) | 43 (9.9%) | 0.723 |
| Alcohol intake (%) | 44 (10.1%) | 42 (9.7%) | 41 (9.4%) | 42 (9.7%) | 0.988 |
| Physical activity (%) | 272 (62.7%) | 277 (63.7%) | 276 (63.4%) | 296 (68.2%) | 0.312 |
| Total intake (g/day) | 1455.72 ± 33.66 | 1453.13 ± 30.85 | 1412.77 ± 31.97 | 1400.72 ± 34.32 | 0.533 |
| Total energy intake (kcal/day) | 2275.36 ± 43.76 | 2283.16 ± 42.33 | 2288.13 ± 43.19 | 2282.06 ± 47.83 | 0.998 |
| Protein intake (g/day) | 81.27 ± 2.24 | 81.30 ± 1.92 | 83.13 ± 1.94 | 81.67 ± 2.01 | 0.907 |
| Fat intake (g/day) | 57.90 ± 1.96 | 57.22 ± 1.65 | 60.68 ± 2.07 | 56.70 ± 1.75 | 0.445 |
| Carbohydrate intake (g/day) | 355.57 ± 6.26 | 359.72 ± 6.65 | 352.24 ± 6.15 | 357.28 ± 7.34 | 0.879 |
| Sodium intake (g/day) | 4.82 ± 0.12 | 4.36 ± 0.11 | 4.74 ± 0.13 | 4.70 ± 0.15 | 0.016 |
| Water intake (g/day) | 933.21 ± 26.48 | 932.37 ± 24.29 | 890.92 ± 25.09 | 878.89 ± 26.68 | 0.309 |
The data are shown as the means ± SEs (standard errors).
BMI; body mass index, SDS; standard deviation score, WC; waist circumference, SBP; systolic blood pressure, DBP; diastolic blood pressure, HOMA-IR; homeostatic model assessment of insulin resistance, QUICKI; quantitative insulin sensitivity check index, T-C; total cholesterol, HDL-C; high-density lipoprotein cholesterol, TG; triglyceride, LDL-C; low-density lipoprotein cholesterol.
Adjusted means for MetS components according to the U-Na to U-SG ratio
The adjusted means for MetS components were calculated through an ANCOVA after adjustment for possible confounding factors, including age, BMI SDS, ferritin, vitamin D, house income, smoking, alcohol intake, physical activity, season, total intake, total energy intake, protein intake, fat intake, carbohydrate intake, and water intake according to the U-Na to U-SG ratio (divided into quartiles). The adjusted means for MetS components according to the U-Na to U-SG ratio are presented in Table 2. Significant inverse associations were found for observed between the U-Na to U-SG ratio and HDL-C (P = 0.033 for trend). Significantly lower HDL-C levels were detected in the boys in Q4 than in the boys in Q2 (P = 0.020). A significant positive association was observed between the U-Na to U-SG ratio and TG (P = 0.001 for trend). The TG concentrations were significantly higher in boys in Q4 compared with the boys in Q1 (P = 0.001) and Q2 (P = 0.033). Although the trends did not reach statistical significance, a higher U-Na to U-SG ratio tended to be associated with higher SBP (P = 0.086 for trend), DBP (P = 0.063 for trend), and glucose (P = 0.099 for trend) values.
Table 2. Adjusted means for metabolic syndrome (MetS) components according to the urinary sodium to urinary specific gravity ratio in Korean boys aged 10–18 years (n = 1,738).
| Urinary sodium to urinary specific gravity ratio | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P for trend | |
| WC SDS | -0.30 ± 0.02 | -0.25 ± 0.02 | -0.25 ± 0.02 | -0.22 ± 0.02 | 0.173 |
| SBP (mmHg) | 106.95 ± 0.50 | 107.72 ± 0.50 | 107.47 ± 0.51 | 108.76 ± 0.51 | 0.086 |
| DBP (mmHg) | 66.15 ± 0.45 | 66.93 ± 0.44 | 66.26 ± 0.45 | 67.69 ± 0.46 | 0.063 |
| Glucose (mg/dL) | 89.56 ± 0.31 | 89.67 ± 0.30 | 90.12 ± 0.31 | 90.55 ± 0.32 | 0.099 |
| HDL-C (mg/dL) | 53.11 ± 0.52 | 54.29 ± 0.51 | 52.98 ± 0.53 | 52.14 ± 0.53b | 0.033 |
| TG (mg/dL) | 77.89 ± 2.66 | 82.23 ± 2.63 | 85.29 ± 2.71 | 92.79 ± 2.72a,b | 0.001 |
The data are shown as the means ± SEs (standard errors).
WC; waist circumference, SDS; standard deviation score, SBP; systolic blood pressure, DBP; diastolic blood pressure, HDL-C; high-density lipoprotein cholesterol, TG; triglyceride.
Adjusted means for WC SDS, SBP, DBP, glucose, HDL-C, and TG were determined after adjustment for age, body mass index (BMI) SDS, ferritin, vitamin D, house income, smoking, alcohol intake, physical activity, season, total intake, total energy intake, protein intake, fat intake, carbohydrate intake, sodium intake, and water intake through an analysis of covariance (ANCOVA) according to the urinary sodium to urinary specific gravity ratio.
a: P<0.05 vs. the first quartile
b: P<0.05 vs. the second quartile
c: P<0.05 vs. the third quartile.
Adjusted ORs of MetS and its components according to the U-Na to U-SG ratio
The overall prevalence of MetS was 4.2% in this study. The prevalences of MetS according to a U-Na to U-SG ratio in the lowest, second, third and highest quartiles were 2.8%, 3.7%, 3.7%, and 6.7%, respectively (P = 0.024). The risks of MetS components and MetS according to the U-Na to U-SG ratio (divided into quartiles) were assessed through a multivariate logistic regression analysis after controlling for the abovementioned possible confounders. Table 3 shows the adjusted ORs for MetS and its components according to the U-Na to U-SG ratio. Boys with a U-Na to U-SG ratio in the highest quartile had a 1.73-fold increased risk for elevated TG (95% CI, 1.19–2.51) and a 2.66-fold increased risk for MetS (95% CI, 1.11–6.35) compared with those with a ratio in the lowest quartile. Additionally, significant positive linear associations were observed for the quartiles of the U-Na to U-SG ratio with elevated TG (P = 0.004 for trend) and MetS (P = 0.039 for trend).
Table 3. Adjusted odds ratio (95% CI) of metabolic syndrome (MetS) and its components according to the urinary sodium to urinary specific gravity ratio in Korean boys aged 10–18 years (n = 1,738).
| Urinary sodium to urinary specific gravity ratio | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P for trend | |
| Elevated WC | Reference | 0.97 (0.38–2.45) | 0.35 (0.13–0.94) | 0.72 (0.29–1.78) | 0.249 |
| Elevated BP | Reference | 1.10 (0.79–1.52) | 0.87 (0.62–1.22) | 1.27 (0.91–1.76) | 0.352 |
| Elevated glucose | Reference | 1.01 (0.13–7.58) | 0.79 (0.10–5.99) | 1.38 (0.23–8.43) | 0.741 |
| Reduced HDL-C | Reference | 0.97 (0.57–1.65) | 1.18 (0.70–1.99) | 1.32 (0.79–2.21) | 0.209 |
| Elevated TG | Reference | 1.34 (0.92–1.95) | 1.44 (0.99–2.10) | 1.73 (1.19–2.51) | 0.004 |
| MetS | Reference | 1.74 (0.68–4.42) | 1.28 (0.49–3.34) | 2.66 (1.11–6.35) | 0.039 |
WC, waist circumference; BP, blood pressure; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride, MetS; metabolic syndrome.
The adjusted odds ratios (ORs) of MetS and its components were calculated through multivariate logistic regression analyses after adjustment for age, body mass index (BMI) standard deviation scores (SDS), ferritin, vitamin D, house income, smoking, alcohol intake, physical activity, season, total intake, total energy intake, protein intake, fat intake, carbohydrate intake, sodium intake, and water intake.
Elevated WC was defined as values greater than or equal to the 90th percentile for age and gender. Elevated BP was defined as SBP or DBP greater than or equal to the 90th percentile for age, gender, and height or current administration of antihypertensive drug. Elevated glucose was defined as glucose greater than or equal to 110 mg/dL or medication for diabetes. Elevated TG was defined as TG greater than or equal to 110 mg/dL or current administration of drugs for dyslipidemia. Reduced HDL-C was defined as HDL-C less than 40 mg/dL or current administration of drugs for dyslipidemia.
Discussion
In the current nationally representative study, the boys with a U-Na to U-SG ratio (which was assessed as a surrogate for sodium intake) in the highest quartile exhibited increased mean weight SDS, BMI SDS, and WC SDS values. Covariance analyses revealed a significant positive association between the U-Na to U-SG ratio and TG and a significant inverse association between the U-Na to U-SG ratio and HDL-C in Korean boys aged 10–18 years after adjustment for possible confounders. An ANCOVA showed that a higher U-Na to U-SG ratio tended to be associated with higher SBP, DBP, and glucose values after controlling for possible confounders, but the trends did not reach statistical significance. After adjusting for confounders, multivariate logistic regression analyses revealed that boys with a U-Na to U-SG ratio in the highest quartile exhibited significantly increased risks for elevated TG and MetS compared with those with a ratio in the lowest quartile.
A previous study suggested that high sodium intake is related to MetS, which is characterized by a set of cardiometabolic risk factors, including central obesity, high blood pressure, impaired fasting glucose, and dyslipidemia (elevated triglyceride concentration and low HDL-C concentration) [21]. This relationship may be predominantly explained by previous findings that high sodium intake is closely linked to increased blood pressure [22]. A Brazilian study demonstrated that high sodium intake is associated with BP but not with other MetS components [12]. A study conducted by Hoffmann et al. in Venezuela found that higher urinary sodium is associated with obesity and higher BP but not dyslipidemia or fasting glucose [23]. However, there is evidence indicating that sodium intake is independently associated with insulin resistance-related diseases, such as dyslipidemia, metabolic syndrome and T2DM, in adults. The OPERA (Oulu Project Evaluating the Risk of Atherosclerosis) study found that high sodium intake is associated with MetS components, such as WC and high fasting glucose [24], and Baudrand et al. reported that a high sodium diet is associated with dyslipidemia in Chilean adults [15]. A recent Korean study demonstrated that the estimated 24-hour urine excretion of sodium, which was used as a surrogate of sodium intake, is associated with MetS and its components [25]. Some studies conducted in pediatric fields have indicated that high sodium intake is related to obesity and hypertension [15,26,27], but few studies have evaluated the association of sodium intake with MetS and its components with the exception of elevated BP. In this study, after adjusting for confounders, we performed multivariate logistic regression analyses and demonstrated that boys with a U-Na to U-SG ratio in the highest quartile exhibited significantly higher risks for elevated TG and MetS compared with those with a U-Na to U-SG ratio in the lowest quartile. Our results are in line with previous reports demonstrating that a high sodium intake is related to MetS.
The pathophysiology of the association between sodium intake and insulin resistance is not fully understood. A possible explanation is that high sodium intake is associated with obesity [28], which is predominantly related to insulin resistance, a key mechanism of MetS. It has been demonstrated that increased salt intake is related to stimulation of thirst and appetite and that there is a positive association between salt intake and sugar-sweetened soft drink consumption [29]. Accordingly, a high salt intake is thought to lead to high energy intake and obesity. An Australian study reported that dietary salt intake predicts total fluid consumption and sugar-sweetened beverage consumption, which are associated with obesity risk [30]. However, in the present study, the U-Na to U-SG ratio, a surrogate for sodium intake, was found to be significantly independently associated with MetS after controlling for possible confounding factors.
Other explanations should be considered. Animal studies have yielded evidence indicating that a high salt diet may be independently associated with fat metabolism and insulin resistance, which is a key mechanism of MetS. A high salt diet induces higher adiposity and adipocyte hypertrophy, although no significant changes in the rat’s body weight were detected [31]. In a rat model of MetS, salt restriction induced improvement in insulin resistance without reducing obesity [32]. An animal study suggested that insulin resistance induced by a high sodium diet is related to impaired insulin-stimulated microvascular recruitment [33]. In addition, human studies have indicated that high sodium intake may be independently associated with MetS and its components. A US study suggested that high sodium intake is independently positively associated with adiposity and inflammation in adolescents after adjusting for total energy intake and sugar-sweetened soft drink consumption [34]. It has been reported that high sodium intake is independently related to MetS components, including insulin resistance, dyslipidemia, and hypertension, even after adjusting for confounding variables including age, gender, and BMI [25,35]. Alternatively, a low dietary sodium intake reduces insulin secretion in humans independently of insulin resistance [36]. This study demonstrated that high sodium intake was significantly independently associated with TG and MetS, which is in line with the results from previous studies.
The gold standard for the assessment of sodium intake is urinary sodium excretion from 24-hour urine collection [37], which is more inconvenient than spot urine collection in clinical settings, and approximately 30% of individuals submit under-collections and underestimations of their actual sodium intake [38]. Spot urine samples can be used in large population studies. There are clinically applicable 24-hour sodium excretion equations using spot urinary sodium and urinary creatinine for adults [37,38]. In addition, population-based studies have validated the estimation of 24-hour urinary sodium secretion in adults [22,39]. However, no studies have assessed the medical formula using spot urinary sodium to estimate 24-hour urinary sodium for children and adolescents. A Korean study in adolescents showed that the U-Na to U-SG ratio may be used as a surrogate for sodium intake [35]. In the current study, we utilized the U-Na to U-SG ratio as a surrogate for sodium intake and showed a significant positive association between the U-Na to U-SG ratio and MetS. Nevertheless, a valid and reliable formula for estimating 24-hour urinary sodium using spot urine samples from children and adolescents should be investigated.
A potential limitation of the present study is its cross-sectional nature because causality cannot be proven. Second, we could not adjust the data for pubertal status, familial history of T2DM, or levels of several adipokines. Third, we analyzed the data for dietary intake of nutrients based on a nutritional survey using 24-hour dietary recall, but with dietary recall, many subjects are truly unaware of the amount and type of nutrients they have consumed [40], which may have affected the accuracy of the data, potentially leading to recall bias. However, this study was performed using data from a nationwide examination survey, and the dietary intake of nutrients was adjusted for as a confounder in the ANCOVA and multivariate logistic regression analyses. Finally, we could not adjust for various confounding factors that can influence sodium homeostasis, including past medical history and medications, such as diuretics and inhibitors of the renin–angiotensin system.
The present nationwide, population-based, cross-sectional study showed that the U-Na to U-SG ratio, as a surrogate for sodium intake, was significantly positively associated with TG and significantly inversely associated with HDL-C in Korean boys aged 10–18 years, as demonstrated through an ANCOVA after adjustment for possible confounders. Although the trends did not have statistical significance, a higher U-Na to U-SG ratio tended to be associated with higher SBP, DBP, and glucose values, as demonstrated through an ANCOVA after controlling for confounders. The highest quartile of the U-Na to U-SG ratio was significantly related to increased risks for elevated TG and MetS compared with the lowest quartile among Korean boys aged 10–18 years, as demonstrated through multivariate logistic regression analyses after adjusting for confounders. Our results suggest that high sodium intake, which is determined as the U-Na to U-SG ratio, may be significantly independently associated with MetS in Korean boys aged 10–18 years.
Data Availability
This study used third-party data from the Korean National Health and Nutrition Examination Survey (KNHANES). All relevant data are available from the KNHANES database. which is available to the public via the KNHANES website at the following link: http://knhanes.cdc.go.kr. Others would be able to access these data in the same manner. The authors did not have any special access privileges that others would not have.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Yu SH, Song Y, Park M, Kim SH, Shin S, Joung H. Relationship between adhering to dietary guidelines and the risk of obesity in Korean children. Nutr Res Pract. 2014;8(6):705–12. doi: 10.4162/nrp.2014.8.6.705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li L, de Moira AP, Power C. Predicting cardiovascular disease risk factors in mid-adulthood from childhood body mass index: utility of different cut-offs for childhood BMI. Am J Clin Nutr. 2011;93(6):1204–11. doi: 10.3945/ajcn.110.001222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reaven PD, Traustadóttir T, Brennan J, Nader PR. Cardiovascular Risk Factors Associated With Insulin Resistance in Children Persist Into Late Adolescence. Diabetes Care. 2005;28(1):148–50. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd LJ, Langley-Evans SC, McMullen S. Childhood obesity and risk of the adult metabolic syndrome: a systematic review. Int J Obes (Lond). 2012;36(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juhola J, Magnussen CG, Viikari JS, Kahonen M, Hutri-Kahonen N, Jula A, et al. Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: the Cardiovascular Risk in Young Finns Study. J Pediatr. 2011;159(4):584–90. doi: 10.1016/j.jpeds.2011.03.021 [DOI] [PubMed] [Google Scholar]
- 6.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350(23):2362–74. doi: 10.1056/NEJMoa031049 [DOI] [PubMed] [Google Scholar]
- 7.Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152(2):201–6. doi: 10.1016/j.jpeds.2007.09.010 [DOI] [PubMed] [Google Scholar]
- 8.Kim S, So W-Y. Prevalence of Metabolic Syndrome among Korean Adolescents According to the National Cholesterol Education Program, Adult Treatment Panel III and International Diabetes Federation. Nutrients. 2016;8(10):588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beltran-Sanchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J Am Coll Cardiol. 2013;62(8):697–703. doi: 10.1016/j.jacc.2013.05.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Q, Zhang Z, Kuklina EV, Fang J, Ayala C, Hong Y, et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130(4):611–9. doi: 10.1542/peds.2011-3870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371(7):612–23. doi: 10.1056/NEJMoa1311889 [DOI] [PubMed] [Google Scholar]
- 12.Rodrigues SL, Baldo MP, de Sa Cunha R, Andreao RV, Del Carmen Bisi Molina M, Goncalves CP, et al. Salt excretion in normotensive individuals with metabolic syndrome: a population-based study. Hypertens Res. 2009;32(10):906–10. doi: 10.1038/hr.2009.122 [DOI] [PubMed] [Google Scholar]
- 13.Won JC, Hong JW, Noh JH, Kim D-J. Association Between Estimated 24-h Urinary Sodium Excretion and Metabolic Syndrome in Korean Adults: The 2009 to 2011 Korea National Health and Nutrition Examination Survey. Medicine. 2016;95(15):e3153 doi: 10.1097/MD.0000000000003153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Libuda L, Kersting M, Alexy U. Consumption of dietary salt measured by urinary sodium excretion and its association with body weight status in healthy children and adolescents. Public Health Nutr. 2012;15(3):433–41. doi: 10.1017/S1368980011002138 [DOI] [PubMed] [Google Scholar]
- 15.Baudrand R, Campino C, Carvajal CA, Olivieri O, Guidi G, Faccini G, et al. High sodium intake is associated with increased glucocorticoid production, insulin resistance and metabolic syndrome. Clin Endocrinol (Oxf). 2014;80(5):677–84. [DOI] [PubMed] [Google Scholar]
- 16.Yi KH, Hwang JS, Kim EY, Lee SH, Kim DH, Lim JS. Prevalence of insulin resistance and cardiometabolic risk in Korean children and adolescents: a population-based study. Diabetes Res Clin Pract. 2014;103(1):106–13. doi: 10.1016/j.diabres.2013.10.021 [DOI] [PubMed] [Google Scholar]
- 17.Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol. 2014;43(1):69–77. doi: 10.1093/ije/dyt228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 19.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157(8):821–7. doi: 10.1001/archpedi.157.8.821 [DOI] [PubMed] [Google Scholar]
- 20.Moon JS, Lee SY, Nam CM, Choi JM, Choe BK, Seo JW, et al. 2007 Korean National Growth Charts: review of developmental process and an outlook. Korean J Pediatr. 2008;51(1):1–25. [Google Scholar]
- 21.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366(9491):1059–62. doi: 10.1016/S0140-6736(05)67402-8 [DOI] [PubMed] [Google Scholar]
- 22.Mente A, O'Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, Wielgosz A, et al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med. 2014;371(7):601–11. doi: 10.1056/NEJMoa1311989 [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann IS, Cubeddu LX. Salt and the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2009;19(2):123–8. doi: 10.1016/j.numecd.2008.02.011 [DOI] [PubMed] [Google Scholar]
- 24.Raisanen JP, Silaste ML, Kesaniemi YA, Ukkola O. Increased daily sodium intake is an independent dietary indicator of the metabolic syndrome in middle-aged subjects. Ann Med. 2012;44(6):627–34. doi: 10.3109/07853890.2011.585657 [DOI] [PubMed] [Google Scholar]
- 25.Oh SW, Han KH, Han SY, Koo HS, Kim S, Chin HJ. Association of Sodium Excretion With Metabolic Syndrome, Insulin Resistance, and Body Fat. Medicine (Baltimore). 2015;94(39):e1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellison RC, Sosenko JM, Harper GP, Gibbons L, Pratter FE, Miettinen OS. Obesity, sodium intake, and blood pressure in adolescents. Hypertension. 1980;2(4 Pt 2):78–82. [PubMed] [Google Scholar]
- 27.Woodruff SJ, Fryer K, Campbell T, Cole M. Associations among blood pressure, salt consumption and body weight status of students from south-western Ontario. Public Health Nutr. 2014;17(5):1114–9. doi: 10.1017/S1368980013000335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grimes CA, Riddell LJ, Campbell KJ, He FJ, Nowson CA. 24-h urinary sodium excretion is associated with obesity in a cross-sectional sample of Australian schoolchildren. Br J Nutr. 2016;115(6):1071–9. doi: 10.1017/S0007114515005243 [DOI] [PubMed] [Google Scholar]
- 29.He FJ, Marrero NM, MacGregor GA. Salt intake is related to soft drink consumption in children and adolescents: a link to obesity? Hypertension. 2008;51(3):629–34. doi: 10.1161/HYPERTENSIONAHA.107.100990 [DOI] [PubMed] [Google Scholar]
- 30.Grimes CA, Riddell LJ, Campbell KJ, Nowson CA. Dietary salt intake, sugar-sweetened beverage consumption, and obesity risk. Pediatrics. 2013;131(1):14–21. doi: 10.1542/peds.2012-1628 [DOI] [PubMed] [Google Scholar]
- 31.Fonseca-Alaniz MH, Brito LC, Borges-Silva CN, Takada J, Andreotti S, Lima FB. High dietary sodium intake increases white adipose tissue mass and plasma leptin in rats. Obesity (Silver Spring). 2007;15(9):2200–8. [DOI] [PubMed] [Google Scholar]
- 32.Hattori T, Murase T, Takatsu M, Nagasawa K, Matsuura N, Watanabe S, et al. Dietary salt restriction improves cardiac and adipose tissue pathology independently of obesity in a rat model of metabolic syndrome. J Am Heart Assoc. 2014;3(6):e001312 doi: 10.1161/JAHA.114.001312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Premilovac D, Richards SM, Rattigan S, Keske MA. A vascular mechanism for high-sodium-induced insulin resistance in rats. Diabetologia. 2014;57(12):2586–95. doi: 10.1007/s00125-014-3373-y [DOI] [PubMed] [Google Scholar]
- 34.Zhu H, Pollock NK, Kotak I, Gutin B, Wang X, Bhagatwala J, et al. Dietary Sodium, Adiposity, and Inflammation in Healthy Adolescents. Pediatrics. 2014;133(3):e635–e42. doi: 10.1542/peds.2013-1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chun YH, Han K, Kim DH, Park YG, Cho KH, Choi YS, et al. Association of Urinary Sodium Excretion With Insulin Resistance in Korean Adolescents: Results From the Korea National Health and Nutrition Examination Survey 2009–2010. Medicine (Baltimore). 2016;95(17):e3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luther JM, Byrne LM, Yu C, Wang TJ, Brown NJ. Dietary Sodium Restriction Decreases Insulin Secretion Without Affecting Insulin Sensitivity in Humans. J Clin Endocrinol Metab. 2014;99(10):E1895–E902. doi: 10.1210/jc.2014-2122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Boer IH, Kestenbaum B. Invited commentary: Quantifying salt in urine—a complex solution. Am J Epidemiol. 2013;177(11):1193–5; discussion 6–8. doi: 10.1093/aje/kwt064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawasaki T, Itoh K, Uezono K, Sasaki H. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin Exp Pharmacol Physiol. 1993;20(1):7–14. [DOI] [PubMed] [Google Scholar]
- 39.McLean R, Williams S, Mann J. Monitoring population sodium intake using spot urine samples: validation in a New Zealand population. J Hum Hypertens. 2014;28(11):657–62. doi: 10.1038/jhh.2014.10 [DOI] [PubMed] [Google Scholar]
- 40.Bentley B. A review of methods to measure dietary sodium intake. J Cardiovasc Nurs. 2006;21(1):63–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study used third-party data from the Korean National Health and Nutrition Examination Survey (KNHANES). All relevant data are available from the KNHANES database. which is available to the public via the KNHANES website at the following link: http://knhanes.cdc.go.kr. Others would be able to access these data in the same manner. The authors did not have any special access privileges that others would not have.