Graphical abstract
Keywords: Age, Sex, Pregnancy, Menopause, Physiologic, Pathologic, Hypermanganesemia
Highlights
-
•
The interaction of iron with manganese (Mn) is the major factor affecting blood Mn level in general population.
-
•
Children and pregnant women had no adverse effects from blood Mn levels associated with adverse effects in adult workers.
-
•
The differences between a physiological and a pathological hypermanganesemia complicate the dose-response relationship.
Abstract
The objective of the present study was to evaluate sex, menopause, pregnancy, and age-specific differences of blood manganese (Mn) levels in relation to iron status, and to assess the toxicological implications of these relationships. Females of childbearing age have higher concentrations of blood Mn than males because women have lower concentrations of ferritin. Previous studies indicated significant increases in blood Mn levels throughout pregnancy, and that the geometric mean of blood Mn was significantly higher in premenopausal women than postmenopausal women. This may be due to the enhanced absorption of Mn because of upregulation of iron absorption, which is especially important during late pregnancy. Mn concentrations are highest in infancy, decreased with age up to adolescence, and did not change during adulthood. Thus, the relationship of iron with Mn may be the major factor affecting blood Mn levels according to menstrual stage, reproductive status, menopausal factors, and age. However, Mn absorbed via the gastrointestinal system seems to be less neurotoxic than inhaled or parenteral Mn, due to the tight enterohepatic homeostatic control of this essential element. Furthermore, children and pregnant women had no adverse health effects from blood levels of Mn that were associated with adverse effects in adult workers. In conclusion, the differences between a physiological and a pathological hypermanganesemia complicate interpretation of the dose-response relationship.
1. Background
Manganese (Mn) is a substance with a dual role in organism. Mn is an essential trace element that is required for maintaining proper function and regulation of numerous biochemical and cellular reactions. Despite its essentiality, at excessive concentrations Mn is highly toxic to the central nervous system [29], [33]. Animal studies demonstrated that intestinal absorption of Mn is markedly greater under conditions of iron deficiency. The gastrointestinal absorption of Mn appears to require intestinal iron transporters, such as apical divalent metal transporter 1 (DMT1), which also mediates the uptake of cadmium. DMT1 expression is greater in the presence of low iron stores, and this explains the increased Mn uptake and blood Mn under conditions of iron deficiency [7], [11], [15], [16], [22], [25]. Emerging new evidence indicates that the iron exporter ferroportin also transports Mn [12], [24].
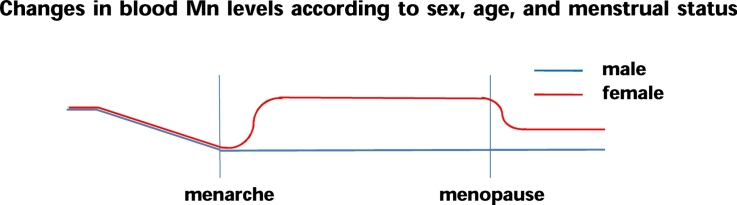
Females of childbearing age have higher concentrations of blood Mn than males because women have lower concentrations of ferritin [19]. Premenopausal women have a higher geometric mean (GM) concentration of blood Mn than postmenopausal women. Increased ferritin may not affect blood Mn levels at menopause, because DMT1 may be inactivated at this time [23]. Previous studies consistently reported significant increases in the mean blood Mn levels throughout pregnancy [34], [35], [36]. Rükgauer [32] showed that serum Mn concentration was highest during infancy, decreased with age up to adolescence, and did not change during adulthood.
The objective of the present study was to evaluate sex, menopause, pregnancy, and age-specific differences of blood Mn levels in relation to iron status, and to assess the toxicological implications of these findings. A literature review was performed on relevant articles and their references in the field of toxicity, physiology, and toxicology of Mn using PubMed from 1965 until 2016.
2. Sex, menopause, pregnancy, and age-specific differences of blood Mn levels in relation to iron status
2.1. Sex
My previous study showed that females of childbearing age had higher concentrations of blood Mn than males in general population [23] and residents with environmental exposure to Mn [20], in agreement with other studies [2], [16], [25], [27] (Table 1). DMT1 expression is greater in the presence of low iron stores, and this may explain the increased Mn uptake and blood Mn levels of females, because females of childbearing age have lower concentrations of ferritin than males [7], [11], [15], [16], [22], [25].
Table 1.
Studies that examined the effect of sex, menopause, pregnancy, and age on blood Mn concentration.
| Variables | No. (n) | Study subjects and findings |
|---|---|---|
| Sex | (n = 2005) | Korean general population 20 y or more; KNHANES 2008/GM of blood Mn in female vs. male: 1.403 vs. 1.192 μg/dL* [19] |
| (n = 297) | Canadian general population/GM of blood Mn in female vs. male: 0.750 vs. 0.675 μg/dL* [2] | |
| (n = 7720) | USA general population (NHANES 2010-11)/GM of blood Mn in female vs. male: 0.99 vs. 0.87 μg/dL* [27] | |
| Menopause | (n = 1826) | Korean general population KNHANES 2008–2009/GM of blood Mn in pre- vs. post-menopause: 1.443 vs. 1.296 μg/dL* [23] |
| Pregnancy | (n = 34) | Australian general population/Maternal blood Mn during pregnancy from 10 to 20 wk vs. 25 vs. 34 wk; 0.82 vs. 0.94 vs. 1.26 μg/dL [34] |
| (n = 290) | Canadian general population/Maternal blood GM Mn during pregnancy at 3rd, vs. 2nd, vs. 1 st trimester vs. non-pregnant 1.56 vs. 0.95 vs. 0.85 and 0.746 μg/dL [35] | |
| (n = 66) | Swedish general population/Maternal blood median Mn during pregnancy at 3rd, vs. 2nd, vs. 1 st trimester 1.26 vs. 1.04 vs. 0.85 μg/dL [36] | |
| (n = 470) | Canadian general population/Maternal blood AM Mn during pregnancy at delivery vs. non-pregnant; 2.4 vs. (0.8–1.2) μg/dL [37] | |
| (n = 1085) | USA general population/blood GM Mn in pregnancy vs. non-pregnant; 1.19 vs. 1.02 μg/dL [27] | |
| (n = 265) | Korean general population/blood GM Mn in pregnancy; 2.25 μg/dL [8] | |
| Age | (n = 2005) | Korean general population 20 y or more; KNHANES 2008/No significant change between 20′s and 40′s [19] |
| (n = 7720) | US general population/blood GM Mn in age groups (1–5 years-old: 1.07 μg/dL; 6–11 years-old: 1.03 μg/dL; 12–19 years-old: 1.01 μg/dL; 20+ years-old: 0.91 μg/dL) [4] | |
| (n = 205) | German population/serum mean Mn in age groups (1, 4–6, 10–14, 22–75): 0.21, 0.14, 0.12, 0.08 μg/dL) [32] | |
GM, geometric mean; KNHANES, Korea National Health and Nutrition Examination Survey; NHANES, National Health and Nutrition Examination Survey. * Statistically significant.
2.2. Menopause
Previous research showed that premenopausal women had a significantly higher GM of blood Mn than postmenopausal women (1.436 vs. 1.30 μg/dL) in general population [23] (Table 1) and residents with environmental exposure to Mn [20]. Increased ferritin may not affect Mn levels during menopause, because DMT1 may be inactivated at this time. Multivariable linear regression analyses showed that serum ferritin and menopausal status were significant predictors of blood Mn (after adjusting for various covariates), and that menopausal status modified the effect of ferritin on blood Mn levels [23].
2.3. Pregnancy
Previous studies consistently reported significant increases in the mean blood Mn levels throughout pregnancy [34], [35], [36] (Table 1). This increase in blood Mn may be related to the enhanced absorption of Mn due to upregulation of iron absorption, particularly during late pregnancy [3], [21], because more iron is needed during the third trimester.
2.4. Age
Rükgauer [32] showed that serum Mn concentration was highest during infancy, decreased with age up to adolescence, and did not change during adulthood. A study of the general population in the United States also showed that the GM blood Mn level decreased with age (1–5 years-old: 1.07 μg/dL; 6–11 years-old: 1.03 μg/dL; 12–19 years-old: 1.01 μg/dL; 20+ years-old: 0.91 μg/dL) [4] (Table 1). In agreement, my research reported no age-related changes in blood Mn of individuals from their 20′s to 40′s [19], because homeostatic mechanisms control the absorption, deposition, and biliary excretion of this essential element. My previous study found that infants with iron deficiencies had a higher mean blood Mn concentration than controls (2.550 vs. 1.499 μg/dL), and that administration of iron therapy to iron-deficient infants significantly decreased their blood Mn levels, and significantly increased their hemoglobin and ferritin levels [28]. Interestingly, the mean blood Mn level in the control group of infants from this previous study (1.499 μg/dL) was higher than the reference value (1.215 μg/dL) reported for the general adult population of Korea [19] and the normal reference range (0.4–1.4 μg/dL) by the Agency for Toxic Substances and Disease Registry (ATSDR) [1]. Dorner et al. [13] showed that infants, especially premature infants, have higher levels of Mn than adults. Animal studies showed that absorption and/or retention of Mn is greater in neonates, but returns to the level of older animals at approximately post-gestational day 17–18 [30]. Mn requirements vary by life stage, and infants typically have higher levels than children or adults [31]. This is mainly due to the high need for iron and the concurrent absorption of Mn in infants, who experience rapid growth, and often suffer from inadequate intake of dietary iron between 6 months and 3 years of age [28].
In summary, the relationship of iron with Mn may be the major factor affecting changes in blood Mn due to menstrual stage, reproductive status, menopausal factors, and age in general population and residents with environmental exposure to Mn. However, whether negative associations between blood Mn concentration and low serum ferritin levels are observed in workers with occupational exposure, and their temporal relationship are not yet determined [14], [10].
3. Physiologic vs. pathologic hypermanganesemia
My previous study showed that patients with iron-deficiency anemia had higher blood Mn concentrations than controls (2.04 μg/dL vs. 1.28 μg/dL) [18]. However, the mean pallidal index (PI) in anemic patients was similar to that of controls, and neither group had high signal intensity of the globus pallidus in T1-weighted MRI. This finding is in contrast to those of my previous study of males with liver cirrhosis. In this cirrhosis study, we observed a significantly increased PI in 27 of 33 patients (81.8%) with liver cirrhosis (mean blood Mn concentration: 2.34 μg/dL) relative to controls without cirrhosis (mean blood Mn concentration: 1.44 μg/dL) [6]. Similarly, 18.6% of welders exposed to Mn (mean blood Mn: 1.53 μg/dL) had an increased PI, and the mean PI in welders was significantly different from that of controls (mean blood Mn: 1.14 μg/dL) (p < 0.001) [5]. Thus, although intestinal absorption of Mn is greater in patients with iron-deficiency anemia, MRI signal intensity in the globus pallidus is minimally affected, in contrast to individuals with liver cirrhosis or occupational Mn exposure. These findings are consistent with those of Newland et al. [26], who studied Mn toxicity in long-tailed macaques. They found that parenteral exposure increased the Mn concentration in the basal ganglia, and this led to a higher PI, but oral exposure did not increase Mn concentration in the brain and did not increase the PI. Mena et al. [25] showed that increased Mn excretion ameliorated the increased Mn absorbed due to anemia, thereby preventing toxic effects. Taken together, normal physiologic Mn exposure via intestinal absorption seems to be less neurotoxic than parenteral or inhaled Mn, due to the tight enterohepatic homeostatic control of this essential element.
Blood Mn levels in children and pregnant women are higher than in adults. In particular, the mean blood Mn level (1.499 μg/dL) of control infants was higher than the reference value (1.215 μg/dL) for the general adult population of Korea [19] and was also higher than control adults [5], [6], [18] of my previous studies. A study of the general population in the United States also showed that the GM blood Mn level decreased with age [4]. Data on pregnant women indicated that blood Mn levels at delivery was at least 1.5-2 times higher than that of non-pregnant women and women in early pregnancy [8], [34], [35], [36].
The blood Mn concentrations that are typical in children and pregnant women (from oral or non-occupational exposures) are similar to the critical concentrations reported for welders exposed to Mn, who have at most 1.5-fold higher blood Mn levels than controls, and also have poor neurobehavioral performance, and higher PI scores [5], [17]. Claus Henn et al. [9] reported an inverted U-shape relationship between blood Mn (mean: 2.43 μg/dL, SD: 4.5) and neurodevelopment in 12 month olds, in that Mn deficiency (1.5-2.0 μg/dL) and Mn excess (more than 3.0 μg/dL) were associated with lower scores. Chung et al. [8] suggested an inverted U-shaped relation between maternal blood Mn at term and neurodevelopmental indexes of infants 6 months after birth. They found that maternal blood Mn concentration up to approximately 2.4–2.8 μg/dL was positively associated with 6-month psychomotor development index (PDI) score, but higher maternal blood Mn concentration was associated with a lower PDI score, suggesting adverse neurodevelopmental effects of low levels (<2.0 μg/dL) and high levels (≥3.0 μg/dL) of maternal blood Mn. The results of these 2 studies of non-occupational exposure to Mn may seem perplexing, because blood Mn levels indicative of normal physiologic concentration in infants and pregnant women would be considered over threshold levels in adult workers. In other words, children and pregnant women do not exhibit adverse health effects when they have blood Mn levels associated with toxicity in adult workers. Taken together, these results suggest that the route or mechanism of exposure to Mn is more important than the blood Mn concentration.
The presence of different blood Mn levels in healthy subjects of different age, sex, and pregnancy and menopausal status makes it difficult to compare findings among studies, especially those related to dose-effect relationships. The differences between a physiological and a pathological hypermanganesemia, further complicates interpretation of the dose-response relationship. Thus, the interpretation of pathogenic values should be based on normal levels or threshold concentrations in different groups of study subjects while taking into account the pathway of Mn into the organism. Details of the dose-response relationships in different study subjects remain to be clarified.
Disclosure
The authors have no potential conflicts of interest to disclose.
References
- 1.ATSDR . ATSDR; 2012. Toxicological Profile for Manganese. [PubMed] [Google Scholar]
- 2.Baldwin M., Mergler D., Larribe F., Bélanger S., Tardif R., Bilodeau L., Hudnell K. Bioindicator and exposure data for a population based study of manganese. Neurotoxicology. 1999;20:343–353. [PubMed] [Google Scholar]
- 3.Bothwell T.H. Iron requirements in pregnancy and strategies to meet them. Am. J. Clin. Nutr. 2000;72:257S–264S. doi: 10.1093/ajcn/72.1.257S. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC), Fourth National Report on Human Exposure to Environmental Chemicals (Updated Tables). Atlanta (GA): CDC. (2015) 239.
- 5.Chang Y., Kim Y., Woo S.-T., Song H.-J., Kim S.H., Lee H., Kwon Y.J., Ahn J.H., Park S.-J., Chung I.-S., Jeong K.S. High signal intensity on magnetic resonance imaging is a better predictor of neurobehavioral performances than blood manganese in asymptomatic welders. Neurotoxicology. 2009;30:555–563. doi: 10.1016/j.neuro.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Choi Y., Park J.K., Park N.H., Shin J.W., Yoo C.-I., Lee C.R., Lee H., Kim H.K., Kim S.-R., Jung T.-H., Park J., Yoon C.S., Kim Y. Whole blood and red blood cell manganese reflected signal intensities of T1-weighted MRI better than plasma manganese in liver cirrhotics. J. Occup. Health. 2005;47:68–73. doi: 10.1539/joh.47.68. [DOI] [PubMed] [Google Scholar]
- 7.Chua A.C., Morgan E.H. Effects of iron deficiency and iron overload on manganese uptake and deposition in the brain and other organs of the rat. Biol. Trace. Element Res. 1996;55:39–54. doi: 10.1007/BF02784167. [DOI] [PubMed] [Google Scholar]
- 8.Chung S.E., Cheong H.-K., Ha E.-H., Kim B.-N., Ha M., Kim Y., Hong Y.-C., Park H., Oh S.-Y. Maternal blood manganese and early neurodevelopment: the mothers and children’s environmental health (MOCEH) study. Environ. Health Perspect. 2015;123:717–722. doi: 10.1289/ehp.1307865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claus Henn B., Ettinger A.S., Schwartz J., Téllez-Rojo M.M., Lamadrid-Figueroa H., Hernández-Avila M., Schnaas L., Amarasiriwardena C., Bellinger D.C., Hu H., Wright R.O. Early postnatal blood manganese levels and children's neurodevelopment. Epidemiology. 2010;21:433–439. doi: 10.1097/ede.0b013e3181df8e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowan D.M., Fan Q., Zou Y., Shi X., Chen J., Aschner M., Rosenthal F.S., Zheng W. Manganese exposure among smelting workers: blood manganese–iron ratio as a novel tool for manganese exposure assessment. Biomarkers. 2009;14:3–16. doi: 10.1080/13547500902730672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis C.D., Wolf T.L., Greger J.L. Varying levels of manganese and iron affect absorption and gut endogenous losses of manganese by rats. J. Nutr. 1992;122:1300–1308. doi: 10.1093/jn/122.6.1300. [DOI] [PubMed] [Google Scholar]
- 12.Donovan A., Lima C.A., Pinkus J.L., Pinkus G.S., Zon L.I., Robine S., Andrews N.C. The iron exporter ferroportin/Sc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Dörner K., Dziadzka S., Höhn A., Sievers E., Oldigs H.D., Schulz-Lell G., Schaub J. Longitudinal manganese and copper balances in young infants and preterm infants fed on breast-milk and adapted cow's milk formulas. Br. J. Nutr. 1989;61:559–572. doi: 10.1079/bjn19890143. [DOI] [PubMed] [Google Scholar]
- 14.Ellingsen D.G., Haug E., Ulvik R.J., Thomassen Y. Iron status in manganese alloy production workers. J. Appl. Toxicol. 2003;23:239–247. doi: 10.1002/jat.913. [DOI] [PubMed] [Google Scholar]
- 15.Erikson K.M., Shihabi Z.K., Aschner J.L., Aschner M. Manganese accumulates in iron-deficient rat brain regions in a heterogeneous fashion and is associated with neurochemical alterations. Biol. Trace Element Res. 2002;87:143–156. doi: 10.1385/BTER:87:1-3:143. [DOI] [PubMed] [Google Scholar]
- 16.Finley J.W. Manganese absorption and retention by young women is associated with serum ferritin concentration. Am. J. Clin. Nutr. 1999;70:37–43. doi: 10.1093/ajcn/70.1.37. [DOI] [PubMed] [Google Scholar]
- 17.Kim Y., Kim K.S., Yang J.S., Park I.J., Kim E., Jin Y. Increase in signal intensities on T1-weighted magnetic resonance images in asymptomatic manganese-exposed workers. Neurotoxicology. 1999;20:901–907. [PubMed] [Google Scholar]
- 18.Kim Y., Park J.K., Choi Y., Yoo C.-I., Lee C.R., Lee H., Lee J.-H., Kim S.-R., Jung T.-H., Yoon C.S., Park J.-H. Blood manganese concentration is elevated in iron deficiency anemia patients, whereas globus pallidus signal intensity is minimally affected. Neurotoxicology. 2005;26:107–111. doi: 10.1016/j.neuro.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y., Lee B.K. Iron defiency increases blood manganese level in the Korean general population according to KNHANES 2008. Neurotoxicology. 2011;32:247–254. doi: 10.1016/j.neuro.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y., Lobdell D.T., Wright C.W., Gocheva V., Hudgens E., Bowler R.M. Blood metal concentrations of manganese: lead and cadmium in relation to serum Ferritin levels in Ohio residents. Biol. Trace Elem. Res. 2015;165:1–9. doi: 10.1007/s12011-014-0223-1. [DOI] [PubMed] [Google Scholar]
- 21.Kirchgessner M., Sherif Y.S., Schwarz F.J. Changes in absorption of manganese during gravidity and lactation. Ann. Nutr. Metab. 1982;26:83–89. doi: 10.1159/000176549. [DOI] [PubMed] [Google Scholar]
- 22.Kwik-Uribe C.L., Gietzen D., German J.B., Golub M.S., Keen C.L. Chronic marginal iron intakes during early development in mice result in persistent changes in dopamine metabolism and myelin composition. J.Nutr. 2000;130:2821–2830. doi: 10.1093/jn/130.11.2821. [DOI] [PubMed] [Google Scholar]
- 23.Lee B.K., Kim Y. Effects of menopause on blood manganese levels in women: analysis of 2008–2009 Korean National Health and Nutrition Examination Survey data. Neurotoxicology. 2012;33:401–405. doi: 10.1016/j.neuro.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Madejczyk M.S., Ballatori N. Biochim. Biophys. Acta. 2012;1818:651–657. doi: 10.1016/j.bbamem.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mena I., Oscar M., Fuenzalida S., Cotizias G. Chronic manganese poisoning. Clinical pictures and manganese turnover. Neurology. 1967;17:128–136. doi: 10.1212/wnl.17.2.128. [DOI] [PubMed] [Google Scholar]
- 26.Newland M.C., Ceckler T.L., Kordower J.H., Weiss B. Visualizing manganese in the primate basal ganglia with magnetic resonance imaging. Exp. Neurol. 1989;106:251–258. doi: 10.1016/0014-4886(89)90157-x. [DOI] [PubMed] [Google Scholar]
- 27.Oulhote Y., Mergler D., Bouchard M.F. Sex- and age-differences in blood manganese levels in the U.S. general population: national health and nutrition examination survey 2011–2012. Environ. Health. 2014;13:87. doi: 10.1186/1476-069X-13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park S., Sim C.-S., Lee H., Kim Y. Blood manganese concentration is elevated in infants with iron deficiency. Biol. Trace Element Res. 2013;155:184–189. doi: 10.1007/s12011-013-9782-9. [DOI] [PubMed] [Google Scholar]
- 29.Peres T.V., Schettinger M.R., Chen P., Carvalho F., Avila D.S., Bowman A.B., Aschner M. Manganese-induced neurotoxicity: a review of its behavioral consequences and neuroprotective strategies. BMC Pharmacol. Toxicol. 2016;17:57. doi: 10.1186/s40360-016-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rehnberg G.L., Hein J.F., Carter S.D., Linko R.S., Laskey J.W. Chronic ingestion of Mn3O4 by rats: tissue accumulation and distribution of manganese in two generations. J. Toxicol. Environ. Health. 1982;9:175–188. doi: 10.1080/15287398209530153. [DOI] [PubMed] [Google Scholar]
- 31.Rice D.C., Lincoln R., Martha J., Parker L., Pote K., Xing S., Smith A.E. Concentration of metals in blood of Maine children 1–6 years old. J. Expo. Sci. Environ. Epidemiol. 2010;20:634–643. doi: 10.1038/jes.2010.42. [DOI] [PubMed] [Google Scholar]
- 32.Rükgauer M., Klein J., Kruse-Jarres J.D. Reference values for the trace elements copper, manganese, selenium, and zinc in the serum/plasma of children, adolescents, and adults. J. Trace Elements Med. Biol. 1997;11:92–98. doi: 10.1016/S0946-672X(97)80032-6. [DOI] [PubMed] [Google Scholar]
- 33.Sidoryk-Wegrzynowicz M., Aschner M. Manganese toxicity in the CNS: the glutamine/glutamate-γ-aminobutyric acid cycle. J. Intern. Med. 2013;273:466–477. doi: 10.1111/joim.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spencer A. Whole blood manganese levels in pregnancy and the neonate. Nutrition. 1999;15:731–734. doi: 10.1016/s0899-9007(99)00144-6. [DOI] [PubMed] [Google Scholar]
- 35.Takser L., Lafond J., Bouchard M., St-Amour G., Mergler D. Manganese levels during pregnancy and at birth: relation to environmental factors and smoking in a Southwest Quebec population. Environ. Res. 2004;95:119–125. doi: 10.1016/j.envres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Tholin K., Sandström B., Palm R., Hallmans G. Changes in blood manganese levels during pregnancy in iron supplemented and non supplemented women. J. Trace Elem. Med. Biol. 1995;9:13–17. doi: 10.1016/S0946-672X(11)80003-9. [DOI] [PubMed] [Google Scholar]
- 37.Zota A.R., Ettinger A.S., Bouchard M., Amarasiriwardena C.J., Schwartz J., Hu H., Wright R.O. Maternal blood manganese levels and infant birth weight. Epidemiology. 2009;20:367–373. doi: 10.1097/EDE.0b013e31819b93c0. [DOI] [PMC free article] [PubMed] [Google Scholar]