Graphical abstract
Keywords: Chloroquine, Biomarker, GOT, GPT, LDH, Histopathology, Acute, Sublethal, C.carpio
Highlights
-
•
Chloroquine (CQ) toxicity on fresh water fingerlings Cyprinus carpio was studied.
-
•
Median lethal concentration (96 h) was noted.
-
•
Acute (96 h) and sub-lethal (35 days) treatments was performed.
-
•
Enzymological activity and histological alteration was analysed.
-
•
Drug CQ has a toxic effect on non-target organism.
Abstract
In this study the toxicity of antimalarial drug chloroquine (CQ) on certain enzymological (GOT, GPT and LDH) and histopathological alterations (Gill, liver and kidney) of a freshwater fish Cyprinus carpio was studied after acute (96 h) and sublethal (35 days) exposure. The median lethal concentration (96 h) of CQ was 31.62 mg/ml. During acute treatment (CQ at 31.62 mg/ml) the treated fish groups showed a significant increase in GOT and GPT activities in blood plasma; whereas LDH activity was decreased when compare to control groups. To analyse the effects of drug at the lowest concentration, the fish were exposed to 3.16 mg/ml (1/10th of 96 h LC50 value) for 96 h. In sublethal treatment (3.16 mg/ml) GOT activity increased up to 14th day and decreased during the rest of the exposure period (21, 28 and 35th day). A biphasic response in GPT activity was observed. LDH activity was found to be increased throughout the study period (35 days) compare to control groups. The alterations in enzyme activities in blood plasma were found to be significant at p < 0.05 (DMRT). Many histopathological changes in vital organs such as gill, liver and kidney of fish were observed in CQ treated group (acute and sub-lethal) compare to normal group. The alterations in the enzymological and histopathological study in the present investigation indicate that the drug CQ has toxic effects on non-target organisms. We conclude that the alterations in enzymological parameters and histopathological changes can be used as biomarker to assess the health of the aquatic organism/environment. Further data on molecular studies are needed to define the mode of action and toxicity of these emerging pollutants.
1. Introduction
Quinoline is known for its bactericidal, antiseptic and antipyretic action. Chloroquine (CQ) belongs the quinoline group. It is a white or slightly yellow crystalline powder with bitter taste. It is a lysosomotropic weak base, soluble in water at pH 4.5 with molecular formula of C18H26CIN3. The derivatives of CQ includes chloroquine diphosphate (C18H29ClN3.2H3PO4), chloroquine phosphate (C18H29ClN3.H3PO4), chloroquine sulfate (C18H26ClN3. H2SO4) and chloroquine dihydrochloride (C18H26ClN3.2HCl) [1]. CQ has been used as a primary antimalarial drug since 1930s due to its tolerability, effectiveness against malaria and inexpensive synthesis [2]. In addition to serving as a malarial drug, CQ is now used in cancer therapy due to its enhancement property against tumour activity [3]. CQ is also shown to significantly improve insulin levels in type 2 diabetes (T2D) [4]. In addition CQ is used as an antifungal [5], it is used in the treatment of rheumatic and immune-mediated diseases [6], management of HIV, SARS-CoV and influenza A/H5N1 virus [7].
Unfortunately, CQ use could have various side effects in mammals such as cardiac arrest, blindness, arrhythmias, hypokalemia, retinopathy, renal failure and cerebral oedema. In addition, CQ treatment during pregnancy is extremely toxic to embryo and overdose could lead to death [8]. The negative effect of CQ is due to the fact that it inhibits diastolic depolarization, which slows down conduction and alters the intracellular transport of ionic transport. It also inhibits glucose 6-phosphate dehydrogenase activity, enzyme synthesis in nucleic acids; cyclic AMP pathway and it also increase oxidative stress in the organs [1], [9]. Due to its high affinity towards nucleates and nucleoproteins, CQ could accumulate in lysosomes, adrenal glands and in epithelial cells of kidney which alters the secretion of aldosterone [10].
Over production and extensive use of pharmaceuticals including CQ may reach the aquatic ecosystem mainly through sewage effluents, washing out of faecal materials by rain, domestic wastewater and STPs. The presence of these pharmaceutical drugs or their residues in the aquatic environment is a serious issue throughout the world. Due to their resistance to degradation and lipophilic property they persist in the aquatic environment and could have negative effects on the biota [11]. So far, more than 100 pharmaceuticals have been identified the aquatic ecosystem [12], recently Ramaswamy et al. [13] and Shanmugam et al. [14] have detected pharmaceutical and personal care products such as carbamazepine, triclosan, parabens, diclofenac, ketoprofen, naproxen, ibuprofen and acetylsalicylic acid in Indian major rivers such as Kaveri, Vellar and Thamiraparani.
Ecological risks by manmade chemicals are a potential subject of concern. Toxicity of any chemical can be determined by using bioassay methods. Specifically fish bioassay is considered as crucial in the field of eco-toxicology. As fish are one of the most organisms of the aquatic food web, and as they are a chief sources of food all over the world and as they are highly sensitive to slight environmental changes [15], [16] it is important to conduct fish bioassay. Bioassays play an important role in providing information about the impact of emerging chemicals [17]. In addition to bioassay, biomarkers are considered as early warning signals in the field of environment risk assessment. The biomarker response reveals the health status of an organism, population and ecosystem [18]. Biochemical and histological biomarkers are known to be sensitive tools to detect direct effects of pollutants in the specific organ [19]. These biomarkers may provide information from the starting point of biological effects to the impact on cell physiology [20].
Among the biochemical biomarkers enzymes are commonly used as a marker of pathological alterations of the organ, as they rapidly respond to chemicals. Glutamate oxaloacetate transaminase (GOT or AST), glutamate pyruvate transaminase (GPT or ALT) and lactate dehydrogenase (LDH) are the enzymes found in heart, liver, kidney, skeletal muscles and erythrocytes. GOT and GPT participate in transamination reactions. Likewise, LDH is an oxidative enzyme which is important for glycolytic activity. The alterations in these enzymes are used as organ health indicators of chemical exposure. GOT, GPT and LDH are widely used enzymological parameters in toxicology and in clinical chemistry to know the status of organs [21]. Similarly, histopathological changes provide the direct effects of the toxicant in organs [22] and also reveal the difference between damage induced by toxicant and other factors in organs/tissues [23]. In fish, gills are the primary site of toxicant exposure and their structural changes indicate the impact of toxicant. Liver is the second largest organ in the body and are known to be a defense organ. Antoine et al. [24] reported that liver is the major target area of human pharmaceuticals. Likewise kidney is a target organ for many pharmaceutical drugs. Hence, histological observation of vital organs such as gill, liver and kidney are important biomarkers in determining the toxic effect of human pharmaceuticals.
The present study was carried out to evaluate the acute and sublethal toxicity of chloroquine (CQ), an antimalarial drug in a freshwater fish Cyprinus carpio using certain biomarkers. The experimental model C. carpio is a common carp cultured widely in India
2. Materials and methods
2.1. Procurement of experimental fish and laboratory setup
Fingerlings of C. carpio (mean body weight of 6.0 ± 0.2 g and body length of 7.0 ± 0.3 cm) were collected/transported to the laboratory from Tamil Nadu Fisheries Development Corporation Limited, Aliyar, Tamilnadu, India in aerated polythene bags. The fingerlings were transported in the early morning to minimize the heat stress. After arrival to the laboratory, they were transferred into large size water tanks and stocked for a minimum period of 30 days to acclimatize. During acclimation period, C. carpio were fed with rice bran and ground nut oil cake in a dough form. Excess amount of feed and faecal materials were removed to avoid contamination of water. In the present study dechlorinated tap water was used. The physico-chemical parameter (temperature 27.1 ± 1.0 °C, pH 7.1 ± 0.10, DO 6.8 ± 0.05 mg/L, total alkalinity 18.2 ± 7.0 mg/L, total hardness 17.8 ± 0.6 mg/L, salinity 0.3 ± 0.06ppt, calcium 4.0 ± 0.1 mg/L, magnesium 2.5 ± 0.4 mg/L) of water was estimated according to procedure framed by APHA [25]. At the end of the acclimatization period, healthy fingerlings were divided randomly into two groups and housed separately in 200 L aquaria tank. The study was performed under a photoperiod of 12:12 light-dark cycle. The fingerlings in the aquaria served as the experimental stock (control and CQ exposed groups).
2.2. Protocol for determination of 96 h LC50
Stock solution was prepared by dissolving 1 g of CQ in appropriate quantity of tap water. A series of tests were carried out to determine the 96 h LC50 value of CQ to the fish C. carpio. Four-day acute static toxicity tests were performed to determine the median lethal concentration of CQ on C. carpio. Two plastic tubs (20L water capacity each) were taken and 8L of water was added to each tub. Fingerlings from the stock were randomly collected and 4 fish was introduced in each tub. A known concentration of CQ was added to each tub. Two tubs are separated and marked as control (not treated with CQ). At the end of every 12 h the numbers of dead and alive fingerlings were noted up to 96 h. The dead fingerlings were removed instantly. The concentration of the CQ in each test was calculated based upon the result obtained in the previous test. Finally a conformation test will be carried out after obtaining the LC50. In the conformation test 10 fingerlings from stock were introduced into tub (50 L water capacity) with 20 L of water. At the same time the control group was also maintained. The LC50 of CQ on C. carpio was calculated by the probit analysis method [26] and noted as 31.62 mg/L. During the test behavioural changes and signs were closely followed up. Homogenicity of the population in the study was calculated by using chi-square test (Table S1).
2.3. Acute toxicity studies
For acute toxicity study, six plastic tubs (50 L water capacity) were taken and to each tub 40 L of water was added. Then three tubs marked as CQ treated and remaining marked as control (C). Then 31.62 mg/L of CQ was added to tubs marked as CQ. 20 fingerlings from the stock were introduced in to each tub. At the end of 96 h blood and organs (gill, liver and kidney) were collected from C and CQ groups.
2.4. Sublethal toxicity studies
For the sub lethal study, 200 healthy fingerlings were randomly collected from the stock and separated into two groups (control and CQ treated). The fingerlings were introduced into two large size aquarium tanks marked C and CQ treated. 1/10 of LC 50 of CQ (3.16 mg/ml) was added and mixed well in the tank marked CQ treated. During the sub lethal study fingerlings were fed ad libitum, excess of feed was removed and the water was replaced with same concentration of CQ. At the end of every 7 day of sublethal exposure, blood and organs (gill, liver and kidney) were collected from the C and CQ groups.
2.5. Sampling
Cardiac blood was collected in a plastic disposable syringe fitted with 26 gauge needle. The syringe was pre-rinsed with the anticoagulant heparin. The collected blood was filled in a heparin rinsed vials and kept in ice cold condition. Whole blood was centrifuged for 15 min at 10 000 rpm and plasma was collected in separate vials. Plasma was used for analysis of biochemical parameters (GOT, GPT and LDH). Then the fingerlings were washed thoroughly with double distilled water and dried with Whattman filter paper. Then 100 mg of gill, liver and kidney of the fingerlings were removed and kept in the vials for morphological studies.
2.6. Plasma GOT, GPT and LDH activity
GOT and GPT activity of fingerlings was estimated according to the method of Reitmen and Frankel [27], LDH was estimated by Tietz [28] using the kit manufactured by Span Diagnostics Ltd. 173-B, New Industrial Estate, Road No. 6-G, Udhna, Surat - 394 210, INDIA.
2.7. Histological changes
Gill, liver and kidney of the fingerlings were dissected and fixed in Bouin’s fluid, dehydrated in graded alcohol, infiltrated with xylene, embedded in paraffin wax, mounted on glass slides and stained in haematoxylin and eosin (HE). Photomicrograph of gill, liver and kidney of C and CQ fingerlings were examined under computer based Trinocular Microscope with image analysis system (Labomed make).
2.8. Statistics
96 h LC50 value of CQ with 95% confidence was calculated by probit analysis method of Finney [26]. Differences between C and CQ groups were calculated for statistical significance at p < 0.05 by Student’s t-test, which is represented by an asterisk symbol. All the values were analysed from one way analysis of variance (software SPSS 6.0) and Duncan’s multiple range tests were analysed to measure the statistical differences between CQ groups. Triple asterisks symbol*** represents significant at <0.001 level.
3. Results
No mortality was observed during the acclimation period, before CQ treatment and in control groups. When the fingerlings exposed to various concentrations of CQ showed a sign of behavioural anomalies like fast swimming, rapid opercula movements, wide open of mouth and operculum, excess mucus secretion, convulsions and jerky movement. Finally the fish became lethargic, there was reduction in opercula movements, and they were at static condition usually at bottom and dead. These changes were more severe when the concentration of CQ increased. The fingerlings in the control groups were active and no anomaly was found. The 96 h LC50 value of CQ to C. carpio was calculated as 31.62 mg/ml. The Chi-square test indicated that the fish population used in this study were found homogenous. To assess the acute and sub-lethal toxicity, C. carpio fingerlings were exposed to 31.32 mg/ml and 3.16 mg/ml (1/10th of the 96 h LC50 value) of CQ respectively.
3.1. Acute effect of CQ on GOT, GPT and LDH activity
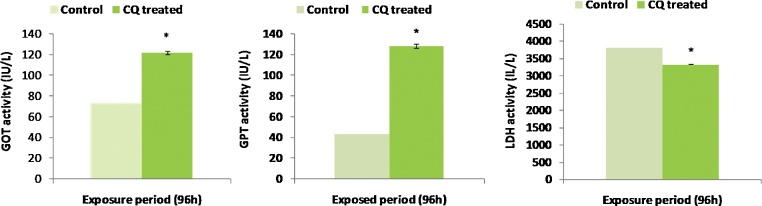
GOT (Fig. 1A) and GPT (Fig. 1B) activity in the plasma of C. carpio exposed to acute concentration of CQ increased significantly (p < 0.05) compared to the control group. The percentage change of GOT and GPT activity of CQ treated group were 67.01 and 28.70% respectively. However plasma LDH (Fig. 1C) activity of fingerlings exposed to acute concentration of CQ was found to be decreased significantly (p < 0.05) compared to control group after acute exposure.
Fig. 1.
Reveals the acute response of plasma enzyme (GOT (A), GPT (B) and LDH (C)) activity of C. carpio to CQ.
3.2. Activity of plasma GOT, GPT and LDH at sublethal concentration of CQ
3.2.1. Plasma GOT activity
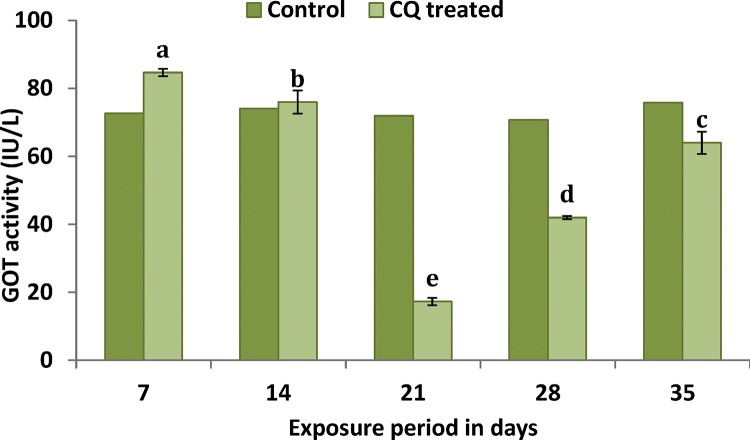
When compared to control group plasma GOT activity significantly (F4,24 = 1.522***) increased at the end of 7 and 14th days of exposure (Fig. 2). However after 21, 28 and 35th day of exposure GOT activity decreased significantly (F4,24 = 1.522***) in CQ treated groups when compare to control group.
Fig. 2.
Illustrates the sublethal response of plasma enzyme GOT activity of C. carpio to CQ. Value followed by the different letter are significantly different at p < 0.05 (DMRT).
3.2.2. Plasma GPT activity
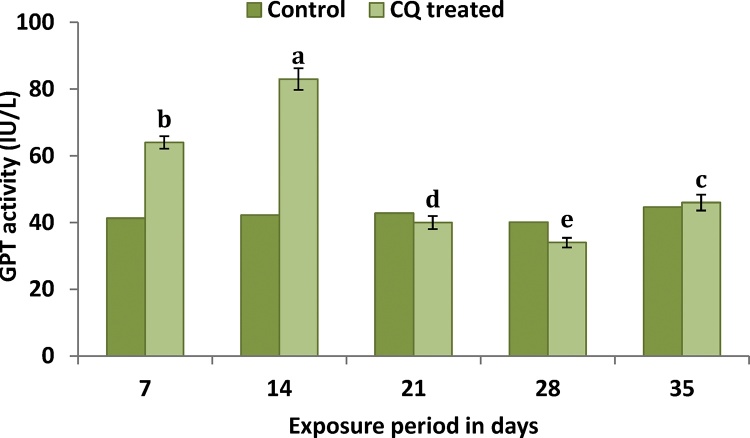
There was a significant (F4,24 = 8443***) increase in GPT activity at the end of 7, 14 and 35th days of exposure in CQ treated groups when compare to control groups (Fig. 3). A maximum increase of 96.68% was noted at the end of 14th day. However on day 21 and 28th the GPT activity was found to be inhibited in CQ treated groups when compare to control groups.
Fig. 3.
Illustrates the sublethal response of plasma enzyme GPT activity of C. carpio to CQ. Value followed by the different letter are significantly different at p < 0.05 (DMRT).
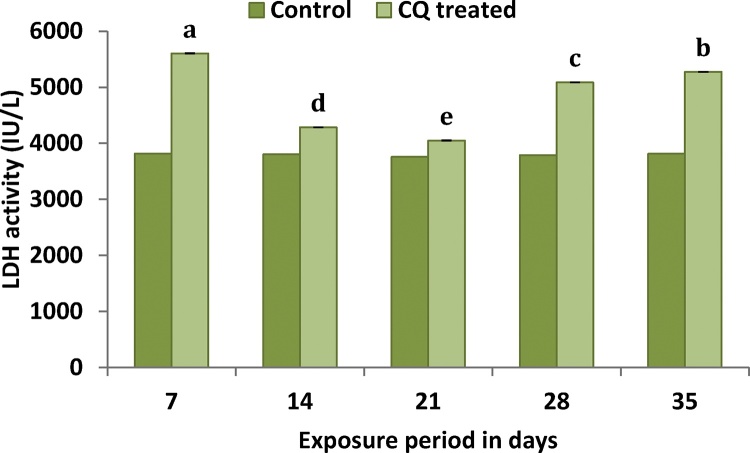
3.2.3. Plasma LDH activity
LDH activity in the plasma of fish exposed to sublethal concentration of CQ was found to be increased throughout the study period when compared to that of the control groups (Fig. 4). The data were statistically significant (F4,24 = 1.744***) among the treatments.
Fig. 4.
Illustrates the sublethal response of plasma enzyme LDH activity of C. carpio to CQ. Value followed by the different letter are significantly different at p < 0.05 (DMRT).
3.3. Histopathological changes in C. carpio exposed to CQ
3.3.1. Gill
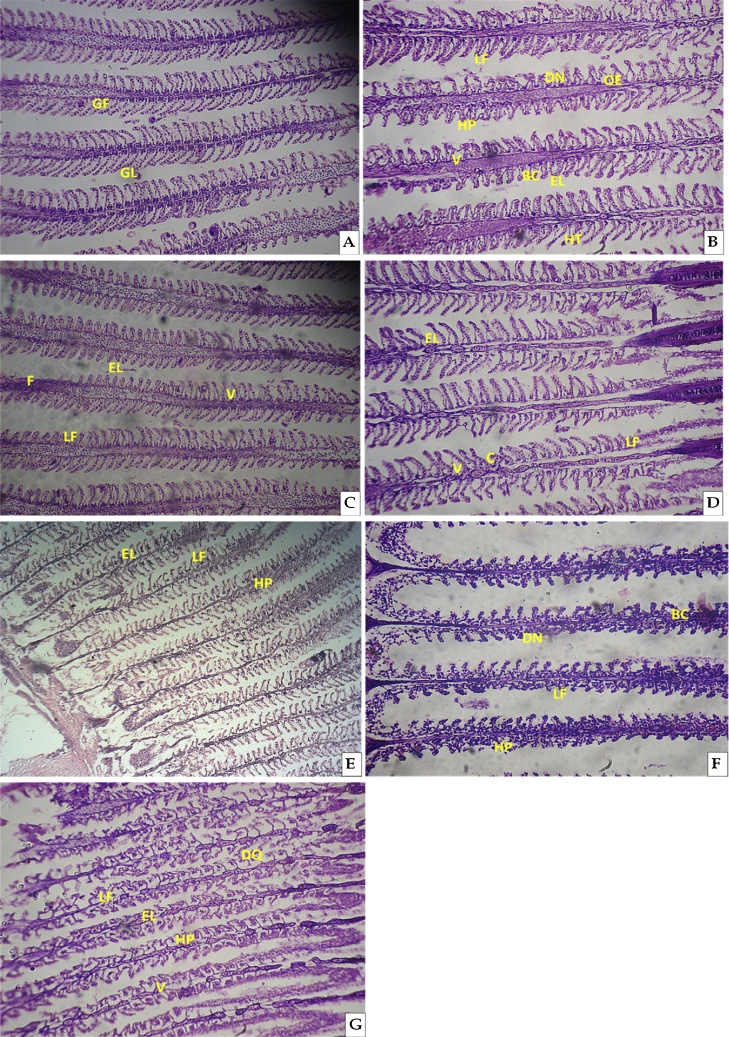
No morphological anomalies were noted in the gill tissue of fingerlings in control groups throughout the study period (Fig. 5A). In the gill tissue of CQ treated fingerlings severe morphological anomalies such as lamellar fusion LF, degenerative and necrotic changes in the epithelium of gill filaments DN, edema OE, vacuolization V, hyperplasia HP, blood congestion BC, epithelial lifting EL, hypertrophy HT, fusion F, Curling C and desquamated DQ were observed. The lesions are summarised in Table 1. Gill tissue in acute treatment had numerous LF, DN, OE, V, HP, BC, EL and HT (Fig. 5B). In sublethal treatment F, EL, LF and V were noticed at the end of 7th day (Fig. 5C) in which numerous of F followed by LF, EL and V were observed. At the end of 14th day of CQ exposure gill tissue had EL, V, LF and C (Fig. 5D). At the end of 21st day of exposure period gill tissue showed EL, LF, HP, V and C (Fig. 5E). At the end of 28th day gill tissue showed LF, HP, BC and DN (Fig. 5F). A numerous of DQ, EL, LF, HP and V were shown at the end of 35th day in the CQ treated fingerlings (Fig. 5G). The anomalies occurred in the gill tissues at acute and sublethal CQ treatment reveals that the drug CQ is toxic and has effects on the gill morphology.
Fig. 5.
Photomicrographs (2 mm) of the H&E-stained gill tissue of C. carpio (A) Control group showing regular shaped GF gill filament and GL lamellae, (B) Acute exposure, (C–G) Sublethal exposure of CQ. LF, lamellar fusion; DN degenerative and necrotic changes in the epithelium of gill filaments; OE edema; V vacualization; HP hyperplasia; BC blood congestion; EL epithelial lifting; HT hypertrophy; F fusion; C Curling; DQ desquamated were found in the CQ exposed fingerlings.
Table 1.
Morphological analysis of gill tissues of C. carpio exposed for acute and sub-lethal study. The morphological anomalies were represented in symbol based on their severity.
| Gill tissues morphological anomalies | Control | 96h | 7th | 14th | 21st | 28th | 35th |
|---|---|---|---|---|---|---|---|
| Lamellar fusion | − | ++ | + | + | + | + | + |
| Degenerative and necrotic changes in the epithelium of gill filaments | − | ++ | − | − | − | + | + |
| Edema | − | ++ | − | − | − | − | − |
| Vacuolization | − | ++ | + | + | + | + | |
| Hyperplasia | − | ++ | − | − | − | − | − |
| Blood congestion | − | + | − | − | − | + | − |
| Epithelial lifting | − | ++ | + | + | + | + | |
| Hypertrophy | − | + | − | − | + | + | + |
| Fusion | − | − | + | − | − | − | − |
| Curling | − | − | − | + | + | − | − |
| Desquamated | − | − | − | − | − | − | − |
Note: The anomalies visible in the gill morphology were divided based on the severity into three grades (−) no anomalies; (+) anomalies in <20% of the fields; (++) anomalies in <20–60% of the fields.
3.3.2. Liver
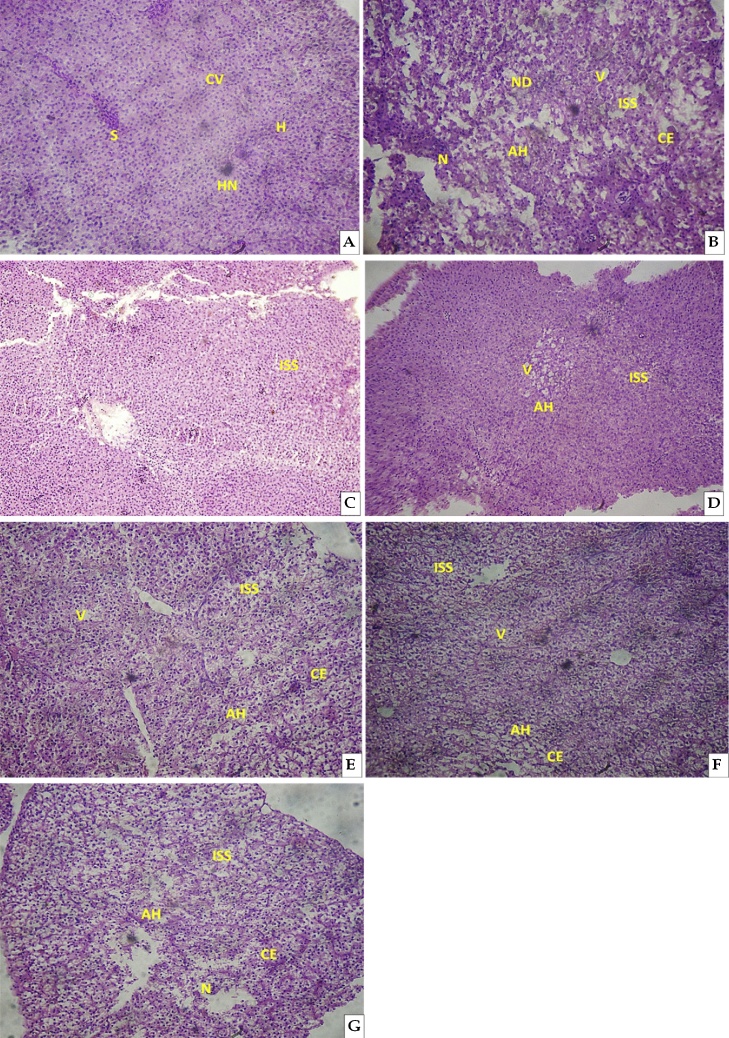
Normal morphology of the liver tissues with central vein CV; sinusoid S; hepatocytes H; hepatocyte nucleus HN were shown in the control group during the study period (96 h and 35 d) (Fig. 6A). Severe morphological anomalies such as nuclear degeneration ND, vacuolization V, cellular edema CE, increased sinusoidal space ISS, necrosis N and altered hepatocyte AH were appeared in the CQ treated fingerlings and are summarised in Table 2. The liver tissue of C. carpio during acute study showed numerous ND, V, CE, ISS, N and AH (Fig. 6B). When compared to the control liver there was denature in the liver morphology of fingerlings exposed to acute treatment.
Fig. 6.
Photomicrographs (2 mm) of the H&E-stained liver tissue of Cyprinus carpio (A) Control group showing regular shaped CV central Vein; S sinusoid; H hepatocytes; HN hepatocyte nucleus (B) Acute exposure, (C-G) Sublethal exposure of CQ. ND nuclear degeneration; V Vacuolation; CE cellular edema; ISS increased sinusoidal space; N necrosis; AH altered hepatocyte were found in the CQ exposed fingerlings.
Table 2.
Morphological analysis of liver tissues of C. carpio exposed for acute and sub-lethal study. The morphological anomalies were represented in symbol based on their severity.
| Liver tissues morphological anomalies | Control | 96h | 7th | 14th | 21st | 28th | 35th |
|---|---|---|---|---|---|---|---|
| Nuclear degeneration | − | ++ | − | − | − | − | − |
| Vacuolization | − | ++ | − | + | + | + | − |
| Cellular edema | − | ++ | − | − | + | + | + |
| Increased sinusoidal space | − | ++ | + | + | + | + | + |
| Necrosis | − | + | − | − | − | − | + |
| Altered hepatocyte | − | + | − | + | + | + | + |
Note: The anomalies visible in the liver morphology were divided based on the severity into three grades (−) no anomalies; (+) anomalies in <20% of the fields; (++) anomalies in <20–60% of the fields.
During sublethal treatment the liver tissues of CQ treated fingerlings showed numerous V, CE, ISS, N and AH (Fig. 6C–G). At the end of 7th day of CQ exposure ISS was noted (Fig. 6C). Morphological changes such as AH, V and ISS were observed in the liver tissue of CQ treated fingerlings at the end of 14th day of exposure (Fig. 6D). At the end of 21st day of exposure period, some anomalies such as ISS, V, AH and CE were noticed (Fig. 6E). At the end of 28th day of exposure period morphological anomalies of V, AH, CE and ISE were observed (Fig. 6F) in which numerous of V, ISS and AH were appeared. Likewise, morphological anomalies of ISS, CE, AH and N were appeared at the end of 35th day of exposure period (Fig. 6G). The morphological anomalies resulted in the acute and sub-lethal treatment clearly indicates that the CQ has a capable to alter the liver morphology in fish.
3.3.3. Kidney
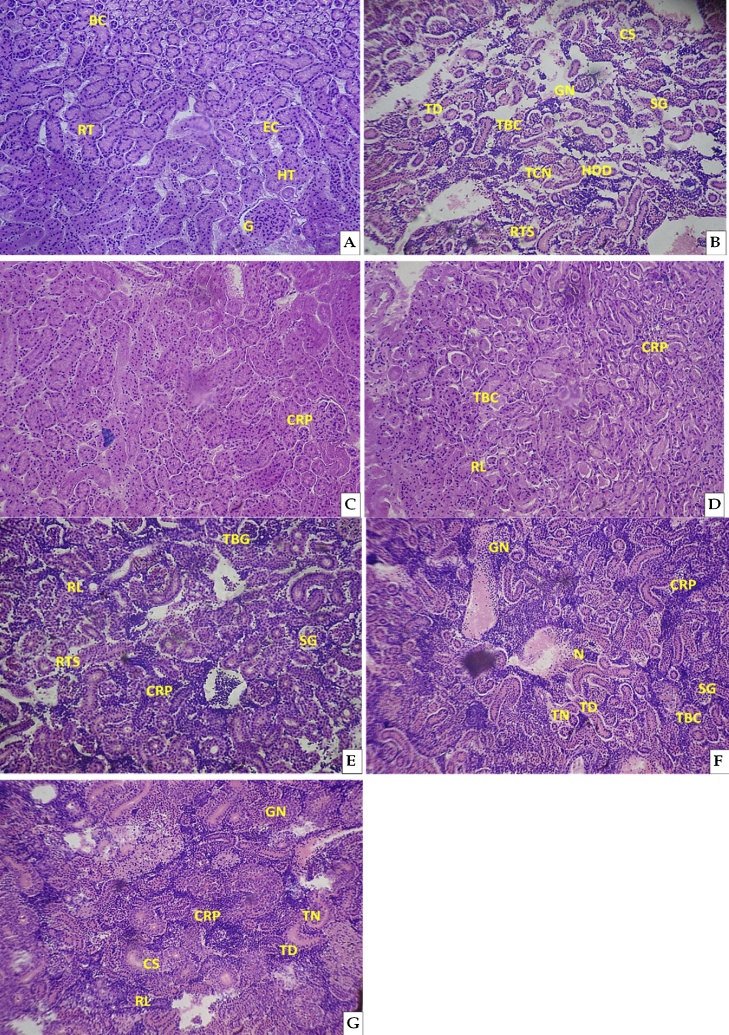
A normal morphology of Bowman’s capsule BC, renal tubule RT, epithelial cell EC, hematopoietic tissue HT, glomerului G were shown in the kidney tissues of control groups (Fig. 7A). Fingerlings in the control groups did not show any morphological anomalies throughout the study period (acute and sublethal). In CQ treatment fingerlings (acute and sublethal) several anomalies such as thickening of Bowman’s capsule TBC, tubular cell necrosis TCN, shrinkage of glomeruli SG, tubular degeneration TD, glomerular necrosis GN, hyaline droplets degeneration HDD, cloudy swelling CS, congestion in renal parenchyma CRP, reduction of lumens RL and renal tubular separation RTS were noticed and are summarised in Table 3. Morphological anomaly such as CS, GN, TD, TBC, TCN, HDD, SG and RTS were shown in the kidney tissues of acute treated fingerlings (Fig. 7B). In sublethal treatment fingerlings exhibit a morphological anomaly of CRP at the end of the 7th day of exposure period (Fig. 7C). Morphological anomalies like CRP, TBC and RL were observed at the end of 14th day of CQ exposure (Fig. 7D) in which there was numerous of TBC than RL and CRP. At the end of the 21st day of exposure period fingerlings exhibits several morphological changes of TBC, RL, SG, CRP and RTS (Fig. 7E). Similarly anomalies of GN, CRP, N, TN, TD, SG and TBC were appeared in the kidney tissues at the end of 28st day of exposure period (Fig. 7F). On 35th day of exposure period fingerlings exhibits morphological anomalies such as GN, N, CRP, TN, TD, CS and RL (Fig. 7G). The kidney morphological changes in the acute and sublethal treatment indicate that the drug CQ has impact and could cause anomalies in the morphology of kidney.
Fig. 7.
Photomicrographs (2 mm) of the H&E-stained kidney tissue of Cyprinus carpio (A) Control group showing regular shaped bowman’s capsule BC; renal tubule RT; epithelial cell EC; hematopoietic tissue HT; glomerului G; (B) Acute exposure, (C–G) Sublethal exposure of CQ. Thickening of Bowman’s capsule TBC; tubular cell necrosis TCN; shrinkage of glomeruli SG; tubular degeneration TD; glomerular necrosis GN; hyaline droplets degeneration HDD; cloudy swelling CS; congestion in renal parenchyma CRP; reduction of lumens RL; renal tubular separation RTS were found in CQ treated fingerlings.
Table 3.
Morphological analysis of kidney tissues of C. carpio exposed for acute and sub-lethal study. The morphological anomalies were represented in symbol based on their severity.
| Kidney tissues morphological anomalies | Control | 96h | 7th | 14th | 21st | 28th | 35th |
|---|---|---|---|---|---|---|---|
| Thickening of Bowman’s capsule | − | ++ | − | + | + | + | − |
| Tubular cell necrosis | − | ++ | − | − | − | + | + |
| Shrinkage of glomeruli | − | ++ | − | − | + | + | − |
| Tubular degeneration | − | + | − | − | − | + | + |
| Glomerular necrosis | − | ++ | − | − | − | + | + |
| Hyaline droplets degeneration | − | + | − | − | − | − | − |
| Cloudy swelling | − | + | − | − | − | − | + |
| Congestion in renal parenchyma | − | − | + | + | + | + | + |
| Reduction of lumens | − | − | − | + | + | − | + |
| Renal tubular separation | − | ++ | − | − | + | − | − |
| Necrosis | − | − | − | − | − | + | + |
Note: The anomalies visible in the kidney morphology were divided based on the severity into three grades (−) no anomalies; (+) anomalies in <20% of the fields; (++) anomalies in <20–60% of the fields.
4. Discussion
Pharmaceuticals in the aquatic environment are a serious concern worldwide because their environmental fate is not clearly understood. Even though their concentrations in the aquatic environment are low (ng L−1 to μg L−1) [11], they could have adverse effects on the aquatic biota. The first report on occurrence of pharmaceuticals in the environment was reported by Garrison et al. [29]. Recently many reports on the effects of pharmaceuticals on non-target organisms such as freshwater mussel [30], fish [17], [31], cladocerans [32], prawn [33], micro alga [34], and invertebrates [35] have been studied. Acute toxicity tests of chemical on organisms are related to non-specific mode of actions, but it provides rapid responses at short duration [16], [21]. The reports and data on acute ecotoxicity of pharmaceuticals on non-target organisms alone could not fulfil the risk assessment [36]. In this line sub lethal toxicity test are important management tool to measure the long term effect of toxicants at low concentration of chemical.
Acute toxicity studies of pharmaceuticals are predominant than the long term toxicity test. Acute toxicity data will be useful only when there will be accidental discharge of drugs [37], in which there will be no mortality in the organism but morphological, behavioural and metabolic alterations could occur. The data on acute toxicity of chloroquine to aquatic organisms are very limited. Zurita et al. [38] reported that the 48 h median effective concentration (EC50) of CQ to Daphnia magna, Chlorella vulgaris, fish cells from Poeciliopsis lucida and the bacterium Vibrio fischeri was 9, 27, 43 and 126 mg/L respectively. Similarly Rendal et al. [39] reported that the toxicity of CQ to Salix viminalis and Daphnia magna was more at pH 9 than at pH 6 indicating that the toxicity of CQ depends on the pH of the media. In the present study the 96 h LC 50 of CQ to C. carpio was found to be 31.32 mg/ml. Our result more or less similar to reports reported on the LC 50 of other pharmaceutical drugs such as sulfadimethoxine, carbamazepine, propranolol, ibuprofen, mefenamic acid, levofloxacin, triclosan to O. latipes was >100, 35.4, 11.40, >100, 8.04, >100 and 0.60 mg/l, respectively [40], [41]. Similarly, Henschel et al. [42] reported the LC 50 of paracetamol to B. rerio was 378 mg/l and clofibric acid to D. rerio was 86 mg/l.
In many aquaculture farms CQ is administrated with a dose of 10–20 mg/L as a safe dose [43]. In the present study the mortality of fish exposed to CQ at higher concentrations may be due to inhibition of various metabolic functions caused by CQ. CQ binds with DNA and inhibits the metabolic functions, interfere with haemoglobin and it also causes cell mediated death [6]. Similar to our findings MacPhee and Ruelle [44] observed mortality of fish salmon upon exposure to 20 μM of CQ, whereas only behavioural changes were noticed in rainbow trout exposed to 388 μM of CQ after 24 h [45]. To analyse the impact of CQ at lower concentration in the present investigation 1/10th value of 96 h LC 50 value (3.16 mg/ml) was taken.
GOT and GPT are the liver guiding enzymes and function as catalyse in transfering amino groups to alpha-keto acids and aspartic acid to α-ketoglutaric acid in interconversion of carbohydrate and protein. In the present investigation GOT, GPT and LDH activity in plasma of fish was altered during acute and sublethal exposure. Van der et al. [46] stated that changes in the activity of plasma GOT and GPT act as a sensitive indicator to know the health status of organs of fish exposed to chemicals. Decrease in transaminase activity was due to the deficiency of amino acids and reduction of α-ketoglutaric acid. When there is minor cell damage, the enzyme activity may increase in the blood (extracellular fluid) [21]. Increase in plasma GOT and GPT activity is an indication of functional damage of muscular, hepatic, and renal cell damage. However, GOT and GPT levels in an organism is depends on the protein and carbohydrate metabolism [47]. Any changes in the protein and carbohydrate metabolism may also leads to a change in the transaminase activity. In the present study also the accumulation of CQ in tissues/organs leads to damage of these organs which results release of these enzymes in to blood. In general an increase in transaminases activity indicates tissue damage whereas the inhibition of these enzymes indicates disturbance in the structure of cell organelles or death of cell organelles. The inhibition of lysosomal functions in PLHC-1 cell lines may be due to selective accumulation of CQ in the lysosomes [38]. Tetrameric enzyme, LDH is located in the cytoplasm and plays an important role in the energy metabolism under oxygen demand. Alteration in LDH activity indicates the release of isozymes from damaged cells, changes in protein and carbohydrate metabolism and leakage from white muscle and red blood cells [47], [46]. Furthermore changes in the activity of LDH could be used as a good marker of membrane permeability and apoptosis [48]. In the present study the significant increase in LDH activity during acute study indicate impaired carbohydrate metabolism caused by the drug chloroquine. However, the observed increase of LDH activity during sublethal treatment might have resulted from tissue damage due to accumulation and toxicity of CQ. In the present study, the alterations in the GOT, GPT and LDH activities clearly indicate that the fish is under stress condition. Alterations in GOT,GPT and LDH activity has been reported in L. rohita exposed to selenium [21], D. magna exposed to carbamazepine [48], C. carpio exposed to clofibric acid and diclofenac [49] and in C. mrigala exposed to ibuprofen [50]. The alteration in these enzyme activities are generally used as sensitive biomarkers for the monitoring of xenobiotics in the aquatic environment. Zurita et al. [38] reported a dose dependent inhibition of total protein content and significant increase in SOD and G6PDH activities in PLHC-1 cells due to CQ toxicity. In the present study also the alterations of the GOT, GPT and LDH activities during acute and sublethal toxicity depends on the dose and exposure period.
Histological studies of the fish provide rapid detection of health status of various organs. Gill, liver and kidney are the vital organs perform various functions such as exchange of gases, osmotic and ionic balance, detoxification mechanism, metabolism and excretion respectively [51]. Gill is the primary organ to contact the waterborne xenobiotics, so examination of the morphology of gill is mandatory in the field of toxicology. In the present study the histology of the control group showed a normal structure with GF and GL. But in the CQ treated group several anomalies such as LF, DN, OE, V, HP, BC, EL, HT, F, C and DQ were occurred. Exposure of fish to xenobiotics could cause various anomalies in the gill morphology [52]. Nascimento et al. [53] observed structural alterations in the fish Oligosarcus hepsetus, Hypostomus auroguttatus and Geophagus brasiliensis collected from the polluted Paraıba do Sul River.
The appearance of EL, HP and OE in the gill of stressed fish indicates the defence responses of the fish exposed to xenobiotics [52], [54]. Likewise, epithelial lifting and hypertrophy may occur by the formation of edema and induced proliferation of cellular component (endoplasmic reticulum) respectively [55]. These changes in the gill morphology may inhibit the entry of xenobiotics into the fish. Morphological alterations in the gill lamellae could cause blood congestion (BC) in the gill of fish exposed to toxic substance [56]. Vacuolisation and lamellar fusion could occur as a response of stress condition, which may disrupt the normal physiological function in fish [57]. Fish in the highly polluted environment could have necrosis in their gill morphology as a result of direct effect of toxicant [58]. In the present study the alterations in the gill morphology may leads to entry of CQ which may disrupt the respiratory mechanism and adverse effects.
Hepatic histopathology provides the toxic effects of chemicals and other substances. Hepatic cells are powerful to withstand to high level of chemicals, hence its histopathological alterations could be used as an ideal indicator for knowing the nature of chemicals [59]. Ahmed et al. [60] reported that liver is the major site of the teleost fish which could show alterations in metabolic mechanism and physiology to waterborne pollutants. In the present study during acute and sublethal CQ exposed group certain structural anomalies such as ND, V, CE, ISS, N and AH were noted. A similar structural alteration in the liver of fish exposed to various chemicals has been reported; Bucher and Hofer [61] in Salmo trutta, Capkin et al. [62] and Uguz et al. [63] in Oncorhynchus mykiss, Miranda et al. [64] in Hoplias malabaricus and Ahmed et al. [60] in Oreochromis mossambicus.
Jarrar and Taib [65] reported that nuclear degeneration, cellular oedema, necrosis and alteration of hepatocytes are the indication of the hyperactivity of nucleus and severe and irreversible damage of liver. Vacuolation could occur as a result of anoxia, irregular synthesis of parenchymal cell substances, defence mechanism and various biochemical alterations such as inhibition of protein synthesis and ionic regulation, denaturation of enzymes, energy depletion, disaggregation of microtubules, or shifts in substrate utilization in the liver [66]. The histological alterations occurred in the liver of fish exposed to CQ treatment could result in several abnormal metabolic action which may leads to failure of other organs also. A reduction in cell number, hydropic degeneration of the cytoplasm and pyknotic nuclei has been reported in PLHC-1 cells due to CQ toxicity [38].
Kidney is the vital organ in maintaining the internal ionic and water balance and excretion of unwanted digested food stuffs from the body. Renal histology is a powerful parameter in assessing the effect of chemicals in the organism. According to Ortiz et al. [67] kidney could be used as a good indicator in toxicology research because it is the organ which receives high of post-brachial blood. In the present study the fish in control groups showed a regular shape of BC, RT, EC, HT and G in their kidney structure. Structural anomalies such as TBC, TCN, SG, TD, GN, HDD, CS, CRP, RL and RTS were observed in the kidney of fish exposed to CQ. Similar result was found by Veiga et al. [68] in Prochilodus lineatus, Pacheco and Santos [69] in Anguilla anguilla, Capkin et al. [62] in Oncorhynchus mykiss, Das and Mukherjee [70] in Labeo rohita, Gill et al. [71] in Puntius conchonius and Cengiz [72] Cyprinus carpio exposed to various chemicals.
Tubular, granular and hyaline droplet degeneration and necrosis are the common structural changes that occur in the presence of toxic substance in the kidney [73]. Rand [74] reported that the accumulation of irregular size of eosinophilic granules, degradation of hyaline droplet in the cytoplasm causes necrosis. Cloudy swelling could be occurring in the fish exposed to toxic substance by the hypertrophy and fine granules in cytoplasm. Flow of blood through the kidney is higher than other organs of the body; Bowman’s capsule in the kidney often gets pathological effects due to presence of chemicals in the blood [75]. The structural changes occurred in the kidney of fish exposed to CQ treatment indicates the functional impairment, which could alter the metabolic mechanisms of the fish.
5. Conclusion
The present study concludes that CQ may affect the health condition of the fish by altering the enzymological and histological parameters of the fish C. carpio both at acute and sublethal concentrations tested in this study. The observed enzymological and histological alterations during acute toxicity may be due to high dose of the CQ, whereas the alterations during sublethal treatment indicates that CQ may remains active in aquariums and cause adverse effects. The alterations in the enzymological and histopathological parameters can be used as ideal biomarker in aquatic toxicology. Furthermore, the enzymological and histological alterations are very much essential to precede further toxicity study for the better define about the pharmaceuticals toxicity in aquatic organisms.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.toxrep.2017.11.006.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Shen H., Wu N., Wang Y., Zhao H., Zhang L., Li T., Zhao M. Chloroquine attenuates paraquat-induced lung injury in mice by altering inflammation, oxidative stress and fibrosis. Int. Immunopharmacol. 2017;46:16–22. doi: 10.1016/j.intimp.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Li J., Li S., Bai C., Liu H., Gramatica P. Structural requirements of 3-carboxyl-4(1H)-quinolones as potential antimalarials from 2D and 3D QSAR analysis. J. Mol. Graph Model. 2013;44:266–277. doi: 10.1016/j.jmgm.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Lin Y.C., Lin J.F., Wen S.I., Yang S.C., Tsai T.F., Chen H.E., Chou K.Y., Hwang T.I.S. Chloroquine and hydroxychloroquine inhibit bladder cancer cell growth by targeting basal autophagy and enhancing apoptosis. Kaohsiung J. Med. Sci. 2017;33:215–223. doi: 10.1016/j.kjms.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Halaby M.J., Kastein B.K., Yang D.Q. Chloroquine stimulates glucose uptake and glycogen synthase in muscle cells through activation of Akt. Biochem. Biophys. Res. Commn. 2013;435:708–713. doi: 10.1016/j.bbrc.2013.05.047. [DOI] [PubMed] [Google Scholar]
- 5.Shinde R.B., Raut J.S., Chauhan N.M., Karuppayil S.M. Chloroquine sensitizes biofilms of Candida albicans to antifungal azoles. Braz. J. Infect. Dis. 2013;17(4):395–400. doi: 10.1016/j.bjid.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomé R., Lopes S.C.P., Costa F.T.M., Verinaud L. Chloroquine Modes of action of an undervalued drug. Immunol. Lett. 2013;153:50–57. doi: 10.1016/j.imlet.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Takano T., Katoh Y., Doki T., Hohdatsu T. Effect of chloroquine on feline infectious peritonitis virus infection in vitro and in vivo. Antiviral. Res. 2013;99:100–107. doi: 10.1016/j.antiviral.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wichmann O., Eggelte T.A., Gellert S., Osman M.E., Mylius F., Ehrhardt S., Anemana S.D., Bienzle U., Mockenhaupt F.P. High residual chloroquine blood levels in African children with severe malaria seeking healthcare. Trans. R. Soc. Trop. Med. Hyg. 2007;101:637–642. doi: 10.1016/j.trstmh.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Stanton R.C. Glucose-6-Phosphate dehydrogenase, NADPH and cell survival. IUBMB Life. 2012;64(5):362–369. doi: 10.1002/iub.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Back D.J., Purba H.S., Park B.K., Ward S.A., Orme M.L. Effect of chloroquine and primaquine on antipyrine metabolism. Br. J. Clin. Pharmacol. 1983;16:497–502. doi: 10.1111/j.1365-2125.1983.tb02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandão F.P., Rodrigues S., Castroa B.B., Gonçalves F., Antunes S.C., Nunes B. Short-term effects of neuroactive pharmaceutical drugs on a fish species: biochemical and behavioural effects. Aquat. Toxicol. 2013;144–145:218–229. doi: 10.1016/j.aquatox.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Zenker A., Cicero M.R., Prestinaci F., Bottoni P., Carere M. Bioaccumulation and biomagnification potential of pharmaceuticals with a focus to the aquatic environment. J. Environ. Manage. 2014;133:378–387. doi: 10.1016/j.jenvman.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Ramaswamy B.R., Shanmugam G., Velu G., Rengarajan B., Joakim Larsson D.G. GC–MS analysis and ecotoxicological risk assessment of triclosan, carbamazepine and parabens in Indian rivers. J. Hazard. Mater. 2011;186(2–3):1586–1593. doi: 10.1016/j.jhazmat.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 14.Shanmugam G., Sampath S., Selvaraj K.K., Larsson D.G., Ramaswamy B.R. Non-steroidal anti-inflammatory drugs in Indian rivers. Environ. Sci. Pollut. Res. Int. 2014;21(2):921–931. doi: 10.1007/s11356-013-1957-6. [DOI] [PubMed] [Google Scholar]
- 15.Sathya V., Ramesh M., Poopal R.K., Dinesh B. Acute and sublethal effects in an Indian major carp Cirrhinus mrigala exposed to silver nitrate: gill Na+/K+-ATPase, plasma electrolytes and biochemical alterations. Fish Shellfish Immunol. 2012;32:862–868. doi: 10.1016/j.fsi.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Poopal R.K., Ramesh M., Dinesh B. Short-term mercury exposure on Na+/K+-ATPase activity and ionoregulation in gill and brain of an Indian major carp, Cirrhinus mrigala. Trace Elem. Med. Biol. 2013;27:70–75. doi: 10.1016/j.jtemb.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Ambili T.R., Saravanan M., Ramesh M., Abhijith D.B., Poopal R.K. Toxicological Effects of the antibiotic oxytetracycline to an Indian major carp Labeo rohita. Arch. Environ. Contam. Toxicol. 2013;64:494–503. doi: 10.1007/s00244-012-9836-6. [DOI] [PubMed] [Google Scholar]
- 18.Tkachenko H., Kurhaluk N. Pollution-induced oxidative stress and biochemical parameter alterations in the blood of white stork nestlings Ciconia ciconia from regions with different degrees of contamination in Poland. J. Environ. Monit. 2012;14:3182–3191. doi: 10.1039/c2em30391d. [DOI] [PubMed] [Google Scholar]
- 19.Capkin E., Birincioglu S., Altinok I. Histopathological changes in bow trout (Oncorhynchus mykiss) after exposure to sublethal composite nitrogen fertilizers. Ecotoxicol. Environ. Saf. 2009;72:1999–2004. doi: 10.1016/j.ecoenv.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Viarengo A., Lowe D., Bolognesi C., Fabbri E., Koehler A. The use of biomarkers in biomonitoring: a 2-tier approach assessing the level of pollutant-induced stress syndrome in sentinel organisms. Comp. Biochem. Physiol. 2007;146C:281–300. doi: 10.1016/j.cbpc.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Ramesh M., Sankaran M., Veera-Gowtham V., Poopal R.K. Hematological, biochemical and enzymological responses in an Indian major carp Labeo rohita induced by sublethal concentration of waterborne selenite exposure. Chem. Biol. Interact. 2014;207:67–73. doi: 10.1016/j.cbi.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Figueiredo-Fernandes A., Ferreira-Cardoso J.V., Garcia-Santos S., Monteiro S.M., Carrola J., Matos P., Fontaínhas-Fernandes A. Histopathological changes in liver and gill epithelium of Nile tilapia, Oreochromis niloticus, exposed to waterborne copper. Pesq. Vet. Bras. 2007;27(3):103–109. [Google Scholar]
- 23.Javed M., Ahmad I., Usmani N., Ahmad M. Studies on biomarkers of oxidative stress and associated genotoxicity and histopathology in Channa punctatus from heavy metal polluted canal. Chemosphere. 2016;151:210–219. doi: 10.1016/j.chemosphere.2016.02.080. [DOI] [PubMed] [Google Scholar]
- 24.Antoine D.J., Harrill A.H., Watkins P.B., Kevin Park B. Safety biomarkers for drug-induced liver injury–current status and future perspectives. Toxicol. Res. 2014;3:75–85. [Google Scholar]
- 25.APHA . Twentieth ed. American Public Health Association; Washington DC: 1998. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- 26.Finney D.J. Third ed. Griffin Press; London, UK: 1978. Statistical Methods in Biological Assay; p. 508. [Google Scholar]
- 27.Reitmen S., Frankel S. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminase. Am. J. Clin. Pathol. 1957;2:8–56. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 28.Tietz W. Ascorbic acid. In: Tietz W., editor. Fundamentals of Clinical Chemistry. W.B. Saunders company; Britain: 1976. pp. 419–422. [Google Scholar]
- 29.Garrison A.W., Pope J.D., Allen F.R. Analysis of organic compounds in domestic wastewater. In: Keith C.H., editor. Identification and Analysis of Organic Pollutants in Water. Ann Arbor Science Michigan; USA: 1976. pp. 517–566. [Google Scholar]
- 30.Hazelton P.D., Gregory Cope W., Mosher S., Pandolfo T.J., Belden J.B., Christopher Barnhart M., Bringolf R.B. Fluoxetine alters adult freshwater mussel behavior and larval metamorphosis. Sci. Total Environ. 2013;445–446:94–100. doi: 10.1016/j.scitotenv.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 31.Carlsson G., Patring J., Kreuger J., Norrgren L., Oskarsson A. Toxicity of 15 veterinary pharmaceuticals in zebrafish (Danio rerio) embryos. Aquat. Toxicol. 2013;126:30–41. doi: 10.1016/j.aquatox.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Han S., Choi K., Kim J., Ji K., Kim S., Ahn B., Yun J., Choi K., Khim J.S., Zhang X., Giesy J.P. Endocrine disruption and consequences of chronic exposure to ibuprofen in Japanese medaka (Oryzias latipes) and freshwater cladocerans Daphnia magna and Moina macrocopa. Aquat. Toxicol. 2010;98(3):256–264. doi: 10.1016/j.aquatox.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Gerhardt A., Janssens de Bisthoven L., Mo Z., Wang C., Yang M., Wang Z. Short-term responses of Oryzias latipes (Pisces: adrianichthyidae) and Macrobrachium nipponense (Crustacea: palaemonidae) to municipal and pharmaceutical waste water in Beijing, China: survival, behaviour, biochemical biomarkers. Chemosphere. 2002;47(1):35–47. doi: 10.1016/s0045-6535(01)00223-5. [DOI] [PubMed] [Google Scholar]
- 34.Yang L.H., Ying G.G., Su H.C., Stauber J.L., Adams M.S., Binet M.T. Growth-inhibiting effects of 12 antibacterial agents and their mixtures on the freshwater microalga Pseudokirchneriella subcapitata. Environ. Toxicol. Chem. 2008;27(5):1201–1218. doi: 10.1897/07-471.1. [DOI] [PubMed] [Google Scholar]
- 35.Pascoe D., Karntanut W., Müller C.T. Do pharmaceuticals affect freshwater invertebrates? A study with the cnidarian Hydra vulgaris. Chemosphere. 2003;51(6):521–528. doi: 10.1016/S0045-6535(02)00860-3. [DOI] [PubMed] [Google Scholar]
- 36.Fent K., Weston A.A., Caminadaa D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006;76:122–159. doi: 10.1016/j.aquatox.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Santos L.H., Araujo A.N., Fachini A., Pena A., Delerue-Matos C., Montenegro M.C. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Hazard. Mater. 2010;175:45–95. doi: 10.1016/j.jhazmat.2009.10.100. [DOI] [PubMed] [Google Scholar]
- 38.Zurita J.L., Jos A.B., del Peso A., Salguero M., Lopez-Artıguez M., Repetto G. Ecotoxicological evaluation of the antimalarial drug chloroquine. Aquat. Toxicol. 2005;75:97–107. doi: 10.1016/j.aquatox.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Rendal C., Kusk K.O., Trapp S. The effect of pH on the uptake and toxicity of the bivalent weak base chloroquine tested on Salix viminalis and daphnia magna. Environ. Toxicol. Chem. 2011;30(2):354–359. doi: 10.1002/etc.391. [DOI] [PubMed] [Google Scholar]
- 40.Kim Y., Choi K., Jung J., Park S., Kim P.G., Park J. Aquatic toxicity of acetaminophen, carbamazepine, cimetidine, diltiazem and six major sulfonamides, and their potential ecological risks in Korea. Environ. Int. 2007;33:275–370. doi: 10.1016/j.envint.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 41.Kim J.W., Ishibashi H., Yamauchi R., Ichikawa N., Takao Y., Hirano M., Koga M., Arizono K. Acute toxicity of pharmaceutical and personal care products on freshwater crustacean (Thamnocephalus platyurus) and fish (Oryzias latipes) J. Toxicol. Sci. 2009;34:227–232. doi: 10.2131/jts.34.227. [DOI] [PubMed] [Google Scholar]
- 42.Henschel K.P., Wenzel A., Diedrich M., Fliedner A. Environmental hazard assessment of pharmaceuticals. Regul. Toxicol. Pharm. 1997;25:220–225. doi: 10.1006/rtph.1997.1102. [DOI] [PubMed] [Google Scholar]
- 43.Hemdal J.F. TFH, Publications; Neptune City: 2006. Advanced Marine Aquarium Techniques. [Google Scholar]
- 44.MacPhee C., Ruelle R. Wild l. Range Exp. Station Bull., No.3. University of Idaho Forest; Moscow, ID: 1969. Lethal effects of 1888 chemicals upon four species of fish from western North America; p. 112. [Google Scholar]
- 45.Tojo J., Santamarina M.T., Ubeira F.M., Leiro J., Sanmartin M.L. Efficacy of antiprotozoal drugs against gyrodactilosis in rainbow trout (Oncorhynchus mykiss) Bull. Eur. Assoc. Fish Pathol. 1993;13:79–82. [Google Scholar]
- 46.Van der R.O., Jonny B., Vermeulen N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment, a review. Environ. Toxicol. Pharmacol. 2003;13:57–149. doi: 10.1016/s1382-6689(02)00126-6. [DOI] [PubMed] [Google Scholar]
- 47.Alarifi S., Al-Doaiss A., Alkahtani S., Al-Farraj S.A., Saad Al-Eissa M., Al-Dahmash B., Al-Yahya H., Mubarak M. Blood chemical changes and renal histological alterations induced by gentamicin in rats. Saudi J. Biol. Sci. 2012;19:103–110. doi: 10.1016/j.sjbs.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jos A., Repetto G., Riosc J.C., Hazen M.J., Molero M.L., del Peso A., Salguero M., Fernández-Freire P., Pérez-Martín J.M., Cameán A. Ecotoxicological evaluation of carbamazepine using six different model systems with eighteen endpoints. Toxicol. In Vitro. 2003;17:525–532. doi: 10.1016/s0887-2333(03)00119-x. [DOI] [PubMed] [Google Scholar]
- 49.Saravanan M., Karthika S., Malarvizhi A., Ramesh M. Ecotoxicological impacts of clofibric acid and diclofenac in common carp (Cyprinus carpio) fingerlings: hematological, biochemical, ionoregulatory and enzymological responses. J. Hazard. Mater. 2011;195:188–194. doi: 10.1016/j.jhazmat.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 50.Saravanan M., Usha Devi K., Malarvizhi A., Ramesh M. Effects of Ibuprofen on hematological, biochemical and enzymological parameters of blood in an Indian major carp, Cirrhinus mrigala. Environ. Toxicol. Pharmacol. 2012;34:14–22. doi: 10.1016/j.etap.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Evans D.H. CRC Press; Boca Raton: Florida: 1993. The Physiology of Fishes. [Google Scholar]
- 52.Heath A.G. CRC Press; Boca Raton : FL: 1987. Water Pollution and Fish Physiology. [Google Scholar]
- 53.Nascimento A.A., Araujo F.G., Gomes I.D., Mendes R.M., Sales A. Fish gills alterations as potential biomarkers of environmental quality in a eutrophized tropical River in South-Eastern Brazil. Anat. Histol. Embryol. 2012:1–8. doi: 10.1111/j.1439-0264.2011.01125.x. [DOI] [PubMed] [Google Scholar]
- 54.Martinez C.B.R., Nagae M.Y., Zaia C.T., Zaia D.A. Acute morphological and physiological effects of lead in the neotropical fish Prochilodus lineatus. Braz. J. Biol. 2004;64(4):S1519. doi: 10.1590/s1519-69842004000500009. (/S1519-69842004000500009) [DOI] [PubMed] [Google Scholar]
- 55.Pane E.F., Haque A., Wood C.M. Mechanistic analysis of acute, Ni induced respiratory toxicity in the rainbow trout, Oncorhynchus mykiss: an exclusively branchial phenomenon. Aquat. Toxicol. 2004;69:11–24. doi: 10.1016/j.aquatox.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Poleksic V., Mitrovic-Tutundzic V. Fish gills as a monitor of subletal and chronic effects of pollution. In: Muller R., Lloyd R., editors. Sub Liver Is the Important Organ, Which Lethal and Chronic Effects of Pollutants on Freshwater Fish. Fishing News Books; Oxford: 1994. pp. 339–352. [Google Scholar]
- 57.Lima L.C., Ribeiro L.P., Leite R.C., Melo D.C. Stress in fishes. Rev. Bras. Repr. Anim. 2006;30:113–117. [Google Scholar]
- 58.Camargo M.M., Martinez C.B. Histopathology of gills, kidney and liver of a Neotropical fish caged in an urban stream. Neotrop. Ichthyol. 2007;5:327–336. [Google Scholar]
- 59.Fernandes C., Fontainhas-Fernandes A., Rocha E., Salgado M.A. Monitoring pollution in Esmoriz-Paramos lagoon, Portugal: liver histological and biochemical effects in Liza saliens. Environ. Monit. Assess. 2008;145:315–322. doi: 10.1007/s10661-007-0041-4. [DOI] [PubMed] [Google Scholar]
- 60.Ahmed K., Al-Mamun H., Parvin E., Akter M.S., Khan M.S. Arsenic induced toxicity and histopathological changes in gill and liver tissue of freshwater fish, tilapia (Oreochromis mossambicus) Exp. Toxicol. Pathol. 2013;65:903–909. doi: 10.1016/j.etp.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Bucher F., Hofer R. The effects of treated domestic sewage on three organs (gills, kidney, liver) of brown trout (Salmo trutta) Water Res. 1993;27:255–261. [Google Scholar]
- 62.Capkin E., Terzi E., Boran H., Yandi I., Altinok I. Effects of some pesticides on the vital organs of juvenile rainbow trout (Oncorhynchus mykiss) Tissue Cell. 2010;42:376–382. doi: 10.1016/j.tice.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 63.Uguz C., Iscan M., Erguven A., Isgor B., Togan I. The bioaccumulation of nonylphenol and its adverse effect on the liver of rainbow trout (Oncorhynchus mykiss) Environ. Res. 2003;92:262–270. doi: 10.1016/s0013-9351(03)00033-1. [DOI] [PubMed] [Google Scholar]
- 64.Miranda A.L., Roche H., Randi M.A.F., Menezes M.L., Oliveira Ribeiro C.A. Bioaccumulation of chlorinated pesticides and PCBs in the tropical freshwater fish Hoplias malabaricus: histopathological, physiological, and immunological findings. Environ. Int. 2008;34:939–949. doi: 10.1016/j.envint.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 65.Jarrar B.M., Taib N.T. Histological and histochemical alterations in the liver induced by lead chronic toxicity. Saudi J. Biol. Sci. 2012;19:203–210. doi: 10.1016/j.sjbs.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gingerich W.H. Hepatic toxicology of fishes. In: Weber L.J., editor. Aquatic Toxicology. Raven Press; New York: 1982. pp. 55–105. [Google Scholar]
- 67.Ortiz J.B., de Canales M.L.G., Sarasquete C. Histopathological changes induced by lindane (gamma-HCH) in various organs of fishes. Sci. Mar. 2003;67:53–61. http://scimar.icm.csic.es/scimar/pdf/67/sm67n1053. pdf [Google Scholar]
- 68.Veiga M.L., Rodrigues E.L., Pacheco F.J., Ranzani-Paiva M.J.T. Histopathologic changes in the kidney tissue of Prochilodus lineatus, 1836 (Characiformes, Prochilodontidae) induced by sublethal concentration of Trichlorfon exposure. Braz. Arch. Biol. Technol. 2002;45:171–175. [Google Scholar]
- 69.Pacheco M., Santos M.A. Biotransformation, genotoxic and histopathological effects of environmental contaminants in European eel (Anguilla anguilla L.) Ecotoxicol. Environ. Saf. 2002;53:331–347. doi: 10.1016/s0147-6513(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 70.Das B.K., Mukherjee S.C. A histopathological study of carp (Labeo rohita) exposed to hexachlorocyclohexane. Vet. Archiv. 2000;70(4):169–180. http://www-staro.vef.unizg.hr/vetarhiv/papers/70-4/das.pdf [Google Scholar]
- 71.Gill T.S., Pant S.C., Tewari H. Cadmium nephropathy in a freshwater fish Puntius conchonius Hamilton. Ecotoxicol. Environ. Saf. 1989;18:165–172. doi: 10.1016/0147-6513(89)90077-8. [DOI] [PubMed] [Google Scholar]
- 72.Cengiz E.I. Gill and kidney histopathology in the freshwater fish Cyprinus carpio after acute exposure to deltamethrin. Environ. Toxicol. Pharmacol. 2006;22(2):200–204. doi: 10.1016/j.etap.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 73.Silva A.G., Martinez C.B.R. Morphological changes in the kidney of a fish living in an urban stream. Environ. Toxicol. Pharmacol. 2007;23:185–192. doi: 10.1016/j.etap.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 74.Rand G.M. second ed. Taylor and Francis; Washington: USA: 1995. Fundamentals of Aquatic Toxicology: Effects, Environmental Fate, and Risk Assessment; p. 1125. [Google Scholar]
- 75.Schwaiger J., Wanke R., Adam S., Pawert M., Honnen W., Triebskorn R. The use of histopathological indicators to evaluate contaminant-related stress in fish. Arch. Environ. Contam. Toxicol. 1997;6:75–86. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.