Abstract
The aim of our study was to evaluate efficacy and reliability of currently available ventilators for mouthpiece ventilation (MPV). Five life-support home ventilators were assessed in a bench test using different settings simulating the specificities of MPV, such as intermittent circuit disconnection and presence of continuous leaks. The intermittent disconnection of the circuit caused relevant swings in the delivered tidal volume (VT), showing a VT overshoot during the disconnection periods and a VT decrease when the interface was reconnected to the test lung. The five ventilators showed substantial differences in the number of respiratory cycles necessary to reach a stable VT in the volume-controlled setting, ranging from 1.3 ± 0.6 to 7.3 ± 1.2 cycles. These differences were less accentuated in the volume-assisted setting (MPV-dedicated mode, when available). Our data show large differences in the capacity of the different ventilators to deal with the rapidly changing respiratory load features that characterize MPV, which can be further accentuated according to the used ventilator setting. The dedicated MPV modes allow improvement in the performance of ventilators only in some defined situations. This has practical consequences for the choice of the ventilator to be used for MPV in a specific patient.
Keywords: Non-invasive ventilation, mouthpiece ventilation, home ventilators, bench evaluation, neuromuscular disease
Introduction
Long-term home mechanical ventilation (HMV) was introduced in the clinical practice in the 1950s under the pressure of the polio epidemic and is currently one of the few available treatments improving the clinical course of neuromuscular disease patients.1–4 In the current clinical practice, HMV is initially applied using a non-invasive interface during night-time, but in the course of the disease, as respiratory muscle weakness progresses, the extension of HMV during daytime may become necessary.3,5–7 Mouthpiece ventilation (MPV) can be used as an alternative to nasal or naso-buccal HMV interfaces for the daytime ventilation, and it has been reported to improve cough, speech, dyspnoea, survival and patient quality of life.6,8–10 MPV has been developed using volumetric ventilators, and a volumetric ventilation mode is usually chosen also with the new turbine-based home ventilators.6,11,12 Unlike bellow ventilators which deliver a constant tidal volume (VT) for each respiratory cycle, independently of the respiratory system load, the turbine-based ventilators perform a volume-targeted pressure ventilation, using the measured VT of the previous respiratory cycles to adapt the work pressure of the delivered cycle, in order to reach the target VT.13 This modality has been proved to be reliable in situations of constant ventilator load,14,15 but its efficacy may be affected by changes in the characteristics of the respiratory system and by the presence of leaks.15–18 The specificities of MPV, such as the intermittent disconnection of the patient and the presence of continuous leaks, may thus represent a challenge for turbine-based home ventilators. In the last few years, some ventilator manufacturers have integrated several improvements for MPV in their software, or even a dedicated ventilation modality to deal with the particularities of MPV, but we are unaware of any studies investigating the usefulness of these technical advances.
The aim of our bench study was to evaluate the efficacy and reliability of currently available ventilators for MPV according to different settings.
Methods
Ventilators
The five most recent life-support ventilators available in France for home ventilation were tested: VIVO 60 (Firmware 3.05; BREAS Medical, Mölnlycke, Sweden), Astral 150 (SR 1.1; ResMed, Saint-Priest, France), Puritan Bennet 560 (LX010101/AL020002; Covidien, Mansfield, Massachusetts, USA), VentiLogic LS (3.5.0; Weinmann, Hamburg, Germany) and Trilogy 100 (13.2.04; Philips Respironics, Murrysville, Pennsylvania, USA). The last two cited ventilators have specific MPV mode and were tested with this configuration for the assisted ventilation tests (Trilogy 100 with MPV-dedicated ‘kiss’ trigger).
Experimental set-up
The test ventilator was connected via a standard circuit and a 15-mm angled, rigid plastic mouthpiece (Philips Respironics) with a heat-and-moisture exchange filter (Hygroflux 1; Vygon, Ecouen, France; Figure 1), which showed the best results in the recently published bench evaluation,11 to the main chamber of a two-chamber test lung (Michigan Dual Adult Test Lung TTL 2600i; Michigan Instruments, Grand Rapids, Michigan, USA). The compliance of the lung model was set at 30 mL cm−1 H2O, and the airway resistance at 5 cm H2O/L s−1 (Pneuflo airway resistor Rp5; Michigan Instruments), corresponding to typical values for a neuromuscular disease adult.11 A master ventilator (BREAS VIVO 50) was connected to the second chamber of the test lung (driving chamber), which was connected to the main chamber by a small metal component, allowing the driving chamber to lift the main chamber, mimicking the patient’s contribution to inspiration. Because the metal component was not secured to the main chamber, the latter could expand freely above the driving chamber as dictated by the tested ventilator. Flow and pressure signals were captured on the circuit both near the ventilator and near the test lung, using Fleisch pneumotachographs (Fleisch, Lausanne, Switzerland) and an analogue/digital system (MP100; Biopac Systems, Goleta, California, USA) and recorded on a microcomputer for further analysis.
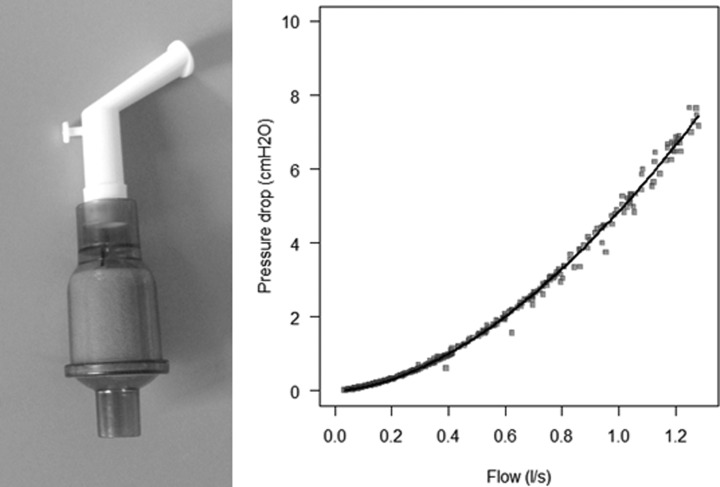
Figure 1.
Mouthpiece and filter used for the bench study. The test mouthpiece was a 15-mm angled, rigid plastic mouthpiece (Philips Respironics) with a heat-and-moisture exchange filter (Hygroflux 1, Vygon). The graph represents the resistance (flow-pressure slope) of the mouthpiece. Pressure drop was measured as pressure at the proximal end of the mouthpiece, the tip of which was open to the atmosphere.
Test protocol
For each ventilator, two volume-controlled settings (VC-CMV with VT 500 and 1000 mL) and one pressure-controlled setting (PC-CMV with an inspiratory pressure set at 20 cm H2O) were tested, at a respiratory frequency of 15 bpm and with an inspiratory time (TI) of 1.3 seconds. For each setting, the delivered VT was initially determined in the stable, leak-free situation over 1 minute (15 respiratory cycles). To simulate the clinical picture of intermittent MPV, the mouthpiece was then intermittently disconnected and connected from the test lung during periods of 10 respiratory cycles and subsequently obstructed during 10 respiratory cycles.
The same measurements were repeated using an assisted volumetric mode (VC-CMV with VT 500 mL, with respiratory frequency set at the lowest possible value, TI 1.3 seconds and inspiratory trigger at the lowest level avoiding auto-triggering), using the MPV mode when available; the master ventilator was set at a frequency of 15 minute−1 and used only to trigger the beginning of the inspiration (PC-CMV mode at 5 cm H2O, TI 0.4 seconds).
A third series of measurements was performed with this last configuration, alternating 10 controlled and 10 assisted cycles without debranching the interface from the circuit. During the assisted cycles, the master ventilator was set to simulate an inspiratory muscular effort of the patient along the whole TI (PC-CMV mode at 10 cm H2O, TI 1.3 seconds).
For the first two tested situations, the number of respiratory cycles necessary to reach a stable targeted VT or pressure according to the chosen mode, defined as the VT or pressure measured in the leak-free test ± 5%, was computed as the mean value of three disconnection/connection tests.
Statistical analysis
Statistical analysis was conducted using R 3.1.2 statistical software (R Core Team 2014, GNU General Public License). Continuous variables were described by mean and standard deviation. We used t-test to compare expected and measured values for the same ventilator, and analysis of variance to assess differences between the five test ventilators.
Results
Controlled setting
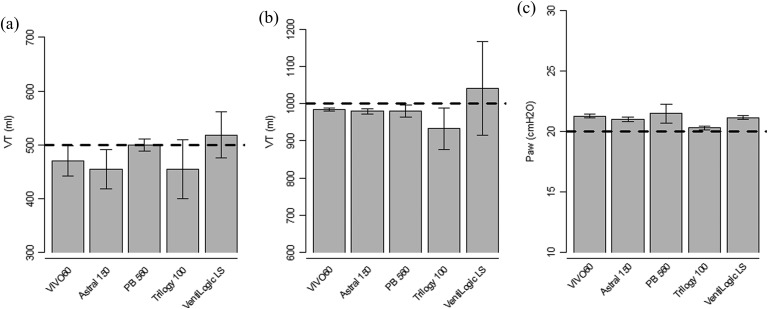
In the stable, leak-free tests the five ventilators delivered the set VT accurately, with less than 10% differences between the desired VT and the measured VT, which is considered as clinically acceptable. The gap ranged from −45 mL (−9%, p < 0.001) to +19 mL (+4%, p < 0.001) for the 500 mL setting and from −67 mL (−7%, p < 0.001) to +41 mL (+4%, p = 0.04) for the 1000 mL setting. In the PC-CMV setting, the measured airway pressures (Paw) ranged between +0.3 cm H2O (1.5%, p < 0.001) and +1.5 cm H2O (7.4%, p < 0.001) above the set pressure of 20 cm H2O (Figure 2).
Figure 2.
Delivered tidal volume and airway pressure in stable state without leaks. Bars represent mean tidal volume (VT), respectively, airway pressure (Paw), whiskers represent the 95% confidence interval. (a) Volume-controlled setting (VC-CMV) with VT 500 mL; (b) VC-CMV with VT 1000 mL; (c) pressure-controlled setting (PC-CMV) with inspiratory pressure set at 20 cm H2O.
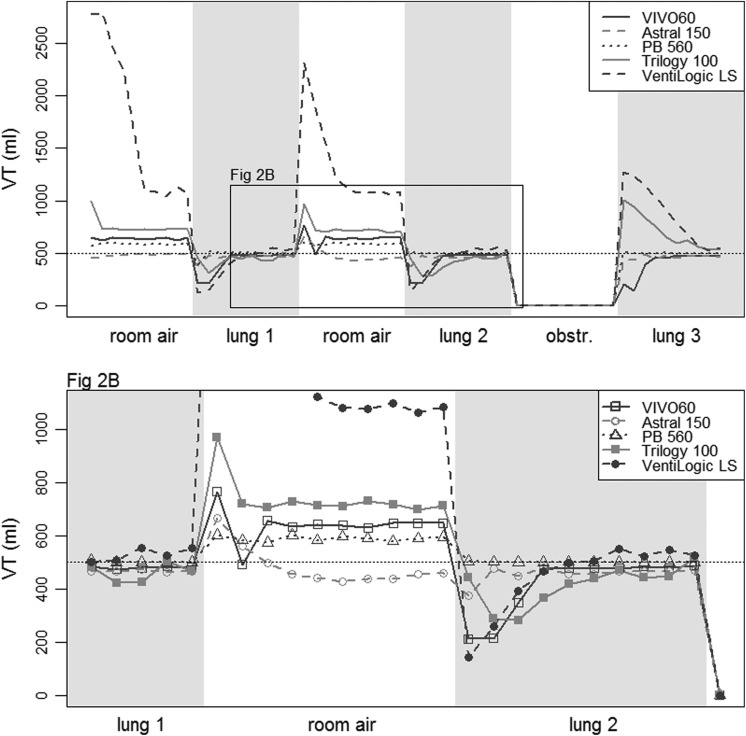
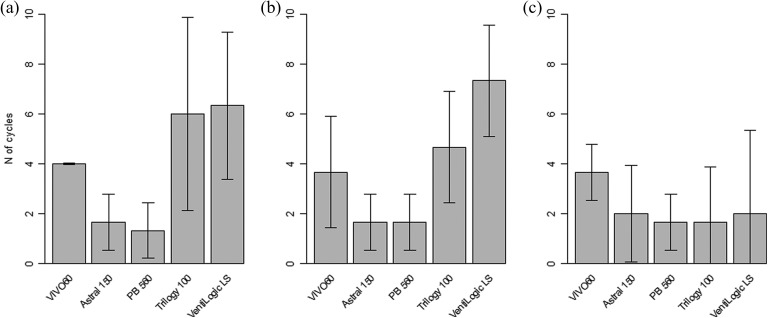
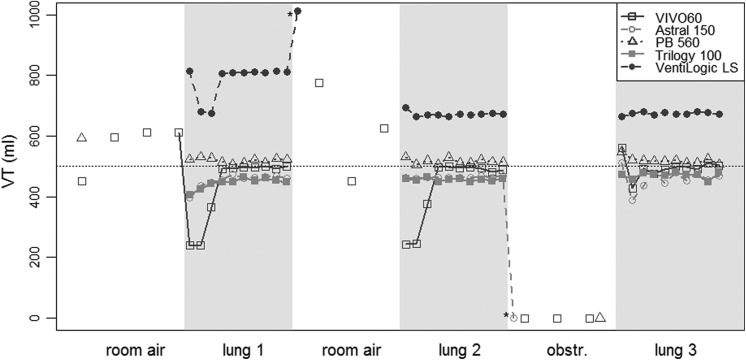
Figure 3 shows the VT provided cycle by cycle by the five ventilators in the volume-controlled settings (VC-CMV) with VT 500 mL, when the mouthpiece was then intermittently disconnected and connected from the test lung. The VT increased during the disconnection periods (with decrease of the respiratory system load) and decreased when the interface was connected to the test lung. A similar trend was obtained in the VC-CMV 1000 mL and in the pressure-controlled (PC-CMV) 20 cm H2O tests (data not shown). The five ventilators showed substantial differences in the number of respiratory cycles necessary to reach a stable VT in the volume-controlled setting (p < 0.001 for both the VT 500 mL and VT 1000 mL settings), ranging from 1.3 ± 0.6 to 7.3 ± 1.2 cycles (Figure 4, panels A and B). In the PC-CMV mode, the mean stabilization durations ranged between 1.0 ± 0.0 and 1.3 ± 0.6 cycles (p < 0.001).
Figure 3.
Intermittent disconnection test in the volume-controlled setting with VT 500 mL. VT: delivered tidal volume; test periods (of 10 respiratory cycles): room air: mouthpiece disconnection; lung: mouthpiece connection to the test lung; obstr.: mouthpiece obstruction.
Figure 4.
Number of cycles necessary to reach a stable VT after reconnection. Bars represent mean values; whiskers represent the 95% confidence interval. (a) Volume-controlled setting (VC-CMV) with VT 500 mL; (b) VC-CMV with VT 1000 mL; (c) volume-assisted/controlled setting (VC-CMV) with VT 500 mL and mouthpiece-dedicated mode when available.
Spontaneous (volume-assisted) setting
The same protocol was repeated using the volume-assisted setting, with a VT of 500 mL and a backup rate as low as possible, and is presented in Figure 5. Three of the ventilators (Astral 150, Trilogy 100 and VentiLogic LS) allowed setting a respiratory rate of 0 minute−1, whilst the respiratory rate was set to 1 for the PB 560 and to 4 for the VIVO 60. In this setting, most of the ventilators delivered no respiratory cycles during the disconnection period (except for two episodes of auto-triggering at the time of the circuit disconnection), and the VT variations were smaller when test lung ventilation was resumed, requiring less cycles to reach the set VT (Figure 4, panel C). It should be noted that the VentiLogic LS delivered a mean VT of 674 ±3 mL in the stable state (+35% of the set volume of 500 mL, p < 0.001).
Figure 5.
Intermittent disconnection test in the volume-assisted setting with VT 500 mL. VT: delivered tidal volume; test periods (of 10 respiratory cycles): room air: mouthpiece disconnection; lung: mouthpiece connection to the test lung; obstr.: mouthpiece obstruction; *auto-triggering at mouthpiece disconnection.
Intermittent-assisted/controlled setting
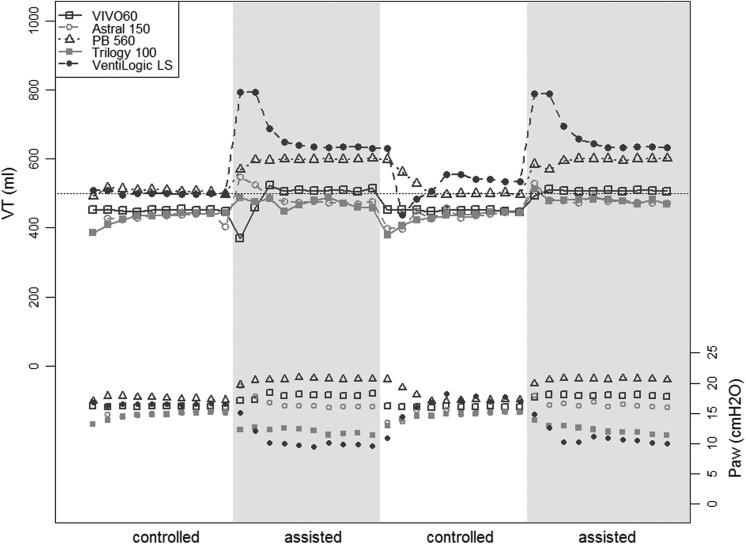
The third test was performed with an assisted volumetric mode (VC-CMV with VT 500 mL), alternating 10 controlled and 10 assisted cycles, during which an inspiratory muscular effort of the patient was simulated, and is shown in Figure 6. During the assisted periods, the ventilators had to reduce the respiratory support to compensate for the simulated muscular work of the patient. All the ventilators delivered a higher VT during the assisted period than during the controlled period, with differences ranging from 31 ± 1 to 114 ± 5 mL (+6% to +23% of the VT set at 500 mL, all p < 0.001), and with differences between the five ventilators in the amount of cycles needed to stabilize the VT.
Figure 6.
Intermittent controlled-assisted test (VC-CMV setting with VT 500 mL). VT: delivered tidal volume; Paw: airway pressure; test periods (of 10 respiratory cycles): controlled: volume-controlled setting; assisted: volume-assisted setting.
Discussion
The present data highlight relevant differences between the five currently available life-support home ventilators in their accuracy to provide the set VT in the typical settings of MPV.
All the last generation home life-support ventilators have a pneumatic system based on a turbine and perform volumetric ventilation using an algorithm to adjust the working pressure based on the previous cycles (volume-targeted pressure ventilation). The detailed descriptions of the used algorithms are not explicitly disseminated, and the performance of these ventilators can only be evaluated in terms of their output for any particular input, whilst how that output is determined remains hidden in a kind of ‘black box’. According to previous observations, current life-support ventilators obtain good performances in the stable situation.13–15 However, this operating mode may be challenged when facing rapidly changing load conditions (resistance and compliance of the respiratory system), as it is the case during MPV, because of intermittent disconnection of the patient from the mouthpiece, changing leaks and changing inspiratory muscular work of the patient. In our tests setting, we reproduced some of these features, highlighting some particularities of the tested ventilators that may help clinicians to choose the best ventilator for individual clinical situations.
When a respiratory rate was set on the ventilator (controlled setting), as it is recommended for the management of the more severe and ventilation-dependent patients,6,19 the delivered volume was lower than expected following each circuit disconnection. This resulted from a VT overshoot during the phases of circuit disconnection, causing a down-regulation of the ventilator’s working pressure in the subsequent cycles. When the test lung was reconnected to the mouthpiece, the delivered VT was lower than the set VT until the ventilators compensated for the changing condition. The rapidity to compensate was the main characteristic differentiating the tested ventilators, with two ventilators (Astral 150 and PB 560) needing less than two respiratory cycles and two ventilators (Trilogy 100 and VentiLogic LS) needing up to seven respiratory cycles.
As expected, the use of a barometric ventilation mode reduced the drop in delivered VT at the moment of the circuit reconnection in our test model, which alternated between two constant respiratory loads (room air and test lung). This ventilation modality is however less used in the home ventilation of dependent patients, since it doesn’t guarantee a stable VT in the case of changing resistance or compliance of the respiratory system (e.g. in case of airways obstruction due to secretions).6,11
Three of the tested ventilators (Astral 150, Trilogy 100 and VentiLogic LS) have been improved to deal with MPV and allow (among other features) the use of a volume-assisted ventilation mode without minimal respiratory frequency. In our test, the use of this ventilation mode reduced the phenomenon of rise and fall of the VT, since it avoided the delivery of respiratory cycles during the phases of circuit disconnection. This was particularly beneficial for the Trilogy 100 and VentiLogic LS ventilators. It should however be stressed that the MPV-dedicated volumetric mode of VentiLogic LS is actually a volume-cycled barometric (pressure support) mode, where a fixed inspiratory pressure is set by the user and the TI is controlled by the software basing on the delivered VT. This mode has the advantages of the barometric mode for the rapidity in adjusting VT delivery in the situation of intermittent circuit disconnection, but, similarly to the barometric modes, does not ensure a set VT. Furthermore, the test ventilator delivered a 35% higher VT than the set volume in this modality, suggesting that the response time to cycle into expiration was too long.
The simulated situation of a patient being intermittently active during inspiration showed relevant differences between the tested ventilators in their ability to reduce the respiratory support to compensate for the muscular work of the patient. As a consequence, the spontaneous effort during assisted periods led to a VT up to 27% higher than the set VT.
Summarizing our findings, the two ventilators with a dedicated MPV mode (Philips Trilogy 100 and Weinmann VentiLogic LS) showed the poorest performances in the controlled setting, the usual ventilation mode used for the most severe, ventilator-dependent patients. In the assisted setting, the Trilogy 100 showed good performances thanks to the MPV mode, whilst the reduction in VT instability obtained by the VentiLogic LS using the MPV modality was counterbalanced by a +35% VT overshoot. The ResMed Astral 150 has no MPV ventilation mode but has been conceived with some features allowing to deal with MPV (such as the possibility to set the respiratory backup rate to zero and to customize the alarms) and showed good performances in both the controlled and the assisted setting. Out of the two ventilators without dedicated MPV features, the BREAS VIVO 60 was slower in adapting to the changing load conditions, whilst the Covidien PB 560 showed good performances in both the controlled and the assisted setting.
The described differences between the study ventilators have an impact for the daily clinical practice, and our data may help clinicians to make an informed choice about using a specific ventilator in individual clinical situations. First of all, the choice of the ventilator should take into account the advantages and limitations of each machine, which also depend on the planned ventilator mode. For example, the MPV-dedicated mode without backup respiratory rate may be beneficial in less-dependent patients who use MPV in an intermittent manner with frequent disconnections from the interface, whilst the most severe, ventilator-dependent patients may take greater advantage of a more reactive ventilator, with greater rapidity in adjusting VT delivery following changing load conditions. Furthermore, any change of ventilator model or modification of ventilatory mode requires an adjustment of the ventilator settings and a new assessment of the delivered parameters. Last but not least, patients should be informed about the fact that the delivered VT following disconnection from the mouthpiece may be smaller than expected and that recovery to the usual VT may take several respiratory cycles, since this may be perceived as uncomfortable.
The main limitation of our study is linked to the need to standardize the test situations, whilst trying to reproduce the potentially problematic features of MPV. As a consequence, we had to simplify the multitude of possible MPV use pattern, which represents a wide spectrum of rapidly changing load conditions for the used ventilators. We selected the worst conditions considering that the transient changes were the largest when going from no leaks to total disconnection. Our work’s purpose was to help caregivers to recognize the potential problems related to MPV set up, but it should be stressed that the individual clinical context of the patients, their pattern of MPV use and the ventilator settings play a central role and must be taken into account in the choice of a ventilator for MPV.
In conclusion, our data show large differences in the capacity of the different life-support ventilators to deal with the rapidly changing respiratory load features that characterize MPV, which can be further accentuated according to the choice of ventilator settings. Furthermore, the newly developed MPV modes allow to improve the performance of ventilators only in some definite situations. This has practical consequences, since the choice of the ventilator to be used for MPV in a specific patient should also contemplate the advantages and limitations of each machine, which depend on the planned ventilator mode.
Footnotes
Author Contributions: AO, HP, LF, KL and FL designed the experiment; AO, LF, KL, DS and FL conducted the research; AO, IV and FL analyzed the data and performed the statistical analyses; AO, HP, DO and FL wrote the manuscript; AO and FL have primary responsibility for the integrity of the data and the accuracy of the data analysis. All authors had full access to all of the data in the study, revised the manuscript for important intellectual content and approved the final version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Simonds AK. Chronic hypoventilation and its management. Eur Respir Rev 2013; 22: 325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eagle M, Baudouin SV, Chandler C, et al. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord 2002; 12: 926–929. [DOI] [PubMed] [Google Scholar]
- 3. Simonds AK, Muntoni F, Heather S, et al. Impact of nasal ventilation on survival in hypercapnic Duchenne muscular dystrophy. Thorax 1998; 53: 949–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Annane D, Orlikowski D, Chevret S. Nocturnal mechanical ventilation for chronic hypoventilation in patients with neuromuscular and chest wall disorders. Cochrane Database Syst Rev 2014; 12: CD001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bach JR, Goncalves MR, Hon A, et al. Changing trends in the management of end-stage neuromuscular respiratory muscle failure: recommendations of an international consensus. Am J Phys Med Rehabil 2013; 92: 267–277. [DOI] [PubMed] [Google Scholar]
- 6. Garuti G, Nicolini A, Grecchi B, et al. Open circuit mouthpiece ventilation: concise clinical review. Rev Port Pneumol 2014; 20: 211–218. [DOI] [PubMed] [Google Scholar]
- 7. Bach JR, O’Brien J, Krotenberg R, Alba AS. Management of end stage respiratory failure in Duchenne muscular dystrophy. Muscle Nerve. 1987; 10: 177–182. [DOI] [PubMed] [Google Scholar]
- 8. Toussaint M, Steens M, Wasteels G, et al. Diurnal ventilation via mouthpiece: survival in end-stage Duchenne patients. Eur Respir J 2006; 28: 549–555. [DOI] [PubMed] [Google Scholar]
- 9. Bach JR, Alba AS, Saporito LR. Intermittent positive pressure ventilation via the mouth as an alternative to tracheostomy for 257 ventilator users. Chest 1993; 103: 174–182. [DOI] [PubMed] [Google Scholar]
- 10. Bach JR. A comparison of long-term ventilatory support alternatives from the perspective of the patient and care giver. Chest 1993; 104: 1702–1706. [DOI] [PubMed] [Google Scholar]
- 11. Khirani S, Ramirez A, Delord V, et al. Evaluation of ventilators for mouthpiece ventilation in neuromuscular disease. Respir Care 2014; 59: 1329–1337. [DOI] [PubMed] [Google Scholar]
- 12. Boitano LJ, Benditt JO. An evaluation of home volume ventilators that support open-circuit mouthpiece ventilation. Respir Care 2005; 50: 1457–1461. [PubMed] [Google Scholar]
- 13. Rabec C, Rodenstein D, Leger P, et al. Ventilator modes and settings during non-invasive ventilation: effects on respiratory events and implications for their identification. Thorax 2011; 66: 170–178. [DOI] [PubMed] [Google Scholar]
- 14. Thille AW, Lyazidi A, Richard JC, et al. A bench study of intensive-care-unit ventilators: new versus old and turbine-based versus compressed gas-based ventilators. Intensive Care Med 2009; 35: 1368–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fauroux B, Leroux K, Pepin JL, et al. Are home ventilators able to guarantee a minimal tidal volume? Intensive Care Med 2010; 36: 1008–1014. [DOI] [PubMed] [Google Scholar]
- 16. Lofaso F, Fodil R, Lorino H, et al. Inaccuracy of tidal volume delivered by home mechanical ventilators. Eur Respir J 2000; 15: 338–341. [DOI] [PubMed] [Google Scholar]
- 17. Khirani S, Louis B, Leroux K, et al. Harms of unintentional leaks during volume targeted pressure support ventilation. Respir Med 2013; 107: 1021–1029. [DOI] [PubMed] [Google Scholar]
- 18. Oto J, Chenelle CT, Marchese AD, et al. A comparison of leak compensation in acute care ventilators during noninvasive and invasive ventilation: a lung model study. Respir Care 2013; 58: 2027–2037. [DOI] [PubMed] [Google Scholar]
- 19. Benditt JO. Full-time noninvasive ventilation: possible and desirable. Respir Care 2006; 51: 1005–1012. [PubMed] [Google Scholar]