Abstract
Objective
To describe the behavior of 45 discs of dental amalgam of known dimension prepared from three commercially available brands of dental amalgam (Contour® Kerr®–USA, Admix® SDI®–Australia and Nu Alloy® Newstethic®–Colombia) when subjected to the action of high temperatures (200°C, 400°C, 600°C, 800°C, 1000°C). It was hoped to establish parameters that could be used for human dental identification in cases of charred, burned or incinerated human remains.
Materials and methods
A pseudo-experimental descriptive in-vitro study was designed to describe the macroscopic physical changes to the surface of 45 discs of pre-prepared amalgam of three commercially available brands exposed to a range of high temperatures.
Results
Characteristic and repetitive physical changes were a noticeable feature of the discs of amalgam of each brand of amalgam subjected to the different temperature ranges. These physical changes included changes in dimensional stability, changes in texture, changes in colour, changes in the appearance of fissures and cracks and changes in the fracture and fragmentation of the sample.
Conclusions
The characteristics of dental amalgam may be of assistance in cases of human identification where charred, burned or incinerated human remains are a feature and where fingerprints or other soft tissue features are unavailable.
KEYWORDS: Forensic sciences, forensic dentistry, dental identification, dental amalgam, high temperatures
Introduction
The importance of identification of the deceased has been embraced and accepted across society since time immemorial. Identification of the deceased is paramount for social, cultural, religious, legal and financial reasons.
Post mortem investigations are often multi-disciplinary. The variety of disciplines involved in an investigation may include data collection, analysis and interpretation. Input from various forensic experts, including forensic odontologists, is usually routine. Input from the appropriate legal authority is, in the majority of cases, mandatory.
Post mortem examination of both hard and soft tissues of the stomatognathic system is appropriate in the process to establish definitive identity of the deceased. Positive identification is dependant upon reliable coincidence between ante-mortem and post-mortem dental records.
In cases of human identification involving charred, burned or incinerated human remains where visual identification is problematic and where fingerprints or other soft tissue features are unavailable the stomatognathic system may provide distinctive and circumstantial evidence to achieve a positive identification using dental, anthropological and DNA analysis (1).
In circumstances such as those described above the forensic odontologist relies on comparison of the ante-mortem and post-mortem records of the deceased (2, 3).
It is well recognized that the resilience of dental tissues and that of dental restorative materials, including amalgam, both play a key part in human identification (4, 5).
The American Board of Forensic Odontology (6), with national and international support, has listed four categories of certainty of identity when comparing ante- mortem and post-mortem dental records; positive identification (total coincidence), possible identification (compatibility), insufficient evidence (inadequate information available) and exclusion (incoherence and incompatibility) (7).
Dental amalgam is characterized by being a low cost biocompatible material, with good physical and mechanical properties. These properties include good thermal and electrical conductivity, together with good compressive and flexural strength and a low capacity of permanent deformation. However dental amalgam is non-adhesive to tooth substrate, is opaque, of low aesthetic specification and is not anti-cariogenic. Amalgam is used frequently to restore Class I and II cavities where restoration of the structure of the tooth is of a higher priority than any aesthetic consideration (8-10). Globally, dental caries is the most prevalent disease presenting in adults.
Over the last 150 years dental amalgam has been the material of choice in restorative dentistry because of its durability and cost-effectiveness, despite current trends toward the development and use of polymeric materials with superior aesthetic properties (11-15).
The purpose of this study is to establish in vitro parameters regarding the behavior of dental amalgam when subjected to high temperatures. Forensic experts could then use this information to determine the range of temperature in which the changes occurred. It is hoped that this may contribute to the process of forensic dental identification in case of burned, charred or incinerated human remains.
MATERIALS AND METHODS
This is a pseudo-experimental descriptive transversal observational study that analyzed, through stereomicroscopy, the behavior of 45 discs of dental amalgam of known dimension prepared from three commercially available brands (Contour® Kerr®–USA, Admix® SDI®–Australia and Nu Alloy® Newstethic®–Colombia) when subjected to the action of high temperatures (200°C, 400°C, 600°C, 800°C, 1000°C). In the interests of comparison and standardisation, three dental amalgam systems with similar proportional composition were used in the study, Two of the brands are used commonly on a global basis (Contour® Kerr®–USA and Admix® SDI®–Australia) whilst the other is used commonly in Latin America (Nu Alloy® Newstethic®–Colombia).
PRODUCTION OF THE DISCS OF DENTAL AMALGAM USED IN THE STUDY
Aluminum matrix, acetate sheets and glass slabs were used to simultaneously prepare 5 discs of amalgam, each one 10 millimeters in diameter and 4 millimeters thick. Four mono-dose dental amalgam capsules were used for each disc. Each capsule was triturated in a Variamix® Dentsply® amalgamator for 12 seconds. The discs of amalgam were prepared by the conventional technique of packing (placement of the amalgam in the matrix), condensation (compaction of the amalgam in the matrix) and polishing (adaptation of the amalgam with the edge of the matrix) (16, 17). The surfaces of the discs were not polished (with the aim of not altering the composition of the surface relative to the center of the disc). Once the crystallization phase was complete, the discs were removed from the matrix (Figure 1).
Fig.1.

Disc elaborated in dental amalgam.
HANDLING AND CONSERVATION OF THE SAMPLE
Once the discs of dental amalgam had been produced, each specimen was stored individually in a plastic opaque container and maintained at a relative humidity and ambient temperature. Before the application of high temperatures, a digital photography was taken to each of the specimens through a Leuchtturm® digital stereomicroscopy of 1.3 mega pixels at 15X and 50X. The discs were distributed randomly on each of the six groups (one control group –ambient temperature– and five intervention groups) according to temperature ranges (Table 1).
Table 1. Sample distribution.
| Amalgam brands | Temperature | |||||
|---|---|---|---|---|---|---|
| Control group | Intervention group | |||||
| 28şC | 200şC | 400şC | 600şC | 800şC | 1000şC | |
| Contour® Kerr® | 3 | 3 | 3 | 3 | 3 | 3 |
| Admix® SDI® | 3 | 3 | 3 | 3 | 3 | 3 |
| Nu Alloy® Newstethic® | 3 | 3 | 3 | 3 | 3 | 3 |
APPLICATION OF HIGH TEMPERATURES
This procedure was performed based on the scientific and technical protocol established by the Odontostomatology Department of University of Pavia (Italy) (18) and based on studies carried out by the School of Dentistry of Universidad del Valle (Colombia) (19). The in vitro model as proposed in this study was carried out in an oven and not on direct flame. From previous studies it was noted that the highest temperature reached had been 1000°C that was achieved within 25 or 30 minutes and later maintained at approximately 500°C until all of the oxygen was depleted or all the organic contents reduced to coal (carbonization) or to calcium compounds, phosphates, silica or other trace elements (incineration) (20). This “muffle effect” in situ, was performed to simulate the effect of perioral tissues, facial musculature, bone tissue and periodontal and dental tissues (3).
The intervention group specimens corresponding to each range of temperature were placed in individual trays of refractory coating (Cera-Fina® Whipmix®) to facilitate their handling. They were then subjected to direct heat inside a muffle type oven (Thermolyne®) previously calibrated to five different temperature ranges (200°C, 400°C, 600°C, 800°C, 1.000°C) with a climbing rate of 10°C per minute from an initial temperature of 28°C until each of the proposed temperatures were reached. For example, nine specimens were introduced (three discs of every brand) according to the 200°C group, each one on their respective tray, at a temperature range starting at 28°C to 200°C, The oven was then left cooling to ambient temperature before removal of the trays with the specimens. So on for 600°C, 800°C and 1.000°C group specimens.
By being subjected to high temperatures, the experimental discs of dental amalgam may be found to demonstrate changes in color, texture, fissures and cracks, fractures, dimensional stability and explosion (20).
SAMPLE OBSERVATION
Before describing the changes in the characteristics of the pre-prepared discs of dental amalgam subjected to high temperatures, two of the authors underwent training regarding the observation of these changes (variables) in the control group (color, texture, fissures and cracks, fractures, dimensional stability and fragmentation), and had to unify the criteria for observation. In order to estimate the intraobserver and interobserver agreement, the Kappa test was performed in the Stata® software ver. 6.0.
STATISTICAL ANALYSIS
IBM SPSS Statistics® Ver. 22.0 software was used to calculate the prevalence (%) of macroscopic changes in the sample. The variables that were considered were temperature, color, texture, fissures and cracks, fractures, dimensional stability and explosion.
RESULTS
Respect standardization of observers, Kappa test determined the intraobserver standardization (0.88 and 0.86) and interobserver (0.89 and 0.90) of the two observers, respectively.
The three commercial brands (Contour® Kerr®–USA, Admix® SDI®–Australia and Nu Alloy® Newstethic®–Colombia) of dental amalgam had a different behavior in each temperature range (Table 2). At 15X (Figure 2) and at 50X (Figure 3) changes in dimensional stability, texture, color, fissures and cracks, fracture and explosion changes were seen in the dental amalgam discs that were subjected to the action of high temperatures. In general, at 200°C the dental amalgam discs of the three commercial brands lost brightness and the surface seemed rough, much more in the Admix® SDI®–Australia sample discs. At 400°C numerous rounded nodules with a porous appearance together with fissures and cracks appeared (these nodules in the Admix® SDI®–Australia discs appeared at 200°C). At 600°C the dental amalgam discs of the three commercial brands turned opaque and had a dark grey color. Deep fracture lines were observed in the places where the nodules had been previously present and there was evidence of loss of dimensional stability that appeared as a convex shape to the surface of the disc; at 800°C there was fragmentation of each disc where the appearance of rough, opaque and fragile pieces were observed. They tended to pulverize by touch. Shiny, bright and compact fragments grouped into rounded structures. The same changes at 8000C were seen at 10000C.
Table 2. Frequency of the changes of the discs of dental amalgam subjected to high temperatures.
| Characteristics |
Contour® Kerr® |
Admix® SDI® |
Nu Alloy® Newstethic® |
|---|---|---|---|
| 200şC | |||
| Color | Bright gray color remains (100%) | Bright gray color remains (100%) | Bright gray color remains (100%) |
| Texture | The surface appearance is porous (100%) | The surface appearance is porous (100%) Superficial nodules appear (100%) | The surface appearance is rough and porous (100%) |
| Fissures and cracks | Are not presented (None) | Are not presented | Are not presented |
| Fracture | It’s not presented (None) | It’s not presented | It’s not presented |
| Dimensional stability | It’s not observed | It’s not observed | It’s not observed |
| Fragmentation | It’s not observed | It’s not observed | It’s not observed |
| 400şC | |||
| Color | Gray color remains but not as bright (100%) | Gray color remains but not as bright (100%) | Gray color remains but not as bright (100%) |
| Texture | The surface appearance is porous (100%) Superficial nodules appear (100%) | The surface appearance is compact (100%) Superficial nodules appear (100%) | The surface appearance is porous (100%) Superficial nodules appear (100%) |
| Fissures and cracks | Pores and cracks are observed on the nodules (100%) | Pores and cracks are observed on the nodules (100%) | Pores and cracks are observed on the nodules (100%) |
| Fracture | It’s not presented | It’s not presented | It’s not presented |
| Dimensional stability | It’s not observed | It’s not observed | It’s not observed |
| Fragmentation | It’s not observed | It’s not observed | It’s not observed |
| 600şC | |||
| Color | Changes to a dark opaque gray (100%) | Changes to a dark opaque gray (100%) | Changes to a dark opaque gray (100%) |
| Texture | The surface appearance is compact (100%) and the nodules disappear (100%) | The surface appearance is compact (100%) and there’s traces of nodules (100%) | The surface appearance is compact (100%) and the nodules disappear (100%) |
| Fissures and cracks | There are fissures and cracks where the nodules were (100%) | There are fissures and cracks where the nodules were (100%) | There are fissures and cracks where the nodules were (100%) |
| Fracture | Deep fracture lines are observed (66.6%) | It’s not presented | Deep fracture lines are observed (33.3%) |
| Dimensional stability | Thermic expansion is observed –shape alteration– (100%) | Thermic expansion is observed –shape alteration– (66.6%) | Thermic expansion is observed –shape alteration– (33.3%) |
| Fragmentation | It’s not observed | It’s not observed | It’s not observed |
| 800şC and 1.000şC | |||
| Color | Changes to black opaque with bright gray spheres (100%) | Changes to black opaque with bright gray spheres (100%) | Changes to black opaque with bright gray spheres (100%) |
| Texture | A pulverized phase and a compact phase shaped like little spheres are observed | A pulverized phase and a compact phase shaped like little spheres and bands are observed | A pulverized phase and a compact phase shaped like little spheres are observed |
| Fissures and cracks | Fragmentation and pulverization are observed (100%) | Fragmentation and pulverization are observed (100%) | Fragmentation and pulverization are observed (100%) |
| Fracture | Fragmentation is observed (100%) | Fragmentation is observed (100%) | Fragmentation is observed (100%) |
| Dimensional stability | Fragmentation and pulverization by thermic expansion are observed–shape alteration– (100%) | Fragmentation and pulverization by thermic expansion are observed –shape alteration– (100%) | Fragmentation and pulverization by thermic expansion are observed–shape alteration– (100%) |
| Fragmentation | Fragmentation and destruction of the disc is observed (100%) | Fragmentation and destruction of the disc is observed (100%) | Fragmentation and destruction of the disc is observed (100%) |
Fig. 2.
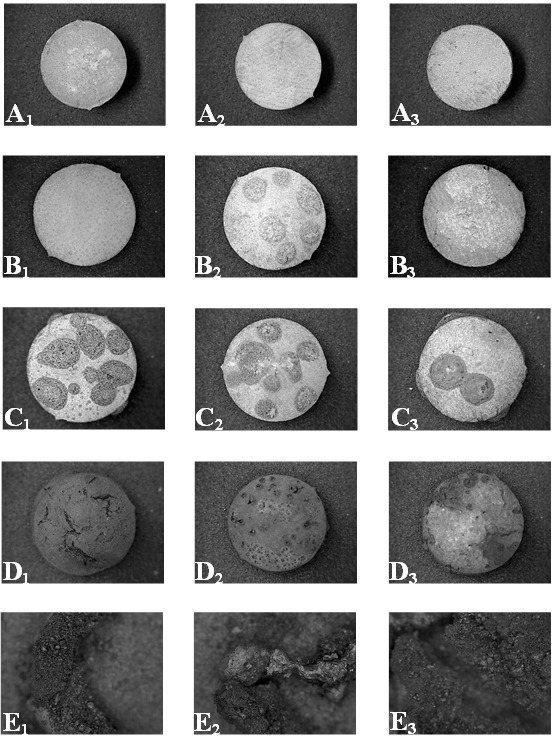
Amalgam discs observed at 15X. A1 Contour® Kerr®–USA amalgam disc, A2 Admix® SDI®–Australia amalgam disc and A3 Newstethic®–Colombia amalgam disc. B1 Contour® Kerr® amalgam disc, B2 Admix® SDI®–Australia amalgam disc in which silver nodules were observed and B3 Newstethic®–Colombia amalgam disc subjected to a temperature of 200°C. C1 Contour® Kerr®–USA amalgam disc, C2 Admix® SDI®–Australia amalgam disc and C3 Newstethic®–Colombia amalgam disc subjected to a temperature of 400°C in which silver nodules were observed. D1 Contour® Kerr®–USA amalgam disc, D2 Admix® SDI®–Australia amalgam disc and D3 Newstethic®–Colombia amalgam disc subjected to a temperature of 600°C in which the silver nodules disappear. E1 Contour® Kerr®–USA amalgam disc, E2 Admix® SDI®–Australia amalgam disc and E3 Nu Alloy® Newsthetic®–Colombia amalgam disc subjected to a temperature of 800°C in which the conformation of silver spheres and bands was observed.
Fig. 3.

Amalgam discs at 50X in which the appearance of silver nodules was notice between 200° and 400°C, the disappearance of the same at 800°C, and the conformation of silver spheres (E1 and E3) and bands (E2) at 1000°C.
DISCUSSION
Before discussing the behavior of the three commercial brands of dental amalgam used in this study it should be noted that they are “latest generation” dental materials with different sizes of mixed particles and contain a high copper content to eliminate the “gamma 2 phase”. The components of Contour® Kerr®–USA are 41% silver, 31% tin, 28% copper and 41% mercury; those for Admix® SDI®–Australia are 40% silver, 31% tin, 29% copper and 47,4% mercury; and for Nu Alloy® Newsthetic®–Colombia are 45% silver, 31% tin, 24% copper and 50% mercury. It is important to notice the proportions of silver, copper and tin that are used in the overall composition the alloy (17).
There are many publications in the forensic literature in respect of the behavior of dental materials when subject to the action of high temperatures. Merlati et al (18), subjected teeth restored with amalgam to high temperatures. The study showed that bubbles appeared in the surface of the amalgam at 200°C and mercury evaporated at 6000C. The same authors in other study, subjected 75 teeth (25 class I amalgam restored teeth and 25 class V amalgam restored teeth) to high temperatures, observing that in each temperature range there were repeated changes in the samples and at 1000°C the amalgam disintegrated (22).
Moreno et al (19), performed a study where 200 teeth were subjected to high temperatures. 50 teeth were filled with dental amalgam (GS-80 SDI®). The purpose of the study was to describe the subsequent behavior of the amalgam fillings whilst being subjected to the action of high temperatures and to establish parameters that could be used in cases of forensic dental identification where burned, charred or incinerated human remains were a feature of the case. The authors described that at 200°C and at 400°C the amalgam suffered loss of brightness and nodule formation on the surface. At 600°C the amalgam developed an opaque black color together with the loss of morphological features. At 800°C the texture became roughened and some specimens were fragmented. Finally between 1000°C and 1200°C the amalgam cracked into fragments.
Patidar et al (23), subjected teeth filled with different materials, including dental amalgam, to the action of high temperatures. His study found that after 200°C the appearance of surface of the amalgam fillings presented as a rough and fissured. These features became more evident with increasing temperature: at 800°C the appearance of globular structures in the restorations surface was noted.
Aramburo et al (24), performed an in vitro pseudo-experimental study in order to observe the micro structural physical changes in dental tissues (enamel, dentin and cementum) and in dental materials commonly used in endodontics in 124 human teeth. Forty two teeth were restored with dental amalgam (GS-80® SDI®), and then subjected to high temperatures. The authors found that at 200°C the amalgams turned opaque and rough by forming surface nodules. At 400°C the amalgam suffered loss of brightness and fissures were also observed. At 600°C the amalgam turned black. At 800°C the black colour was still present together with rounded nodules and internal fissures. At 1000°C the amalgam fragmented and pulverized.
Moreno and Mejia (25, 26) carried out a pilot study, using scanning electron microscopy, to standardize the technique used to observe teeth subjected to high temperatures. In the case of the dental amalgam at 200°C the superficial texture and structure changes are related to the melting points of the metals that form the alloy. At 400°C the amalgam presented with roughness in the occlusal surface associated with the development of nodules that arise when the mercury evaporates through gas bubbles. When the temperature lowers by action of the environmental pressure, the other elements of the alloy are grouped and driven by the mercury to form these nodules.
All of these in vitro studies described the changes that occurred in dental amalgam restorations by subjecting extracted restored teeth to the action of high temperatures. All of the studies found the formation of rounded or globular structures in the surface of the amalgam, a fact that was first described by Günther and Schmidt –and referenced by Rötzscher et al (27)– as “silver bullets” that develop at 800°C due to mercury evaporation.
The formation of “rounded or globular structures” and “silver bullets” is now well recognized in the literature. This in vitro study was designed to further investigate and to progress the process of the formation of these “rounded or globular structures” and “silver bullets” in dental amalgam.
Against this background the study was based on the use of pre-prepared discs of amalgam rather than the use of extracted teeth that had been previously been restored with amalgam.
Dental amalgam subjected to high temperatures presents with superficial texture and structural changes related to melting points of the metals that form the alloy. Between 200°C and 400°C the amalgam presents with roughness apparent on the occlusal surface related to the development of nodules that arise when the mercury evaporates through gas bubbles. These gas bubbles drive with them silver particles to the amalgam surface and because of decreases in pressure and temperature the gas bubbles and silver particles coalesce to form the so called “silver bullets”. Temperature affects the gamma 1 phase so that the mercury as it passes from solid to liquid state sweeps silver particles to the surface and evaporates and leaves the silver particles to form a superficial nodule. At temperatures between 800°C and 1.000°C, the silver particles reach melting point and evaporate, resulting in the disappearance of the nodules. As the temperature decreases little spheres or (betas) are formed composed of either copper or tin. They are fragmented and pulverized as a result of the oxidation of the copper (from 600°C) and due to the early fusion of tin (since 200°C) (28-30). These changes result in dimensional instability, fractures and further disintegration (fragmentation and pulverization) of the dental amalgam (21).
It is recognized that this study has certain limitations associated with the choice of using pre-prepared “in vitro” discs of amalgam rather than the choice of using “in vivo” extracted teeth that had been previously been restored with dental amalgam. The behavior of amalgam restorations in extracted teeth subjected to high temperatures has been reported in previous studies. However in this study it was decided to use pre-prepared discs of amalgam to standardize the macroscopic changes that occur when amalgam is free from the dimensional changes associated with the dental tissues.
CONCLUSIONS
Dental amalgam when subjected to high temperatures suffers changes in color, texture, the development of fissures and cracks, fractures, dimensional stability and explosion. The most distinctive change is the development of silver nodules in the surface of the amalgam from 200°C to 400°C and the subsequent disappearance of these silver nodules to form spheres and bands at temperatures of up to 1000°C.
The development of silver nodules, and their subsequent disappearance, is a regular feature of the “latest generation” of current commercially available brands of dental amalgam (rich in copper) when they are subjected to high temperatures. This paper provides further evidence of how changes in the appearance of the surface of dental amalgam restorations subject to high temperatures may facilitate determination of the maximum temperature to which the burned, charred or incinerated human remains were subjected.
Further studies are recommended including a wider range of commercially available brands of dental amalgam. These additional studies should incorporate data obtained from scanning electron microscopy, mass spectrometry and atomic force microscopy to identify the presence (qualitative analysis) and the quantity (quantitative analysis) of the components of the dental amalgam. The use of extracted teeth containing dental amalgam of a known brand would be beneficial.
ACKNOWLEDGMENTS
This research was financed by the internship grant of the Vice-President of Research at Universidad del Valle.
Footnotes
The authors declare that they have no conflict of interest.
References
- 1.Rothwell BR. Principles of dental identification. Dent Clin North Am. 2001;45:253–70. [PubMed] [Google Scholar]
- 2.Norrlander AL. Burned and incinerated remains, In Bowers CM, Bell GL (ed.). Manual of Forensic Odontology, 3th edition. Colorado Springs. American Society of Forensic Odontology; 1997. [Google Scholar]
- 3.Delattre VF. Burned beyond recognition: Systematic approach to the dental identification of charred human remains. J Forensic Sci. 2000;45(3):589–96. 10.1520/JFS14733J [DOI] [PubMed] [Google Scholar]
- 4.Sweet D. Why a dentist for the identification? Dent Clin North Am. 2001;45:237–51. [PubMed] [Google Scholar]
- 5.Pretty IA, Sweet D. A look at forensic dentistry. Part 1: The role of teeth in the determination of human identity. Br Dent J. 2001;190:359–66. [DOI] [PubMed] [Google Scholar]
- 6.American Board of Forensic Odontology ABFO Body identification guidelines. J Am Dent Assoc. 1994;125(9):1244–46. 10.14219/jada.archive.1994.0160 [DOI] [PubMed] [Google Scholar]
- 7.Marín L, Moreno F. Forensic dentistry: dental identification of burned corpses. Report of two cases. Revista Estomatología. 2004;12(2):57–70. [Google Scholar]
- 8.Chain MC, Baratieri LN. Aesthetic restorations with composite resins in posterior teeth. Editora Artes Médicas: San Pablo; 2001.
- 9.Guzmán H. Dental biomaterials in clinical use. 3th edition, Bogotá. Ediciones ECOE; 2003. [Google Scholar]
- 10.Nocchi C. Restorative dentistry: Health and Aesthetic, 2nd edition. Buenos Aires. Editorial Médica Panamericana; 2008. [Google Scholar]
- 11.Cook A. Amalgam: Dead or alive? Dent Update. 2006;33:94–8. [DOI] [PubMed] [Google Scholar]
- 12.Yengopal V, Harneker SY, Patel N, Siegfried N. Dental fillings for the treatment of caries in the primary dentition. Cochrane Database Syst Rev. 2009; (2):CD004483. [DOI] [PubMed] [Google Scholar]
- 13.Maserejian NN, Hauser R, Tavares M, Trachtenberg FL, Shrader P, McKinlay S. Dental composites and amalgam and physical development in children. J Dent Res. 2012;91(11):1019–25. 10.1177/0022034512458691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips RW. Skinner's Science of Dental Materials, 9th Edition. Philadelphia WB. Saunders Company; 1992. [Google Scholar]
- 15.Craig RG, Powers JM, Wataha JC. Dental Materials: Properties and Manipulation, 7th Edition, Mosby St Louis; 2000. [Google Scholar]
- 16.Stratis S, Bryant RW. The influence of modified cavity design and finishing techniques on the clinical performance of amalgam restorations: a 2-year clinical study. J Oral Rehabil. 1998;25(4):269–78. 10.1111/j.1365-2842.1998.00227.x [DOI] [PubMed] [Google Scholar]
- 17.Gómez CA, Arismendi JA. Preclinical and clinical evaluation of a commercial dental amalgam. Rev Fac Odontol Univ Antioq. 2010;22(1):63–71. [Google Scholar]
- 18.Merlati G, Danesino P, Savio C, Fassina G, Osculati A, Menghini P. Observations of dental prostheses and restorations subjected to high temperatures: experimental studies to aid identification processes. J Forensic Odontostomatol. 2002;20(2):17–24. [PubMed] [Google Scholar]
- 19.Moreno S, León M, Marín L, Moreno F. In vitro behavior of the dental tissues and some dental materials subjected to high temperatures with forensic purposes. Colomb Med. 2008;39 Supl 1:28–46. [Google Scholar]
- 20.Symes SA, Dirkmaat DC, Ousley S, Chapman E, Cabo L. Recovery and interpretation of burned human remains. US Department of Justice Office of Justice Programs United States of America: Rockville; 2012 [Update 2012 March; cited 2014 April 29]. https://www.ncjrs.gov/pdffiles1/nij/grants/237966.pdf
- 21.Moreno S, Merlati G, Marín L, Savio C, Moreno F. Effects of high temperatures on different dental restorative systems: Experimental study to aid identification processes. J Forensic Dent Sci. 2009;1(1):17–23. 10.4103/0974-2948.50883 [DOI] [Google Scholar]
- 22.Merlati G, Savio C, Danesino P, Fassina G, Menghini P. Further Study of restored and unrestored teeth subjected to high temperatures. J Forensic Odontostomatol. 2004;22(2):34–39. [PubMed] [Google Scholar]
- 23.Patidar KA Parwani R, Wanjari S. Effects of high temperature on different restorations in forensic identification: Dental samples and mandible. J Forensic Dent Sci. 2010;2(1):37–43. 10.4103/0974-2948.71056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aramburo J, Zapata A, Zúńiga S, Moreno F. Stereomicroscopic analysis of dental materials employed in endodontic exposed to high temperatures. Rev Estomat. 2011;19(2):8–15. [Google Scholar]
- 25.Moreno F, Mejía C. Scanning electron microscopy analysis of two endodontically treated teeth subjected to high temperatures. A pilot study. Rev Fac Odontol Univ Antioq. 2011;23(19):22–36. [Google Scholar]
- 26.Vásquez L, Rodríguez P, Moreno F. In vitro behavior of dental tissues and some dental materials of endodontics use, submitted to high temperatures with forensic applications. Rev Odont Mex. 2012;16(3):171–81. [Google Scholar]
- 27.Rötzscher K, Grundmann C, Benthaus S. The effects of high temperatures on human teeth and dentures. Int Poster J Dent Oral Med 2004; 6: Poster 213.
- 28.Odanov Z, Djurdjev M. Investigation of the mechanism of mercury removal from a silver dental amalgam alloy. J Serb Chem Soc. 2004;69(12):1111–20. 10.2298/JSC0412111O [DOI] [Google Scholar]
- 29.Mrowec S, Stokosa A. Oxidation of copper at high temperatures. Oxid Met. 1971;3:291–311. 10.1007/BF00603530 [DOI] [Google Scholar]
- 30.Kerl B, Forbeck F. Mercurio. En: Stohmann F, Editor. Great Encyclopedia of Industrial Chemistry Tomo X. Barcelona F. Soix; 1956. [Google Scholar]


