Abstract
A newly engineered infrared fluorescent protein will allow microscopists to peer more deeply into living animals.
Less than two decades after the introduction of green fluorescent protein as a genetically encoded fluorescent marker1, biologists have at their disposal a veritable rainbow of fluorescent proteins, with colors that extend across most of the visible spectrum2. However, fluorescence imaging in live animals using these proteins remains hampered by the limited penetration depths of visible light in the body. In this issue, Filonov et al.3 present a fluorescent protein that brightly labels live mammalian cells and has emission and excitation spectra at near-infrared wavelengths that undergo substantially less scattering and absorption than visible light in most tissues. This protein represents a noteworthy improvement over prior infrared fluorescent protein markers and will enhance researchers’ capabilities to peer deep inside the mammalian body using light.
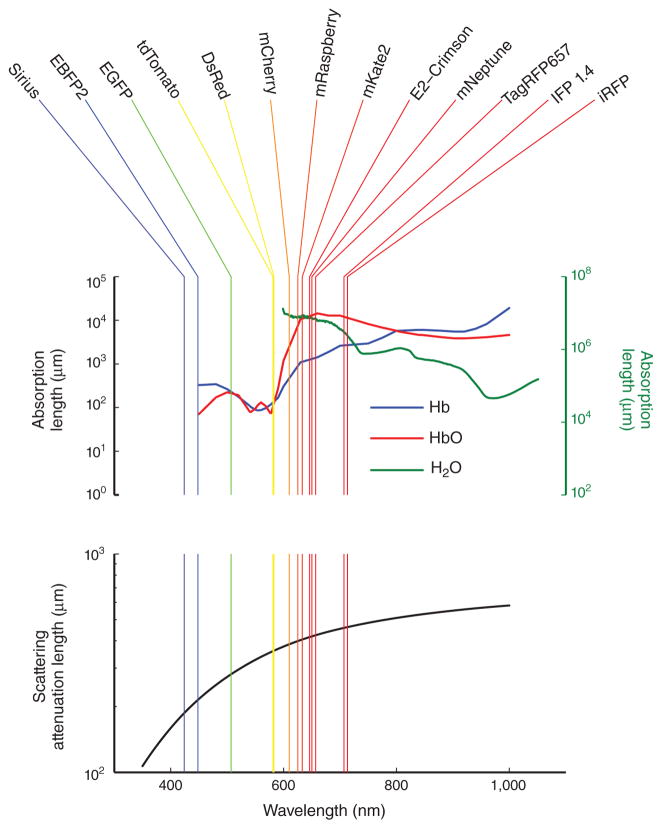
Imaging depth is restricted by both the scattering and absorption of light, with the relative impact of the two depending on the particular imaging modality, the optical wavelength and the tissue type. Both of these optical processes determine the so-called attenuation length. Within a uniform medium, the odds of a photon traveling unimpeded decline exponentially with the distance traveled; the attenuation length is the characteristic distance over which this decline occurs. (Over one attenuation length, a propagating photon has a ~37% probability of traveling unhindered, but over six attenuation lengths, that probability drops to ~0.25%.) A reduction in either scattering or absorption increases the attenuation length. For visible light in biological tissue, a typical attenuation length can be as short as ~100 μm or less. With near-infrared light, however, both scattering and absorption in biological tissue are generally less severe, and attenuation lengths are correspondingly longer (Fig. 1).
Figure 1.
Absorption and scattering lengths are longer for near-infrared compared with visible light. Fluorescence emission peaks for an assortment of fluorescent proteins are shown in the top panel atop plots of the absorption lengths of deoxy-hemoglobin8 (Hb; blue curve, left axis), oxy-hemoglobin8 (HbO; red curve, left axis) and water9 (green curve, right axis). (Note that emission peaks do not necessarily convey a fluorophore’s perceived color.) The absorption lengths for hemoglobin were calculated from the molar extinction coefficients assuming a concentration of 2.2 mM hemoglobin, which is representative of human and mouse blood. The bottom panel shows the wavelength dependence of the scattering attenuation length of a tissue sample of human skin10 (black trace).
Because of the exponential dependence on distance, moderate improvements in attenuation length can lead to substantial gains in imaging performance. A doubling of the attenuation length increases by ~20-fold the number of emitted photons that travel unimpeded from a depth of six (original) attenuation lengths—that is, from ~0.25% to ~5%. Such manifold improvements in signal power imply that a seemingly modest shift to the use of near-infrared markers offers substantial advantages for in vivo imaging.
Filonov et al.3 are not the first to explore the use of phytochromes for infrared fluorescence imaging. Phytochromes are bacterial and plant photopigments that naturally absorb red and near-infrared light. They also fluoresce, although generally too weakly to be used as fluorescent markers. An engineered version of a phytochrome from the bacteria Deinococcus radiodurans was recently developed4 into an infrared fluorescent marker called IFP 1.4. In that study, the fluorescence efficiency of the native phytochrome was improved by reducing the chances that the energy from an absorbed photon decays nonproductively (that is, without emission of a fluorescence photon) and by increasing the binding affinity to its chromophore, biliverdin, which is a product of heme metabolism and endogenous to mammalian cells. But the binding affinity of IFP 1.4 to biliverdin is still insufficient to allow maximal fluorescence in mammalian cells, and addition of exogenous biliverdin is typically required to boost the infrared fluorescence signal.
To improve upon IFP 1.4, Filonov et al.3 take a similar optimization approach but start from a different protein. They use a phytochrome from the bacterium Rhodopseudomonas palustris and improve the protein’s properties through multiple rounds of mutagenesis. The resulting protein, called infrared fluorescent protein (iRFP), appears to be considerably superior to IFP 1.4. The fluorescence spectra of the two molecules are similar, with an excitation maximum of 684 nm for IFP 1.4 versus 690 nm for iRFP, and with emission maxima of 708 nm and 713 nm, respectively. The molecular brightness of iRFP is estimated to be ~20% greater than that of IFP 1.4 when the two are compared in vitro. However, the big gains from iRFP occur in live cells, where the effective brightness of iRFP is approximately tenfold greater.
Physiological levels of biliverdin appear to fully activate iRFP fluorescence while leaving a substantial fraction of IFP 1.4 molecules unbound to their biliverdin chromophore. iRFP also seems more stable in live cells than IFP 1.4, yielding higher accumulated expression levels. Even in the presence of exogenous biliverdin, the fluorescence of IFP 1.4 is still approximately sevenfold weaker than that of iRFP. Crucially, physiological levels of biliverdin in cells from a variety of tissues are capable of fully activating iRFP.
Of course, the ability to look deeply into tissue by fluorescence imaging depends not just on the properties of the fluorescent marker but also on the optical instrumentation. In parallel to the improvement and color expansion of fluorescent proteins that have occurred over the past 15 years, optical physicists have developed new, noninvasive fluorescence imaging modalities for looking deeper into living bodies. These advances include intra-vital, laser-scanning two-photon microscopy as a means for imaging cells lying hundreds of microns deep within scattering tissue5 and several molecular imaging techniques for use in whole animals, such as fluorescence tomography6. Two-photon microscopy is limited in resolution by the diffraction of light and thus its wavelength. Whole-animal fluorescence imaging techniques typically operate in a very different regime, in which photons originating millimeters deep within the body undergo multiple scattering events before exiting the tissue. This can degrade resolution to the millimeter-scale for deeper tissues.
To illustrate the potential of iRFP for whole-body imaging techniques, Filonov et al.3 show that in polyurethane mouse phantoms iRFP yields higher ratios of fluorescence signal to background fluorescence than the best available red-shifted fluorescent proteins. They also show that iRFP can be detected in the liver of living mice at a depth of several millimeters. Thus, iRFP should be very useful for whole-animal imaging applications, which have become important in many preclinical disease studies, such as for tracking fluorescently labeled cancer cells. Detecting small populations of these or other labeled cells deep within a whole animal is often challenging, but iRFP is poised to help. The reduced autofluorescence at infrared wavelengths lowers the levels of nonspecific, background fluorescence. Moreover, the reduction in scattering achieved by using infrared light should improve the effective resolution and raise the spatial concentration of fluorescence signals that emerge from the animal, further improving the ability to detect these signals against the fluorescence background. In addition, most charge-coupled device (CCD) camera chips are maximally sensitive to light of wavelengths near ~700 nm.
Fluorescence microscopy techniques whose resolution is limited by diffraction will incur a drop in resolution with iRFP compared with visible fluorophores, owing to iRFP’s longer wavelength. For two-photon microscopists interested in improving the penetration depth into tissue, this drop in resolution should pale against the benefits of reduced light scattering. The two-photon absorption spectrum of iRFP has a local maximum of ~1.3 μm, which is potentially an attractive excitation wavelength because, in this spectral neighborhood, both scattering and absorbance in tissue have attenuation lengths in the 1 mm range. (High-resolution imaging modalities that use light scattering for contrast, such as optical coherence tomography, have already employed 1.3 μm light to attain far superior penetration depths at the millimeter-scale than those achieved by fluorescence microscopy.) The commercial options for tunable, ultrashort-pulsed laser sources that emit at 1.3 μm for two-photon microscopy have also recently expanded and could be affordable for many institutes. Although more work is needed to characterize the two-photon excitation properties of iRFP, microscopists should closely monitor progress on this front as substantial gains in imaging depth might be achievable.
Just as visible fluorescent proteins led to many different fluorescent indicators for monitoring cellular dynamics, we hope that iRFP will similarly prompt the development of infrared indicators. For example, an effective near-infrared Ca2+ indicator would be a boon for monitoring cells’ physiological activation in vivo. Infrared indicators of the future might even employ fluorescence resonance energy transfer (FRET) between two infrared fluorescent proteins, akin to the many visible FRET indicators in current use7. Overall, researchers’ prospects for looking deep are looking ever brighter.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Contributor Information
Jérôme Lecoq, James H. Clark Center, Stanford University, Stanford, California, USA.
Mark J Schnitzer, James H. Clark Center, Stanford University, Stanford, California, USA. Howard Hughes Medical Institute and the CNC Program, Stanford University, Stanford, California, USA.
References
- 1.Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 2.Shaner NC, Steinbach PA, Tsien RY. Nat Methods. 2005;2:905–909. doi: 10.1038/nmeth819. [DOI] [PubMed] [Google Scholar]
- 3.Filonov GS, et al. Nat Biotechnol. 2011;29:757–761. doi: 10.1038/nbt.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shu X, et al. Science. 2009;324:804–807. doi: 10.1126/science.1168683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helmchen F, Denk W. Nat Methods. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- 6.Ntziachristos V. Annu Rev Biomed Eng. 2006;8:1–33. doi: 10.1146/annurev.bioeng.8.061505.095831. [DOI] [PubMed] [Google Scholar]
- 7.Tsien RY. Cold Spring Harb Protoc. 2009. pdb top57. [DOI] [PubMed] [Google Scholar]
- 8.Zijlstra WG, Buursma A, Meeuwsen-van der Roest WP. Clin Chem. 1991;37:1633–1638. [PubMed] [Google Scholar]
- 9.Matcher SJ, Cope M, Delpy DT. Phys Med Biol. 1994;39:177–196. doi: 10.1088/0031-9155/39/1/011. [DOI] [PubMed] [Google Scholar]
- 10.Bashkatov ANG, Kochubey EA, Tuchin VI. Appl Phys (Berl) 2005;38:2543–2555. [Google Scholar]