Abstract
UNAIDS, and the WHO, plan to use “treatment as prevention” (TasP) to eliminate HIV. The rationale is that treating HIV-infected individuals reduces their infectivity. We present a novel geostatistical framework for implementing the rollout of TasP in sub-Saharan Africa. We focus on Lesotho, because UNAIDS has identified their epidemic as a priority for elimination. Our framework is based on a density of infection (DoI) map that we generate by gridding high-resolution demographic data, and spatially smoothing georeferenced HIV-testing data. The map reveals the geographic dispersion pattern of HIV-infected individuals, both diagnosed and undiagnosed. We use the map to design a treatment allocation strategy that optimizes, under resource constraints, the efficiency of resource utilization. Using this strategy (rather than the current treatment allocation strategy) would make it easier to find, diagnose and treat individuals. We also use our framework to evaluate the feasibility of UNAIDS’ elimination plan. We show the feasibility of reaching specific treatment coverage levels depends upon the geographic dispersion pattern of HIV-infected individuals, and that this pattern reflects the spatial demographics of Lesotho. Only 20% of HIV-infected individuals live in urban areas; most live in rural settlements where the DoI is less than six infected individuals/km2. Given these conditions, it will be almost impossible to reach a very high coverage of treatment. Therefore, the UNAIDS elimination plan is unlikely to succeed in Lesotho. Taken together, our results show that the spatial demographics of populations will significantly hinder, and may even prevent, the elimination of HIV in sub-Saharan Africa.
Introduction
The WHO (1) and UNAIDS (2) plan to use “treatment as prevention” (TasP) to achieve the global elimination of HIV. In sub-Saharan Africa (SSA), ~25 million individuals are infected with HIV (3). The rationale behind using TasP is straightforward: treating HIV-infected individuals, by reducing their viral load, makes them less infectious than untreated individuals (4). Therefore a high coverage of treatment could, potentially, prevent a substantial number of new HIV infections (5-7).The elimination plan proposed by UNAIDS is described as the 90-90-90 strategy: to diagnose 90% of HIV-infected individuals, treat 90% of the diagnosed, and achieve viral suppression in 90% of patients (8). The aim is to achieve these goals by 2020. TasP is now being used in many resource-rich countries, where HIV epidemics are concentrated in “high-risk groups” and the 90-90-90 goals appear attainable (7, 9). However, TasP has not yet been introduced into resource-constrained countries in SSA, where HIV epidemics are generalized: i.e., the prevalence of HIV, predominantly due to heterosexual transmission, is high in the general population. As a consequence of these epidemiological conditions, to eliminate HIV, TasP will need to be used throughout entire countries. Notably, the majority (~60%) of the population in SSA lives in rural areas (10), where settlements are often widely dispersed, and population density is low (11). The potential effect of these spatial demographics on hindering, or even preventing, the TasP-based elimination of HIV in SSA has not been considered by either UNAIDS or the WHO. Here we present a novel geostatistical framework for designing TasP-based elimination strategies for resource-constrained countries in SSA. Our framework also enables their feasibility to be evaluated. The strategies take into account the characteristic spatial demographics of populations in SSA, the countrywide spatial diffusion of generalized HIV epidemics, and resource constraints. The framework is based on the construction of a map that reveals the geographic dispersion pattern of all HIV-infected individuals in an entire country. As an example, we design TasP-based HIV elimination strategies for Lesotho, a country where 73% of the population live in rural areas (10). We also use our framework to evaluate the feasibility, and potential success, of the 90-90-90 elimination strategy.
UNAIDS has identified the HIV epidemic in Lesotho as a priority for elimination (8). The epidemic is one of the most severe worldwide: prevalence in the general population is ~25% (12). Healthcare is decentralized, and the country is divided into ten healthcare districts (HCDs), Fig. 1A. In each district there is one major urban center; in two HCDs, Maseru and Leribe, there are also several large towns. Currently, only 36% of HIV-infected individuals in Lesotho are receiving treatment (13); ~200 clinics are dispersed throughout the country (Fig. 1B).
Fig. 1. Maps of Lesotho.
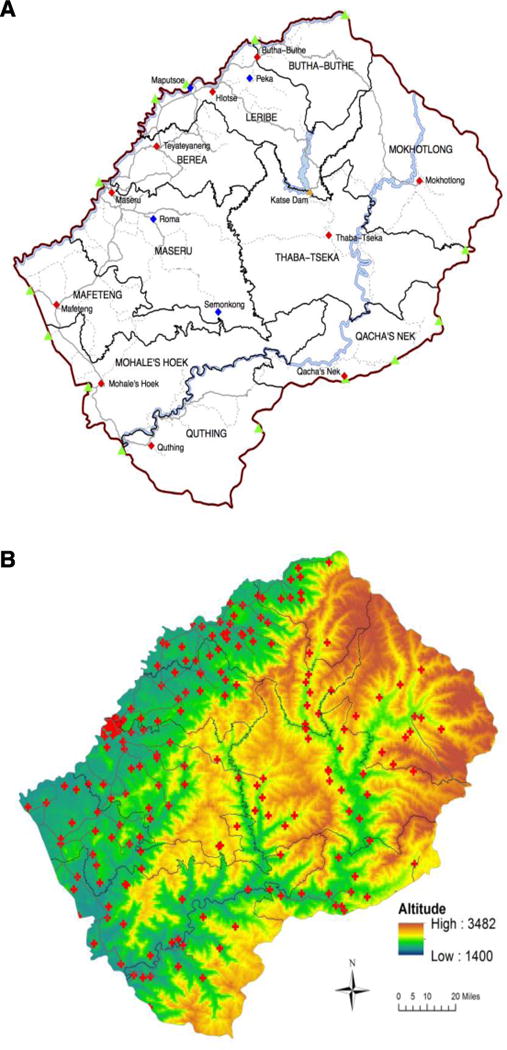
(A) Map of Lesotho showing the national border (red line), border crossings into South Africa (green triangles), the major urban center in each healthcare district, HCD (red diamonds), major towns (blue diamonds), the road network (paved roads, solid gray lines; tracks, dotted gray lines), major water bodies (light blue), and the Katse Dam (orange triangle). The black lines delimit the ten HCDs. (B) Map showing the topography of Lesotho: the black lines show the administrative boundaries of the ten HCDs, the red crosses show the ~200 clinics dispensing HIV treatment. Altitude is given in meters.
Our geostatistical framework consists of two components: a country-level Density of Infection (DoI) map and Epidemic Concentration Curves (ECCs). The map reveals the number of infected individuals/km2: both diagnosed and undiagnosed individuals. An ECC is a new theoretical construct that we are introducing for the study of infectious diseases; it is a semi-logarithmic plot that enables an epidemic to be characterized within a spatial demographic context. The ECC provides a quantitative estimate of both the degree of urbanization of the epidemic (the percentage of the epidemic that is concentrated in urban areas), and the degree of dispersion (and number) of HIV-infected individuals in rural areas.
The DoI map reflects the spatial diffusion of the epidemic in the general population, the dispersion patterns (and size) of rural and urban settlements, and geographic variation in population density. To construct the map we use geostatistical techniques to integrate three large datasets: (i) gridded high-resolution demographic data from the WorldPop Project (14), (ii) age-structure census data, and (iii) georeferenced HIV-testing data collected from ~7,000 individuals (15–49 years old) who participated in the 2010 Lesotho Demographic Health Survey (LDHS) (15). The LDHS is a nationally representative population-level survey; see Materials and Methods and fig. S1.
Our analyses focus on the initial stage of the rollout of TasP-based elimination strategies. Specifically, we use our geostatistical framework to design a treatment allocation strategy for the implementation stage. We design, under resource constraints, an “efficient” treatment allocation strategy that aims to optimize the efficiency of resource utilization. The strategy ensures that TasP is used in the communities with the highest DoI and minimizes the area that needs to be covered to find and treat infected individuals.
We then compare the efficient strategy with the current treatment allocation strategy. Strategies are compared in terms of how they divide a limited supply of treatment within a decentralized healthcare system, specifically, among the ten HCDs in Lesotho. Notably, we do not predict the epidemiological outcomes of either treatment allocation strategy. To make such predictions would require developing a complex spatially-explicit transmission model based on the DoI map, and taking population mobility and all major forms of migration into account; transmission modeling is the focus of our ongoing research.
Finally, we use our geostatistical framework to evaluate the feasibility of achieving the 90-90-90 treatment goals of the UNAIDS TasP-based elimination strategy. The belief that this strategy will succeed is based, in part, on the implicit assumption that people pick their sex partners from the surrounding community. We assess the potential for individuals to have sex partners outside their home communities by constructing two countrywide connectivity maps and a mobility map. The connectivity maps reveal the proportion of households that have family members living, on a temporary basis, elsewhere: either in Lesotho or in South Africa. The mobility map reveals the percentage of the population that has made at least one overnight trip in the past twelve months.
Results
Spatial Demographics
The gridded WorldPop data shows the settlement dispersion patterns in Lesotho and the geographic variation in population density, at a resolution of 0.01 km2 (Fig. 2A); the total population size of Lesotho is ~2 million. The settlements were identified by using satellite data on surface imagery of land cover patterns to reallocate population census data (11). Approximately 27% of the population lives in one of the twelve urban centers in Lesotho (10); the population density is highest (~4,000 individuals/km2) in the capital city, Maseru (Fig. 2A and 2B). The majority live in small, dispersed, rural settlements where (on average) there are ≤ 100 individuals/km2 (Fig. 2B); this includes individuals of all ages. There is relatively little variation in age-structure among HCDs, but an urban-rural difference is apparent (fig. S2): ~60% of the population in urban centers are 15 to 49 years old versus ~50% in rural areas.
Fig. 2. Demographic and HIV prevalence data.
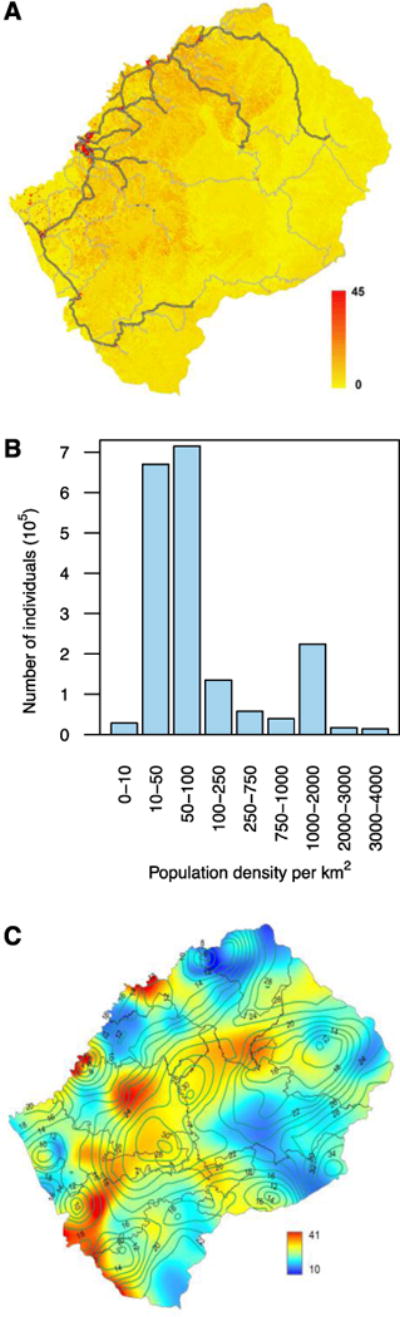
(A) Map of gridded WorldPop data for Lesotho showing settlement patterns and geographic variation in population density. The population density ranges from 0 (yellow) to 45 (red) individuals (of any age) per 100 meters by 100 meters. Gray lines show the road network, paved roads are outlined in black. (B) Histogram of the gridded WorldPop data for Lesotho. (C) Epidemic Surface Prevalence (ESP) map for Lesotho. Color scale indicates HIV prevalence (%) in individuals 15 to 49 years old. The isoclines show the size (in km) of the radii of the smoothing circles used in the spatial interpolation to calculate the ESP map; these circles contain 200 individuals between 15 and 49 years old.
The epidemic surface prevalence (ESP) Map
The ESP map (Fig. 2C) provides a spatial visualization of the HIV epidemic throughout Lesotho. Specifically, it shows the percentage of the population (aged 15 to 49 years old) that is infected with HIV. The map was constructed using spatial interpolation techniques (16), and georeferenced HIV-testing data from the LDHS; see Materials and Methods. There is relatively little variation in HIV prevalence among the HCDs, except in Butha-Buthe in the north where the prevalence is lower (16%) than in the rest of the country. Notably, an individual’s probability of HIV infection is higher in urban centers than in rural areas; HIV prevalence (on average) is 27% in urban centers versus 21% in rural settlements.
The DoI Map
The DoI map (Fig. 3A) is a combination of the maps in Fig. 2A, 2C, and S2; see Materials and Methods. It shows the geographic dispersion pattern of all HIV-infected individuals (diagnosed and undiagnosed) in Lesotho who are between 15 and 49 years old. The map reveals that the epidemic has spread to almost every community in the country. Additionally, since prevalence is relatively uniform (Fig. 2C), it shows that the geographic dispersion pattern of HIV-infected individuals (15 to 49 years old) directly reflects the spatial demographics of the population. Specifically, it reflects the dispersion pattern (and size) of settlements, and the geographic variation in population density (Fig. 2A and 2B). All subsequent estimates of the DoI are given in terms of the number of infected individuals/km2; this refers to individuals between 15 and 49 years old.
Fig. 3. Results showing the geographic dispersion of HIV-infected individuals throughout Lesotho.
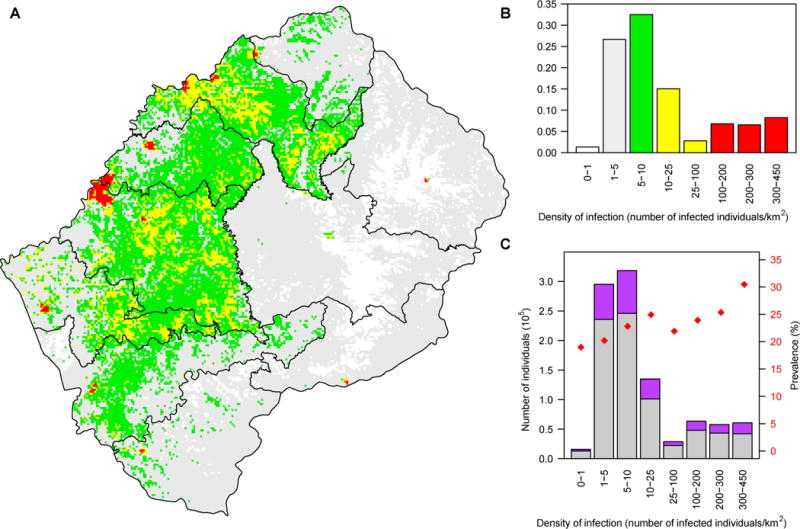
(A) Density of Infection (DoI) map shows the dispersion pattern and the geographic variation in the number of HIV-infected individuals (15 to 49 years old) per km2: white (≤1), gray (1-5), green (5-10), yellow (10-100), and red (>100). (B) Histogram shows the distribution of the HIV epidemic (number of HIV-infected 15 to 49 year olds) as a function of the DoI. The color code is the same as in (A). (C) Histogram shows the distribution of the population (15 to 49 year olds), and the prevalence of HIV, as a function of the DoI: purple data represent HIV-infected individuals, gray data uninfected individuals, and red dots HIV prevalence.
The DoI in the twelve urban areas in Lesotho ranges from 100 to 450 infected individuals/km2; the density is one to two orders of magnitude lower in rural settlements. Notably, the vast majority of infected individuals live in rural areas, many where the DoI is extremely low. Almost a third live in rural settlements where there are less than six infected individuals/km2 (Fig. 3A and 3B). The HIV prevalence is high (~20%), even when the DoI is extremely low (Fig. 3C). Most of the HIV-infected individuals in rural settlements live close (within one kilometer) to ~2-20 uninfected individuals of the opposite gender, whereas (on average) most of the infected individuals in urban centers live in close proximity to ~350-500 uninfected adults of the opposite gender (Fig. 3C). The DoI varies substantially within, as well as among, the ten HCDs (Fig. 4).
Fig. 4. Geographic dispersion pattern of HIV-infected individuals for each healthcare district (HCD).
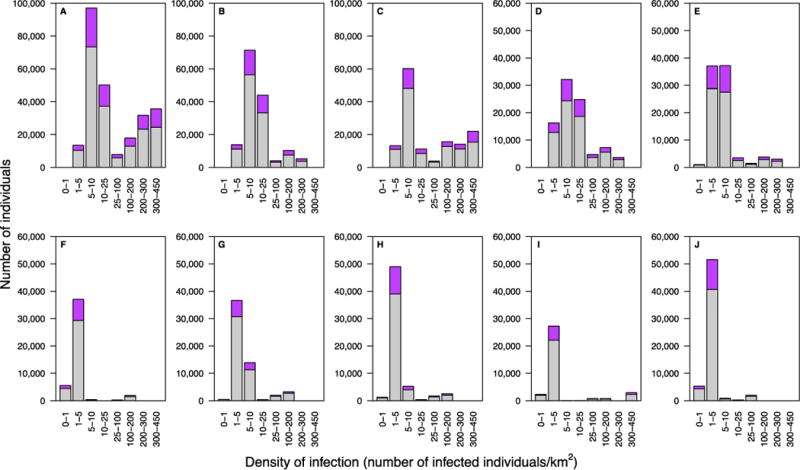
Histograms show the distribution of the population (15 to 49 year olds) as a function of the DoI: purple data represent HIV-infected individuals, gray data uninfected individuals. (A) Maseru HCD, (B) Leribe HCD, (C) Berea HCD, (D) Mafeteng HCD, (E) Mohale’s Hoek HCD, (F) Mokhotlong HCD, (G) Butha-Buthe HCD, (H) Quthing HCD, (I) Qacha’s Nek HCD, and (J) Thaba-Tseka HCD.
Based on the DoI map, we estimate there are 223,505 HIV-infected individuals (15-49 years old) living in Lesotho; this includes both diagnosed and undiagnosed individuals. This estimate lies within the 95% Confidence Interval (CI) obtained when estimating the number of infected individuals (15-49 years old) without spatially smoothing the prevalence data (CI: 213,540-243,229); see Materials and Methods.
The national-level ECC
The national-level ECC shows that the HIV epidemic in Lesotho is extremely rural: only ~20% of infected 15 to 49 year olds live in urban areas (Fig. 5A). Outside the urban areas, the DoI drops precipitously. The shape of the ECC demonstrates that finding, testing, and treating, HIV-infected individuals will become progressively more challenging as treatment coverage increases.
Fig. 5. Epidemic Concentration Curves (ECCs) quantifying the HIV epidemic within a demographic context.
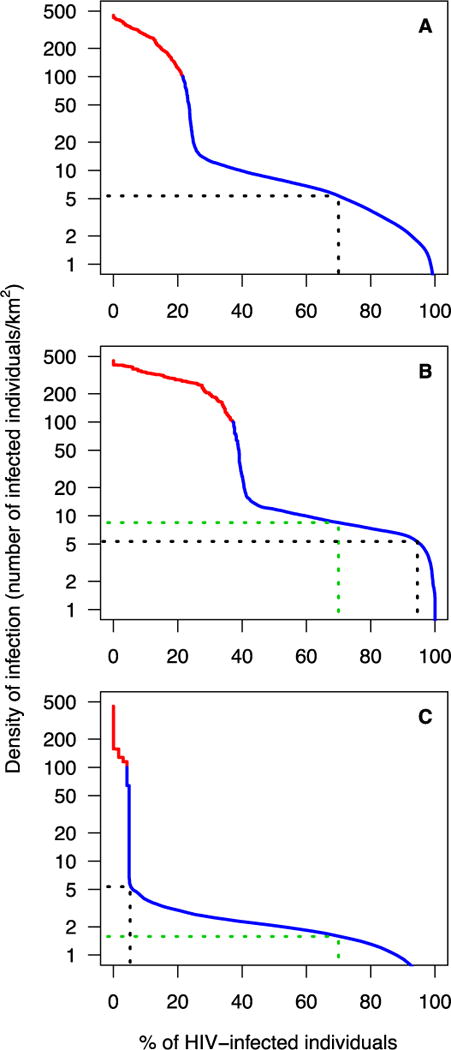
The red part of each curve shows the degree of urbanization of the epidemic, the blue part of each curve shows the degree of dispersion of HIV-infected individuals (15-49 year olds) in rural areas. The dotted black line in (A), (B), and (C) shows the percentage of HIV-infected individuals who would receive treatment if the efficient treatment allocation strategy is used; the dotted green line in (B) and (C) shows the percentage of HIV-infected individuals who would receive treatment if the current treatment allocation strategy is used. Both strategies result in an overall treatment coverage of 70%. (A) The ECC for Lesotho. (B) The ECC for Maseru healthcare district (HCD). (C) The ECC for Mokhotlong HCD.
Designing an efficient treatment allocation strategy for implementing the rollout of TasP
The national-level ECC, in combination with the DoI map, can be used to design an efficient treatment allocation strategy for implementing the rollout of TasP. Specifically, it can be used to identify which communities should use TasP when there is not enough treatment to achieve universal coverage throughout the country, i.e., given resource constraints. The level of resource constraints will determine the amount of treatment that is available. Our approach can be used for any level of constraints.
The elimination strategy proposed by UNAIDS requires treating ~80% of HIV-infected individuals (i.e., diagnosing 90% of infected individuals, and then treating 90% of the diagnosed), and achieving viral suppression in 90% of the treated individuals. This treatment goal could also be reached by treating ~70% of infected individuals, and achieving viral suppression in essentially all of them. Therefore, based on the UNAIDS strategy, we consider ~70% as the minimum treatment coverage needed for elimination.
To achieve 70% coverage, and use resources efficiently (i.e., employ the efficient treatment allocation strategy), TasP should only be used in communities where there are at least five infected individuals/km2 (Fig. 5A). TasP should first be rolled out in urban communities in the red areas of the DoI map (where there are 100-450 infected individuals/km2), then in rural communities in the yellow areas (where there are 10-100 infected individuals/km2), and finally in rural communities in green areas (where there are 5-10 infected individuals/km2), Fig. 3A. This efficient treatment allocation strategy would ensure universal coverage in all urban areas in all ten HCDs, and in the largest rural communities in the country. Under this strategy, TasP would not be used in the grey and white areas of the DoI map.
Implementing TasP-based elimination strategies in a decentralized healthcare system
Lesotho has, as do many other countries in SSA, a decentralized healthcare system. Therefore, decisions will need to be made – if treatment is limited – as to how to divide the available supply among the HCDs in order to implement TasP-based elimination strategies. To design treatment allocation strategies for a decentralized healthcare system, we constructed an ECC for each of the ten HCDs. As previously, this approach can be used for any given level of resource constraints.
The ECC for the most urbanized HCD, Maseru, is shown in Fig. 5B; the ECC for one of the most rural HCDs, Mokhotlong, is shown in Fig. 5C. ECCs for the other eight HCDs are shown in fig. S3. The variation in the shape of the ECCs reflects differences among the HCDs in the urbanization of their epidemic, and the degree of geographic dispersion of infected individuals in their rural areas. These differences reflect the differences in their spatial demographics, i.e., in the dispersion patterns (and size) of rural and urban settlements, and geographic variation in population density.
If the efficient allocation strategy is used for implementation, the treatment supply would be divided among the ten HCDs to reflect the geographic dispersion pattern of HIV-infected individuals throughout Lesotho. HCD-specific coverage would vary considerably, due to the substantial differences (among the HCDs) in their spatial demographics. With a national coverage of ~70%, HCD-specific coverage would vary from 4% in Thaba-Tseka to 94% in Maseru. In Thaba-Tseka very few individuals live in urban areas, and rural settlements are small and widely dispersed. In Maseru ~40% live in urban areas, and rural settlements are relatively large and clustered. Results for all 10 HCDs are shown in Fig. 5B, 5C, and S3.
The current strategy for allocating treatment aims to achieve treatment equity. If this strategy is used for rolling out TasP, spatial demographics would not be considered. The treatment supply would be divided among the ten HCDs in proportion to the burden of disease, i.e., in proportion to the number of infected individuals in each HCD. Under this strategy, the treatment coverage in every HCD would be the same as at the national level. Results for all 10 HCDs (based on a national coverage of ~70%) are shown in Fig. 5B, 5C, and S3.
Our results show that – due to the geographic dispersion pattern of HIV-infected individuals in Lesotho – the amount of treatment that each HCD would receive would differ considerably depending on which allocation strategy was used to implement a TasP-based elimination strategy. The six most rural HCDs in Lesotho would receive more treatment under the current allocation strategy than under the efficient allocation strategy (Fig. 5C and S3D-H). Conversely, the four most urbanized HCDs would receive more treatment under the efficient than under the current strategy (Fig. 5B and S3A-C).
Feasibility, and likely success, of the UNAIDS TasP-based elimination strategy
The national-level ECC, coupled with the DoI map, indicates that achieving UNAIDS’ 90-90-90 treatment goals is unlikely to be possible in Lesotho – even if the efficient treatment allocation strategy is used. To reach the 90% diagnosis goal would require finding many HIV-infected individuals who are living in widely dispersed rural settlements, where there may be only two infected individuals/km2 (Fig. 3A and 5A). To treat 90% of these diagnosed individuals would require treating many who are living in fairly inaccessible rural areas, where there may be as few as four infected individuals/km2 (Fig. 3A and 5A). Notably, to achieve universal access to treatment (as proposed by the WHO (1)) would require treating many individuals in areas where there is likely to be only one infected individual/km2 (Fig. 3A and 5A).
Even treating ~70% of infected individuals in Lesotho, the minimum (according to UNAIDS) treatment coverage needed to eliminate HIV, may not be feasible (Fig. 5A).
The success of a TasP-based elimination strategy will depend, in part, upon migration rates and population mobility. TasP will not be an effective elimination tool if individuals “import” HIV into, and/or “export” HIV out of, their home communities. Both the connectivity maps (Fig. 6A and 6B), and the mobility map (Fig. 6C) show that there is a potential for people to have sex partners from outside their home community; see Materials and Methods for a description of how the maps were constructed. The connectivity maps show that many families are geographically separated on a temporary basis; ~40% have a household member living, part time, in another region of Lesotho (Fig. 6A), and ~30% in South Africa (Fig. 6B). The maps show distinct geographic patterns that differ based on where the household member (who is away from their home community) is living. The population in Lesotho is also extremely mobile (Fig. 6C); overall, ~50% of 15 to 49 year olds have made one or more overnight trip(s) in the past 12 months. Notably, travel is extremely high along the border with South Africa.
Fig. 6. Connectivity and mobility maps.
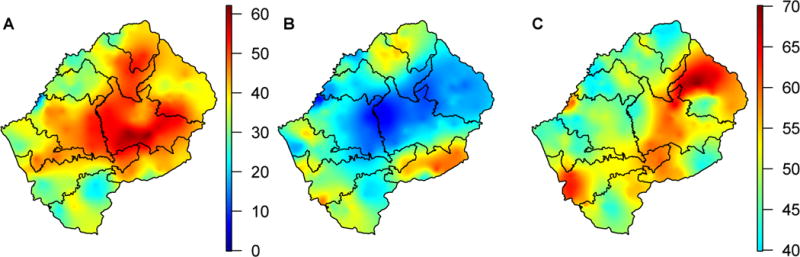
(A) Connectivity map shows the percentage of households with adult members living elsewhere in Lesotho. (B) Connectivity map shows the percentage of households with adult members living in South Africa. (C) Mobility map shows the percentage of adults who made at least one overnight trip in the past 12 months.
Discussion
By using geostatistical techniques to view a generalized HIV epidemic in SSA within a demographic context, we have gained a greater understanding of one of the most severe HIV epidemics in the world. Although there is relatively little geographic variation in HIV prevalence throughout the country, the DoI varies over two orders of magnitude. The geographic dispersion pattern of HIV-infected individuals reflects the spatial demographics of the country, and highlights the significance of the urban-rural divide. Only ~20% of the epidemic is concentrated in the urban centers. The vast majority of HIV-infected individuals live in small, widely dispersed, rural settlements. Notably, most rural settlements appear to contain at least one infected individual – even those in the most remote mountainous regions.
The spatial diffusion of the epidemic reflects the high mobility of the population, and the high levels of circular and cross-border migration. Temporary migration in Lesotho is related to employment; there is circular migration within the country to work in agriculture or the textile industry, and cross-border migration to work in the mines in South Africa. Previous studies of Lesotho have shown that (on average) residents who travel have higher numbers of sex partners than those who do not, and men who travel frequently have an increased risk of HIV infection (17). Permanent migration may also have facilitated the spatial diffusion of HIV. In Lesotho, between 2001 and 2011, ~45% of the population changed their permanent residency (18), moving from rural areas to urban centers, or within urbanized regions. The high mobility of the population, and high migration rates, are likely to be important in driving ongoing transmission. These factors, and the high degree of connectivity with South Africa will increase the difficulty of eliminating HIV in Lesotho.
To date, TasP has only been shown to be effective in preventing HIV infections in one clinical trial (5) and, in a few, real-world settings (6, 7). Four clinical trials are currently studying the effectiveness of TasP in decreasing incidence (19). One study has reported preliminary results: TasP was not found to be effective in reducing incidence, but treatment coverage only reached 40% (20). There are many reasons that TasP may not be effective in controlling HIV epidemics in SSA: resource constraints may limit the treatment supply (and hence the achievable coverage levels), viral suppression rates may not reach high enough levels, and mobility/migration may result in the continuous importation/exportation of HIV into/out of communities within a country, or between countries. However, the decision to use TasP has already been made (1, 2), and TasP will soon be rolled out in SSA (21). As a consequence, our study focuses on the design and evaluation of potential implementation strategies. Specifically, it focuses on how to divide a limited supply of treatment among HCDs in a decentralized healthcare system, and the feasibility (and likely success) of the UNAIDS 90-90-90 elimination strategy.
Even if TasP is ineffective in reducing incidence in SSA, rolling out TasP will be extremely beneficial for those who receive treatment, in that they will gain a substantial increase in life expectancy (22). The number of individuals who will directly benefit from the rollout will be determined by the level of resource constraints, but not by the treatment allocation strategy. Both the efficient and the current treatment allocation strategies will cause the same overall reduction in the AIDS mortality rate at the national level. However, using the efficient strategy rather than the current strategy would result in lower AIDS mortality rates in the more urbanized HCDs and higher rates in the most rural HCDs. This would exacerbate already significant urban-rural disparities in healthcare. Notably, the efficient strategy is likely to be more cost-effective (in terms of costs per life-year saved) than the current strategy because it would make it easier to find, diagnose and treat individuals, and require an overall smaller catchment area for treatment programs. In addition – if TasP is effective – implementing the rollout using the efficient strategy may be more likely to succeed in eliminating HIV than using the current treatment allocation strategy, as TasP may be more effective at a high DoI than at a low DoI. We stress that the governments of the countries that roll out TasP should choose the treatment allocation strategy that is used for implementation.
Our geostatistical framework can be used to design implementation strategies for other types of interventions for controlling HIV epidemics in SSA, e.g., circumcision (23-25) and pre-exposure prophylaxis (26). It could be used to evaluate their feasibility, as well as improve their efficiency and cost-effectiveness. Additionally, it could be used for increasing the efficiency and cost-effectiveness of large-scale HIV-testing campaigns. Our approach identifies both the geographic dispersion pattern of HIV-infected individuals and the number of uninfected individuals living in close proximity to them. Consequently, it could be used to decide which of the available prevention modalities (including TasP) are most appropriate for each community. The DoI map that we have developed for Lesotho highlights the necessity of developing new prevention tools for uninfected individuals living in inaccessible areas of the country, and/or where the DoI is low, and/or where mobility/migration rates are high.
Many other countries in SSA have generalized epidemics, similar spatial demographics to Lesotho, and a significant percentage of their population living in rural areas (table S1); many also have highly mobile population and high rates of circular migration (27-29). The data that are necessary to construct DoI maps and ECCs for 24 of these countries are publicly available (30). The geographic dispersion pattern of HIV-infected individuals in each country would vary, due to country-specific differences in epidemiology and spatial demographics. However, the dispersion patterns may be qualitatively similar (in terms of the urban-rural divide) to the pattern in Lesotho. The DoI maps and ECCs for the 24 countries could be used in the same manner as we have shown for Lesotho. By analyzing the specific patterns, it could be determined in which, if any, of these countries TasP could succeed in eliminating HIV. Additionally, our approach could be used to develop country-specific diagnosis and treatment coverage goals.
As with all studies, ours has limitations. It is not possible to obtain a quantitative assessment of the accuracy of the ESP map (16), i.e., to generate a map of standard errors. Methods to generate such maps are under development (31). The ESP map is based on spatially smoothing prevalence data, therefore, the degree to which the data are smoothed is important. The map is likely to be imprecise if prevalence is low. In the case of Lesotho, the ESP map is likely to be fairly accurate as the data are smoothed based on a sample size of 200 individuals (see Materials and Methods), and prevalence is very high throughout the country. The ESP map that we have generated, in order to construct our DoI map, shows the prevalence of HIV infection in adults (i.e., in both women and men) who are between 15 to 49 years old. Elsewhere we have shown that gender-based HIV ESP maps of Lesotho differ both quantitatively and qualitatively (32). Their spatial patterns differ, and HIV prevalence is always substantially higher in women than in men. Consequently, the associated gender-specific DoI maps would also be considerably different. This demonstrates that it would be essential to construct gender-specific DoI maps if the objective is to develop health policies for gender-based prevention tools, e.g., vaginal microbicides. Notably, we have analyzed the most up-to-date data sets that were available: spatial demographic and HIV-testing data from 2010. However more recent demographic data will soon become available. The Bureau of Statistics in Lesotho has just completed the 2016 census; data from this census will be used to update the spatial demographic estimates in the WorldPop database. In addition, HIV-testing data from the 2014 LDHS will soon become available. HIV prevalence is unlikely to have changed significantly over four years, but some small settlements in remote areas have been reported to have been abandoned in the past few years. Any demographic changes would need to be included in a DoI map before using it to make detailed health policy decisions.
Our results have significant implications for global health policies. UNAIDS did not consider the spatial demographics of populations in SSA when they designed their TasP-based HIV elimination plan and set their 90-90-90 goals. We have evaluated the feasibility of implementing their plan in Lesotho, where one in four adults is infected with HIV. We have shown that the feasibility directly depends upon the geographic dispersion pattern of HIV-infected individuals, and that the pattern reflects the spatial demographics of the population. In Lesotho, infected individuals are widely dispersed and many live in rural settlements in areas where there is a very low DoI. These conditions will make it extremely challenging to reach the diagnosis goal of the 90-90-90 strategy, and essentially impossible to reach the treatment coverage goal. Resource constraints will limit the availability of treatment for TasP. However, our results show that the spatial demographics of populations will also hinder - and may even prevent - the elimination of HIV in SSA.
Materials and Methods
Study design
The study presents a novel geostatistical framework for designing, and evaluating, TasP-based elimination strategies for countries in SSA. The approach is based on two components: a DoI map and ECCs. The DoI map provides a high-resolution visualization of the number of 15-49 year old HIV-infected individuals/km2 throughout Lesotho. The data from the DoI map is used to construct the ECCs. To construct the DoI map, geostatistical methods are used to combine three “types” of data: gridded high-resolution demographic data from the WorldPop Project (33), age-structure data collected during the 2010 census in Lesotho, and georeferenced HIV-testing data collected from ~7,000 individuals (15-49 years old) who participated in the 2010 Lesotho Demographic Health Survey (LDHS) (15).
WorldPop Project Dataset
The WorldPop database contains estimates for the geographic distribution of the population in terms of population density and settlement patterns for 126 countries in Africa, America, and Asia (33). Fifty African countries, including Lesotho, are represented in the database. For our analyses we used the alpha version of the WorldPop dataset for Lesotho; this version contains 2010 estimates of the number of people per 100 meters by 100 meters.
The WorldPop dataset for Lesotho is in the form of a raster image; i.e., it is a dataset of population estimates presented in a set of discrete uniform cells (pixels) that are based on a gridded surface, where each pixel on the grid represents a defined square area in a specific geographic location. The WorldPop dataset for Lesotho has a resolution of 100 meters by 100 meters.
The WorldPop database was constructed by using satellite data on surface imagery, specifically imagery on land cover patterns, to map the settlement patterns. The surface imagery data were used to reallocate the population census data to settlements; settlements in Lesotho vary from cities to small rural homesteads. Satellite data were taken from NASA’s Landsat spacecraft, which uses Enhanced Thematic Mapper imagery to monitor the earth’s land cover. The details of the methodologies used by Linard et al. (11) to construct the database are described on the WorldPop website (14). These data are available online (33).
Lesotho 2010 Demographic and Health Survey Dataset
The Lesotho Demographic and Health Survey (15) is a nationally representative population-level survey that was conducted between 2009 and 2010. The survey utilized a two-stage cluster sample design. The number and location of clusters was determined based on a list of 33 enumeration areas so that the population sample was geographically proportional to the most recent census estimate. Four hundred clusters were sampled: 94 urban and 306 rural. Geographic coordinates that specify the location of each cluster were collected in the field using handheld GPS receivers; cluster locations are shown in fig. S1. Geographic coordinates were measured during the survey sample listing process, or during the administration of the survey itself; further methodological details are provided online (30). Five clusters did not have geographic coordinates so they were not used in our analyses.
After the locations for the 400 clusters had been chosen, a complete listing of households was created for each cluster. Households were then randomly selected for participation in the survey; the response rate was 94% (9,391 households participated). Three types of questionnaires were used to collect data in the LDHS: the Household Questionnaire, the Woman’s Questionnaire, and the Man’s Questionnaire.
One representative from each of the participating households completed the Household Questionnaire where they provided data for: themselves, all of the individuals who were regular members of their household, and any visitors who were staying at their house. The representative specified (for each individual) their age, gender and relationship to the head of the household. Data were collected on 44,546 individuals (from the 9,391 households), infants to 96 year olds; identifiers linked the data for each individual to the household (and the cluster location) where the Household Questionnaire was completed. The LDHS considered an individual to be a household member if they had slept in the house the night before the survey. Based on this definition 33,719 individuals (newborns to 96 year olds) were considered to be household members: 18,233 women and 15,486 men.
All of the women (between 15 to 49 years old) in the 9,391 households were eligible to participate in the individual-level survey; i.e., to complete the Woman’s Questionnaire. This survey collected data on demographics, economics, behavior and health; 98% of the 7,786 eligible women participated. Then ~50% of the 9,391 households were randomly selected. All of the men in the selected households (between 15 to 59 years old) were eligible to participate in the individual-level survey, i.e., to complete the Men’s Questionnaire. Ninety-five percent of the 3,493 eligible men participated in the individual-level survey.
In the households where men were interviewed, all 15 to 59 year old men and all 15 to 49 year old women were eligible for HIV-testing; 94% of the eligible women, and 88% of the eligible men were tested. This resulted in a total of 7,099 HIV test results: 3,928 from women and 3,171 from men. For each individual, their HIV test result was linked to their data collected in the individual-level survey and the household-level survey. The testing data were georeferenced by using the geographic coordinates for the cluster location.
A more detailed description of the LDHS datasets and HIV-testing data can be found elsewhere (12). These data are also available online (15).
Estimating the number of HIV-infected individuals
We estimated the burden of disease by calculating the number of individuals in Lesotho who are between 15-49 years old, and then multiplying this number by the average HIV prevalence level in Lesotho. We calculated the number of 15-49 years olds by multiplying the total number of individuals of all ages living in Lesotho (from the WorldPop database) with the proportion of individuals who are aged 15-49 years old (from the 2010 Census data). We estimated the average HIV prevalence in Lesotho from the HIV-testing data collected in the 2010 LDHS. Using this procedure, there were 228,384 (mean, 95% CI: 213,540-243,229) HIV-infected individuals (aged 15 to 49 years old) living in Lesotho. This includes both diagnosed and undiagnosed individuals.
Notably, it has been shown using Heckman-type selection models (34) that HIV prevalence estimates that are determined using data from the 2010 LDHS are not susceptible to selection bias. They do not need adjusting due to non-participation in testing; this is due to the extremely high response rate (94% of eligible women, and 88% of eligible men, were tested for HIV).
Constructing the DOI map
We first constructed a high-resolution demographic map of Lesotho by plotting gridded data from the WorldPop Project (33). Next, we constructed a second map by plotting age-structure data from the 2010 Census; data were stratified at the level of the HCD, and then between urban and rural areas. Then we constructed the ESP map; we used the HIV-testing data from the LDHS, and spatial interpolation techniques (16). All three maps were saved as raster images.
To construct the DoI map we used raster multiplication to combine the three maps; this procedure was performed in ArcGIS (35) (using ArcMap Advanced 10.1 software) and R (36).
Constructing the ESP map
We generated the ESP map by using an adaptive bandwidth kernel density (ABKD) estimation method (16) to smooth spatially and interpolate georeferenced HIV-testing data from 7,099 individuals (15 to 49 years old) who participated in the 2010 LDHS; the bandwidth specifies the degree of smoothing. Each individual is linked by an identifier to one of the households in one of the clusters in the dataset. The ABKD estimation method smoothed the localized HIV prevalence around each cluster (16).
The ESP map is defined, when using the ABKD estimation method, as a density surface. The ABKD method prevents under-smoothing in areas with sparse observations, and over-smoothing in areas with many observations. We used the R programming package, prevR, for implementation (36). This software package first constructs an intensity surface for the infected population and an intensity surface for the total population (i.e., infected and uninfected individuals). It then generates the ESP map by dividing the intensity surface for the infected population by the intensity surface for the total population. The intensity ŝ (i.e., the number of individuals per unit area) at spatial location (x,y) is determined by the equation:
where N is the number of individuals included in the smoothing circle, di is the geometric distance between cluster i and the geographic location point (x,y), K is the kernel density function, and hi is the smoothing bandwidth used at cluster i. A two-dimensional Gaussian function is used for the kernel density function. The smoothing bandwidth hi used at cluster i is proportional to the radius r of the smoothing circle that is drawn around cluster i in order to encompass N individuals.
We used an N of 200. The size of the smoothing circles reflects population density. With an N of 200 the smoothing circle around each cluster encompasses at least four clusters and, even when prevalence is low (~10%), encompasses at least 20 infected individuals.
Constructing the national-level ECC
First, we used the DoI map to obtain estimates ϕk for the number of 15-49 year old HIV-infected individuals in each square kilometer k of the country. These were ordered from low to high as ϕ(1), ϕ(2),…, ϕ(n). The ECC was then constructed by plotting cj for on a semi-log scale where:
Constructing the connectivity and mobility maps
To construct the connectivity maps we used data from the 2010 LDHS and spatial interpolation techniques (16) to map the percentage of households which had a family member living (on a temporary basis) in: (i) another part of Lesotho, or (ii) South Africa. To construct the mobility map we used data from the 2010 LDHS and spatial interpolation techniques (16) to map the percentage of individuals (15-49 years old) who made one or more overnight trips in the past 12 months.
Supplementary Material
Fig. S1. Map of cluster sample sites for the 2010 Lesotho Demographic and Health Survey.
Fig. S2. Map of the population’s age-structure.
Fig. S3. Epidemic Concentration Curves (ECCs) for eight of the ten healthcare districts (HCDs) in Lesotho.
Table S1. Sub-Saharan African countries that have georeferenced individual-level HIV-testing data collected in a Demographic and Health Survey (DHS).
One sentence summary.
Demographic characteristics of populations in sub-Saharan Africa will significantly hinder, and may even prevent, the elimination of HIV.
Acknowledgments
The authors are grateful to Laurence Palk, Katie Sharp, and Nelson Freimer for discussions throughout the course of this research. B.J.C., J.T.O., and S.B. acknowledge the financial support of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants R01 AI116493 and R56 AI041935). B.J.C., J.T.O., and S.B. designed the project, interpreted the results, and jointly wrote the manuscript. B.J.C. and J.T.O. performed all of the geostatistical analyses and coding.
Footnotes
The authors declare no competing financial interests. Information about data access is detailed in the Materials and Methods.
References
- 1.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. World Health Organization; Geneva: 2015. [PubMed] [Google Scholar]
- 2.UNAIDS. 2016-2021 strategy: on the fast-track to end AIDS. UNAIDS; Geneva: 2015. [Google Scholar]
- 3.UNAIDS. The GAP report. UNAIDS; Geneva: 2014. [Google Scholar]
- 4.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, Meehan MO, Lutalo T, Gray RH. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JHS, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, De Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339:966–971. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okano JT, Robbins D, Palk L, Gerstoft J, Obel N, Blower S. Testing the hypothesis that treatment can eliminate HIV: a nationwide, population-based study of the Danish HIV epidemic in men who have sex with men. Lancet Infect Dis. 2016;16:789–796. doi: 10.1016/S1473-3099(16)30022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.UNAIDS. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. UNAIDS; Geneva: 2014. [Google Scholar]
- 9.Hill A, Pozniak A. HIV treatment cascades: how can all countries reach the UNAIDS 90-90-90 target? AIDS. 2015;29:2523–2525. doi: 10.1097/QAD.0000000000000864. [DOI] [PubMed] [Google Scholar]
- 10.World Bank. Urban population (% of total) 2015 available at http://data.worldbank.org/indicator/SP.URB.TOTL.IN.ZS.
- 11.Linard C, Gilbert M, Snow RW, Noor AM, Tatem AJ. Population distribution, settlement patterns and accessibility across Africa in 2010. PLoS ONE. 2012;7:e31743. doi: 10.1371/journal.pone.0031743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coburn BJ, Okano JT, Blower S. Current drivers and geographic patterns of HIV in Lesotho: implications for treatment and prevention in Sub-Saharan Africa. BMC Med. 2013;11:224. doi: 10.1186/1741-7015-11-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Wolock TM, Carter A, Nguyen G, Kyu HH, Gakidou E, Hay SI, Mills EJ, Trickey A, Msemburi W, Coates MM, Mooney MD, Fraser MS, Sligar A, Salomon J, Larson HJ, Friedman J, Abajobir AA, Abate KH, Abbas KM, Razek MM, Abd-Allah F, Abdulle AM, Abera SF, Abubakar I, Abu-Raddad LJ, Abu-Rmeileh NM, Abyu GY, Adebiyi AO, Adedeji IA, Adelekan AL, Adofo K, Adou AK, Ajala ON, Akinyemiju TF, Akseer N, Lami FH, Al-Aly Z, Alam K, Alam NK, Alasfoor D, Aldhahri SF, Aldridge RW, Alegretti MA, Aleman AV, Alemu ZA, Alfonso-Cristancho R, Ali R, Alkerwi A, Alla F, Mohammad R, Al-Raddadi S, Alsharif U, Alvarez E, Alvis-Guzman N, Amare AT, Amberbir A, Amegah AK, Ammar W, Amrock SM, Antonio CA, Anwari P, Arnlov J, Artaman A, Asayesh H, Asghar RJ, Assadi R, Atique S, Atkins LS, Avokpaho EF, Awasthi A, Quintanilla BP, Bacha U, Badawi A, Barac A, Barnighausen T, Basu A, Bayou TA, Bayou YT, Bazargan-Hejazi S, Beardsley J, Bedi N, Bennett DA, Bensenor IM, Betsu BD, Beyene AS, Bhatia E, Bhutta ZA, Biadgilign S, Bikbov B, Birlik SM, Bisanzio D, Brainin M, Brazinova A, Breitborde NJ, Brown A, Burch M, Butt ZA, Campuzano JC, Cardenas R, Carrero JJ, Castaneda-Orjuela CA, Rivas JC, Catala-Lopez F, Chang HY, Chang JC, Chavan L, Chen W, Chiang PP, Chibalabala M, Chisumpa VH, Choi JY, Christopher DJ, Ciobanu LG, Cooper C, Dahiru T, Damtew SA, Dandona L, Dandona R, das Neves J, de Jager P, De Leo D, Degenhardt L, Dellavalle RP, Deribe K, Deribew A, Des Jarlais DC, Dharmaratne SD, Ding EL, Doshi PP, Driscoll TR, Dubey M, Elshrek YM, Elyazar I, Endries AY, Ermakov SP, Eshrati B, Esteghamati A, Faghmous ID, Farinha CS, Faro A, Farvid MS, Farzadfar F, Fereshtehnejad SM, Fernandes JC, Fischer F, Fitchett JR, Foigt N, Fullman N, Furst T, Gankpe FG, Gebre T, Gebremedhin AT, Gebru AA, Geleijnse JM, Gessner BD, Gething PW, Ghiwot TT, Giroud M, Gishu MD, Glaser E, Goenka S, Goodridge A, Gopalani SV, Goto A, Gugnani HC, Guimaraes MD, Gupta R, Gupta V, Haagsma J, Hafezi-Nejad N, Hagan H, Hailu GB, Hamadeh RR, Hamidi S, Hammami M, Hankey GJ, Hao Y, Harb HL, Harikrishnan S, Haro JM, Harun KM, Havmoeller R, Hedayati MT, Heredia-Pi IB, Hoek HW, Horino M, Horita N, Hosgood HD, Hoy DG, Hsairi M, Hu G, Huang H, Huang JJ, Iburg KM, Idrisov BT, Innos K, Iyer VJ, Jacobsen KH, Jahanmehr N, Jakovljevic MB, Javanbakht M, Jayatilleke AU, Jeemon P, Jha V, Jiang G, Jiang Y, Jibat T, Jonas JB, Kabir Z, Kamal R, Kan H, Karch A, Karema CK, Karletsos D, Kasaeian A, Kaul A, Kawakami N, Kayibanda JF, Keiyoro PN, Kemp AH, Kengne AP, Kesavachandran CN, Khader YS, Khalil I, Khan AR, Khan EA, Khang YH, Khubchandani J, Kim YJ, Kinfu Y, Kivipelto M, Kokubo Y, Kosen S, Koul PA, Koyanagi A, Defo BK, Bicer BK, Kulkarni VS, Kumar GA, Lal DK, Lam H, Lam JO, Langan SM, Lansingh VC, Larsson A, Leigh J, Leung R, Li Y, Lim SS, Lipshultz SE, Liu S, Lloyd BK, Logroscino G, Lotufo PA, Lunevicius R, Razek HM, Mahdavi M, Majdan M, Majeed A, Makhlouf C, Malekzadeh R, Mapoma CC, Marcenes W, Martinez-Raga J, Marzan MB, Masiye F, Mason-Jones AJ, Mayosi BM, McKee M, Meaney PA, Mehndiratta MM, Mekonnen AB, Melaku YA, Memiah P, Memish ZA, Mendoza W, Meretoja A, Meretoja TJ, Mhimbira FA, Miller TR, Mikesell J, Mirarefin M, Mohammad KA, Mohammed S, Mokdad AH, Monasta L, Moradi-Lakeh M, Mori R, Mueller UO, Murimira B, Murthy GV, Naheed A, Naldi L, Nangia V, Nash D, Nawaz H, Nejjari C, Ngalesoni FN, de Dieu Ngirabega J, Nguyen QL, Nisar MI, Norheim OF, Norman RE, Nyakarahuka L, Ogbo FA, Oh IH, Ojelabi FA, Olusanya BO, Olusanya JO, Opio JN, Oren E, Ota E, Padukudru MA, Park HY, Park JH, Patil ST, Patten SB, Paul VK, Pearson K, Peprah EK, Pereira CC, Perico N, Pesudovs K, Petzold M, Phillips MR, Pillay JD, Plass D, Polinder S, Pourmalek F, Prokop DM, Qorbani M, Rafay A, Rahimi K, Rahimi-Movaghar V, Rahman M, Rahman MH, Rahman SU, Rai RK, Rajsic S, Ram U, Rana SM, Rao PV, Remuzzi G, Rojas-Rueda D, Ronfani L, Roshandel G, Roy A, Ruhago GM, Saeedi MY, Sagar R, Saleh MM, Sanabria JR, Santos IS, Sarmiento-Suarez R, Sartorius B, Sawhney M, Schutte AE, Schwebel DC, Seedat S, Sepanlou SG, Servan-Mori EE, Shaikh MA, Sharma R, She J, Sheikhbahaei S, Shen J, Shibuya K, Shin HH, Sigfusdottir ID, Silpakit N, Silva DA, Silveira DG, Simard EP, Sindi S, Singh JA, Singh OP, Singh PK, Skirbekk V, Sliwa K, Soneji S, Sorensen RJ, Soriano JB, Soti DO, Sreeramareddy CT, Stathopoulou V, Steel N, Sunguya BF, Swaminathan S, Sykes BL, Tabares-Seisdedos R, Talongwa RT, Tavakkoli M, Taye B, Tedla BA, Tekle T, Shifa GT, Temesgen AM, Terkawi AS, Tesfay FH, Tessema GA, Thapa K, Thomson AJ, Thorne-Lyman AL, Tobe-Gai R, Topor-Madry R, Towbin JA, Tran BX, Dimbuene ZT, Tsilimparis N, Tura AK, Ukwaja KN, Uneke CJ, Uthman OA, Venketasubramanian N, Vladimirov SK, Vlassov VV, Vollset SE, Wang L, Weiderpass E, Weintraub RG, Werdecker A, Westerman R, Wijeratne T, Wilkinson JD, Wiysonge CS, Wolfe CD, Won S, Wong JQ, Xu G, Yadav AK, Yakob B, Yalew AZ, Yano Y, Yaseri M, Yebyo HG, Yip P, Yonemoto N, Yoon SJ, Younis MZ, Yu C, Yu S, Zaidi Z, Zaki Mel S, Zeeb H, Zhang H, Zhao Y, Zodpey S, Zoeckler L, Zuhlke LJ, Lopez AD, Murray CJ. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980-2015: the Global Burden of Disease Study 2015. Lancet HIV. 2016;3:e361–387. doi: 10.1016/S2352-3018(16)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WorldPop. Methods. 2014 available at http://www.worldpop.org.uk/data/methods/
- 15.Ministry of Health and Social Welfare (Lesotho) Lesotho Demographic and Health Survey 2009. ICF International; Calverton: 2010. [Google Scholar]
- 16.Larmarange J, Vallo R, Yaro S, Msellati P, Meda N. Methods for mapping regional trends of HIV prevalence from Demographic and Health Surveys (DHS) Cybergeo Europ J Geo. 2011:558. [Google Scholar]
- 17.Palk L, Blower S. Brief report: Mobility and circular migration in Lesotho: implications for transmission, treatment, and control of a severe HIV epidemic. J Acquir Immune Defic Syndr. 2015;68:604–608. doi: 10.1097/QAI.0000000000000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palk L, Blower S. Mapping divided households and residency changes: the effect of couple separation on sexual behavior and risk of HIV infection. Sci Rep. 2015;5:17598. doi: 10.1038/srep17598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwuji CC, McGrath N, de Oliveira T, Porter K, Pillay D, Fisher M, M. Newport. Newell ML. The art of HIV elimination: past and present science. J AIDS Clin Res. 2015:6. doi: 10.4172/2155-6113.1000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dabis F. paper presented at the 21st International AIDS Conference; Durban. 18 to 22 July 2016. [Google Scholar]
- 21.UNAIDS. 90-90-90: On the right track towards the global target. UNAIDS; Geneva: 2016. [Google Scholar]
- 22.Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, Avihingsanon A, Cooper DA, Fatkenheuer G, Llibre JM, Molina JM, Munderi P, Schechter M, Wood R, Klingman KL, Collins S, Lane HC, Phillips AN, Neaton JD. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailey RC, Moses S, Parker CB, Agot K, Maclean I, Krieger JN, Williams CF, Campbell RT, Ndinya-Achola JO. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 25.Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, Nalugoda F, Kiwanuka N, Moulton LH, Chaudhary MA, Chen MZ, Sewankambo NK, Wabwire-Mangen F, Bacon MC, Williams CF, Opendi P, Reynolds SJ, Laeyendecker O, Quinn TC, Wawer MJ. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 26.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapía M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernández T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallás EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell JJ, Zheng JH, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abel GJ, Sander N. Quantifying global international migration flows. Science. 2014;343:1520–1522. doi: 10.1126/science.1248676. [DOI] [PubMed] [Google Scholar]
- 28.United Nations. Cross-national comparisons of internal migration: An update on global patterns and trends. United Nations; New York: 2013. [Google Scholar]
- 29.Grabowski MK, Lessler J, Redd AD, Kagaayi J, Laeyendecker O, Ndyanabo A, Nelson MI, Cummings DA, Bwanika JB, Mueller AC, Reynolds SJ, Munshaw S, Ray SC, Lutalo T, Manucci J, Tobian AA, Chang LW, Beyrer C, Jennings JM, Nalugoda F, Serwadda D, Wawer MJ, Quinn TC, Gray RH. The role of viral introductions in sustaining community-based HIV epidemics in rural Uganda: evidence from spatial clustering, phylogenetics, and egocentric transmission models. PLoS Med. 2014;11:e1001610. doi: 10.1371/journal.pmed.1001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demographic and Health Surveys. GPS Data Collection. 2014 available at http://dhsprogram.com/What-We-Do/GPS-Data-Collection.cfm.
- 31.The Subnational Estimates Working Group of the HIV Modelling Consortium. Evaluation of geospatial methods to generate subnational HIV prevalence estimates for local level planning. AIDS. 2016;30:1467–1474. doi: 10.1097/QAD.0000000000001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okano JT, Blower S. Constructing gender-specific maps of HIV epidemics in Sub-Saharan Africa: implications for health policy. Lancet Infect Dis. doi: 10.1016/S1473-3099(16)30451-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WorldPop. Data. 2014 available at http://www.worldpop.org.uk/data/
- 34.Clark SJ, Houle B. Validation, replication, and sensitivity testing of Heckman-type selection models to adjust estimates of HIV prevalence. PLoS ONE. 2014;9:e112563. doi: 10.1371/journal.pone.0112563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ESRI. Environmental Systems Research Institute; Redlands: 2011. [Google Scholar]
- 36.R Development Core Team. R foundation for Statistical Computing; Vienna: 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Map of cluster sample sites for the 2010 Lesotho Demographic and Health Survey.
Fig. S2. Map of the population’s age-structure.
Fig. S3. Epidemic Concentration Curves (ECCs) for eight of the ten healthcare districts (HCDs) in Lesotho.
Table S1. Sub-Saharan African countries that have georeferenced individual-level HIV-testing data collected in a Demographic and Health Survey (DHS).


