Abstract
Study Objectives:
To determine if the type of continuous positive airway pressure (CPAP) mask interface influences CPAP treatment efficacy, adherence, side effects, comfort and sleep quality in patients with moderate-severe obstructive sleep apnea (OSA).
Methods:
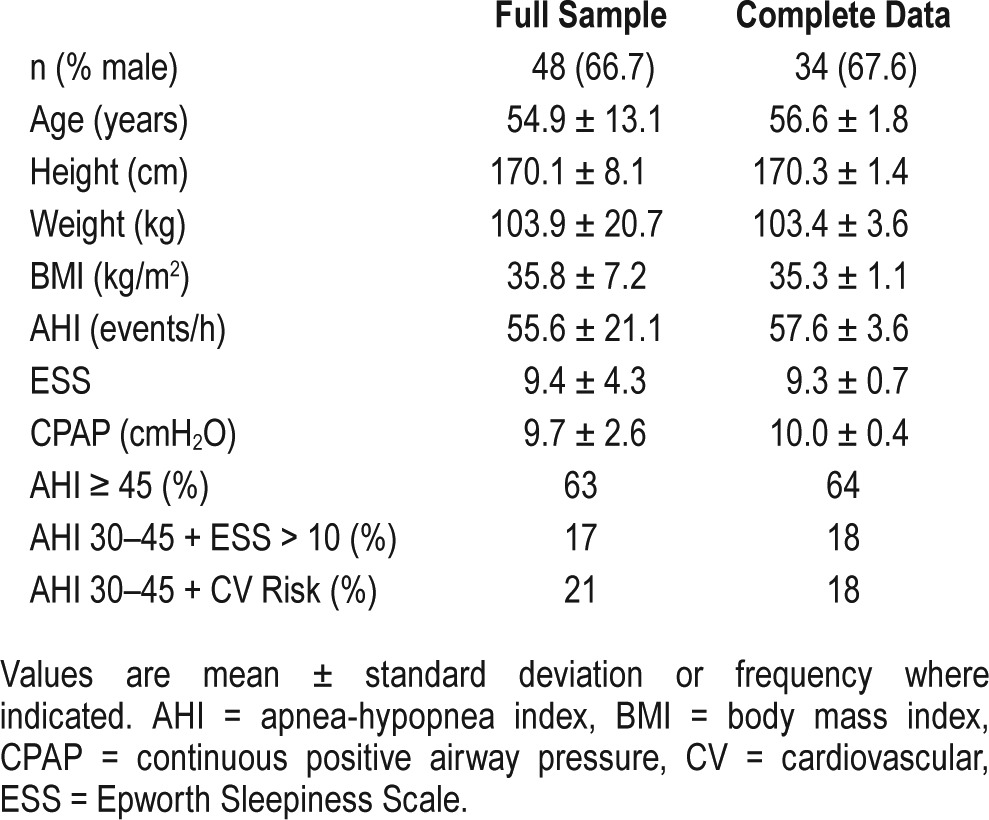
This took place in a hospital-based tertiary sleep disorders unit. It is a prospective, randomized, crossover trial comparing three CPAP interfaces: nasal mask (NM), nasal mask plus chinstrap (NM-CS) and oronasal mask (ONM) each tried in random order, for 4 weeks. After each 4-week period, patient outcomes were assessed. Participants had a new diagnosis of obstructive sleep apneas. Forty-eight patients with moderate-severe OSA (32 males, mean ± standard deviation apnea-hypopnea index (AHI) 55.6 ± 21.1 events/h, age 54.9 ± 13.1 years, body mass index 35.8 ± 7.2 kg/m2) were randomized. Thirty-five participants completed the full study, with complete data available for 34 patients.
Results:
There was no statistically significant difference in CPAP adherence; however, residual AHI was higher with ONM than NM and NM-CS (residual AHI 7.1 ± 7.7, 4.0 ± 3.1, 4.2 ± 3.7 events/h respectively, main effect P = .001). Patient satisfaction and quality of sleep were higher with the NM and NM-CS than the ONM. Fewer leak and mask fit problems were reported with NM (all chi-square P < .05), which patients preferred over the NM-CS and ONM options (n = 22, 9 and 4 respectively, P = .001).
Conclusions:
The CPAP adherence did not differ between the three different mask interfaces but the residual AHI was lower with NM than ONM and patients reported greater mask comfort, better sleep, and overall preference for a NM. A nasal mask with or without chinstrap should be the first choice for patients with OSA referred for CPAP treatment.
Clinical Trial Registration:
Registry: Australian and New Zealand Clinical Trials Registry, URL: https://www.anzctr.org.au, title: A comparison of continuous positive airway pressure (CPAP) interface in the control of leak, patient compliance and patient preference: nasal CPAP mask and chinstrap versus full face mask in patients with obstructive sleep apnoea (OSA), identifier: ACTRN12609000029291
Citation:
Rowland S, Aiyappan V, Hennessy C, Catcheside P, Chai-Coetzer CL, McEvoy RD, Antic NA. Comparing the efficacy, mask leak, patient adherence, and patient preference of three different CPAP interfaces to treat moderate-severe obstructive sleep apnea. J Clin Sleep Med. 2018;14(1):101–108.
Keywords: sleep apnea, continuous positive airways pressure, adherence
BRIEF SUMMARY
Current Knowledge/Study Rationale: The study was conducted as there are few published data regarding the clinical effect of the various types of continuous positive airway pressure (CPAP) interfaces.
Study Impact: Clinicians can use the data obtained in this study to help determine the best option for patients commencing CPAP therapy. Results also identified the effect that a change in interface style may have on the control of obstructive sleep apnea and CPAP pressure requirements.
INTRODUCTION
Obstructive sleep apnea (OSA) is estimated to affect at least 10% of the middle-aged population1,2 and is associated with increased cardiovascular, motor vehicle, and other accident risk, lower workplace productivity, and increased health care expenditure in comparison with the normal population.2,3
Continuous positive airway pressure (CPAP) is the treatment of choice for patients with moderate-severe OSA.3,4 Because long-term patterns of CPAP use are often established in the first few days,5 it is important to find a mask that is acceptable to the patient, seals comfortably, and is associated with minimal side effects.
There are three different types of CPAP delivery: nasal, oral, and oronasal, although oral masks have not found widespread acceptance and are rarely used. A nasal mask (NM) is most commonly used but some studies report side effects in more than 50% of patients.3,6 Mouth and mask leaks are common5,7–9 and may cause increased arousals,10 drying of the nasal and oral mucosa,9,11 secondary nasal congestion and rhinitis,12 sore eyes, and irritating noises and airstreams.13 It is common practice to try to reduce mouth leaks with a chinstrap that supports and restricts movement of the lower jaw (nasal mask with chin-strap [NM-CS]),14,15 but there are limited data to support the efficacy of this approach.16,17
An oronasal mask (ONM)16 covers both the mouth and nose, allowing the patient to breathe through the mouth while maintaining therapeutic CPAP pressure. In theory, this will eliminate the problem of mouth leaks and nasal and upper airway symptoms associated with the use of NMs.18 However, ONMs are generally harder to fit, resulting in more leaks, and some studies suggest they may cause greater patient discomfort and reduce CPAP adherence.19,20 Furthermore, it may be they are less effective in controlling upper airway obstruction in some patients.21 Oronasal masks are also more costly.
CPAP is well established as primary treatment for OSA and it is well recognized that appropriate mask interface selection is a key determinant of treatment efficacy and compliance. However, the data to guide clinicians and patients in the initial choice of a mask interface are lacking.22 Most studies investigating the efficacy of different types of mask interfaces have relied on short-term titration data for comparison.23–25 No studies have compared the efficacy of an NM-CS versus an ONM.19 The need for further randomized studies comparing the different forms of CPAP mask interface has been expressed in two reviews.22,26 In this study we aimed to compare the effects of three commonly used CPAP interface options (NM, NMCS, and ONM) in patients with OSA on efficacy of treatment, CPAP adherence, overall leak (mask and/or mouth), and self-reported side effects, sleep quality, daytime sleepiness, mask comfort, and overall patient preference.
METHODS
The study was approved by the Repatriation General Hospital Research and Ethics Committee and was registered with the Australian and New Zealand Clinical Trials Registry (ACTRN12609000029291).
Participant Selection
Participants who met the following selection criteria were prospectively recruited between June 2007 and July 2008 from the clinics of the Adelaide Institute for Sleep Health, a hospital-based tertiary sleep disorders unit.
Inclusion Criteria
(1) Diagnosis of moderate-severe OSA defined as an apneahypopnea index (AHI) ≥ 45; or ≥ 30 events/h with an Epworth Sleepiness Scale score > 10, or preexisting cardiovascular disease. All patients had full laboratory-based polysomnography scored using the American Academy of Sleep Medicine standards of the time,4 where apnea was defined as a complete cessation of airflow (nasal cannula pressure or in both thoracic and abdominal excursions) for ≥ 10 seconds, and hypopnea as clear (> 50%) reduction in airflow for ≥ 10 seconds, or a discernible reduction in airflow accompanied by either an SaO2 desaturation ≥ 3% or an arousal. (2) Recommended for CPAP therapy by a sleep physician and successfully completed a full-night attended CPAP pressure determination study in which the pressure was manually adjusted until respiratory events and snoring were eliminated. (3) Willing to embark on a 12-week home trial of CPAP.
Exclusion Criteria
(1) Significant nasal resistance (ie, unable to breathe through the nose comfortably at rest with the mouth closed). (2) Previous use of CPAP for > 1 week. (3) Prior upper airway surgery for OSA. (4) Unstable psychiatric or psychological illness that would prevent accurate reporting.
In accordance with the clinical practice of the sleep disorders unit at the time, heated humidification was not used at the commencement of CPAP therapy. Normally a humidifier would have been introduced if the patient had a history of nasal symptoms or if nasal symptoms developed. However, the use of a humidifier may have masked important differences in comfort and side effects between the mask interfaces.9,27 For this reason patients who opted to use a humidifier during the course of the study were excluded from the final analysis.
Study Design
The study was a prospective, randomized, crossover trial. All participants used each of the three mask configurations (NM, NM-CS, ONM), for a period of 4 weeks each, in a random order using a balanced block design, after the therapeutic CPAP was determined during in-laboratory titration. Equal numbers of each possible sequence combination were drawn up prior to study recruitment and then placed in a random fashion into consecutively numbered and sealed opaque envelopes. In sequential order, one envelope was opened at each participant's first appointment. A specialist clinical nurse, aware of the trial, selected and fitted each mask option to obtain a comfortable seal, educated patients regarding CPAP use, ensured they could fit each mask and the chinstrap as they were issued, and monitored progress. There was no washout period between the treatment arms. Patients were reviewed and data collected at 4, 8, and 12 weeks. After all three interface options had been tried, patients nominated and were given their preferred interface option for ongoing use.
Equipment
CPAP
The Respironics M series Pro CPAP (Monroeville, Pennsylvania, United States) was loaned to each patient for the duration of the study. This device was selected for its ability to record adherence data and measure daily leak independent of the mask used.
Chinstrap
The Respironics Premium chinstrap was chosen based on our preliminary experience and commercial availability. It could be adjusted to fit a range of head sizes, and previous patients reported that it stayed in place.
CPAP masks
Masks were selected from the available commercial range produced by Respironics, Fisher and Paykel (Auckland, New Zealand), and ResMed (Sydney, Australia) to provide the best fit in regard to comfort and mask seal. Changes to brand, model, or size of mask (same interface type) were made within the first 2 weeks of each treatment arm upon patient request.
Measurements
Baseline anthropomorphic data (height/weight), age, AHI, prescribed CPAP pressure, and self-assessed daytime sleepiness using the Epworth Sleepiness Scale (ESS) score28 were recorded. Additional data collected at the completion of each treatment arm (ie, at 4, 8, and 12 weeks) included weight, CPAP adherence, time with a large leak (as defined by CPAP machine algorithm set to the appropriate mask type), ESS, a mask comfort questionnaire (reproduced in the supplemental material), and AHI (recorded by the CPAP machine). CPAP data were downloaded using Encore Pro 2 software (Philips Respironics, Murrysville, Pennsylvania, United States) to provide average adherence and leak over weeks 3 and 4 of each treatment arm. This allowed a 2-week period of acclimatization for each new mask setup.
Mask Comfort Questionnaire
We were unable to find a published, validated questionnaire to assess mask comfort and side effects, so we developed our own (reproduced in the supplemental material) based on side effects and complaints reported in earlier studies.7,19,29,30 Patients answered “yes” or “no” to 11 problem items listed in the questionnaire, and could specify an unlisted problem if relevant. Visual analog scales were used to measure overall level of satisfaction with the mask and sleep quality (0–10), 0 being the worst score and 10 the best.
Sample Size and Statistical Analysis
The primary outcome measure was patient CPAP adherence. We estimated that approximately 40 patients were required to detect a 0.5 h/night difference in adherence, which was considered to be the minimal clinically significant difference assuming a two-tailed level of significance of 0.05, 80% power and a standard deviation of repeated adherence measurements in previous trials in our unit of 0.7 h/night.
Baseline characteristics were compared (patients completing versus withdrew) with independent sample Student t tests. The primary CPAP adherence analysis was conducted using IBM SPSS Statistics Version 23 (IBM Corp., Armonk, New York, United States) on an intention-to-treat basis using zero hours of use for patients with missing data, and using all available data from patients who completed each arm of the study in secondary analyses. Continuous variables were assessed for normality via Kolmogorov-Smirnov tests and Box-Cox transformations examined toward normalizing non-normally distributed data. Effects of mask interface on patient outcomes were examined using linear mixed-effects model analysis31 with normally distributed data and Friedman tests with non-normally distributed data. To allow for repeated measures over time with each different mask type, and inter-individual variability, mixed models used an autoregressive covariance structure with mask type as a fixed factor and subjects entered as a random effect, each with a separate intercept. Count data including occurrence of side effects, and mask preference were compared using chi-square tests. Significant main effects were examined using Bonferroni adjusted post hoc pairwise comparisons. Pearson correlation and backward stepwise multivariate and logistic regression analyses were used to explore whether anthropomorphic parameters (age, sex, height, weight and body mass index), baseline AHI or CPAP pressure were predictive of residual AHI and residual AHI ≥ 10 versus < 10 events/h. All data are presented as mean ± standard deviation. A value of P < .05 was considered significant.
RESULTS
Patient Characteristics
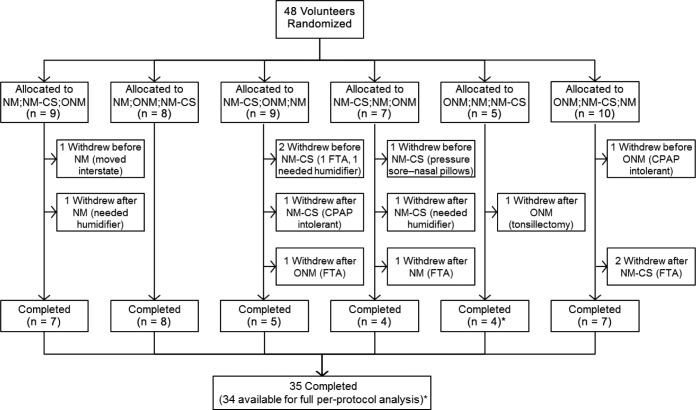
The median (interquartile range) time interval between positive airway pressure titration and enrollment into the trial was 1.1 (0.9 to 1.8) months. Figure 1 shows the number of patients allocated to each treatment condition and the number of study withdrawals and study completions. Forty-eight patients were randomized into the study and 35 completed the study (Figure 1). There was no difference in the proportion of patients withdrawing after any of the three mask conditions (χ2 = 0.221). CPAP treatment data were missing from the NM condition in 1 patient due to a faulty CPAP data card, and from the NM-CS condition in 2 patients due to study withdrawal prior to CPAP download. Patients randomized to and completing the study were predominantly male, middle-aged, and obese, and had moderate to severe OSA (Table 1). There were no statistically significant differences in any baseline characteristic between patients who withdrew in comparison with those who completed the full study protocol.
Figure 1. Study flow diagram showing study mask allocations and withdrawals.
* = a faulty CPAP data card led to missing NM treatment data in 1 patient who completed the full protocol. CPAP = continuous positive airway pressure, FTA = failed to attend subsequent appointment, NM = nasal mask, NM-CS = nasal mask with chinstrap, ONM = oronasal mask.
Table 1.
Patient characteristics at baseline.
Treatment Data
Treatment outcome data are summarized in Table 2. CPAP adherence was similar and not different between interface types in hours of CPAP use per night, the percentage of nights used for at least 4 hours, and number of adherent versus nonadherent patients. More time was spent in CPAP machine detected large leak with the ONM versus NM interface. Participants reported more restful sleep using the NM or NM-CS than with the ONM (Table 2). There was a significant effect of interface type on how refreshed patients felt in the morning, but post hoc tests showed no significant differences in any pairwise contrasts. Daytime sleepiness was reduced from baseline with all interface types (P = .001, all pairwise ESS contrasts versus baseline P < .01) but was not different between interface types. The level of overall satisfaction was higher with the NM and NM-CS than with the ONM. All three treatments reduced AHI; however, the residual AHI was significantly higher with ONM than NM and NM-CS, indicating incomplete control of upper airway obstruction (Table 2 and Figure 2). There was a significant effect of mask interface type on the proportion of patients with a residual AHI ≥ 10 events/h, but no significant difference between mask interfaces in post hoc comparisons.
Table 2.
Treatment data.
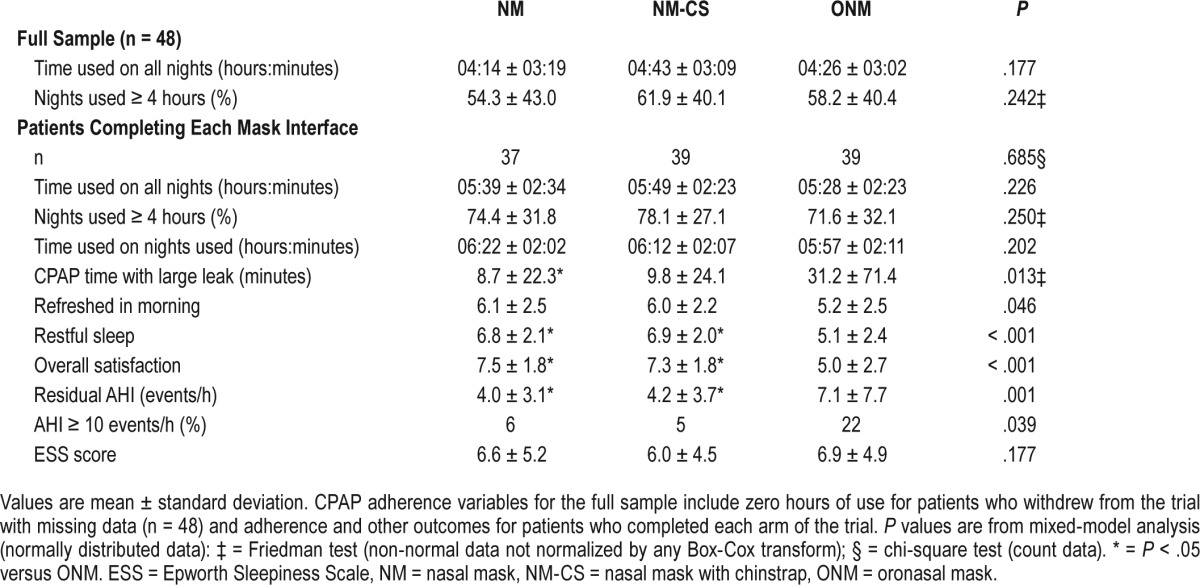
Figure 2. Residual AHI with each mask interface type.
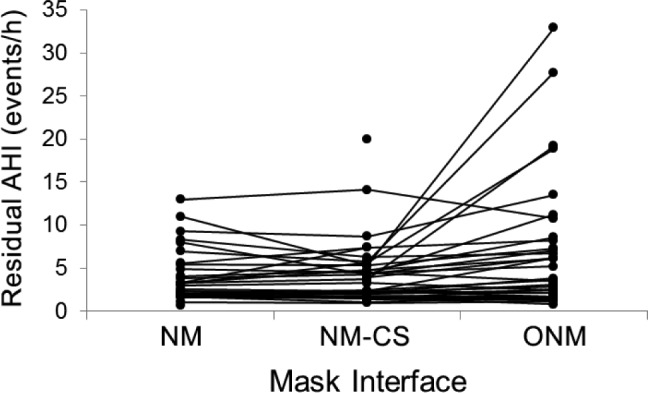
AHI = apnea-hypopnea index, CS = chinstrap, NM = nasal mask, NMCS = nasal mask with chinstrap, ONM = oronasal mask.
In backward stepwise multiple regression, baseline AHI, body mass index, and CPAP pressure were the only significant independent predictors of residual AHI with the ONM (standardized β = 0.28, 0.29 and −0.79; P = .026, .030 and < .001 respectively; adjusted model r2 = 0.53, P < .001).
Mask Comfort Questionnaire
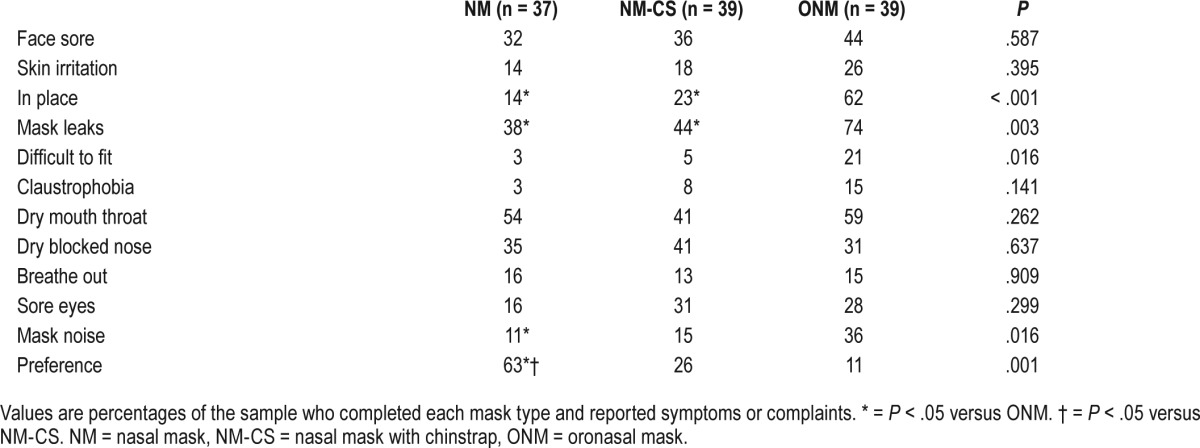
Data on symptoms or complaints with each interface type are presented in Table 3. NM was the easiest mask to fit, caused fewer complaints of mask noise, and was perceived to leak less than the ONM. Other occasional problems included problems fitting the mask, itchiness, hot to wear causing sweating, cold air and teeth sensitivity, and some problems relating to dentures. Patient preference for the NM was significantly greater than for the NM-CS and ONM.
Table 3.
Percentage of patients with symptoms or complaints, and patient preference for mask type.
DISCUSSION
The selection of a CPAP mask interface is made with the objective of achieving maximum adherence and therapeutic benefit, hence patient comfort and an effective seal are critical. There was no significant difference in the level of CPAP adherence between mask types, but time in large leak was higher in the ONM versus NM, and the NM was perceived to be easier to fit and keep in place, to leak less, be quieter, and provide a more restful sleep than the ONM. Patient preference was for the NM.
Given that early CPAP adherence and side effects have been shown to be independent predictors of long-term CPAP adherence,6 it is perhaps surprising that CPAP adherence was not different between the three different CPAP interface types. An early study by Mortimore et al.19 demonstrated a higher nightly adherence (mean difference 1 h/night, 95% confidence interval 1.8 to 0.3, P = .01) with an NM compared to an ONM in a small group of patients with OSA. More recently, Borel et al.20 reported in an observational study of more than 2,300 patients with OSA starting CPAP and followed for an average of 4.5 months that ONMs were associated with a twofold higher risk of CPAP nonadherence. The reason differences in side effects and patient preference between masks did not translate into differences in CPAP adherence in our study may be because of the relatively small sample size and short follow-up.
All three interfaces reduced AHI, but NM was the most effective. Although some previous studies have shown comparable efficacy between mask types,26 emerging data suggest that the therapeutic pressures needed to treat OSA with an ONM might be higher than with NM, and that ONM might be less effective in treating OSA than NM at the same pressure.21,24,32,33 In a randomized controlled trial, Teo et al. demonstrated that the physician- prescribed CPAP pressure was not different between NM and ONM, but the residual AHI and arousal index were higher with ONM. Moreover, most patients preferred NM over ONM.23 The upper airway is known to exhibit Starling resistor behavior, with complete collapse below and partial collapse above a critical closing pressure (Pcrit). Smith et al. showed that inspiratory airflow increases in proportion to positive pressure above Pcrit with an NM, but with no increase in airflow even well above the NM Pcrit when the positive pressure was applied through an ONM.34 This could reflect mask or route of delivery effects on mandible or tongue position and upper airway dimensions.21,34 High residual AHI on ONM in the current study was confined to a relatively small number of patients. It will be important in future studies to investigate potential contributing factors such as mask strap tension and jaw size or position.
CPAP therapy was subjectively beneficial with all interface options with no significant difference in the change in ESS. Although objective sleep quality was not quantified, subjective sleep quality was reported to be less restful with the ONM.
A relatively high percentage of patients reported upper airway symptoms with one or more interface. Interestingly, the NM-CS, an intervention designed to reduce mouth leak and nasal irritation/mouth dryness, did not significantly alter leak or symptoms, possibly because of persistent leak between the lips.17 Also, although ONM is reported to reduce upper airway dryness,9 by preventing mouth leak, we found similar levels of patient-reported mouth dryness with ONM and NM.
Overall satisfaction scores were significantly higher for the NM and NM-CS than the ONM, possibly influenced by patients finding these options were easier to fit, stayed in place better, and leaked less. Additionally, the NM was quieter, when compared to ONM. As in previous studies17,22 patient preference was significantly in favor of the NM, with 21 of the 33 participants selecting the NM option, 8 the NM-CS, and only 4 choosing the ONM.
Methodological Considerations
We estimated that 40 patients were required to detect a 0.5 h/night difference in adherence between interface types. Although 48 patients commenced the trial and contributed to the intention-to-treat analysis, only 35 completed the full protocol, and 1 of these had missing CPAP data. Consequently, although neither intention-to-treat nor per-protocol analyses suggest any systematic treatment adherence differences between mask interface types, type II error could potentially help explain these findings. Our data may have some bias in that 3 patients who experienced severe rhinitis/nasal congestion and required heated humidification were withdrawn at the first review after 4 weeks of CPAP therapy. Two had used an NM-CS and 1 an NM (ie, none of the 3 had tried an ONM).
Each mask was only used for a period of 4 weeks, so data were short-term. It is possible that long-term usage with the ONM option might decrease if side effects such noise, leaks, and discomfort were ongoing. Although we were constrained by our study design to avoid the use of humidification, it will be important in future studies to see whether our results (NM versus ONM) hold up if humidification is used.
Initial experiences during CPAP titration study nights could potentially influence subsequent treatment adherence. Ongoing early experiences associated with mask fit, comfort, and leaks may also interact with more complex psychological and psychosocial factors to influence long-term acceptance and use.35,36 Nevertheless, initial mask comfort and experiences may be among the leading modifiable factors influencing long-term CPAP use. Data concerning the mask type used during the initial CPAP titration were not available, but usual clinical practice in our laboratory is to initiate CPAP with an NM unless this proves to be problematic during the night. Consequently, most patients are likely to have commenced CPAP using an NM. This could reflect a bias, but given the crossover study design and that retitrating CPAP is typically not done or practical in clinical practice, we believe this is unlikely to have had any major effect on study outcomes relevant to common clinical practice.
The efficacy of the treatment was measured based on CPAP machine AHI data and there are very few studies comparing the accuracy of machine-interpreted AHI.37 However, all the patients included in the study used the same CPAP device with the same algorithm. Future studies might include a laboratory-attended CPAP titration after establishing CPAP therapy on different masks to assess control of OSA. The trial was randomized, but given the nature of comparisons between mask types it was not possible to meaningfully blind either patients or nurses supervising CPAP use.
CONCLUSIONS
We found no difference in CPAP adherence levels during 4-week trials of three different CPAP mask interfaces, but CPAP delivered through an NM showed less leak and a greater reduction in AHI compared with an ONM, and patients reported greater mask comfort, better sleep, and overall preference for the NM. These data suggest that unless patients have significant nasal problems, OSA therapy may be better tolerated and more effective if initially commenced with NM treatment. Increasing recognition that variable phenotypes underlie OSA—where route of breathing effects could be important in some patients—suggest that further studies are needed to clarify causes of higher AHI in some patients with an ONM. The data revealed by these studies could help to deliver better individualized mask interface choice for patients and further improve treatment outcomes.
DISCLOSURE STATEMENT
Work for this study was performed at the Adelaide Institute for Sleep Health at the Repatriation General Hospital, Adelaide South Australia. All authors have seen and approved the manuscript. Financial support was obtained through a Research Grant from Foundation Daw Park. This was used to purchase the CPAP equipment and chinstraps used during the trial. Sharn Rowland reports funding from Foundation Daw Park for this study; participation in SMOSA 2 study: an NH&MRC-funded, clinical research trial that received ResMed-donated equipment, Philips Respironics-donated equipment, and SomnoMed-donated equipment. Vinod Aiyappan reports equipment donation from Fisher and Paykel, Philips Respironics and lecture fees from bioCSL, Novartis, Menarini and Boehringer-Ingelheim. Cathy Hennessy reports funding from Foundation Daw Park for this study. Peter Catcheside reports equipment loan agreement with Philips Respironics and by Air Liquide Healthcare to support a trial of supine-avoidance versus CPAP to treat positional OSA. Nick Antic reported receiving a grant from Philips Respironics for a large randomized controlled trial of CPAP therapy for obstructive sleep apnea, with equipment donations from Philips Respironics, ResMed, and Fisher and Paykel. He received additional equipment donations from ResMed, Philips Respironics and SomnoMed, and lecture fees and payment for development of educational presentations from ResMed and GSK. Ching Li Chai-Coetzer reports participation in SMOSA 2 study: an NH&MRC-funded, clinical research trial that received ResMed-donated equipment, Philips Respironics-donated APAP units, and SomnoMed-donated mandibular advancement splints. R. Doug McEvoy reports direct cost sponsorship from Philips Respironics and Fisher Paykel for SAVE trial and equipment donations for SAVE trial from Philips Respironics and Fisher Paykel.
ACKNOWLEDGMENTS
The authors gratefully acknowledge provision of a research grant from Foundation Daw Park SA to support this project.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- CV
cardiovascular
- ESS
Epworth Sleepiness Scale
- FTA
failed to attend
- NM
nasal mask
- NM-CS
nasal mask with chinstrap
- ONM
oronasal mask
- OSA
obstructive sleep apnea
- Pcrit
critical closing pressure
- SaO2
saturation of oxygen in arterial blood
REFERENCES
- 1.Bearpark H, Elliott L, Grunstein R, et al. Snoring and sleep apnea. A population study in Australian men. Am J Respir Crit Care Med. 1995;151(5):1459–1465. doi: 10.1164/ajrccm.151.5.7735600. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 3.Gay P, Weaver T, Loube D, Iber C. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29(3):381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 4.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine task force. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 5.Engleman HM, Asgari-Jirhandeh N, McLeod AL, Ramsay CF, Deary IJ, Douglas NJ. Self-reported use of CPAP and benefits of CPAP therapy: a patient survey. Chest. 1996;109(6):1470–1476. doi: 10.1378/chest.109.6.1470. [DOI] [PubMed] [Google Scholar]
- 6.McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1108–1114. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- 7.Pepin JL, Leger P, Veale D, Langevin B, Robert D, Levy P. Side effects of nasal continuous positive airway pressure in sleep apnea syndrome. Study of 193 patients in two French sleep centers. Chest. 1995;107(2):375–381. doi: 10.1378/chest.107.2.375. [DOI] [PubMed] [Google Scholar]
- 8.Rapoport DM. Methods to stabilize the upper airway using positive pressure. Sleep. 1996;19(9 Suppl):S123–S130. doi: 10.1093/sleep/19.suppl_9.s123. [DOI] [PubMed] [Google Scholar]
- 9.Martins De Araujo MT, Vieira SB, Vasquez EC, Fleury B. Heated humidification or face mask to prevent upper airway dryness during continuous positive airway pressure therapy. Chest. 2000;117(1):142–147. doi: 10.1378/chest.117.1.142. [DOI] [PubMed] [Google Scholar]
- 10.Meyer TJ, Pressman MR, Benditt J, et al. Air leaking through the mouth during nocturnal nasal ventilation: effect on sleep quality. Sleep. 1997;20(7):561–569. doi: 10.1093/sleep/20.7.561. [DOI] [PubMed] [Google Scholar]
- 11.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147(4):887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 12.Richards GN, Cistulli PA, Ungar RG, Berthon-Jones M, Sullivan CE. Mouth leak with nasal continuous positive airway pressure increases nasal airway resistance. Am J Respir Crit Care Med. 1996;154(1):182–186. doi: 10.1164/ajrccm.154.1.8680678. [DOI] [PubMed] [Google Scholar]
- 13.Bachour A, Maasilta P. Mouth breathing compromises adherence to nasal continuous positive airway pressure therapy. Chest. 2004;126(4):1248–1254. doi: 10.1378/chest.126.4.1248. [DOI] [PubMed] [Google Scholar]
- 14.Piper A, Willson G. Nocturnal nasal ventilatory support in the management of daytime hypercapnic respiratory failure. Aust J Physiother. 1996;42(1):17–29. doi: 10.1016/s0004-9514(14)60437-2. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez J, Sharshar T, Hart N, Chadda K, Raphael JC, Lofaso F. Air leaks during mechanical ventilation as a cause of persistent hypercapnia in neuromuscular disorders. Intensive Care Med. 2003;29(4):596–602. doi: 10.1007/s00134-003-1659-5. [DOI] [PubMed] [Google Scholar]
- 16.Willson GN, Piper AJ, Norman M, et al. Nasal versus full face mask for noninvasive ventilation in chronic respiratory failure. Eur Respir J. 2004;23(4):605–609. doi: 10.1183/09031936.04.00051604. [DOI] [PubMed] [Google Scholar]
- 17.Bachour A, Hurmerinta K, Maasilta P. Mouth closing device (chinstrap) reduces mouth leak during nasal CPAP. Sleep Med. 2004;5(3):261–267. doi: 10.1016/j.sleep.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Valentin A, Subramanian S, Quan SF, Berry RB, Parthasarathy S. Air leak is associated with poor adherence to autoPAP therapy. Sleep. 2011;34(6):801–806. doi: 10.5665/SLEEP.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mortimore IL, Whittle AT, Douglas NJ. Comparison of nose and face mask CPAP therapy for sleep apnoea. Thorax. 1998;53(4):290–292. doi: 10.1136/thx.53.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borel JC, Tamisier R, Dias-Domingos S, et al. Type of mask may impact on continuous positive airway pressure adherence in apneic patients. PLoS One. 2013;8(5):e64382. doi: 10.1371/journal.pone.0064382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schorr F, Genta PR, Gregorio MG, Danzi-Soares NJ, Lorenzi-Filho G. Continuous positive airway pressure delivered by oronasal mask may not be effective for obstructive sleep apnoea. Eur Respir J. 2012;40(2):503–505. doi: 10.1183/09031936.00145111. [DOI] [PubMed] [Google Scholar]
- 22.Toraldo DM, De Nuccio F, Nicolardi G. Effects of nCPAP therapy on cardiorespiratory outcomes in obstructive sleep apnea syndrome: compliance and technological advancements. Expert Rev Respir Med. 2011;5(1):41–47. doi: 10.1586/ers.10.86. [DOI] [PubMed] [Google Scholar]
- 23.Teo M, Amis T, Lee S, Falland K, Lambert S, Wheatley J. Equivalence of nasal and oronasal masks during initial CPAP titration for obstructive sleep apnea syndrome. Sleep. 2011;34(7):951–955. doi: 10.5665/SLEEP.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebben MR, Oyegbile T, Pollak CP. The efficacy of three different mask styles on a PAP titration night. Sleep Med. 2012;13(6):645–649. doi: 10.1016/j.sleep.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Bakker JP, Campbell AJ, Neill AM. Increased mortality risk in congestive heart failure patients with comorbid sleep apnoea: 10-year follow up. Intern Med J. 2012;42(11):1264–1268. doi: 10.1111/j.1445-5994.2012.02904.x. [DOI] [PubMed] [Google Scholar]
- 26.Chai CL, Pathinathan A, Smith B. Continuous positive airway pressure delivery interfaces for obstructive sleep apnoea. Cochrane Database Syst Rev. 2006;(4):CD005308. doi: 10.1002/14651858.CD005308.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mador MJ, Krauza M, Pervez A, Pierce D, Braun M. Effect of heated humidification on compliance and quality of life in patients with sleep apnea using nasal continuous positive airway pressure. Chest. 2005;128(4):2151–2158. doi: 10.1378/chest.128.4.2151. [DOI] [PubMed] [Google Scholar]
- 28.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 29.Massie CA, Hart RW. Clinical outcomes related to interface type in patients with obstructive sleep apnea/hypopnea syndrome who are using continuous positive airway pressure. Chest. 2003;123(4):1112–1118. doi: 10.1378/chest.123.4.1112. [DOI] [PubMed] [Google Scholar]
- 30.Anderson FE, Kingshott RN, Taylor DR, Jones DR, Kline LR, Whyte KF. A randomized crossover efficacy trial of oral CPAP (Oracle) compared with nasal CPAP in the management of obstructive sleep apnea. Sleep. 2003;26(6):721–726. doi: 10.1093/sleep/26.6.721. [DOI] [PubMed] [Google Scholar]
- 31.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 32.Bakker JP, Neill AM, Campbell AJ. Nasal versus oronasal continuous positive airway pressure masks for obstructive sleep apnea: a pilot investigation of pressure requirement, residual disease, and leak. Sleep Breath. 2012;16(3):709–716. doi: 10.1007/s11325-011-0564-3. [DOI] [PubMed] [Google Scholar]
- 33.Mysore S, Catcheside P, Aiyappan V, et al. Inadequate control of OSA using oronasal masks [abstract 091] Sleep Biol Rhythms. 2013;11(Suppl 2):29. [Google Scholar]
- 34.Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. J Appl Physiol. 1988;64(2):789–795. doi: 10.1152/jappl.1988.64.2.789. [DOI] [PubMed] [Google Scholar]
- 35.Crawford MR, Espie CA, Bartlett DJ, Grunstein RR. Integrating psychology and medicine in CPAP adherence--new concepts? Sleep Med Rev. 2014;18(2):123–139. doi: 10.1016/j.smrv.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Dzierzewski JM, Wallace DM, Wohlgemuth WK. Adherence to continuous positive airway pressure in existing users: self-efficacy enhances the association between continuous positive airway pressure and adherence. J Clin Sleep Med. 2016;12(2):169–176. doi: 10.5664/jcsm.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prasad B, Carley DW, Herdegen JJ. Continuous positive airway pressure device-based automated detection of obstructive sleep apnea compared to standard laboratory polysomnography. Sleep Breath. 2010;14(2):101–107. doi: 10.1007/s11325-009-0285-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.