Abstract
Study Objectives:
To study the effect of altitude on subjective sleep quality in populations living at high and low altitudes after excluding cases of restless legs syndrome (RLS).
Methods:
This population-based study was conducted at three different altitudes (400 m, 1,900–2,000 m, and 3,200 m above sea level). All consenting subjects available from random stratified sampling in the Himalayan and sub-Himalayan regions of India were included in the study (ages 18 to 84 years). Sleep quality and RLS status were assessed using validated translations of Pittsburgh Sleep Quality Index (PSQI) and Cambridge Hopkins RLS diagnostic questionnaire. Recent medical records were screened to gather data for medical morbidities.
Results:
In the total sample of 1,689 participants included, 55.2% were women and average age of included subjects was 35.2 (± 10.9) years. In this sample, overall 18.4% reported poor quality of sleep (PSQI ≥ 5). Poor quality of sleep was reported more commonly at high altitude compared to low altitude (odds ratio [OR] = 2.65; 95% CI = 1.9–3.7; P < .001). It was more frequently reported among patients with RLS (29.7% versus 17.1% without RLS; P < .001). Other factors that were associated with poor quality of sleep were male sex, smoking, chronic obstructive pulmonary disease (COPD), and varicose veins. Binary logistic regression indicated that COPD (OR = 1.97; 95% CI = 1.36–2.86; P < .001), high altitude (OR = 2.22; 95% CI = 1.55–3.18; P < .001), and RLS (OR = 1.66; 95% CI = 1.12–2.46; P = .01) increased the odds for poor quality of sleep.
Conclusions:
This study showed that poor quality of sleep was approximately twice as prevalent at high altitudes compared to low altitudes even after removing the potential confounders such as RLS and COPD.
Citation:
Gupta R, Ulfberg J, Allen RP, Goel D. Comparison of subjective sleep quality of long-term residents at low and high altitudes: SARAHA Study. J Clin Sleep Med. 2018;14(1):15–21.
Keywords: COPD, high altitude, population, RLS, sleep quality
BRIEF SUMMARY
Current Knowledge/Study Rationale: Many of the available studies that address the issue of quality of sleep at high altitude have methodological limitations (eg, lowlanders were made to ascend to high altitude; quality of sleep was measured using polysomnography, which has little correlation with subjective feeling; and small sample size). It is known that acclimatization to high altitude occurs, especially in populations native to high altitude. This study was conducted to compare subjective quality of sleep between populations native to high altitude and low altitude.
Study Impact: This study found that subjective quality of sleep was poor, compared to lowlanders, in a significant proportion of the population native to moderately high altitude—even after removing potential confounders such as chronic obstructive pulmonary disease and restless legs syndrome, perhaps because of deficient acclimatization. The acclimatization process may involve genetic as well as epigenetic factors.
INTRODUCTION
In the recent past, there has been an increasing interest in the effect of altitude on the quality of sleep.1–3 It has been found that quality of sleep worsens as we ascend to higher altitudes and this has been ascribed to many factors, including periodic breathing secondary to hypobaric hypoxia and also to frequent arousals from sleep thought to be associated with periodic breathing.4,5 However, the relationship between quality of sleep and high altitude is complicated by the individuality of response to hypoxia; all studies do not show clear effects of altitude on periodic breathing.6 Acclimation is a natural process and it has been shown that Tibetans living at very high altitudes maintain optimal oxygen saturation during wakefulness as well as during sleep and have good quality of sleep.7 Even the lowlanders adapt themselves to the high altitude and their quality of sleep improves and sleep disturbances reduce as they spend more time at high altitude.8
Reports assessing the effect of altitude on quality of sleep have shown that some factors may influence the relationship (eg, duration of exposure to the high altitude, the altitude itself, genetic makeup, assessment tools, time of assessment after exposure to altitude, presence of medical morbidities, smoking, sampling methods, and size of study population). Most of the earlier studies have included subjects who were native lowlanders and were made to ascend to various altitudes (ranging from 3,200 m to 7,134 m); they adopted different methods for assessment of quality of sleep (eg, questionnaires or polysomnography), had sample sizes ranging from 9 to 100, and differed in the time provided for acclimatization (1 day to 6 days).1,4,5,9 Although these findings are important for climbers, they cannot be extrapolated to long-term residents at high altitudes because populations native to high altitude become acclimated.7,10 Only a handful of studies have been conducted to assess the quality of sleep of natives staying at high altitude and fewer have compared their quality of sleep with that of lowlanders.10–15 However, small sample size was a limiting factor in these studies, making the generalization of these findings difficult.10–14 Moreover, literature correlating subjective quality of sleep with polysomnography-measured sleep is inconsistent and shows only a weak correlation between subjective quality of sleep and objectively measured polysomnographic data.16,17 Last, restless legs syndrome (RLS) is more prevalent among high-altitude residents compared to those at low altitude, and its effect has not been taken into consideration in any of the earlier studies.18–21 Considering the methodological limitations of earlier studies, this study was planned to compare the subjective quality of sleep between residents residing at moderately high altitude for a long time and residents living at low altitude after controlling for RLS.
METHODS
This study (Sleep and RLS at High Altitude [SARAHA]) was conducted in the Himalayan region of India after obtaining approval from the institutional ethics committee of the Himalayan Institute of Medical Sciences, Dehradun, India. Results showing increased prevalence of RLS at higher altitude have been presented elsewhere21; the current study focuses on subjective quality of sleep. In this cross-sectional study, subjects were recruited via a random stratified sampling method. Populations from three different altitudes—400 m above sea level (masl), 1,900–2,100 masl and 3,200 masl—were included in this study.
The population sample was obtained from villages situated at three altitudes in the state of Uttarakhand located in the foothills of Himalaya. All the villages were grouped in geographical blocks—Doiwala (district Dehradun; 400 masl), Chakrata (district Dehradun; 1,900–2,100 masl) and Joshimath (district Chamoli; 3,200 masl). These places share common culture and speak Hindi language. From each block, four to five villages were chosen randomly; however, for the Joshimath block, only one village could be found. From these villages, houses were chosen in a stratified manner. Two nonmedical field workers were trained by one of the authors (RG) for the data collection. They were blinded to the hypothesis to reduce bias. They collected data through a door-to-door survey using the face-to-face interview method.
Sample
All the members from the chosen household (age 18 to 84 years) were included in the study. This was especially important for the mid and high altitudes because of the small population of each village. The same process was repeated at low altitude to maintain consistency. Subjects provided informed consent for the study that included consent to review their medical records as provided by them. However, subjects suffering from dementia, stroke, Parkinson disease, chronic kidney disease, and those taking psychotropic medication (which was documented in their medical records) were excluded from the study.
Procedure
Demographic data, sleep patterns (time to bed and time to wake up), and smoking status (nonsmoker, ex-smoker, and smoker) were obtained through open-ended questions during the interview. In addition, comorbid medical disorders (eg, chronic obstructive pulmonary disease [COPD], systemic hypertension, congestive heart failure, peripheral neuropathy, varicose veins, anemia) were ascertained by their most recent medical records (from past 3 months). Female subjects of childbearing age were asked about pregnancy history. To assess the consistency of their sleep-wake schedule (fixed sleep schedule) the following question was asked: “Does your time to bed and wake time remain almost same on at least 5 nights a week?” A difference of up to 30 minutes in time to bed and wake time was considered insignificant. To assess the duration of stay subjects were asked “How long have you been staying at this place?” and responses were recorded in years.
Physical Characteristics
Height and weight were measured using a standard stadiometer and an electronic weighing machine. A record of percutaneous O2 saturation was obtained from an index finger with the Nidek finger pulse oximeter 6500 (Nidek Medical India, Kolkata, India) while the subjects were at rest.
Assessment of RLS and Sleep Quality
Subjects with RLS were identified using the Hindi version of the well-validated Cambridge Hopkins RLS diagnostic questionnaire (CH-RLSq).22,23 This Hindi version has been found to have specificity of 72.2% with 87.6% sensitivity and positive predictive value of 83.3% in a Hindi-speaking population in India.22
Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI) that has been validated in an Indian population.24,25 The validated Hindi version used was obtained from the MAPI research trust. PSQI assesses sleep quality during the past month and has 7 subscales—subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medications, and daytime dysfunction. An overall score is calculated and a score of zero to 4 suggests good sleep quality.24
Items from both the questionnaires were verbally presented in a face-to-face meeting and responses were recorded. This was done based on varying literacy status of the subjects included in this study.
Assessment of Physical Activity
Physical ability at leisure time was measured using the Hindi version of the Godin leisure-time exercise questionnaire.26 It assesses frequency of heavy, moderate, and light exercise during the past week along with the frequency of activities that were enough to induce sweating during the past week. Global score was calculated by multiplying the frequency of heavy, moderate, and light activity by 9, 5, and 3, respectively.
Statistical Analysis
Statistical analysis was done using SPSS Statistics for Windows Version 21.0 (IBM Corp., Armonk, New York, United States). Proportions were compared using chi-square analysis. Statistical significance of differences in means between two groups was analyzed using independent-sample t test. Oneway analysis of variance with Tukey post hoc analysis was used to evaluate normally distributed continuous variables among three altitudes. Subjects included in the mid and high altitudes shared many of the characteristics and the sample size at highest altitude was very small; hence, the mid- and high-altitude categories were combined to form a group “high altitude” as reported in the prior study.21 A binary logistic regression was run using the variables that were found significant in univariate analyses comparing good and poor quality of sleep. Model 1 included subjects having RLS, whereas a second model was run after excluding cases having RLS.
RESULTS
For this study, 1,792 eligible subjects were approached. Of these, 103 (21 at low altitude, 65 at mid-altitude and 17 at high altitude) were excluded for various reasons (eg, not willing to participate in the study, taking neuropsychotropic medications, providing inadequate information, and having neurodegenerative or cerebrovascular disorders). There was no difference in the proportion of these exclusion factors at different altitudes. Response rate was 95.7%, 94.05%, and 91.4% at low, mid, and high altitudes, respectively. Thus, a total of 1,689 subjects were included in this study (55.2% female, average age 35.2 years, standard deviation = 10.9, range 18 to 84 years). In this sample, overall 18.4% of subjects reported poor quality of sleep, which reduced to 17.1% after excluding subjects with RLS. Proportion of first-degree relatives of the index subjects included in the study was higher at high altitude than low altitude (17.9% versus 11.7%; P = .001). Prevalence of COPD, hypertension, anemia, varicose veins, and snoring was higher at mid and high altitudes.21 Other details of the study sample have been provided in Table 1. Although the difference in body mass index among three altitudes was statistically significant, it was clinically insignificant (Table 1). There was no difference in the waking oxygen saturation (%) among three altitudes (low altitude 95.8 ± 9.7; mid altitude 95.4 ± 8.7; high altitude 94.6 ± 2.6; P = .31). Mid- and high-altitude groups were similar regarding most of the factors including quality of sleep and timing of sleep (Table 1); hence, these categories were combined.
Table 1.
Characteristics of the study sample at different altitudes.
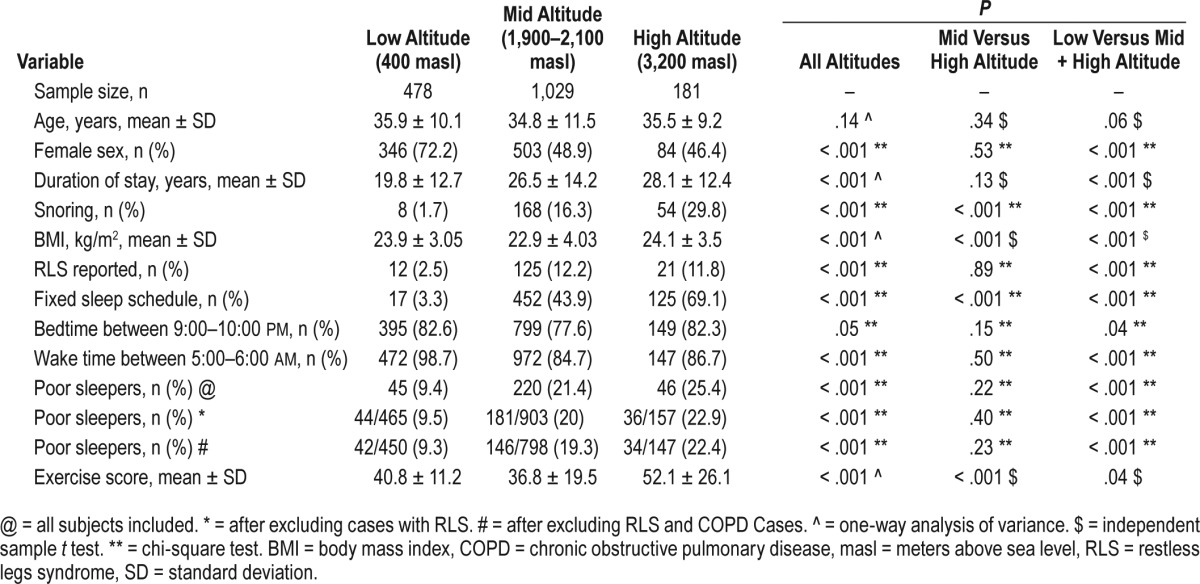
Analysis after combining mid- and high-altitude categories showed that altitude was also an important factor for poor quality of sleep. Poor quality of sleep was reported more commonly at high altitude as compared to low altitude, when all subjects (including those having RLS) were included (OR = 2.65; 95% CI = 1.9–3.7; P < .001). These odds did not change much, even after removing cases in whom RLS was diagnosed (OR = 2.46; 95% CI = 1.7–3.4; P < .001) and further, even after removing those with COPD and RLS (OR = 2.27; 95% CI = 1.5–3.2; P < .001) (Table 1). Thus, the sleep quality worsened independent of RLS at higher altitude.
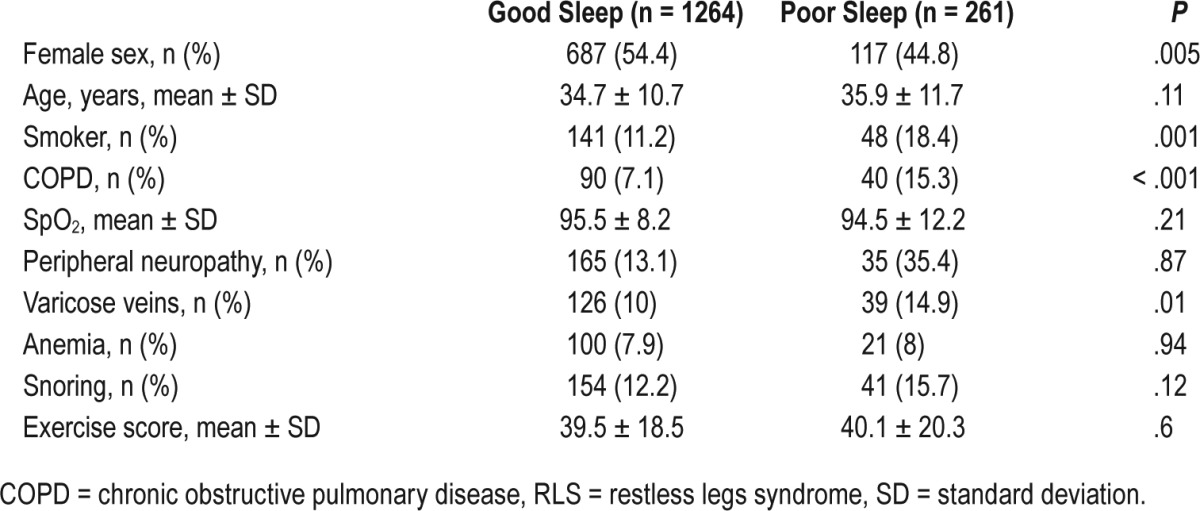
Poor quality of sleep was also more frequently reported among patients with RLS (29.7% versus 17.1% without RLS; P < .001). Male sex (20.5% versus 16.7% females; P = .04), smokers (25.9% versus 17.4% among nonsmokers; P = .003), subjects with COPD (33.3% versus 16.8% among no-COPD; P < .001), and varicose veins (25.1% versus 17.5% without it; P = .007) were factors reportedly associated with a higher prevalence of poor quality of sleep. However, peripheral neuropathy, anemia, and snoring were unrelated to the quality of sleep. Factors associated with good and poor quality of sleep are described in Table 2.
Table 2.
Factors associated with sleep quality of the subjects after excluding RLS cases.
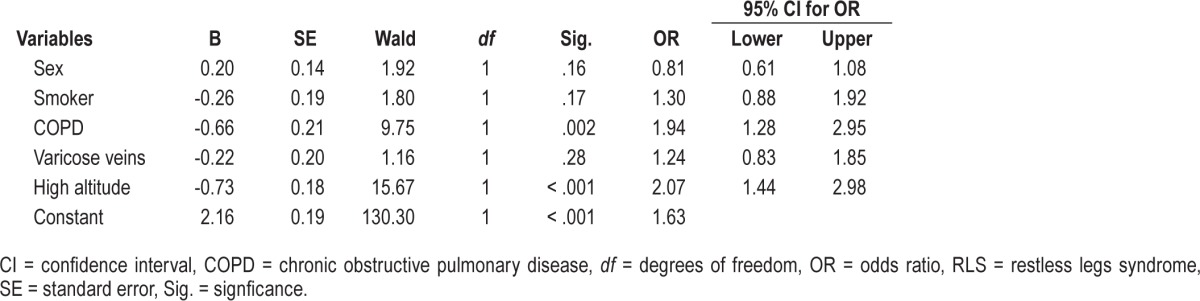
Binary logistic regression was used to find relative contribution of factors that were found to have significant effect on quality of sleep in univariate analysis. Two models were developed, one that included subjects with RLS cases and second, after excluding them. The model without RLS was overall significant (χ2 = 47.54; P < .001) and it classified 82.9% cases correctly (Nagelkerke R Square = 0.05). This model showed that COPD and high altitude significantly increased the odds for poor quality of sleep (Table 3). The second model that included subjects with RLS was also significant overall (χ2 = 68.07; P < .001) and it classified 81.6% cases correctly (Nagelkerke R Square = 0.06). This model indicated that COPD (OR = 1.97; 95% CI = 1.36–2.86; P < .001), high altitude (OR = 2.22; 96% CI = 1.55–3.18; P < .001), and RLS (OR = 1.66; 95% CI = 1.12–2.46; P = .01) increased the odds for poor quality of sleep.
Table 3.
Binary logistic regression model for factors leading to poor sleep quality (excluding RLS cases).
DISCUSSION
This study indicated that sleep quality is poor in the native population residing at high altitudes (1,900–3,200 masl) compared to those living at low altitude (400 masl). Logistic regression showed that COPD and RLS increased the odds for poor sleep quality; however, their exclusion did not change the results. To the best of our knowledge, this is the first study that has assessed sleep quality in native highlanders in a fairly large sample.
Can periodic breathing explain the higher prevalence of poor quality of sleep in the current study? Frequent arousals were observed at high altitude in lowlanders after rapid escalation to 3,200 masl, and a previous study reported wide variation in the proportion of time spent in periodic breathing.6 Interestingly, adaptation to high altitude is fast and it has been reported that lowlanders, after rapid escalation to the high altitude (3,200–3,600 masl), adapt to high altitude in a period as short as 6 days, producing higher mean oxygen saturation during sleep (compared to day 1).6,10 This suggests an improvement in ventilatory mechanisms along with improved objective quality of sleep by the end of 1 week (compared to day 1) without any change in sleep duration.6,10 In addition, not all subjects experienced periodic breathing unless they had a rapid ascent to 4,000 masl; in only one-fourth did periodic breathing develop after rapid ascent to 2,500 masl.27 Thus, these studies suggest that periodic breathing does not develop in all subjects, and there is interindividual variation in the proportion of time spent in periodic breathing due to the ventilatory mechanism that rapidly adapts to high altitude, even in lowlanders made to ascend to high altitude. Though we could not find any data that depicted the periodic breathing at this altitude among the native adult population, 1 study among native Andean infants showed absence of periodic breathing at the altitude of 3,650 masl, which was attributed to genetic changes accrued over generations.11 Periodic breathing occurs in response to inadequate environmental oxygen. In the current study we did not find any difference in oxygen saturation during the waking state. This is a replication of a previous study that reported that oxygen saturation does not drop below 90% in a normal population unless the person goes beyond 3,500 masl.28 Combining the facts that the population in the current study was native to high altitude and had comparable oxygen saturation during wakefulness, it is unlikely that periodic breathing is the reason for the greater prevalence of poor quality of sleep at higher altitude. We found that prevalence of snoring increased linearly with altitude even when body mass index was within normal limits, but we did not screen for obstructive sleep apnea (OSA). However, available literature suggests that it is central sleep apnea, not OSA, that increases with altitude, and snoring is much more prevalent than OSA.27,28 Hence, increasing prevalence of snoring at higher altitude cannot be extrapolated to OSA and poor sleep quality. However, before reaching a conclusion, both issues (ie, periodic breathing and OSA in these populations) require further exploration.
Two other factors that were found to worsen quality of sleep in this study were RLS and COPD. Previous studies have reported that RLS disrupts the initiation and maintenance of sleep, and that sleep improves with the treatment of RLS.29–31 Similarly, sleep quality has been found to be poor among patients with COPD and was reported to be related to the frequent arousals from sleep due to hypoxemia or to direct effects of illness (eg, coughing).32,33 In addition, patients with COPD are at a higher risk for developing RLS, and both conditions together can worsen sleep quality.34,35 Thus, the current study confirms earlier findings. However, in the current study, quality of sleep was poor at high altitude even after removing cases of RLS and COPD (Table 1), suggesting a clear altitude effect, which needs further exploration.
Though nearly one-fifth of the population at high altitude had poor quality of sleep, it must not be ignored that the rest of the population (almost 80%) had normal sleep at high altitude. This is an important finding and requires some discussion. Literature suggests that despite respiratory acclimatization that occurs after a short period of exposure to high altitude, quality of sleep of lowlanders who continue to live at high altitudes remains poorer than that of native highlanders.10,13,36 Perhaps native highlanders develop physiological changes to counter the effects of high altitude. Children of Tibetans, even those who are born and lived throughout childhood at low altitude, had normal sleep when they moved to high altitude during their adulthood for the first time.7 This fact cannot be explained by anything other than genetic/epigenetic factors. It may be possible that with continuous exposure to high altitude for generations, native highlanders (Tibetans) had developed some yet-to-be-discovered genetic changes that did not allow their sleep to be disrupted at high altitude even after prolonged stay at lowland. Another possibility is that there was gene-environment interaction (epigenetic changes) to produce permanent phenotypic changes. For example, it has been reported that Tibetans, native highlanders, have higher oxygen saturation both at rest and during work; they depict changes in the pulmonary physiology (eg, larger lung volume, greater diffusion capacity, higher hypoxic and hyper-capnic responsiveness with lesser nocturnal desaturations and better quality of sleep).7 However, the process of acclimatization to high altitude may be population-specific because those living at comparable altitude in Andes have shown different physiological changes. For example, native highlanders at Andes adjust to high altitude with increased erythrocytosis, increased diffusion capacity, and total lung capacity.37 These results suggest that different populations adapt to high altitude differently. Children residing at high altitude in the Andes showed recurrent desaturations during sleep and poor sleep similar to that of lowlanders.11 This was suggested that because of the marriages between native Amerindians and Europeans, the genetic pool was diluted and therefore had taken away the adaptation effect among Amerindians.11 Together, these data are in accordance with the theory of evolution. It suggests that the human race has adapted to high altitude over a period of time, and this evolution has been genetic as well as phenotypic. Results of the current study may be considered in light of aforementioned facts. The population of the Uttarakhand state where the current study was carried out, irrespective of the altitude, is composed of local inhabitants who have lived there for thousands of years (apparently local inhabitants are not Tibetans) and those who have migrated there a few centuries back. Moreover, in this population, marriages among people with different gene pools (migrants and natives) are not uncommon. Because of this reason, genetic variation has been reported in the population living in this state both at the low as well as high altitude.38,39 This could be one reason for the higher prevalence of poor quality of sleep reported at high altitude in the current study, a finding seen in the Andean population, as mentioned earlier. This hypothesis, however, needs to be tested through future studies.
Like any other scientific investigation, the current study also had a few methodological limitations. First, other factors that might have influenced the quality of sleep (eg, anxiety, depression) were not taken into consideration. Second, females were overrepresented in this sample, in whom insomnia is already more prevalent. Third, more than one person was included from one house, thus increasing the possibility of genetic effect on quality of sleep. Fourth, diagnoses of medical disorders were based on medical records rather than objective examination at the time of study. Last, because of the study design, we could not rule out sleep-related breathing disorders through objective methods, if any, as a cause for poor quality of sleep. However, large sample size, randomization of subjects, subjective measure of quality of sleep, inclusion of population at high altitude that were long-term residents, and use of validated measured to assess conditions in question were the strengths of this study.
In conclusion, this study showed that quality of sleep was poor in the inhabitants of altitudes as low as 2,000 masl even after staying there for years as compared to the population staying at 400 masl. Sleep-related breathing disorders and/or genetic susceptibility leading to poor acclimatization might have played a role in the findings; however, this needs to be examined in future studies.
DISCLOSURE STATEMENT
All authors have seen and accepted the manuscript in its current form. Dr. Allen reports consulting and clinical trial work with Luitpold Pharma, unrelated to the submitted work. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
This study was approved by Swami Rama Himalayan University, Dehradun vide project number Psychiatry/2015/02. The authors thank the MAPI research trust for allowing the use of the Hindi version of PSQI in this study.
ABBREVIATIONS
- BMI
body mass index
- COPD
chronic obstructive pulmonary disease
- masl
meters above sea level
- OR
odds ratio
- OSA
obstructive sleep apnea
- PSQI
Pittsburgh Sleep Quality Index
- RLS
restless legs syndrome
- SARAHA
Sleep and RLS at High Altitude
REFERENCES
- 1.Koziej M, Mańkowski M, Sarybaev AS, et al. [Quality of sleep and periodic breathing during sleep in healthy persons at a height of 3200 meters] Pneumonol Alergol Pol. 1996;64(9-10):651–657. [PubMed] [Google Scholar]
- 2.Weil JV. Sleep at high altitude. High Alt Med Biol. 2004;5(2):180–189. doi: 10.1089/1527029041352162. [DOI] [PubMed] [Google Scholar]
- 3.Wickramasinghe H, Anholm JD. Sleep and breathing at high altitude. Sleep Breath. 1999;3(3):89–102. doi: 10.1007/s11325-999-0089-1. [DOI] [PubMed] [Google Scholar]
- 4.Jafarian S, Gorouhi F, Taghva A, Lotfi J. High-altitude sleep disturbance: results of the Groningen Sleep Quality Questionnaire survey. Sleep Med. 2008;9(4):446–449. doi: 10.1016/j.sleep.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 5.Szymczak RK, Sitek EJ, Sławek JW, Basiński A, Siemiński M, Wieczorek D. Subjective sleep quality alterations at high altitude. Wilderness Environ Med. 2009;20(4):305–310. doi: 10.1580/1080-6032-020.004.0305. [DOI] [PubMed] [Google Scholar]
- 6.Zieliński J, Koziej M, Mańkowski M, et al. The quality of sleep and periodic breathing in healthy subjects at an altitude of 3,200 m. High Alt Med Biol. 2000;1(4):331–336. doi: 10.1089/15270290050502408. [DOI] [PubMed] [Google Scholar]
- 7.Wu T, Kayser B. High altitude adaptation in Tibetans. High Alt Med Biol. 2006;7(3):193–208. doi: 10.1089/ham.2006.7.193. [DOI] [PubMed] [Google Scholar]
- 8.Nussbaumer-Ochsner Y, Ursprung J, Siebenmann C, Maggiorini M, Bloch KE. Effect of short-term acclimatization to high altitude on sleep and nocturnal breathing. Sleep. 2012;35(3):419–423. doi: 10.5665/sleep.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thakur L, Anand JP, Malhotra AS, Sharma YK, Panjwani U. Sleep architecture at 4300 m altitude in a sample of Indian lowlanders. Indian J Physiol Pharmacol. 2012;56(4):295–300. [PubMed] [Google Scholar]
- 10.Roach GD, Schmidt WF, Aughey RJ, et al. The sleep of elite athletes at sea level and high altitude: a comparison of sea-level natives and high-altitude natives (ISA3600) Br J Sports Med. 2013;47(Suppl 1):i114–i120. doi: 10.1136/bjsports-2013-092843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill CM, Carroll A, Dimitriou D, et al. Polysomnography in Bolivian children native to high altitude compared to children native to low altitude. Sleep. 2016;39(12):2149–2155. doi: 10.5665/sleep.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakamoto R, Okumiya K, Norboo T, et al. Sleep quality among elderly high-altitude dwellers in Ladakh. Psychiatry Res. 2016;249:51–57. doi: 10.1016/j.psychres.2016.12.043. [DOI] [PubMed] [Google Scholar]
- 13.Kong F, Liu S, Li Q, Wang L. Sleep architecture in partially acclimatized lowlanders and native Tibetans at 3800 meter altitude: what are the differences? High Alt Med Biol. 2015;16(3):223–229. doi: 10.1089/ham.2014.1058. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz AJ, Sepúlveda MA, Martínez PH, et al. Prevalence of sleep complaints in Colombia at different altitudes. Sleep Sci. 2016;9(2):100–105. doi: 10.1016/j.slsci.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bloch KE, Buenzli JC, Latshang TD, Ulrich S. Sleep at high altitude: guesses and facts. J Appl Physiol. 2015;119(12):1466–1480. doi: 10.1152/japplphysiol.00448.2015. [DOI] [PubMed] [Google Scholar]
- 16.Buysse DJ, Hall ML, Strollo PJ, Jr, et al. Relationships between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and clinical/ polysomnographic measures in a community sample. J Clin Sleep Med. 2008;4(6):563–571. [PMC free article] [PubMed] [Google Scholar]
- 17.Unruh ML, Redline S, An MW, et al. Subjective and objective sleep quality and aging in the Sleep Heart Health Study. J Am Geriatr Soc. 2008;56(7):1218–1227. doi: 10.1111/j.1532-5415.2008.01755.x. [DOI] [PubMed] [Google Scholar]
- 18.Vizcarra-Escobar D, Mendiola-Yamasato A, Risco-Rocca J, et al. Is restless legs syndrome associated with chronic mountain sickness? Sleep Med. 2015;16(8):976–980. doi: 10.1016/j.sleep.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Demirci S, Demirci K, Doğru A, İnal EE, Koyuncuoğlu HR, Şahin M. Restless legs syndrome is associated with poor sleep quality and quality of life in patients with ankylosing spondylitis: a questionnaire-based study. Acta Neurol Belg. 2016;116(3):329–336. doi: 10.1007/s13760-015-0564-3. [DOI] [PubMed] [Google Scholar]
- 20.Sander HH, Eckeli AL, Costa Passos AD, Azevedo L, Fernandes do Prado LB, França Fernandes RM. Prevalence and quality of life and sleep in children and adolescents with restless legs syndrome/Willis-Ekbom disease. Sleep Med. 2017;30:204–209. doi: 10.1016/j.sleep.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Gupta R, Ulfberg J, Allen RP, Goel D. High prevalence of restless legs syndrome/Willis Ekbom Disease (RLS/WED) among people living at high altitude in the Indian Himalaya. Sleep Med. 2017;35:7–11. doi: 10.1016/j.sleep.2017.02.031. [DOI] [PubMed] [Google Scholar]
- 22.Gupta R, Allan RP, Pundeer A, Das S, Dhyani M, Goel D. Hindi translation and validation of Cambridge-Hopkins Diagnostic Questionnaire for RLS (CHRLSq) Ann Indian Acad Neurol. 2015;18(3):303–308. doi: 10.4103/0972-2327.162290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen RP, Burchell BJ, MacDonald B, Hening WA, Earley CJ. Validation of the self-completed Cambridge-Hopkins questionnaire (CH-RLSq) for ascertainment of restless legs syndrome (RLS) in a population survey. Sleep Med. 2009;10(10):1097–1100. doi: 10.1016/j.sleep.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 25.Manzar MD, Moiz JA, Zannat W, et al. Validity of the Pittsburgh Sleep Quality Index in Indian university students. Oman Med J. 2015;30(3):193–202. doi: 10.5001/omj.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godin G, Shepherd R. Godin leisure-time exercise questionnaire. Med Sci Sports Exerc. 1997;29(suppl 6):S36–S38. [Google Scholar]
- 27.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 28.Normal Oxygen Saturations at Various Altitudes. High Altitude Medicine Guide website. [Accessed May 6, 2017]. http://www.high-altitude-medicine.com/SaO2-table.html. Updated May 8, 2000.
- 29.Allen R, Becker PM, Bogan R, et al. Ropinirole decreases periodic leg movements and improves sleep parameters in patients with restless legs syndrome. Sleep. 2004;27(5):907–914. doi: 10.1093/sleep/27.5.907. [DOI] [PubMed] [Google Scholar]
- 30.Ulfberg J, Nyström B, Carter N, Edling C. Prevalence of restless legs syndrome among men aged 18 to 64 years: an association with somatic disease and neuropsychiatric symptoms. Mov Disord. 2001;16(6):1159–1163. doi: 10.1002/mds.1209. [DOI] [PubMed] [Google Scholar]
- 31.Bogan RK. Effects of restless legs syndrome (RLS) on sleep. Neuropsychiatr Dis Treat. 2006;2(4):513–519. doi: 10.2147/nedt.2006.2.4.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNicholas WT, Verbraecken J, Marin JM. Sleep disorders in COPD: the forgotten dimension. Eur Respir Rev. 2013;22(129):365–375. doi: 10.1183/09059180.00003213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McSharry DG, Ryan S, Calverley P, Edwards JC, McNicholas WT. Sleep quality in chronic obstructive pulmonary disease. Respirology. 2012;17(7):1119–1124. doi: 10.1111/j.1440-1843.2012.02217.x. [DOI] [PubMed] [Google Scholar]
- 34.Cavalcante AG, de Bruin PF, de Bruin VM, et al. Restless legs syndrome, sleep impairment, and fatigue in chronic obstructive pulmonary disease. Sleep Med. 2012;13(7):842–847. doi: 10.1016/j.sleep.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 35.Lo Coco D, Mattaliano A, Lo Coco A, Randisi B. Increased frequency of restless legs syndrome in chronic obstructive pulmonary disease patients. Sleep Med. 2009;10(5):572–576. doi: 10.1016/j.sleep.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Gao L, Wuren T, Ga Q, Guan W, Ge R. [Pilot study on the differences of young male's sleep structure and quality between indigenous Tibetans and longtime Han residents in high altitude area] Zhonghua Yi Xue Za Zhi. 2015;95(42):3416–3419. [PubMed] [Google Scholar]
- 37.Hill CM, Baya A, Gavlak J, et al. Adaptation to life in the high Andes: nocturnal oxyhemoglobin saturation in early development. Sleep. 2016;39(5):1001–1008. doi: 10.5665/sleep.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Negi N, Tamang R, Pande V, et al. The paternal ancestry of Uttarakhand does not imitate the classical caste system of India. J Hum Genet. 2016;61(2):167–172. doi: 10.1038/jhg.2015.121. [DOI] [PubMed] [Google Scholar]
- 39.Chahal SM, Singh P, Singh H, Bansal R, Bansal IJ. Genetic variation and structure of the people of Uttarakhand, Central Himalayas, India. Hum Biol. 2008;80(4):409–434. doi: 10.3378/1534-6617-80.4.409. [DOI] [PubMed] [Google Scholar]


