Abstract
Study Objectives:
Sleep disturbances are commonly reported by cancer survivors. Systematic light exposure using bright light has been used to improve sleep in other populations. In this secondary data analysis, the effect of morning administration of bright light on sleep and sleep quality was examined in a mixed group of fatigued cancer survivors.
Methods:
Forty-four cancer survivors screened for cancer-related fatigue were randomized to either a bright white light or a comparison dim red light condition. Participants were instructed to use a light box every morning for 30 minutes for 4 weeks. Wrist actigraphy and the Pittsburgh Sleep Quality Index were administered at 4 time points: prior to light treatment (baseline), 2 weeks into the intervention, during the last week of the intervention, and 3 weeks postintervention. Thirty-seven participants completed the end-of-intervention assessment.
Results:
Repeated-measures linear mixed models indicated a statistically significant time × treatment group interaction effect with sleep efficiency improving more in the bright light condition over time compared with the dim light condition (F3,42 = 5.55; P = .003) with a large effect size (partial η2 = 0.28). By the end of the intervention and 3 weeks postintervention, mean sleep efficiency in the bright light group was in the normal range. Medium to large effect sizes were also seen in sleep quality, total sleep time, and wake after sleep onset for participants favoring the bright light condition.
Conclusions:
The results suggest that systematic bright light exposure in the morning may have beneficial effects on sleep in fatigued cancer survivors. Larger scale efficacy trials are warranted.
Clinical Trial Registration:
Registry: ClinicalTrials.gov, Title: Treating Cancer-Related Fatigue Through Systematic Light Exposure, Identifier: NCT01873794, URL: https://clinicaltrials.gov/ct2/show/NCT01873794
Citation:
Wu LM, Amidi A, Valdimarsdottir H, Ancoli-Israel S, Liu L, Winkel G, Byrne EE, Sefair AV, Vega A, Bovbjerg K, Redd WH. The effect of systematic light exposure on sleep in a mixed group of fatigued cancer survivors. J Clin Sleep Med. 2018;14(1):31–39.
Keywords: actigraphy, cancer, fatigue, light therapy, sleep disturbance, sleep efficiency, sleep quality
BRIEF SUMMARY
Current Knowledge/Study Rationale: Systematic light exposure (sLE) using morning bright light therapy has commonly been used to treat seasonal affective disorder. More recently, it has shown promise in preventing and treating fatigue in cancer patients and survivors. In this secondary analysis, we sought to investigate the effect of sLE on sleep in a mixed group of fatigued cancer survivors following primary cancer treatment.
Study Impact: Given the difficulties that cancer survivors often have in engaging in activity that could treat sleep disturbance, bright light therapy has the potential to provide cancer survivors with an easy-to-use and inexpensive tool that could improve quality of life. Larger-scale studies to test the efficacy of sLE to treat sleep disturbances in cancer survivors are needed.
INTRODUCTION
Sleep disturbances are reported by cancer patients at a significantly higher rate than in the general population.1 Among posttreatment cancer survivors, 23% to 44% experience insomnia symptoms even years after treatment.2 Furthermore, a study that presented results of the 2007 National Health Interview Survey in the United States showed that the prevalence of insomnia symptoms reported by cancer survivors (31%) is significantly greater than that of the general population (17%).3 Sleep disturbances often co-occur with cancer-related fatigue and are sometimes considered part of the cancer symptom cluster that also includes cognitive impairment and depressed mood.4 Although sleep and fatigue are often correlated, there is evidence supporting the hypothesis that they are distinct constructs that ought to be examined separately. For example, in a study of fatigue in breast cancer survivors, although survivors reported greater fatigue compared with age-matched healthy women and women with benign breast problems, there were no group differences in sleep duration. Risk factors for sleep problems among cancer patients include the cancer itself, medications (including chemotherapy), additional treatment factors (eg, corticosteroids), psychosocial factors (eg, cancer worry), and comorbid medical disorders (eg, headaches).5 Fiorentino and Ancoli-Israel suggest that a negative feedback loop occurs in cancer patients whereby the challenges they face may contribute to sleep problems that, in turn, may exacerbate the medical conditions comorbid with cancer.6 It seems likely that this same negative feedback loop applies to cancer survivors after primary treatment as well, especially given that many continue to receive adjuvant treatments for many years (eg, maintenance chemotherapy in individuals living with multiple myeloma; hormone therapy in breast cancer survivors).
Sleep disturbances are most commonly treated with medications but many cancer patients are reluctant to add more medications to their list.7 Recommended nonpharmacologic treatments for sleep disturbances typically include behavioral and cognitive behavioral therapies. These therapies have been shown to be effective8 but not all patients have the discipline to change behaviors and some patients find these treatments to be burdensome.9 Systematic light exposure (sLE; commonly known as light therapy) using bright white light (BWL) is a low-cost and easily disseminable intervention that may be an effective nonpharmacologic method to ameliorate sleep problems in cancer survivors. Light is a powerful synchronizer of the human circadian system because of its effects on the brain via a non-image forming photoreceptor system that is distinct from rods and cones,10 with the potential to improve sleep via its effects on circadian rhythms.11 Indeed, sLE using bright light has been shown to improve sleep quality in noncancer populations12 and has improved correlates of sleep disturbance in cancer patients, including circadian activity rhythms13 and fatigue, both during chemotherapy14 and, in our current sample, after primary cancer treatment for fatigue.15
In this secondary data analysis, we sought to investigate the effect of sLE on various indicators of potential sleep disturbance in a mixed group of fatigued cancer survivors following primary cancer treatment.
METHODS
Study Design
The design for this study consisted of a two-group randomized controlled trial comparing BWL with a comparison dim red light (DRL) condition in cancer survivors participating in a study investigating the effects of sLE on cancer-related fatigue.15 Eligible participants were randomized to 4 weeks of either morning BWL or morning DRL treatment. Outcomes were assessed at baseline, 2 weeks into the intervention (mid-intervention), during the last week of the intervention (end of intervention), and 3 weeks postintervention.
Recruitment and Procedures
Study approval was obtained by Mount Sinai's Program for the Protection of Human Subjects. Adult survivors of breast and gynecologic cancers who had completed all cancer treatment and survivors of hematological malignancy who had completed hematopoietic stem cell transplant were approached for the study during their regular clinic visits at Mount Sinai. Informed consent was obtained from all participants. Detailed inclusion and exclusion criteria have been described elsewhere.15 Briefly, patients had to meet criteria for clinically significant fatigue. Eligible participants were block randomized to BWL or DRL in a 1-to-1 ratio. Among other questionnaires, participants completed a self-report measure of sleep quality at all time points. Sleep/wake activity was assessed with actigraphy.
Apparatus
Light
Litebook 1.2 (Litebook, Ltd. Medicine Hat, Canada) was used to deliver both the BWL and DRL. For the BWL condition, the Litebook used 60 premium white light emitting diode (LED) lights in a 3.875 × 3 inch elliptical display that mimics the visible spectrum of sunlight (full spectrum white light) and emits approximately 1,350 lux with spectral emission peaks at 464 nm and a second peak at 564 nm at a distance of 20 inches.16 For the DRL condition, an identical-appearing device utilizing red LED lights was used that emits < 50 lux. This is a standard comparison condition as circadian photoreceptors are relatively insensitive to the red light frequency. Participants were instructed to self-administer the light treatment by placing the light box at a 45° angle, 18 inches from their face, for 30 minutes upon awakening every morning throughout the 4-week intervention period.
Actigraphy
Sleep/wake activity was recorded with the Actiwatch 2 (Respironics, Inc., a Philips Healthcare company, Murrysville, Pennsylvania, United States), which is similar in size to a watch and was worn by participants on the nondominant wrist. Each participant wore the Actiwatch 2 for 3 consecutive days (72 hours) at each of the 4 time points and completed a sleep log in which they recorded time to bed, time awake, and other information that was used to hand edit and score the data.
Measures
Fatigue
The Functional Assessment of Chronic Illness Therapy – Fatigue, or FACIT-Fatigue, scale was used for screening of participants into the study17 with a clinical cutoff of ≤ 33.18,19 The 13-item scale has excellent test-retest reliability (r = 0.90) and internal consistency (α = 0.93–0.95).17
Sleep Quality
The Pittsburgh Sleep Quality Index (PSQI) is a 19-item self-report measure that is used to assess sleep quality.20 The PSQI consists of 7 components measuring duration of sleep, sleep disturbance, sleep latency, day dysfunction due to sleepiness, sleep efficiency, overall sleep quality, and whether a person needs medications in order to sleep. The components are then summed to compute a global sleep quality score. A global score > 5 indicates that a participant reports severe difficulties in at least 2 domains or moderate difficulties in more than 3 areas. Internal consistency for the PSQI is generally good, ranging between 0.70 and 0.83.21 In the current sample, it was 0.67.
Actigraphy Sleep Outcomes
Actigraphy data were scored with Actiware 6 software. The following sleep outcomes were computed at each time point based on actigraphy: total sleep time (in hours), sleep efficiency (the percentage of time in bed when the person is sleeping), and wake time after sleep onset (the amount of nocturnal sleep time when the person is awake).
Sociodemographic and Medical Data
Sex, age, race/ethnicity, marital status, education level, and household income level were gathered during screening and baseline assessments. Basic medical data, including diagnosis, were gathered through medical chart review.
Data Analysis
Actigraphy data were scored using the same approach used in other recent studies in cancer patients.22,23 In other words, actigraphy data were automatically scored with Actiware version 6.0.9 software (Respironics, Inc., a Philips Healthcare company, Murrysville, Pennsylvania, United States) for sleep/ wake for each minute of recording and hand-edited with additional information from a sleep log completed by the participants. Summary statistics for wake and sleep durations were computed for the in-bed recording times obtained from the patients' diaries (time to sleep and final awakening time).
Descriptive statistics were used to characterize sociodemo-graphic, medical, and sleep variables. Group differences on sociodemographic and medical characteristics at baseline were analyzed with independent t tests and chi-square tests. In order to determine equivalence between the BWL and DRL groups at baseline on outcome variables of interest, General Linear Models were conducted. To examine whether BWL improved sleep outcomes compared with DRL, repeated-measures linear mixed models were used. SAS version 9.4 (SAS Institute Inc., Chicago, Illinois, United States) was used, specifically the SAS procedure MIXED. A dummy-coded group variable (BWL versus DRL) was entered as the independent variable to test main effects and a time by group variable to test interaction effects for each outcome. Effect sizes for each significant effect were calculated using partial η2 (where SS is sum of squares):
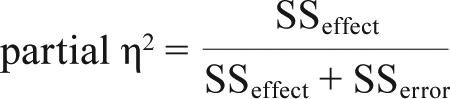 |
ie, the proportion of the total variance attributed to the effect over time. For these exploratory analyses, a significance level of P < .05 was used.
RESULTS
Participant Characteristics
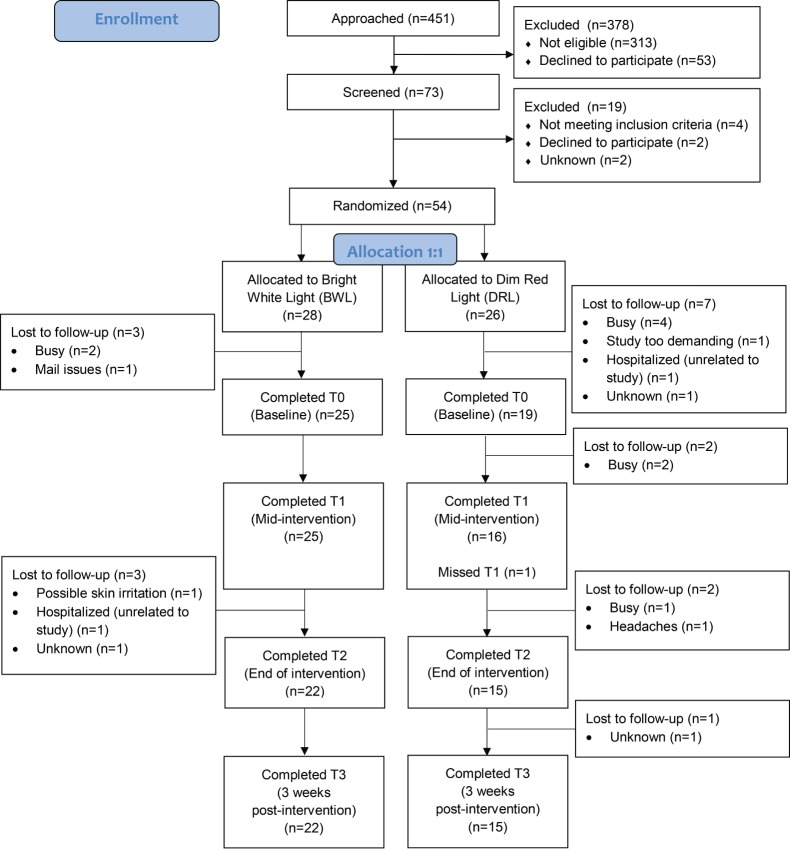
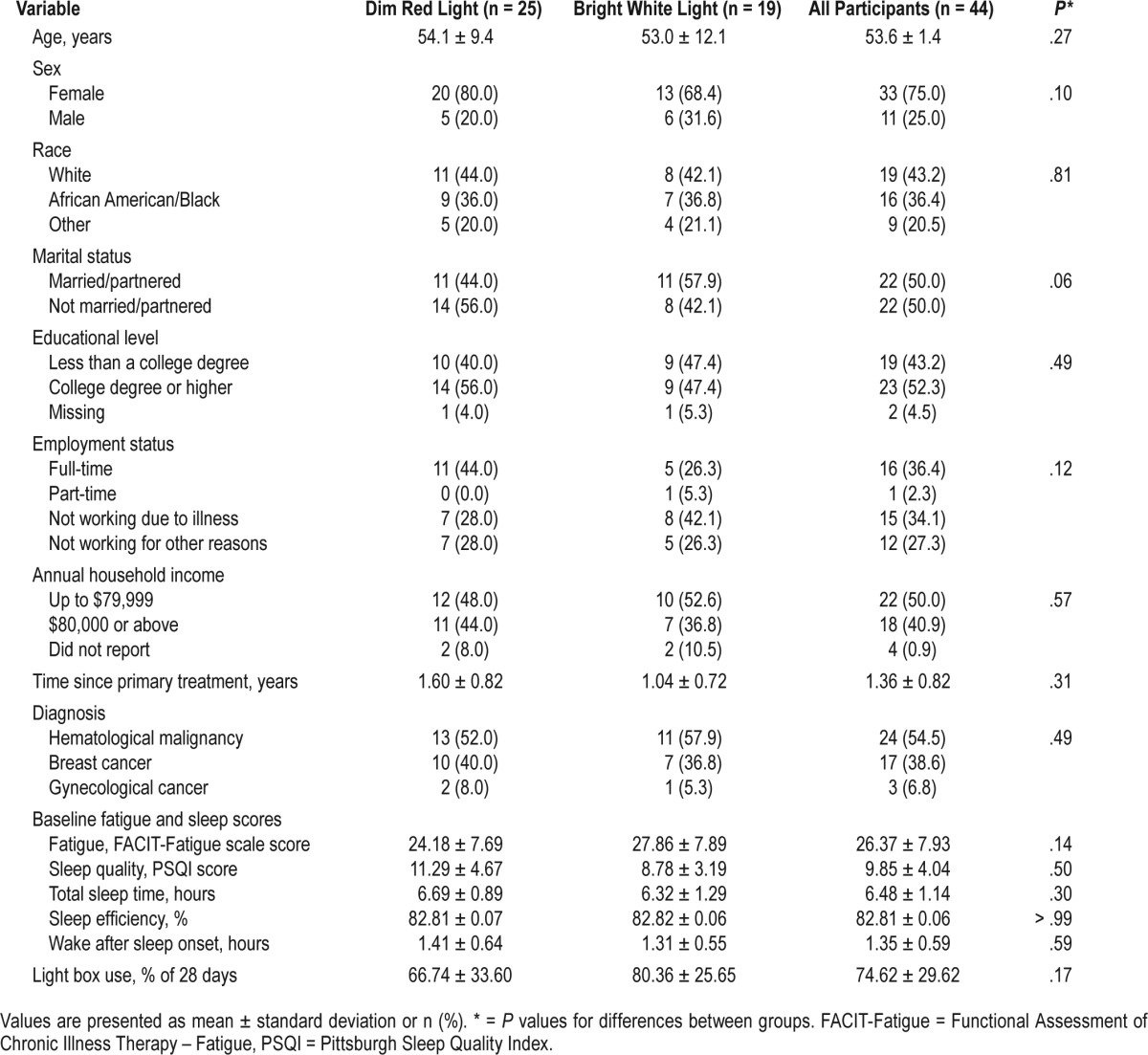
A total of 44 patients participated in the study (see consort flow diagram in Figure 1). Table 1 summarizes participant characteristics. Groups did not differ significantly on sociodemographic characteristics (ie, age, sex, race [White versus other], marital status [married/partnered versus other], educational level [college-educated versus not college-educated], employment status [employed versus not employed], annual household income [< $80,000 versus ≥ $80,000]), or on medical characteristics (ie, time since treatment, diagnosis [hematological malignancy versus other]). To be included in the study, all participants met criteria for clinically significant fatigue at screening. At baseline shortly after screening, 84.2% and 80.0% of those in the BWL and DRL groups, respectively, were clinically significantly fatigued. Mean level of fatigue at baseline was 24.18 and 27.86 for the DRL and BWL groups, respectively. Fatigue levels did not differ significantly between groups (t40 = −1.50, P = .14). DRL and BWL participants used their light box on average 66.74% and 80.36% of the prescribed 28 days, respectively, with no significant difference between groups (t38 = −1.42, P = .17).
Figure 1. Consort diagram.
Table 1.
Participant characteristics.
Subjective Sleep Outcome
Sleep Quality on the PSQI
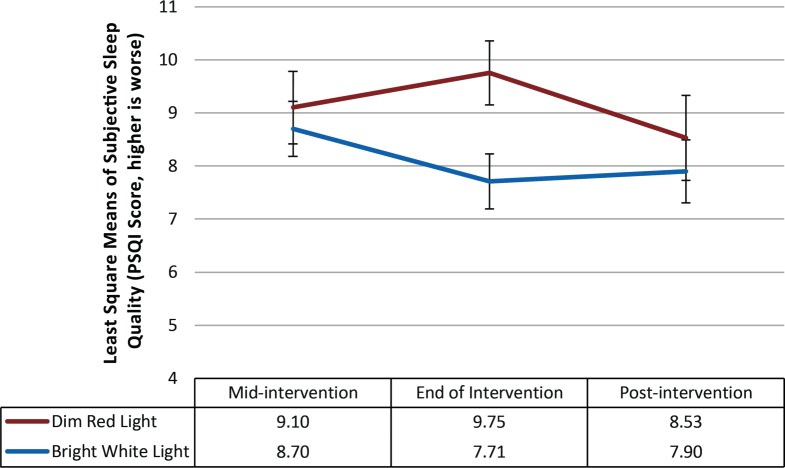
At baseline, the proportion of participants who exceeded the clinical cutoff for poor sleep quality was 76% and 78.9% in the DRL and BWL groups, respectively, and 77.3% overall. There was a marginally significant difference in the mean level of sleep quality between groups at baseline (P = .05) with survivors in the DRL arm reporting poorer sleep quality (mean = 11.29) than survivors in the BWL condition (mean = 8.78). A repeated-measures linear mixed model was used with baseline sleep quality included as a covariate to account for baseline differences. The model indicated that neither the main effect for time (F2,54 = 1.21; P = .31), the main effect for treatment (F1,34 = 2.54, P = .12), nor the time × treatment condition (F2,54 = 2.31, P = .11) was statistically significant. Nevertheless, the effect size was medium to large (partial η2 = 0.08) using Cohen criteria.24 As can be seen in Figure 2, at the end of the intervention, sleep quality in the BWL group (mean = 7.71) improved marginally more than for the survivors in the DRL condition (mean = 9.75) (Tukey-adjusted t54 = −2.66; P = .10). However, this improvement was not sustained 3 weeks after the intervention.
Figure 2. Least squares mean and standard error bars for subjective sleep quality over time.
After controlling for baseline levels of sleep quality, the repeated-measures linear mixed model showed no effects for time (P = .31), treatment condition (P = .12), nor time by treatment condition (P = .11). PSQI = Pittsburgh Sleep Quality Index.
Objective Sleep Outcomes
Total Sleep Time
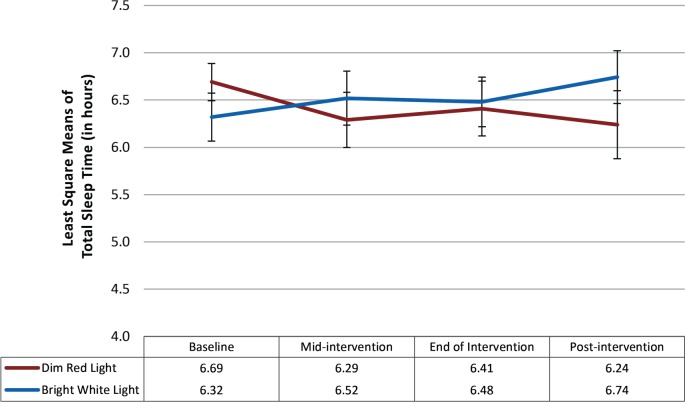
At baseline, there were no significant differences in total sleep time between participants in the BWL (mean = 6.69 hours) and DRL groups (mean = 6.32 hours; P = .30). The repeated-measures linear mixed model indicated that neither the main effect for time (F3,42 = 0.15, P = .93) nor the main effect for treatment condition was statistically significant (F1,42 = 0.12, P = .74). The time × treatment interaction was also not significant (F3,42 = 2.70; P = .06), suggesting that the groups did not differ with respect to change in total sleep time over the study period. Nevertheless, the effect size was large (partial η2 = 0.16) using Cohen criteria.24 As can be seen in Figure 3, total sleep time tended to increase over time for those in the BWL condition whereas total sleep time tended to decrease in the DRL condition.
Figure 3. Least squares mean and standard error bars for total sleep time over time.
The repeated-measures linear mixed model showed that neither the main effect for time (P = .93) nor treatment condition (P = .74) were statistically significant. Time × treatment was also not significant (P = .06).
Sleep Efficiency
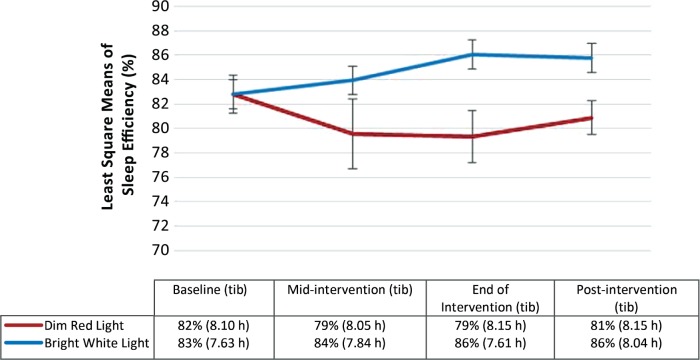
At baseline, the proportion of participants who exceeded the clinical cutoff for poor sleep efficiency (≤ 85%) was 52.6% and 60.0% in the DRL and BWL groups, respectively, and 56.8% overall. There were no significant differences in mean sleep efficiency between participants in the DRL (mean = 81.8%) and BWL groups (mean = 82.8%; P = .62) at baseline. The repeated-measures linear mixed model indicated that the main effect for time was not significant (F3,42 = 0.82; P = .49). The main effect for treatment condition was also not significant (F1,42 = 3.52; P = .07). The time × treatment condition was statistically significant, with sleep efficiency improving more in the BWL condition over time than in the DRL condition (F3,42 = 5.55; P = .003) with a large effect size (partial η2 = 0.28) (see Figure 4). At the end of the intervention and 3 weeks postintervention, sleep efficiency in the BWL condition was in the clinically normal range (mean = 86.06% and 85.77% respectively), but sleep efficiency in the DRL condition remained in the impaired range (mean = 79.35% and 80.88% respectively). In order to inform our understanding of what was driving improvements in sleep efficiency in the BWL group, we undertook a post hoc repeated-measures linear mixed model analysis with time in bed as the outcome variable. The model indicated that neither the main effect for time (F3,42 = 0.09; P = .97) nor treatment condition (F1,42 = 0.60, P = .44) were statistically significant. Time × treatment was also not significant (F3,42 = 0.36, P = .78).
Figure 4. Least squares mean and standard error bars for sleep efficiency over time with mean levels of time in bed (in hours) included for reference.
The repeated-measures linear mixed model showed that the main effect for time was not significant (P = .49). The main effect for treatment condition was also not statistically significant (P = .07). However, the time × treatment condition was statistically significant (P = .003). tib = time in bed in hours.
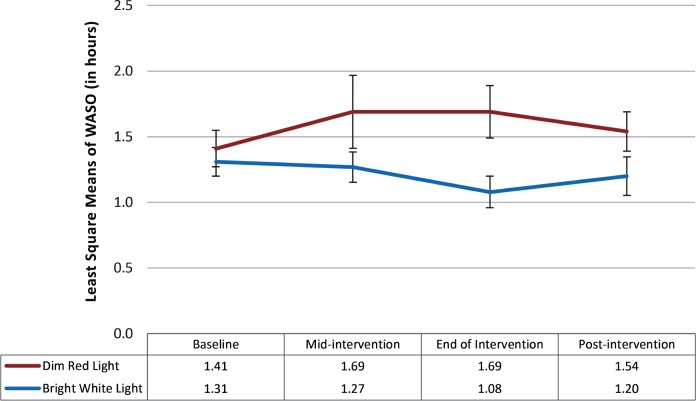
Wake After Sleep Onset
At baseline, there were no significant differences in wake after sleep onset (WASO) between participants in the BWL (mean = 1.41 hours) and DRL groups (mean = 1.31 hours; P = .59). The repeated-measures linear mixed model indicated that the main effect for time was not significant (F3,42 = 0.54; P = .66) and the main effect for treatment condition was also not significant (F1,42 = 3.43; P = .07). The time × treatment condition was not statistically significant (F3,42 = 2.64; P = .06); nevertheless, the effect size was large (partial η2 = 0.16) (see Figure 5).
Figure 5. Least squares mean and standard error bars for WASO over time.
The repeated-measures linear mixed model showed that the main effects for time (P = .66) and for treatment condition (P = .07) were not statistically significant. The time × treatment condition was also not statistically significant (P = .06). WASO = wake after sleep onset.
DISCUSSION
We have previously shown that sLE using bright light in the morning may improve cancer-related fatigue in a mixed group of cancer survivors.15 In this study, we extend these results by showing that sLE using bright light may also have beneficial effects on sleep. In particular, we found statistically significant improvements in sleep efficiency. Average sleep efficiency in the bright light group improved to clinically normal levels on average (> 85%) by the end of the intervention and this improvement remained even 3 weeks afterward. The dim light group remained at low sleep efficiency levels on average for the entire study. Given that time in bed remained consistent across time for both groups, and that there were marginal time × treatment effects favoring the BWL group with respect to total sleep time and WASO, it appears that the favorable improvements to sleep efficiency in the BWL group were not due to reductions in time in bed. Although there were no statistically significant improvements detected in subjective sleep quality or other sleep outcomes, receiving morning BWL conferred greater benefit than morning DRL on sleep quality, total sleep time, and WASO with medium to large effects.
Importantly, despite participants being screened for fatigue, a large proportion of participants evidenced clinically signifi-cant levels of sleep disturbance at baseline (77% had poor sleep quality; 57% had low sleep efficiency) corroborating the view that fatigue and sleep problems in cancer survivors are interrelated symptoms4 and highlighting that interventions to improve fatigue may also have beneficial effects on sleep.
Our findings corroborate studies in non-cancer populations (ie, nursing home residents and elderly patients with dementia) that have shown that sLE using bright light can improve sleep efficiency, reduce nocturnal wake time, and increase nocturnal sleep time.25,26 Moreover, this study highlights the potential for sLE as a treatment for sleep problems in cancer survivors.
Although there were indications of a medium to large effect size difference between groups in change in sleep quality over time favoring the BWL group, average sleep quality remained, in both groups, in the clinically impaired range for the entire study (ie, above 5 on the PSQI). There is research to show that, among older adults of similar age to those in the current sample, perceived sleep quality can be quite different from “objective” reality.27 Hence, it is possible that subjective versus objective measures of sleep assess different aspects of sleep, or that it may take time for individuals to recognize improvements in their own sleep. Indeed, self-reported sleep quality, whether or not there is correlation with actigraph measures, should not be ignored due to known associations with pain, fatigue, distress, and well-being in cancer patients.28,29 Thus, it is important for future sLE research to include both self-report and actigraph measures of sleep and/or to follow up with participants over a longer period of time.
This study is not without limitations. First, it was a secondary analysis of a small sample and therefore we did not adjust for multiple comparisons; the results serve more to inform future larger-scale studies than to provide us with definitive conclusions about the efficacy of sLE using bright light to treat sleep disturbances. Second, the sample was screened for clinical levels of cancer-related fatigue and not for sleep problems. However, it should be noted that at baseline, mean sleep quality for the sample was poor and 77% of the sample exceeded the clinical cutoff. Third, due to the dependent and exploratory nature of these analyses, correction for multiple testing was not undertaken and, thus, these results should be interpreted with caution. Fourth, the sample was too heterogeneous and small for us to be able to determine whether sLE is differentially effective across different disease and treatment groups. Fifth, we did not examine potential underlying mechanisms of the effect of sLE on sleep.
Despite these limitations, this study had a number of important strengths. The sample was diverse with respect to ethnicity and socioeconomic status. Along with the clinical heterogeneity of the sample, this diversity may actually demonstrate the potentially broad reach of systematic light exposure as an effective treatment. Finally, this study demonstrates the promise of sLE as a treatment not only for cancer-related fatigue but also for cancer-related sleep problems that affect a large number of cancer survivors. Additional large-scale studies are necessary.
DISCLOSURE STATEMENT
This work was supported by the National Cancer Institute of the National Institutes of Health (W.H.R., grant# R21CA158954 and K05CA108955; and L.W., grant #5K07CA184145-03). Dr. Amidi's work was supported by the Danish Council of Independent Research (DFF – 5053-00220). Content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. All authors have seen and approved the final version of this manuscript. Dr. Ancoli-Israel is a scientific consultant for Acadia, Eisai, Merck, Perdue, and Pfizer. The remaining authors report no conflicts of interest.
ABBREVIATIONS
- BWL
bright white light
- DRL
dim red light
- FACIT-Fatigue
Functional Assessment of Chronic Illness Therapy – Fatigue
- LED
light emitting diode
- PSQI
Pittsburgh Sleep Quality Index
- sLE
systematic light exposure
- WASO
wake after sleep onset
REFERENCES
- 1.Malone M, Harris AL, Luscombe DK. Assessment of the impact of cancer on work, recreation, home management and sleep using a general health status measure. J R Soc Med. 1994;87(7):386–389. doi: 10.1177/014107689408700705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19(3):895–908. doi: 10.1200/JCO.2001.19.3.895. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JG, Taylor AG. Use of complementary therapies for cancer symptom management: results of the 2007 National Health Interview Survey. J Altern Complement Med. 2012;18(3):235–241. doi: 10.1089/acm.2011.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fiorentino L, Rissling M, Liu L, Ancoli-Israel S. The symptom cluster of sleep, fatigue and depressive symptoms in breast cancer patients: severity of the problem and treatment options. Drug Discov Today Dis Models. 2011;8(4):167–173. doi: 10.1016/j.ddmod.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ancoli-Israel S. Recognition and treatment of sleep disturbances in cancer. J Clin Oncol. 2009;27(35):5864–5866. doi: 10.1200/JCO.2009.24.5993. [DOI] [PubMed] [Google Scholar]
- 6.Fiorentino L, Ancoli-Israel S. Insomnia and its treatment in women with breast cancer. Sleep Med Rev. 2006;10(6):419–429. doi: 10.1016/j.smrv.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiorentino L, Ancoli-Israel S. Sleep dysfunction in patients with cancer. Curr Treat Options Neurol. 2007;9(5):337–346. [PMC free article] [PubMed] [Google Scholar]
- 8.National Institutes of Health. National Institutes of Health state of the science conference statement on manifestations and management of chronic insomnia in adults, June 13-15, 2005. Sleep. 2005;28(9):1049–1057. doi: 10.1093/sleep/28.9.1049. [DOI] [PubMed] [Google Scholar]
- 9.Gotts Z, Deary V, Newton JL, Ellis J. Treatment of insomnia reduces fatigue in chronic fatigue syndrome in those able to comply with the intervention. Fatigue Biomed Heal Behav. 2016;4(4):208–216. [Google Scholar]
- 10.Vandewalle G, Maquet P, Dijk DJ. Light as a modulator of cognitive brain function. Trends Cogn Sci. 2009;13(10):429–438. doi: 10.1016/j.tics.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Monk TH. Sleep and circadian rhythms. Exp Gerontol. 1991;26(2-3):233–243. doi: 10.1016/0531-5565(91)90015-e. [DOI] [PubMed] [Google Scholar]
- 12.Chong MS, Tan KT, Tay L, Wong YM, Ancoli-Israel S. Bright light therapy as part of a multicomponent management program improves sleep and functional outcomes in delirious older hospitalized adults. Clin Interv Aging. 2013;8:565–572. doi: 10.2147/CIA.S44926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neikrug AB, Rissling M, Trofimenko V, et al. Bright light therapy protects women from circadian rhythm desynchronization during chemotherapy for breast cancer. Behav Sleep Med. 2012;10(3):202–216. doi: 10.1080/15402002.2011.634940. [DOI] [PubMed] [Google Scholar]
- 14.Ancoli-Israel S, Rissling M, Neikrug A, et al. Light treatment prevents fatigue in women undergoing chemotherapy for breast cancer. Support Care Cancer. 2012;20(6):1211–1219. doi: 10.1007/s00520-011-1203-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redd WH, Valdimarsdottir H, Wu LM, et al. Systematic light exposure in the treatment of cancer-related fatigue: A preliminary study. Psychooncology. 2014;23(12):1431–1434. doi: 10.1002/pon.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desan PH, Weinstein AJ, Michalak EE, et al. A controlled trial of the Litebook light-emitting diode (LED) light therapy device for treatment of Seasonal Affective Disorder (SAD) BMC Psychiatry. 2007;7(1):38. doi: 10.1186/1471-244X-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith E, Lai JS, Cella D. Building a measure of fatigue: the functional assessment of Chronic Illness Therapy Fatigue Scale. PM R. 2010;2(5):359–363. doi: 10.1016/j.pmrj.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94(2):528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 19.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 20.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 21.Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev. 2016;25:52–73. doi: 10.1016/j.smrv.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Ancoli-Israel S, Liu L, Marler MR, et al. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer. 2006;14(3):201–209. doi: 10.1007/s00520-005-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ancoli-Israel S, Liu L, Rissling M, et al. Sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer before and after treatment: a 1-year longitudinal study. Support Care Cancer. 2014;22(9):2535–2545. doi: 10.1007/s00520-014-2204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen J. Statistical Power Analysis for the Behavioural Sciences. 2nd ed. New York, NY: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 25.Fetveit A, Bjorvatn B. The effects of bright-light therapy on actigraphical measured sleep last for several weeks post-treatment. A study in a nursing home population. J Sleep Res. 2004;13(2):153–158. doi: 10.1111/j.1365-2869.2004.00396.x. [DOI] [PubMed] [Google Scholar]
- 26.Mishima K, Okawa M, Hishikawa Y, Hozumi S, Hori H, Takahashi K. Morning bright light therapy for sleep and behavior disorders in elderly patients with dementia. Acta Psychiatr Scand. 1994;89(1):1–7. doi: 10.1111/j.1600-0447.1994.tb01477.x. [DOI] [PubMed] [Google Scholar]
- 27.Landry GJ, Best JR, Liu-Ambrose T. Measuring sleep quality in older adults: a comparison using subjective and objective methods. Front Aging Neurosci. 2015;7:166. doi: 10.3389/fnagi.2015.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delgado-Guay M, Yennurajalingam S, Parsons H, Palmer JL, Bruera E. Association between self-reported sleep disturbance and other symptoms in patients with advanced cancer. J Pain Symptom Manage. 2011;41(5):819–827. doi: 10.1016/j.jpainsymman.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Rissling M, Natarajan L, et al. The longitudinal relationship between fatigue and sleep in breast cancer patients undergoing chemotherapy. Sleep. 2012;35(2):237–245. doi: 10.5665/sleep.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]