Abstract
Study Objectives:
Few studies have addressed dreaming in patients with sleep apnea. We hypothesized that respiratory events and subsequent oxygen desaturation act as an important physiological trigger and may thus influence dream content in patients with a sleep-related breathing disorder.
Methods:
Seventy-six patients (28 women, mean age 54 years, range 20–82) who underwent polysomnography because of suspected sleep apnea participated in this study. Dream reports and dream questionnaires were collected immediately after first morning awakening, at 5:30 AM, at the sleep laboratory. Dream content analysis with respect to possible respiratory-related content was performed. Patients were stratified into primary snoring, mild, moderate, and severe sleep apnea groups.
Results:
In 63 patients sleep apnea was diagnosed (mild n = 31, 49.2%, moderate n = 13, 20.6%, severe n = 19, 30.2%), and 13 subjects in whom a sleep-related breathing disorder was not confirmed were included as a control group with primary snoring. There was no significant difference in respiratory-related dream topics between patients and controls. Also, no influence of respiratory parameters measured during polysomnography on dream content was detectable.
Conclusions:
We failed to detect a difference in dream content between patients with sleep apnea and controls. Further studies are required to determine whether these results indicate that the incorporation of respiratory events into dreams is absent in patients with sleep apnea or represents a bias due to the collection of dream content in the early morning hours.
Citation:
Di Pauli F, Stefani A, Holzknecht E, Brandauer E, Mitterling T, Holzinger B, Högl B. Dream content in patients with sleep apnea: a prospective sleep laboratory study. J Clin Sleep Med. 2018;14(1):41–46.
Keywords: CPAP, dreaming, polysomnography, REM sleep, sleep, sleep apnea
BRIEF SUMMARY
Current Knowledge/Study Rationale: Data on the influence of sleep apnea on dreaming are rare. We hypothesized that respiratory events in patients with a sleep-related breathing disorder may influence dream content.
Study Impact: In this study, there was no significant incorporation of potential respiratory-related dream content in subjects with sleep apnea compared to controls. Further studies are needed to determine if this indicates that sleep-related breathing disorders do not influence dreaming or if these results are due to the collection of dream content after awakening.
INTRODUCTION
Previous studies have indicated that dream content can be modified by exposure to stimuli during rapid eye movement (REM) sleep.1 External auditory, sensory, or olfactory stimuli during REM sleep have been well studied and an effect on dream content has consistently been shown.2–4 However, less is known about internal stimuli and their influence on the content of dreams; one study has shown that thirst, an essential physiological stimulus, was associated with dream content, because beverage and water themes were found more often in thirsty subjects.5
Few previous studies have addressed dream content in patients with sleep apnea. In a historic study published in 1965, recall of potentially respiratory-related dream content—defined as active speech, laughter, crying, smoking, and choking— seemed to occur significantly more often after awakenings following a short breathing pause than after awakenings following normal breathing.6
More recent evidence for the influence of respiratory events on dreams comes from a study comparing dream content in 33 patients with sleep apnea in nights with and without continuous positive airway pressure (CPAP) treatment. Sixteen patients were treated with CPAP during the first night, whereas the remaining 17 were treated during the second night. Negative dream emotions were increased during untreated nights with apneic episodes.7 Additionally, a prospective study showed that in patients with sleep apnea, an apnea-hypopnea index (AHI) ≥ 15 events/h was associated with emotionally negative dreams, although the variability of dream emotions significantly decreased with increasing AHI.8 In a population survey, subjects with nightmares were reported to suffer more frequently from snoring, interrupted sleep, and daytime somnolence, which are signs of sleep apnea.9
In contrast, Schredl et al. reported that nightmare frequencies were comparable between patients with sleep apnea and healthy controls.10 Furthermore, respiratory parameters did not correlate with occurrence of nightmares in this population. Indeed, an earlier study found a correlation between a higher respiratory disturbance index and dreams with less bizarre and more realistic content.11 Overall, there is evidence of a possible influence of sleep apnea on dream content. Based on these studies and on our own anecdotal clinical experience of patients with obstructive sleep apnea (OSA) spontaneously reporting dream content potentially related to sleep apnea such as suffocating or drowning, we hypothesized that apnea or oxygen desaturation act as an important physiological trigger influencing dream content. The severity of oxygen saturation and number of apneas or hypopneas may increase the frequency and change the incorporation of potential respiratory-related dream content. However, in the aforementioned study cohort, dream analysis showed that potential breathing-related topics were very seldom reported. In line with this, some evidence of respiratory-related dream content has been described in single studies, but was not found to be statistically significant.12 Indeed, incorporation of potential respiratory-related dream content in these small studies was not sufficiently addressed, and negative findings could be related to late dream collection.
In this study, we therefore addressed specific respiratory-related dream content in patients with and without sleep apnea and compared different groups of patients who were stratified for sleep apnea severity in a prospective polysomnographic study.
METHODS
Subjects
Between October 2014 and June 2015, subjects undergoing polysomnography (PSG) for suspected sleep apnea (after clinical evaluation by a neurologist experienced in sleep medicine at the outpatient clinic) at the Sleep Disorders Unit, Department of Neurology, of the Medical University of Innsbruck, were consecutively recruited for this study. Inclusion and exclusion criteria were assessed by chart review and by clinical interview. Subjects were classified as having primary snoring or relevant sleep apnea, and were stratified according to severity (mild AHI 5 to 14 events/h, moderate AHI 15 to 29 events/h, severe AHI > 30 events/h) and whether they had OSA, central sleep apnea (CSA), or mixed sleep apnea (MSA).
Patients suffering from psychiatric disorders (eg, depression) or any disease that could affect dream recall (eg, parasomnia, circadian rhythm disorders, neurodegenerative disease), or who were taking medication that could affect dream recall (eg, hypnotics) were excluded from the study. The Ethics Committee of Innsbruck Medical University approved the study, and the participants' written informed consent was obtained according to the Declaration of Helsinki.
Procedures
At admission, demographic data, Epworth Sleepiness Scale, and Beck Depression Inventory scores were recorded. PSG was performed according to current standards13,14 between 10:00 PM and 5:30 AM. Respiratory events were scored according to the current American Academy of Sleep Medicine criteria.14,15 A sleep medicine expert with board certification scored the PSG data. Patients were awakened at 5:30 AM and asked about their dreams. The time of 5:30 AM was selected to ensure that all included patients were asleep at the wake-up time point and in order to avoid interference with later sleep laboratory procedures. Online PSG scoring to ensure that patients were awaken during REM sleep was not performed. Dream data were collected by one author (EH). Reports were recorded and later literally transcribed. Afterward, patients completed a dream questionnaire concerning their usual dream content.
Because of sleep laboratory procedures (eg, beginning treatment in the second night for those with sleep apnea), all patients were interviewed after the first night. Dream reports and questionnaires were gathered before the PSG data were analyzed and before respiratory parameters were known, so both patients and the examiner were blinded to the final diagnoses.
Dream Report Analysis
Words of dream reports were counted and compared between different groups. A psychologist and dreaming expert (BH) blinded for patients' diagnoses analyzed the free-worded dream reports regarding topics indicating sleep apnea. Reports were classified into “no,” “possible” or “likely” respiratory-related dream content. Examples for likely respiratory-related dream content were: “head under water,” “get no air,” “cannot breathe”; for possible respiratory-related dream content: “to be locked up.”
Dream Questionnaire
Comprehensive instructions on how to correctly complete the questionnaire were provided. The dream questionnaire is a standardized questionnaire consisting of 19 items focusing on frequency of dreaming, dream content, and specific actions occurring in the dream (Dreamland questionnaire16). For this study, specific questions possibly related to respiratory dream content were added: feeling like not getting enough air, feeling of being choked, breathlessness, feeling of suffocation, feeling of choking on something, wheezing, being under water, drowning, being in a closed space.
Statistical Analysis
The statistical analyses were performed with SPSS 23 (IBM Corp., Armonk, New York, United States). Data were tested for normal distribution using the Kolmogorov-Smirnov test. Due to non-normal distribution, nonparametric statistics were used (Mann-Whitney U test for two unrelated variables, Wilcoxon test for two related variables). Median scores and interquartile ranges are reported for demographic and polysomnographic data.
RESULTS
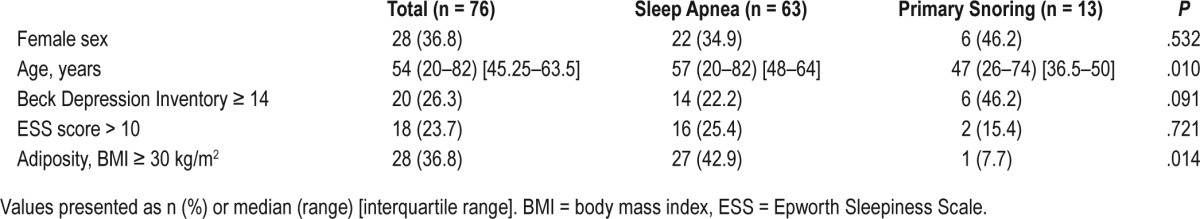
Seventy-six subjects with suspected sleep apnea were included in the study. Sixty-three patients fulfilled the criteria for sleep apnea according to the International Classification of Sleep Disorders, Third Edition. The remaining 13 subjects received a diagnosis of primary snoring. There were no significant sex differences between patients with sleep apnea and primary snoring. Patients with sleep apnea were older than those with primary snoring with a median age of 57 years versus 47 years, respectively (P = .010), and were more often obese (body mass index ≥ 30 kg/m2, 42.9% versus 7.7%, P = .014) (Table 1). Frequency of sleepiness according to the Epworth Sleepiness Scale (score > 10; sleep apnea n = 22, 34.9%; primary snoring n = 6, 46.2%) and frequency of depression measured by the Beck Depression Inventory were equal in both groups (P = .091, P = .721, respectively).
Table 1.
Demographic data.
Most patients (n = 48, 63.1%) received a diagnosis of OSA, 14 were diagnosed with MSA and 1 with CSA (Table 2).
Table 2.
Polysomnographic data.
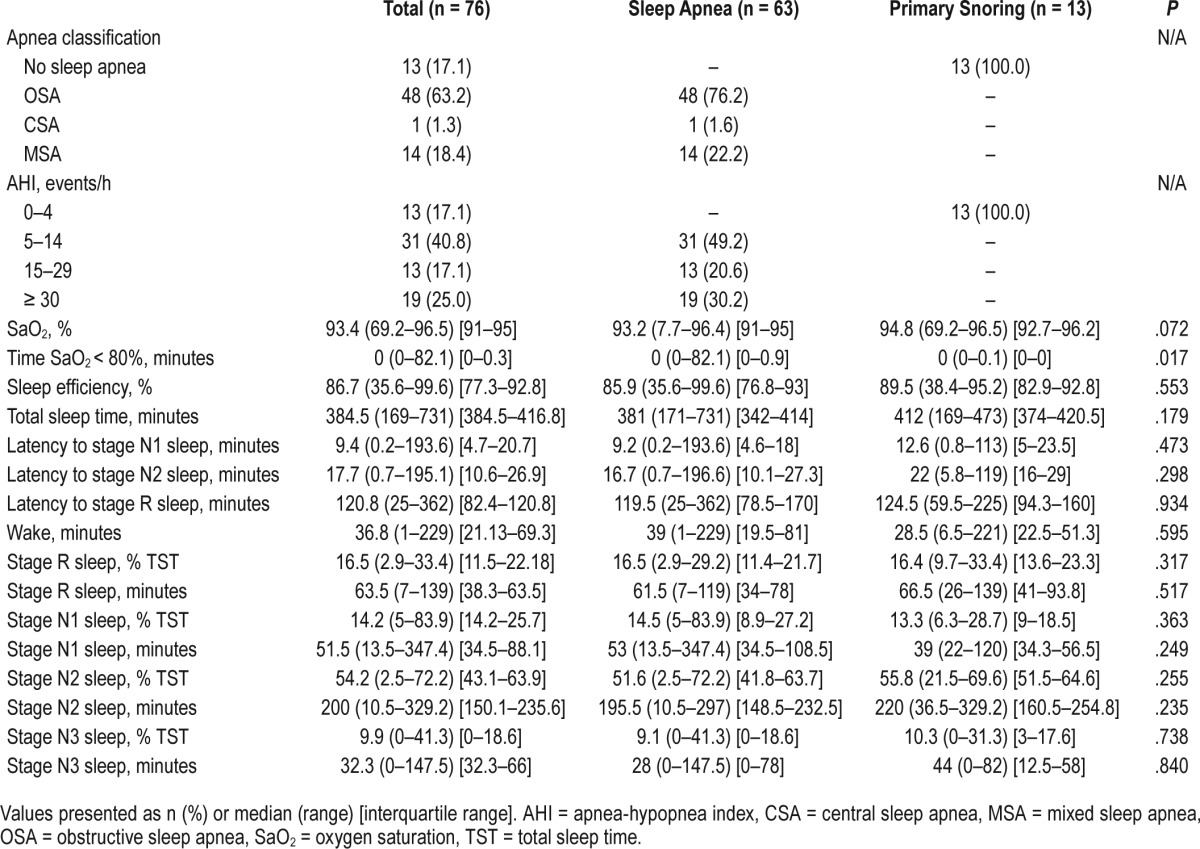
For dream content analyses, patients were stratified according to sleep apnea severity. Most patients had mild (n = 31, 49.2%), 13 (20.6%) had moderate, and 19 (30.2%) had severe sleep apnea. Polysomnographic and respiratory parameters are shown in Table 2. Only 22.4% of the awakenings occurred during REM sleep. There was a nonsignifi-cant trend toward lower REM sleep duration in patients with sleep apnea.
Thirty-three subjects (52.4%) with sleep apnea and 7 controls (46.4%) with primary snoring reported that they had dreamed the previous night. Of these, 82.5% were able to provide a dream report.
The word count of dream reports from the preceding night did not differ significantly between patients with sleep apnea and the group with primary snoring (Table 3).
Table 3.
Respiratory-related dream content.
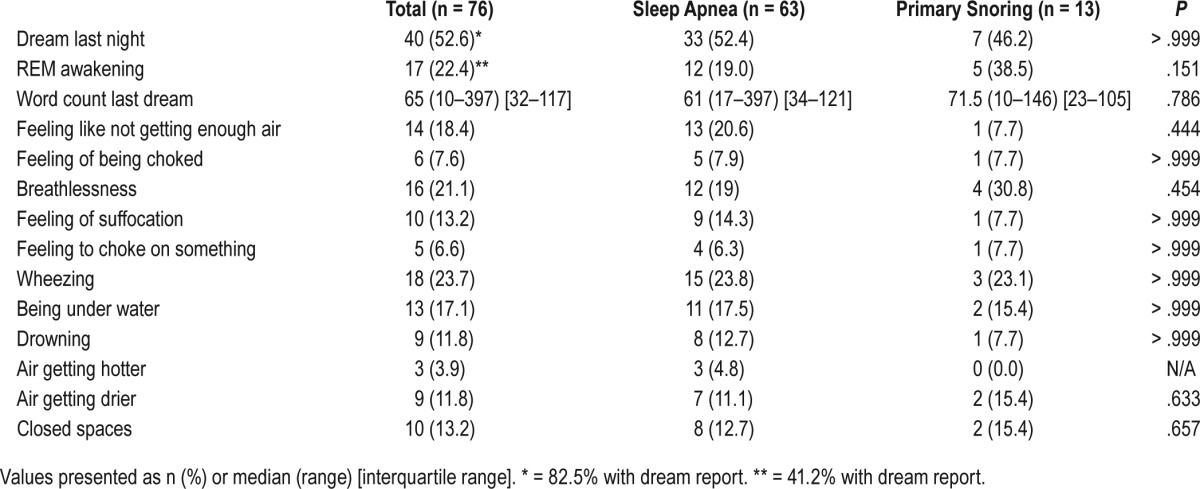
Blinded evaluation of dream reports from the PSG night by an external expert (BH) found no evidence for possibly respiratory-related dream content in 26 patients (76.5%), possible evidence in 8 (23.5%), and likely evidence in 1 (2.9%). This classification could not predict sleep apnea diagnosis.
In the structured questionnaire, the additional questions about possible respiratory-related dream content items added for this study did not show significant differences in patients with sleep apnea and primary snoring (Table 3, all Ps > .05) when comparing single questions. Also, a comparison of subjects with one or more affirmative answers versus subjects who answered all questions negatively was not significant. Only 3 participants complained of nightmares (data not shown), and there was no difference between the sleep apnea and primary snoring groups.
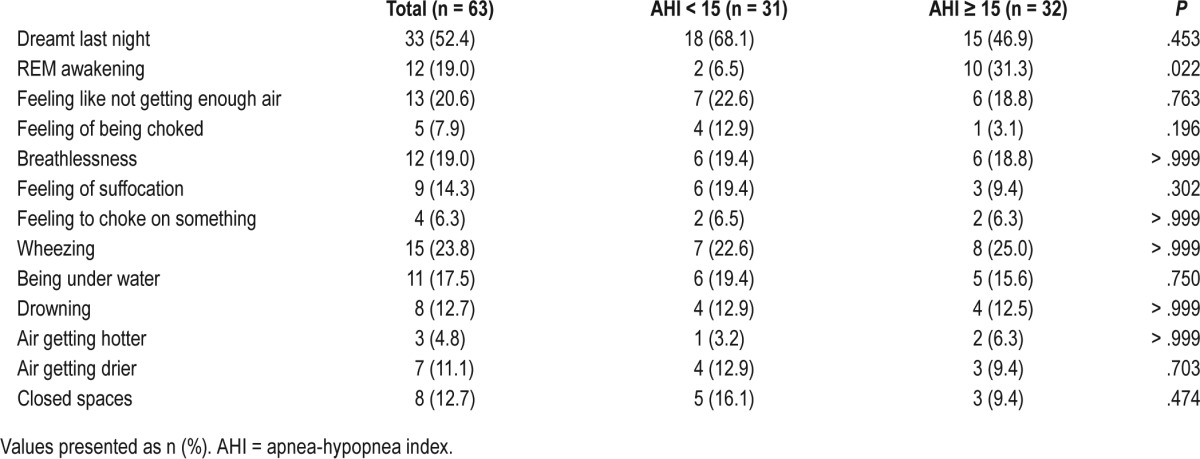
Furthermore, dream content data were compared between patients with different sleep apnea severities: patients with an AHI < 15 events/h were compared to patients with an AHI ≥ 15 events/h and patients with an AHI < 30 events/h to patients with an AHI ≥ 30 events/h. There were no statistical differences regarding dream content in the compared groups (Table 4, AHI < 15 events/h compared with AHI ≥ 15 events/h, further data not shown).
Table 4.
Respiratory-related dream content in patients according to sleep apnea severity.
DISCUSSION
Although some previous studies have reported altered dream content in patients with sleep apnea, in the current prospective study with systematic awakenings after a PSG recording, no significant incorporation of potential respiratory-related dream content was found in subjects with sleep apnea compared to controls.
Few other studies have addressed dream content in patients with respiratory disturbances during sleep. One study compared dreams after REM awakening on nights with and without CPAP treatment in 33 patients with a sleep-related breathing disorder (SRBD) who had undergone at least 2 months of CPAP. Dreams on nights without CPAP were more emotionally negatively compared to dreams from nights with CPAP. Consistent with our results, respiratory-related dream content was not found to be more frequent during treated nights compared to nights with apnea.7 Our data are also in line with those reported by Schredl et al., who did not find an incorporation of breathing-related themes into dreams in patients with a SRBD. Forty-four REM and NREM dreams were analyzed and correlated with respiratory parameters. Among other themes, feelings of being overwhelmed appeared in a few dream reports, but were not associated with the respiratory disturbance index or minimal blood oxygen saturation.11 In this study, a high respiratory disturbance index was related to a marked reduction of dream bizarreness. However, unpleasant dreams were increased in patients with an AHI > 15 events/h, although there was a decrease in emotional variability.8 Breathing-related dream content was not directly addressed in this study. Despite significant oxygen desaturation in patients with an SRBD and a clear association of oxygen desaturation with disease severity in our study, no differences in subgroup analysis according to disease severity and respiratory-related dream content were detected. One possible reason is the development of sleep apnea over a long period and habituation of patients to lower blood oxygen and hypercapnic episodes.
In addition to dream content, it was hypothesized that oxygen desaturation can induce nightmares. In previous studies, it has been hypothesized that oxygen desaturation can induce nightmares. In this study, the occurrence of nightmares was recorded, but they were not analyzed in detail. Only 3 patients complained of nightmares and there were no differences between patients with sleep apnea and controls. Due to the small number of nightmares our analysis is limited, and published data are inconsistent. Whereas Schredl et al.10 found no correlation between respiratory parameters and a higher frequency of nightmares, a study by BaHammam et al.17 found nightmares were associated with a higher REM-AHI, and CPAP treatment was found to decrease the occurrence of nightmares. In contrast, a retrospective study with 393 patients showed lower frequencies of nightmares in patients with OSA and higher AHI.18
The major strength of our study is the well-described cohort with exact AHI and controlled dream assessment immediately after awakening from PSG, because dream recall strongly depends on the time elapsed between awakening and dream report. A potential limitation is that only 53% of our patients reported having had a dream during the night they underwent PSG and only 22% of the awakenings were during REM sleep. Dreams can occur both during NREM and REM awakenings, but are quantitatively and qualitatively different (eg, REM dreams are more bizarre19,20). The fact that only a minor part of the awakenings in this study occurred out of REM sleep may have reduced the probability of dream recall. The early awakening at 5:30 AM was chosen in order to avoid interference with later sleep laboratory procedures, but many patients had not yet entered their final REM episode. It is well known that the first-night effect results in the reduction of REM duration and longer REM latency.21 An influence on dream recall cannot be excluded, but would equally affect all study groups, so we do not believe that it had an effect on our study outcome. Another limitation of our and other studies concerns the lack of standardized dream analysis with respiratory content; therefore, definitions of breathing-related dream content are heterogeneous in the different studies. In our study, only one blinded rater analyzed dream content regarding “no,” “possible” or “likely” respiratory-related dream content. Therefore, no interrater reliability could be determined. A further limitation of this study was the small sample size that resulted from a relatively low number of dream recall in the included patients.
In conclusion, this study showed no incorporation of respiratory-related content in dreams of patients with sleep apnea. Breathing-related content was not increased in those with higher oxygen desaturation.
DISCLOSURE STATEMENT
Work for this study was performed at the Clinical Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria. The authors report no conflicts of interest. All authors declare that they have substantially participated in the work on the manuscript and have taken due care regarding their contribution to ensure the integrity of the work. All authors have seen and approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Heinz Hackner for his excellent technical performance of the polysomnography tests.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CSA
central sleep apnea
- MSA
mixed sleep apnea
- NREM
non-rapid eye movement
- OSA
obstructive sleep apnea
- PSG
polysomnography
- REM
rapid eye movement
- SRBD
sleep-related breathing disorder
REFERENCES
- 1.Eiser AS. Physiology and psychology of dreams. Semi Neurol. 2005;25(1):97–105. doi: 10.1055/s-2005-867078. [DOI] [PubMed] [Google Scholar]
- 2.Schredl M, Atanasova D, Hormann K, Maurer JT, Hummel T, Stuck BA. Information processing during sleep: the effect of olfactory stimuli on dream content and dream emotions. J Sleep Res. 2009;18(3):285–290. doi: 10.1111/j.1365-2869.2009.00737.x. [DOI] [PubMed] [Google Scholar]
- 3.Wollman MC, Antrobus JS. Sleeping and waking thought: effects of external stimulation. Sleep. 1986;9(3):438–448. doi: 10.1093/sleep/9.3.438. [DOI] [PubMed] [Google Scholar]
- 4.Trotter K, Dallas K, Verdone P. Olfactory stimuli and their effects on REM dreams. Psychiatr J Univ Ott. 1988;13(2):94–96. [PubMed] [Google Scholar]
- 5.Bokert EG. The Effect of Thirst and a Related Verbal Stimulus on Dream Reports [dissertation] New York: New York University; 1967. [Google Scholar]
- 6.Hobson JA, Goldfrank F, Snyder F. Respiration and mental activity in sleep. J Psychiatr Res. 1965;3(2):79–90. doi: 10.1016/0022-3956(65)90017-8. [DOI] [PubMed] [Google Scholar]
- 7.Gross M, Lavie P. Dreams in sleep apnea patients. Dreaming. 1994;4(3):195–204. [Google Scholar]
- 8.Fisher S, Lewis KE, Bartle I, Ghosal R, Davies L, Blagrove M. Emotional content of dreams in obstructive sleep apnea hypopnea syndrome patients and sleepy snorers attending a sleep-disordered breathing clinic. J Clin Sleep Med. 2011;7(1):69–74. [PMC free article] [PubMed] [Google Scholar]
- 9.Stepansky R, Holzinger B, Schmeiser-Rieder A, Saletu B, Kunze M, Zeitlhofer J. Austrian dream behavior: results of a representative population survey. Dreaming. 1998;8(1):23–30. [Google Scholar]
- 10.Schredl M, Schmitt J, Hein G, Schmoll T, Eller S, Haaf J. Nightmares and oxygen desaturations: is sleep apnea related to heightened nightmare frequency? Sleep Breath. 2006;10(4):203–209. doi: 10.1007/s11325-006-0076-8. [DOI] [PubMed] [Google Scholar]
- 11.Schredl M, Kraft-Schneider B, Kröger H, Heuser I. Dream content of patients with sleep apnea. Somnologie. 1999;3:319–323. [Google Scholar]
- 12.Schredl M. Dreams in patients with sleep disorders. Sleep Med Rev. 2009;13(3):215–221. doi: 10.1016/j.smrv.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Practice parameters for the indications for polysomnography and related procedures. Polysomnography Task Force, American Sleep Disorders Association Standards of Practice Committee. Sleep. 1997;20(6):406–422. [PubMed] [Google Scholar]
- 14.Berry RB, Brooks R, Gamaldo CE, et al. for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Darien, IL: American Academy of Sleep Medicine; 2015. Version 2.2. [Google Scholar]
- 15.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klösch G, Holzinger B. Dream content analysis: methodological and theoretical approaches. Psychotherapie Forum. 2014;19:121–129. [Google Scholar]
- 17.BaHammam AS, Al-Shimemeri SA, Salama RI, Sharif MM. Clinical and polysomnographic characteristics and response to continuous positive airway pressure therapy in obstructive sleep apnea patients with nightmares. Sleep Med. 2013;14(2):149–154. doi: 10.1016/j.sleep.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Pagel JF, Kwiatkowski C. The nightmares of sleep apnea: nightmare frequency declines with increasing apnea hypopnea index. J Clin Sleep Med. 2010;6(1):69–73. [PMC free article] [PubMed] [Google Scholar]
- 19.Hobson JA, Pace-Schott EF, Stickgold R. Dreaming and the brain: toward a cognitive neuroscience of conscious states. Behav Brain Sci. 2000;23(6):793–842. doi: 10.1017/s0140525x00003976. discussion 904-1121. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen TA. A review of mentation in REM and NREM sleep: “covert” REM sleep as a possible reconciliation of two opposing models. Behav Brain Sci. 2000;23(6):851–866. doi: 10.1017/s0140525x0000399x. discussion 904-1121. [DOI] [PubMed] [Google Scholar]
- 21.Agnew HW, Jr, Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology. 1966;2(3):263–266. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]


