Abstract
Study Objectives:
The degree of neurobehavioral impairment and treatment response in mild-moderate obstructive sleep apnea (OSA) compared to that of an appropriate control group are unclear. This study compared neurobehavioral function and response to continuous positive airway pressure (CPAP) treatment in patients with mild to moderate OSA with those of a non-sleep apneic community sample of similar demography.
Methods:
One hundred ten patients with OSA and 31 asymptomatic community dwellers underwent overnight polysomnography and neurobehavioral testing. Participants with OSA (n = 88) were treated with CPAP for 3 months, and repeat evaluations were performed at the end of the treatment period.
Results:
Compared to the community sample, participants with OSA were significantly sleepier, had impaired mood and quality of life, and showed decrements in neuropsychological function, specifically psychomotor function, working memory and vigilance. Some neuropsychological and mood outcomes were normalized with CPAP, but significant decrements persisted in most outcomes even in those participants with adequate device usage.
Conclusions:
Patients with mild to moderate OSA have significant neurobehavioral morbidity. During “gold standard” treatment, normal function was not achieved, even with adequate device usage. CPAP efficacy for improving sleepiness and neuropsychological function in this milder end of the OSA spectrum may be poor, which may affect CPAP adherence. These findings suggest that there may be neurological changes related to OSA that do not respond to CPAP treatment, the etiology of which requires further investigation.
Citation:
Jackson ML, McEvoy RD, Banks S, Barnes M. Neurobehavioral impairment and CPAP treatment response in mild-moderate obstructive sleep apnea. J Clin Sleep Med. 2018;14(1):47–56.
Keywords: continuous positive airway pressure, neuropsychological function, obstructive sleep apnea, quality of life, sleepiness
BRIEF SUMMARY
Current Knowledge/Study Rationale: This is the largest study to compare neurobehavioral function in patients with mild to moderate OSA to an asymptomatic community sample, and to examine the dose-response relationship between CPAP use and neurobehavioral function.
Study Impact: We have demonstrated that despite significant morbidity in this patient group, the best treatment that we currently have does not return all aspects of neuropsychological function, quality of life, or mood to the functional level of community dwellers of a similar demography, nor is there a dose-response relationship between CPAP use and recovery of function in this milder end of the OSA spectrum.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common condition affecting at least 3% adult females and 10% adult males.1 Some studies have shown that patients with moderate to severe OSA (apnea-hypopnea index [AHI] > 15) had significant impairment of neurobehavioral function including daytime sleepiness, impaired neuropsychological function,2–6 and reduced quality of life,7 which responded to nasal continuous positive airway pressure (CPAP)8–11 and oral appliance12,13 therapy. However, the largest randomized controlled study to date of CPAP versus sham CPAP treatment showed an improvement in subjective and objectively measured sleepiness in patients with moderate to severe OSA but no improvement in any domain of neuropsychological function after 6 months of treatment.8 Others have shown that although neurobehavioral impairment is improved by CPAP treatment, neurobehavioral function may not normalize, even following optimal treatment.14,15 Thus, important questions are raised concerning the effects of OSA on neurobehavioral function and to what extent this is independent of comorbid conditions.
The degree of neurobehavioral impairment in the more numerous group of patients with mild to moderate OSA has been even less well documented. In a study of patients with a wide range of OSA severity and free of major comorbidi-ties and medications, respiratory disturbance index appeared to be independently related to neuropsychological function.16 Because no threshold effect could be identified the authors concluded that even mild-moderate OSA may have a negative effect on neuropsychological function. Others have reported improvements in neurobehavioral dysfunction,16–18 including daytime sleepiness, in mild-moderate OSA with CPAP17–21 and oral appliance17,22 treatment. However, treatment effects appear to be small23 and in none of these studies were baseline or posttreatment results compared directly with a control group. Furthermore, the placebo treatment effect on neuropsychological function appears to be marked in this group.24
Thus, important questions remain as to whether neuropsychological function is actually impaired in patients with mild-moderate OSA, and if so, to what extent this can be attributed to OSA versus comorbid conditions (eg, obesity, depression, cardiometabolic diseases). Also, it is not well understood how CPAP treatment effects compare with those of a placebo intervention, whether there is a “dose-response” effect of increasing levels of CPAP adherence, and if CPAP treatment can be expected to normalize neuropsychological function. To answer these questions, we have examined the neurobehavioral morbidity and the dose-response effect of CPAP versus a placebo pill in a group of participants with mild to moderate OSA and compared the results to those of a healthy, community dwelling group asymptomatic for OSA. These data were collected as part of a large placebo-controlled trial looking at the responses to CPAP and mandibular advancement splint (MAS) in mild to moderate OSA. The intention-to-treat treatment response data of the participants with OSA have been published previously17 and showed that CPAP was more effective, albeit less utilized, than MAS. Therefore, we report here only the CPAP and placebo response; the contemporaneously collected community sample data have not previously been reported.
METHODS
Two Australian centers (Institute for Breathing and Sleep, Austin Health, Melbourne, and the Adelaide Institute for Sleep Health, Repatriation General Hospital, Adelaide) participated in this study. Results of 110 participants with OSA were compared with a group of 31 community dwellers who had no symptoms of sleep-disordered breathing and no significant medical, psychological, or neurological morbidities. Approvals were obtained from both hospitals' Human Research Ethics Committees.
Participant Selection
Participants with OSA were recruited following their first overnight in-laboratory diagnostic sleep study for suspected OSA. They were eligible if they were older than 18 years and had an AHI between 5 and 30.20 Each diagnostic polysomnography (PSG) study required at least 4 hours of sleep, at least 30 minutes of sleep in the supine position, and at least 30 minutes of rapid eye movement (REM) sleep, and was manually scored as previously described.20 Patients with minimum blood oxygen saturation less than 75% in REM sleep and 80% in non-rapid eye movement sleep were excluded, as were patients with clinically significant coexisting disease (eg, diabetes, unstable ischemic heart disease) or sleepiness deemed to be unsafe and requiring urgent treatment (eg, history of falling asleep while driving or working, or in some other unsafe situation). To further exclude comorbidities, a clinical examination, full blood cell count, electrolytes, renal function, fasting blood glucose, and liver function tests were performed. Patients with controlled hypertension and those who had a myocardial infarct at least 6 months prior to the study, without change to their medication for at least 30 days prior to study inclusion, were included in the study. To ensure valid interpretation of the neurobehavioral tests, patients were required to be fluent in English and to have no history of cerebrovascular disease, closed head injury associated with loss of consciousness greater than 15 minutes in duration, stroke, current psychiatric illness, or alcohol or drug abuse.
A healthy community sample with a demographic similar to that of our participants with OSA, (ie, predominantly middle-aged males) was recruited by an independent recruitment agency using a standardized phone interview script and a computer program to randomly select telephone numbers from the telephone book within a 30-km radius of the two research centers. The same inclusions and exclusions were used for the community sample as the participants with OSA regarding medical history. In addition, the community dwellers were excluded if they had significant sleep comorbidities (eg, snoring, insomnia); if they were younger than 30 years or older than 70 years; if English was not their first language; if they were heard to snore more than 1 night/wk; or if they were on any medications for respiratory, cardiovascular, or psychiatric disorders. The selection of healthy community dwellers was based primarily on the sleep and medical history. However, healthy participants who were subsequently found on PSG to have an AHI > 10 were excluded from further evaluation to ensure a comparator population that was as free as reasonable from any clinically relevant sleep disorder.
Study Design and Outcomes (Figure 1)
Figure 1. Progress of participants through the study.
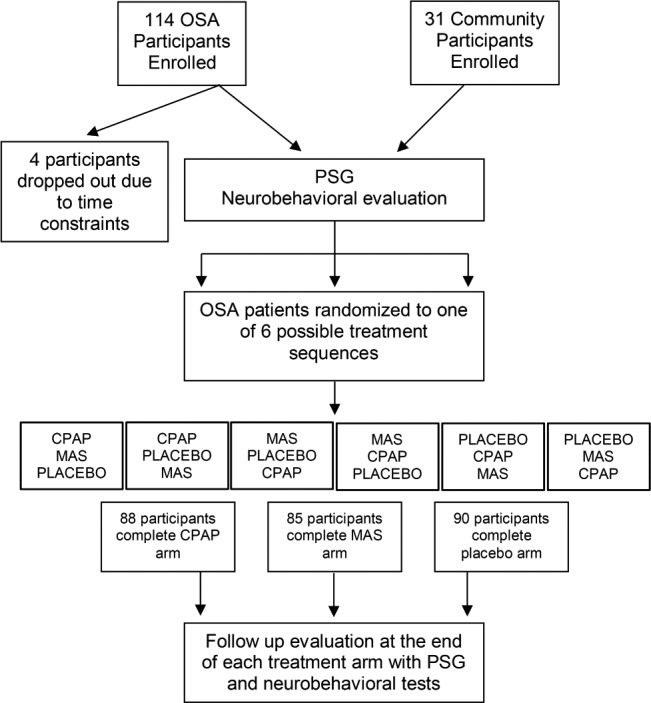
Only participants who completed the CPAP arm are included in the current analysis. CPAP = continuous positive airway pressure, MAS = mandibular advancement splint, OSA = obstructive sleep apnea, PSG = polysomnography.
Following enrollment, all participants underwent baseline testing with overnight PSG followed by comprehensive neurobehavioral testing the next morning. This included standardized measures of subjective and objective daytime sleepiness, mood, quality of life, and neuropsychological function, including memory, information processing and mental flexibility, visual-motor tracking and set shifting (see supplemental material). Height and weight, and neck, waist, and hip circumferences were recorded for each participant. PSG (including analysis and scoring definition) was performed as previously described.17 Interscorer and intrascorer reliability were measured using intraclass correlation coefficients and paired t tests, which were within acceptable published limits.25 All participants were asked to keep a sleep diary for 1 week prior to each session, and to maintain their typical sleep-wake routine during that week.
Participants with OSA were treated for 3 months with each CPAP, MAS and an oral placebo pill in randomized order, with a 2-week washout period between each treatment period. Participants were told that the pill was intended to improve airway function during sleep and were instructed to take it immediately prior to going to bed. Testing was repeated at the end of each treatment period. Our previous study found no order effect of the treatments.17 Only the data from the post-CPAP treatment period and from the placebo condition (n = 84) is included in the current analysis.
Data Analysis and Statistics
Statistical analyses were conducted using SPSS version 22.0 (IBM Corp., Armonk, New York, United States). Baseline differences in neurobehavioral function between participants with OSA (n = 110) and the community sample (n = 31) were analyzed using univariate analysis of variance (ANOVA), controlling for premorbid intelligence, age, and body mass index (BMI) for the neuropsychological measures. To assess the efficacy of CPAP treatment on neurobehavioral function, participants with OSA who completed CPAP treatment (n = 88) were compared to the community sample using univariate ANOVA, controlling for premorbid intelligence, age and BMI for the neuropsychological measures. The CPAP group was then classified according to adequacy of CPAP use, arbitrarily defined as 4 h/night for 70% nights.26 Neurobehavioral function was compared between CPAP users (n = 38) and inadequate users (n = 50), and CPAP users and the community group, using independent samples t tests. To assess the effect of different levels of CPAP use on neurobehavioral function, all participants with OSA were categorized into quartiles according to adequacy of CPAP treatment usage (≤ 0.94, 0.95–3.99, 4.00–5.79, ≥ 5.80 h/night). Mixed-model ANOVAs were conducted to assess differences in mean scores in clinical outcomes across categories of CPAP usage, with post hoc analyses conducted where significant group differences were found. Neurobehavioral function of each CPAP usage category was then compared to their own placebo condition using paired-samples t tests. Three participants did not complete follow-up assessments, leaving n = 85 for all CPAP analyses. Results are given as mean ± standard error of the mean unless otherwise stated.
RESULTS
Participant Characteristics
One hundred fourteen participants with OSA and 41 community sample participants were recruited. Four participants with OSA did not complete the baseline assessment due to family and work time commitments, leaving 110 patients to be included in the current analyses. There was no difference between these 110 participants and the other participants with OSA in terms of age, anthropometry, PSG, or subjective daytime sleepiness measures.17 Ten participants from the community sample had AHI ≥ 10 on their baseline PSG, with associated significant sleep hypoxemia (minimum SaO2 86.0 ± 2.2%, 4% oxygen de-saturation rate 7.9 ± 2.5 events/h of sleep) and sleep fragmentation (arousals 20.3 ± 2.7 events/h, sleep efficiency 76.6 ± 3.0%). These participants were excluded from further analyses, leaving 31 in the community sample.
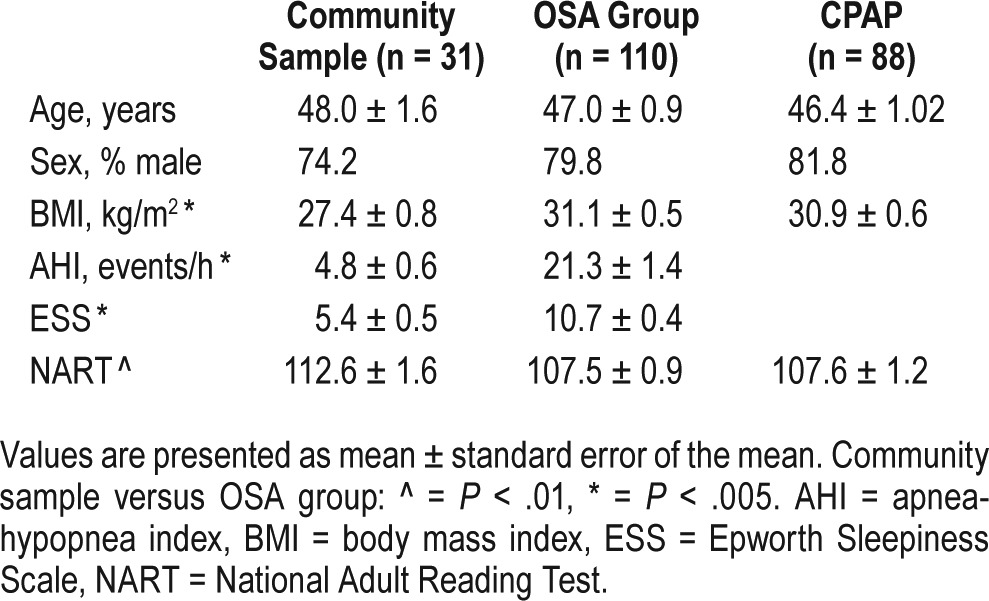
Participants in the community and OSA groups were middle-aged and predominantly male (Table 1). The age range was 23 to 79 years in the OSA group and 31 to 67 years in the community group. In the OSA group, 19 of the 110 participants (17.3%) were older than 55 years, and in the community group, 6 of the 31 (19.4%) were older than 55 years. Participants with OSA had a higher BMI (P = .001), and had significantly lower intelligence scores (National Adult Reading Test (NART) IQ: OSA 107.5 ± 0.89, community 112.6 ± 1.58) than the community sample. Eighty-eight participants with OSA completed the CPAP treatment arm; there was no change in any anthropo-metric measure during this time. The participants who completed CPAP treatment did not differ significantly on age, sex, BMI, or NART from all 110 patients who completed the baseline assessments (Table 1).
Table 1.
Participant characteristics.
Pretreatment Between Group Comparisons
Polysomnography
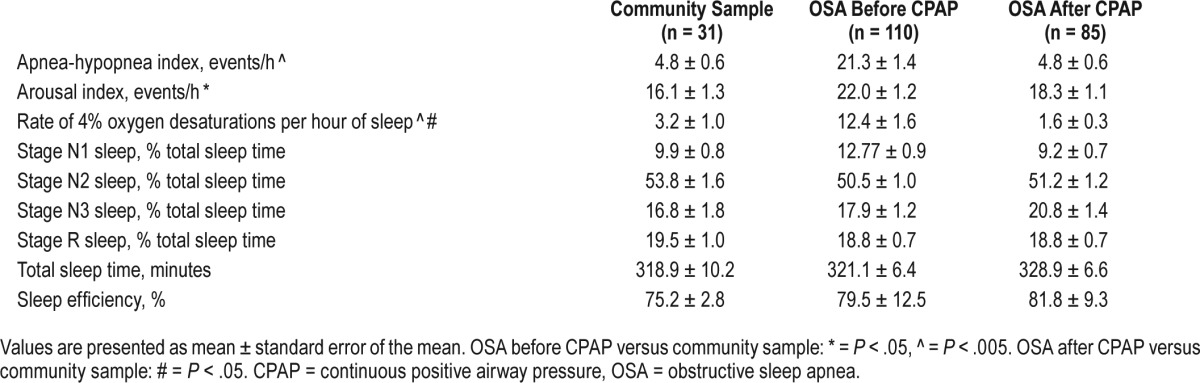
Participants with OSA had more sleep-disordered breathing (AHI, P < .001), sleep fragmentation (arousals, P = .02), and hypoxemia (4% O2 desaturations, P = .003, minimum SaO2, P = .002) than the community group (Table 2). The proportions of sleep stages were not different between OSA and community groups (Table 2).
Table 2.
Polysomnography data for the community sample and participants with OSA before and after CPAP therapy.
Objective and Subjective Sleepiness
Participants with OSA exhibited more daytime sleepiness, both subjectively (Epworth Sleepiness Scale [ESS], P < .001) and objectively (Maintenance of Wakefulness Test [MWT], P = .003; Psychomotor Vigilance Test [PVT] lapses, P < .01), than the community sample (Table 3). We chose an MWT sleep latency below a cutoff level of 36.5 minutes (ie, the mean value for the 31 community dwellers) and an ESS score ≥ 11 to define objective and subjective sleepiness, respectively.27 Using these definitions, 60% of participants with OSA were objectively sleepy, 48.2% were subjectively sleepy, and 34% were both objectively and subjectively sleepy. Two of the community participants (6.5%) were subjectively sleepy and 8 (25.8%) had short MWT sleep latencies.
Table 3.
Sleepiness, neuropsychological performance, mood and quality of life in the community sample, and participants with OSA before and after CPAP therapy.
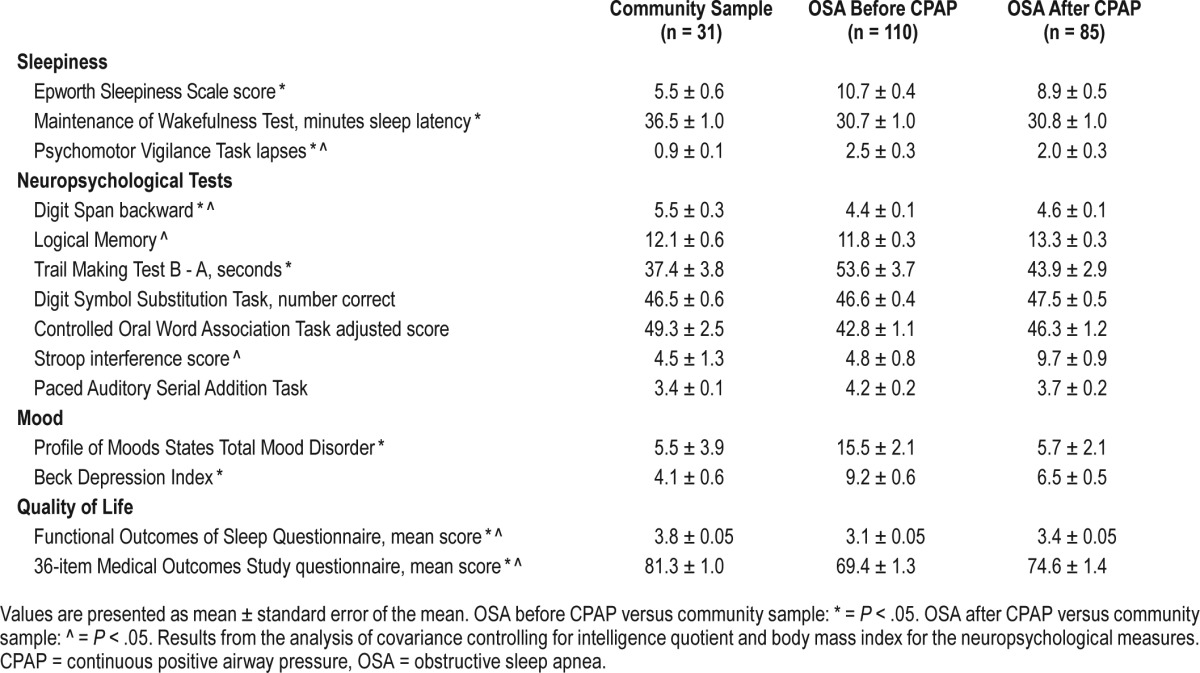
Neuropsychological Function, Mood, and Quality of Life
When controlling for premorbid intelligence, age, and BMI, participants with OSA performed worse in the area of working memory (Digit Span backward, F1,110 = 6.95, P = .01) and psychomotor function (Trailmaking Test [TMT], F1,110 = 4.65, P = .033) compared to the community sample (Table 3). There was a trend for poorer verbal fluency (Controlled Oral Word Association Test [COWAT], P = .11) in the participants with OSA compared to the community sample. There were no group differences on measures of verbal memory (logical memory), set shifting (Stroop), or information processing speed (Digit Symbol Substitution Test [DSST] and Paced Auditory Serial Addition Test [PASAT]).
Minimal to severe depression (Beck Depression Inventory) was observed in 39.1% of the participants with OSA compared to only 12.2% of the community sample. The total mood disorder score (P = .03) and the fatigue (P = .002) domain of the Profile of Mood States (POMS) were higher, and the vigor scores were lower (P = .02), in the OSA group compared to the community sample. Quality of life was impaired in the OSA group compared to the community sample both for the specific sleep questionnaire used (Functional Outcomes of Sleep Questionnaire [FOSQ]) and the generic quality of life questionnaire (36-Item Short Form Health Survey [SF-36]). Scores in all 5 domains of the FOSQ and in 6 of the 8 domains of the SF-36 were lower in the OSA group than the community sample, indicating significant and generalized impairment in quality of life of the participants with OSA.
Pretreatment and Posttreatment Comparisons
Objective and Subjective Sleep
There was a significant reduction in subjective daytime sleepiness (ESS, P < .001), but not objective sleepiness (MWT, P = .99; PVT lapses, P = .22) after CPAP.
Neuropsychological Function, Mood, and Quality of Life
All aspects of verbal fluency (COWAT), psychomotor performance (TMT), complex cognitive function (PASAT, DSST), memory (Logical Memory), and set shifting (Stroop) improved after CPAP (all values of P < .05). Working memory (Digit Span backward) did not improve after CPAP (P = .69). Mood and quality of life measures improved after CPAP (all values of P < .001).
Posttreatment Group Comparisons
PSG variables in the participants with OSA during CPAP treatment were the same (arousal index, total sleep time) or better (AHI, 4% oxygen desaturations) than those of the community group (Table 2). Thus, CPAP therapy effectively normalized breathing during sleep.
Objective and Subjective Sleep
Neither sleepiness measure (ESS or MWT) returned to the level of the community sample following 3 months of CPAP. The ESS score remained ≥ 11 in 35% of the participants with OSA treated with CPAP and the MWT score did not alter with treatment, remaining at < 36.5 minutes in 59% of participants after CPAP. Vigilance showed some improvement with CPAP, but did not return to the level of the community sample (Table 3). In summary, although CPAP was of some benefit, it failed to normalize subjective or objective sleepiness.
Neuropsychological Function, Mood, and Quality of Life
Verbal fluency (COWAT) and psychomotor performance (TMT) improved in the OSA group after CPAP, and did not differ significantly from the community sample (Table 3). Complex cognitive function (PASAT, DSST) remained similar to the community sample level of performance, whereas Logical Memory and Stroop performance were superior in participants with OSA after CPAP than the community sample, possibly due to a learning effect. The domain that was significantly impaired at baseline, working memory (Digit Span backward), showed some improvement with CPAP, but did not return to the level of the community sample.
POMS total score and all subscales did not differ between participants with OSA and the community sample following CPAP. There was some improvement in quality of life with treatment, but scores remained significantly lower than in the community sample. The target FOSQ total score was defined as that of the community sample, which was 3.8 ± 0.05. At baseline, 96.4% of the participants with OSA were below this and 90.9% did not achieve this target with CPAP therapy.
Treatment Adherence
CPAP usage as objectively recorded by a concealed timer at the pressure meter was 3.6 ± 0.3 h/night for 60.3 ± 3.7% nights. Adequate adherence was arbitrarily defined as at least 4 h/night for 70% nights; on this basis, 43.1% of participants (n = 38) had adequate usage of the pump. Participants who were adequate CPAP users had a higher NART score (mean 110.5 ± 1.4 versus 105.4 ± 1.7; P = .03), and tended to be less depressed (P = .10) and older (P = .06) than the inadequate users. Effectiveness analyses for clinical outcomes were conducted comparing adequate versus inadequate CPAP users. There was no significant difference in performance on any of the neuropsychological or sleepiness measures between adequate versus inadequate CPAP users (all values of P > .1).
Compared to the community sample, participants with OSA who had adequate CPAP usage did not differ on the COWAT, Logical Memory and TMT tasks, or the POMS vigor subscale and total mood score. The CPAP users continued to exhibit impaired performance on the PVT (P = .02) and Digit Span backward (P = .02), and significantly higher depressive symptoms, SF-36, and FOSQ scores (all values of P < .001) compared to the community sample. These participants did not recover to the level of the community sample on any measure of sleepiness (ESS and MWT; P < .005). This definition of adequate usage is, however, one that was derived by consensus26; it is unclear how many hours of treatment usage are actually enough to achieve target outcomes. Previous studies have addressed this question and our data provide additional support.14,15
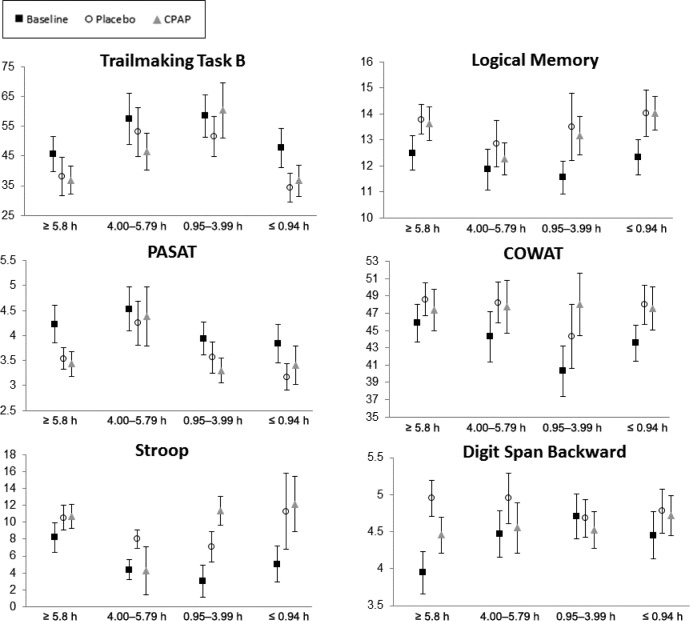
Participants were categorized into quartiles based on mean hours of CPAP usage per night. Figure 2, Figure 3, and Figure 4 present the mean scores across sleepiness, neuropsychological, and mood/quality of life measures for each CPAP quartile for participants at baseline and during CPAP and placebo. Mixed-models ANOVA revealed a significant difference between categories for the changes in MWT between groups (P = .047). The ≤ 0.94 h/night CPAP group had significantly shorter sleep latency compared to the ≥ 5.8 h/night group (P = .009). No significant main effect of CPAP group for any of the other sleepiness neuropsychological or mood and quality of life measures were found.
Figure 2. Mean sleepiness measures (ESS, MWT, PVT) for the participants with OSA at baseline, after placebo, and after CPAP for each CPAP use category.
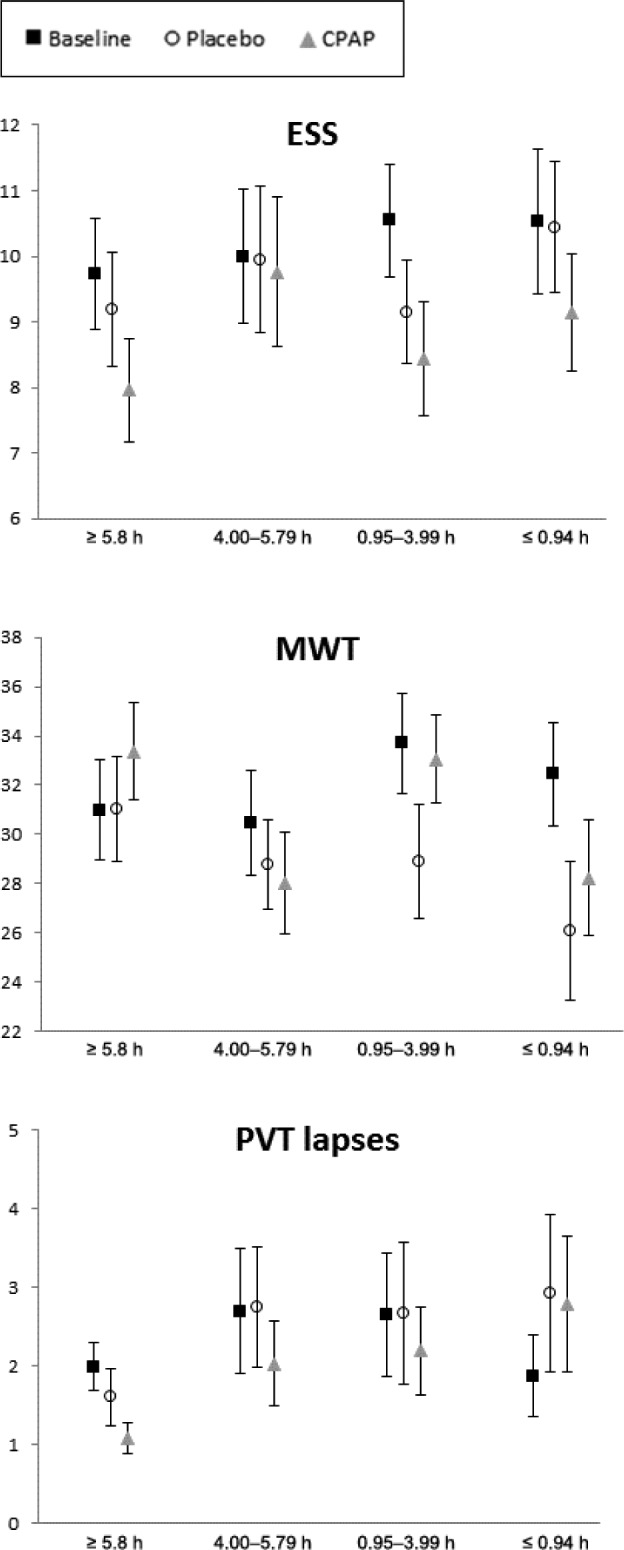
Error bars represent standard error of the mean. CPAP = continuous positive airway pressure, ESS = Epworth Sleepiness Scale, MWT = Maintenance of Wakefulness Test, OSA = obstructive sleep apnea, PVT = Psychomotor Vigilance Task.
Figure 3. Neuropsychological test performance for the participants with OSA at baseline, after placebo and after CPAP for each CPAP use category.
Error bars represent standard error of the mean. COWAT = Controlled Oral Word Association Test, CPAP = continuous positive airway pressure, OSA = obstructive sleep apnea, PASAT = Paced Auditory Serial Addition Test.
Figure 4. Mean quality of life (FOSQ) and mood (BDI, POMS) scores for the participants with OSA at baseline, after placebo and after CPAP for each CPAP use category.
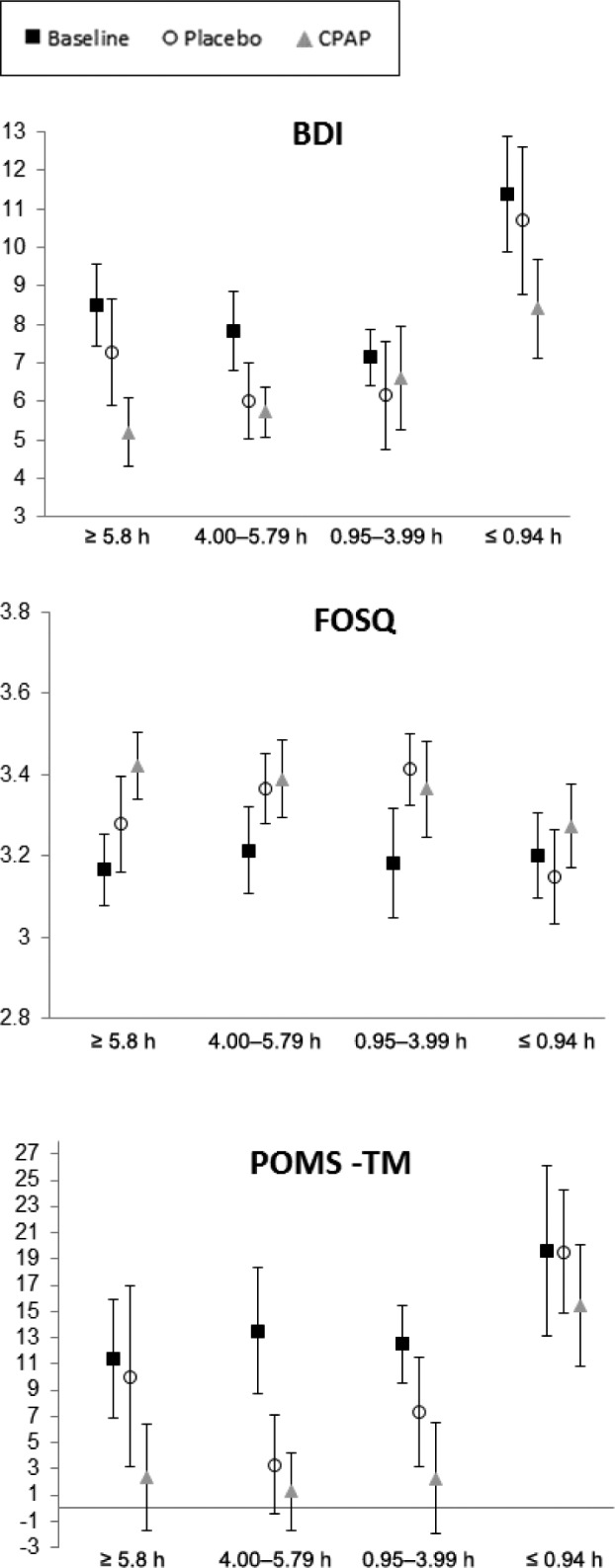
Error bars represent standard error of the mean. BDI = Beck Depression Inventory, CPAP = continuous positive airway pressure, FOSQ = Functional Outcomes of Sleep Questionnaire, OSA = obstructive sleep apnea, POMS = Profile of Mood States.
Neurobehavioral function was compared for each CPAP usage category to their own placebo level. For the ≤ 0.94 h/night group, the ESS score (P = .005) and POMS total mood score (P = .045) were significantly better after CPAP compared to placebo. For the 0.95–3.99 h/night group, the MWT time was significantly longer (P = .004), but Stroop performance was significantly poorer (P = .022) after CPAP compared to placebo. For the 4–5.79 h/night group, the number of PVT lapses was significantly lower after CPAP compared to placebo (2.03 versus 2.75 lapses; P = .028). Digit Span backward performance was marginally better after CPAP compared to placebo in this group (P = .09). For the ≥ 5.8 h/night group, Digit Span backward performance was significantly poorer after CPAP compared to placebo (4.30 versus 4.95; P = .033), and ESS and POMS total mood scores were marginally better after CPAP compared to placebo (P = .08).
DISCUSSION
This is the largest study to compare neurobehavioral function in patients with mild to moderate OSA to an age-matched healthy asymptomatic community sample, an approach that provides a much stronger level of evidence than comparison with population norms.
The results show that, compared to the community sample, untreated patients with mild to moderate OSA have more daytime sleepiness, both objectively and subjectively, and have impaired quality of life, mood, working memory, and psycho-motor function. Lack of impairment in short- and long-term memory is in contrast to some other studies,27,28 but may be due to the milder form of the disease, or a consequence of different tasks used, particularly visual versus verbal memory tasks.29 Similar diffuse impairment across a number of cognitive domains has been reported in other studies of patients with more severe OSA.30–32 Cognitive function has rarely been examined in patients with milder forms of OSA and has been presumed or observed to be absent or less severe,33 possibly because of increased capacity to compensate for impairments in performance through increased cognitive reserve or intelligence.34 However, results of our study suggest that this group of patients is significantly impaired across a range of neurocognitive domains, particularly vigilance and working memory. Potential reasons include neural damage resulting from intermittent hypoxia/re-oxygenation or sleep fragmentation35–37 and comorbidities (eg, depression, obesity). The lower intelligence of our OSA group may also have contributed, although neuro-cognitive test results remained much the same whether or not the analyses included IQ as a covariate.
Following adequate treatment with CPAP, the best currently available therapy, patients with OSA showed signifi-cant improvements in subjective sleepiness, mood, and most neuropsychological domains compared to baseline. However significant residual morbidity in sleepiness, some aspects of neuropsychological function, and mood and quality of life remained, relative to the community sample. This lack of normalization of neurobehavioral function following CPAP treatment is in line with previous studies in patients with moderate to severe OSA.14,27,38 It has previously been argued that a lack of improvement in neurobehavioral function is due to milder disease, inadequate CPAP use, or irreversible damage to specific brain regions. For example, abnormalities in frontal white matter and metabolites have been observed in patients with OSA who have been treated with CPAP for 6 months.28 It is also likely that these residual impairments are a result of comorbidities. In contrast to previous studies that have excluded patients with significant comorbidities, we included patients with OSA with stable comorbidities, and thus are likely to be more representative of the clinical population. A further strength of this study was that the OSA group had a lower IQ and were significantly impaired at baseline, thus a ceiling effect cannot explain the current findings; this has been argued as an explanation for a lack of improvement in neuropsychological function in previous studies.8
Some functions did normalize following CPAP treatment, including verbal fluency and psychomotor function. Positive benefits on mood were also observed. Although mean depression scores were in the normal range (Beck Depression Inventory < 13), 10 participants with OSA exhibited moderate-severe symptoms at baseline, whereas only 1 participant was in the moderate depression category after CPAP treatment. Other neuropsychological functions, however, did not return to the level of the community participants, including vigilance and working memory. This dissociation in improvement in different cognitive domains may reflect temporal difference in restoration of cognitive function with treatment, or improvements in the structure or functionality of brain regions associated with performance on these tasks. However, further analysis of the dose-response relationship between CPAP use and improvement in neurobehavioral function does not support this hypothesis. In those with good CPAP usage, there were incremental improvements in vigilance, DSST performance, and some aspects of mood, although these did not differ significantly between different categories of CPAP use. It is possible that some participants with poor nightly usage may have experienced a placebo effect, as reflected in improvements in self-reported OSA symptom severity (data not presented here) and in some of the neuropsychological tasks (eg, Stroop). In turn, they may have tried harder or felt more capable of performing the neuropsychological tests. It is also plausible that the tasks used were not sensitive enough to detect the small differences that may have existed between the CPAP use categories.
Further analysis comparing each treatment usage group to the placebo condition found no differences in neuropsycho-logical task performance, even in those who were using CPAP for more than 5.8 h/night. Some improvements in mood and sleepiness were observed after CPAP compared to placebo; however, these were not dose dependent. These findings raise questions regarding the efficacy of CPAP to improve or restore neurobehavioral function in patients with mild-moderate OSA. Compared to previous studies that have used a sham CPAP control group, the current study used a placebo pill. One of the issues with sham treatment is that many participants recognize that the treatment is not active,39 which can affect the validity of that control condition. In the current study, all participants underwent 3 months each of CPAP, placebo pill, and oral appliance therapy; therefore, they would have realized the ineffective nature of sham CPAP. Additionally, sham CPAP would not have been a suitable placebo for MAS treatment.
It is possible that 3 months of CPAP is an insufficient time for cognitive dysfunction and sleepiness to resolve, and a longer treatment period is required to see improvements in some measures. Evidence from other studies suggests impairments may continue to improve over time (up to 12 months)40,41; however, whether these functions return to control levels was not evaluated in these studies. Regardless, most studies do not support a dose-response relationship between length of adequate CPAP use and improvement in cognitive function, with improvements after 2 to 3 weeks remaining relatively stable.42
Another possibility is that neurobehavioral impairment is premorbid in some patients with OSA. The community sample was age-matched to our patients with OSA; however, their IQ was estimated to be higher, and their BMI was lower, than the patient group. These findings could equally be due to associated obesity,43 obesity-related comorbidities (cardiovascular and metabolic disease), or the medications used to treat these conditions in the patients with OSA. This raises questions as to whether OSA is a cause of these deficits, or if they are pre-morbid and will not resolve completely with treatment. Future studies would benefit from matching patients with OSA with obese age-matched controls to better understand the role of obesity on neuropsychological dysfunction in OSA.
The lack of a dose-response relationship found for CPAP use and sleepiness, neuropsychological outcomes, mood, or quality of life substantially narrows the reasons for neurobehavioral impairment in patients with mild OSA and the at-best partial response to treatment. These data bring into question the use of CPAP in this mild end of the OSA spectrum, particularly for sleepiness measures (both objective and subjective), which may directly affect adherence to treatment.
One of the limitations of the current study is that the community sample was not reexamined at follow-up, and the patients with OSA were tested multiple times (although the treatment arms were completed in a randomized fashion), thus making the posttreatment results susceptible to the confounding effects of practice. Some of the neuropsychological test performance in the participants with OSA exceeded levels of the community group after CPAP (Stroop and Logical Memory), which may be a result of learning effects. Alternate versions of the tasks were used where possible, and many of the neuropsychological tasks used in the study, such as the PVT, do not carry any learning effects.44
CONCLUSIONS
Previous studies have shown significant neurobehavioral morbidity and hypertension in participants with mild to moderate OSA, which is likely to be independent of age.45 The current study provides further evidence in support of significant neurobehavioral morbidity among patients with mild to moderate OSA by comparing results with those in a carefully chosen healthy community dwelling group. In addition, although we have previously shown that the improvement in neuropsychological function with CPAP and MAS is statistically significant,17 the current study shows that the treatment response is inadequate to return participants to the functional level of healthy individuals who are asymptomatic of sleep-disordered breathing, and that treatment does not improve function in a dose-response manner. Thus, these findings are consistent with the hypothesis that there may be neurologic changes related to OSA that do not respond to CPAP treatment. This lack of symptomatic response likely compromises treatment adherence and therefore further detracts from the efficacy of treatment in this group. Whether these residual deficits are due to irreversible impairment or reflect premorbid levels of function remains to be elucidated.
DISCLOSURE STATEMENT
Work for this study was performed at the Institute for Breathing and Sleep, Austin Health, Melbourne and Adelaide Institute for Sleep Health, Repatriation General Hospital, Adelaide. This study has not been registered with a clinical trial registry because it was conducted prior to 2004. The study was funded by the National Health and Medical Research Council of Australia (APP981031). Equipment was provided by ResMed Australia, RJ and VK Bird. MJ receives grant support from the NHMRC and non-financial support from Air Liquide. RDM receives funding from NHMRC Australia, grant support from Philips Respironics, Fisher & Paykel Healthcare, and RedMed Foundation, and nonfinancial support from ResMed and Philips Respironics. SB receives grant support from the NHMRC. MB receives research support from Air Liquide.
ACKNOWLEDGMENTS
The authors acknowledge the significant contribution to this project of our colleague and friend, Professor Rob Pierce, to whom this manuscript is dedicated. The authors would like to thank the Sleep Unit staff at both institutions for their assistance with data collection. MLJ was supported by a National Health and Medical Research Council (NHMRC) of Australia Early Career Fellowship, MB was supported by an NHMRC Postgraduate Scholarship, and RDMcE by an NHMRC Practitioner Fellowship.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- ANOVA
analysis of variance
- BDI
Beck Depression Inventory
- BMI
body mas index
- CPAP
continuous positive airways pressure
- COWAT
Controlled Oral Word Association Test
- DSST
Digit Symbol Substitution Test
- ESS
Epworth Sleepiness Scale
- FOSQ
Functional Outcomes of Sleep Questionnaire
- MWT
Maintenance of Wakefulness Test
- MAS
mandibular advancement splint
- NART
National Adult Reading Test
- OSA
obstructive sleep apnea
- PASAT
Paced Auditory Serial Addition Test
- POMS
Profile of Mood States
- PSG
polysomnography
- PVT
Psychomotor Vigilance Test
- REM
rapid eye movement
- SF-36
36-Item Short Form Health Survey
- TMT
Trailmaking Test
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedard MA, Montplaisir J, Richer F, Rouleau I, Malo J. Obstructive sleep apnea syndrome: pathogenesis of neuropsychological deficits. J Clin Exp Neuropsychol. 1991;13(6):950–964. doi: 10.1080/01688639108405110. [DOI] [PubMed] [Google Scholar]
- 3.Engleman H, Kingshott R, Martin S, Douglas NJ. Cognitive function in the sleep apnea/hypopnea syndrome (SAHS) Sleep. 2000;23(Suppl 4):S102–S108. [PubMed] [Google Scholar]
- 4.Kim HC, Young T, Matthews CG, Weber SM, Woodward AR, Palta M. Sleep-disordered breathing and neuropsychological deficits. A population-based study. Am J Respir Crit Care Med. 1997;156(6):1813–1819. doi: 10.1164/ajrccm.156.6.9610026. [DOI] [PubMed] [Google Scholar]
- 5.Naegele B, Thouvard V, Pepin J, et al. Deficits of cognitive executive functions in patients with sleep apnea syndrome. Sleep. 1995;18(1):43–52. [PubMed] [Google Scholar]
- 6.Mathieu A, Mazza S, Décary A, et al. Effects of obstructive sleep apnea on cognitive function: a comparison between younger and older OSAS patients. Sleep Med. 2008;9(2):112–120. doi: 10.1016/j.sleep.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Baldwin CM, Griffith KA, Nieto FJ, O'Connor GT, Walsleben JA, Redline S. The association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep. 2001;24(1):96–105. doi: 10.1093/sleep/24.1.96. [DOI] [PubMed] [Google Scholar]
- 8.Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep. 2012;35(12):1593–1602. doi: 10.5665/sleep.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montserrat JM, Ferrer M, Hernandez L, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir. Crit Care Med. 2001;164(4):608–613. doi: 10.1164/ajrccm.164.4.2006034. [DOI] [PubMed] [Google Scholar]
- 10.Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999;353(9170):2100–2105. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- 11.Engleman HM, Cheshire KE, Deary IJ, Douglas NJ. Daytime sleepiness, cognitive performance and mood after continuous positive airway pressure for the sleep apnoea/hypopnoea syndrome. Thorax. 1993;48(9):911–914. doi: 10.1136/thx.48.9.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta A, Qian J, Petocz P, Darendeliler MA, Cistulli PA. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163(6):1457–1461. doi: 10.1164/ajrccm.163.6.2004213. [DOI] [PubMed] [Google Scholar]
- 13.Hoekema A, Stegenga B, De Bont L. Efficacy and co-morbidity of oral appliances in the treatment of obstructive sleep apnea-hypopnea: a systematic review. Crit Rev Oral Biol Med. 2004;15(3):137–155. doi: 10.1177/154411130401500303. [DOI] [PubMed] [Google Scholar]
- 14.Antic NA, Catcheside P, Buchan C, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011;34(1):111–119. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weaver T, Maislin G, Dinges D, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning volume. Sleep. 2007;30(6):711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams N, Strauss M, Schluchter M, Redline S. Relation of measures of sleep-disordered breathing to neuropsychological functioning. Am J Respir Crit Care Med. 2001;163(7):1626–1631. doi: 10.1164/ajrccm.163.7.2004014. [DOI] [PubMed] [Google Scholar]
- 17.Barnes M, McEvoy RD, Banks S, et al. Efficacy of positive airways pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170(6):656–664. doi: 10.1164/rccm.200311-1571OC. [DOI] [PubMed] [Google Scholar]
- 18.Engleman HM, Kingshott RN, Wraith PK, Mackay TW, Deary IJ, Douglas NJ. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep Apnea/Hypopnea syndrome. Am J Respir Crit Care Med. 1999;159(2):461–467. doi: 10.1164/ajrccm.159.2.9803121. [DOI] [PubMed] [Google Scholar]
- 19.Weaver TE, Mancini C, Maislin G, et al. Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea: results of the CPAP Apnea Trial North American Program (CATNAP) randomized clinical trial. Am J Respir Crit Care Med. 2012;186(7):677–683. doi: 10.1164/rccm.201202-0200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnes M, Houston D, Worsnop CJ, et al. A randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165(6):773–780. doi: 10.1164/ajrccm.165.6.2003166. [DOI] [PubMed] [Google Scholar]
- 21.Marshall N, Neill A, Campbell A, Shepperd DS. Randomised controlled crossover trial of humidified continuous positive airway pressure in mild obstructive sleep apnoea. Thorax. 2005;60(5):427–432. doi: 10.1136/thx.2004.032078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez AI, Martinez P, Miro E, Bardwell WA, Buela-Casal G. CPAP and behavioral therapies in patients with obstructive sleep apnea: Effects on daytime sleepiness, mood, and cognitive function. Sleep Med Rev. 2009;13(3):223–233. doi: 10.1016/j.smrv.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Marshall N, Barnes M, Travier N, et al. Continuous positive airway pressure reduces daytime sleepiness in mild-moderate obstructive sleep apnoea: meta-analysis. Thorax. 2006;61(5):430–434. doi: 10.1136/thx.2005.050583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz SW, Cimino CR, Anderson WM. CPAP or placebo-effect? Sleep. 2012;35(12):1585–1586. doi: 10.5665/sleep.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163(1):19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 26.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147(4):887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 27.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 28.O'Donoghue F, Wellard R, Rochford P, et al. Magnetic resonance spectroscopy and neurocognitive dysfunction in obstructive sleep apnea before and after CPAP treatment. Sleep. 2012;35(1):41–48. doi: 10.5665/sleep.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Twigg GL, Papaioannou I, Jackson ML, et al. Obstructive sleep apnea syndrome is associated with deficits in verbal but not visual memory. Am J Respir Crit Care Med. 2010;182(1):98–103. doi: 10.1164/rccm.200901-0065OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naëgele B, Launois S, Mazza S, Feuerstein C, Pépin JL, Lévy P. Which memory processes are affected in patients with obstructive sleep apnea? An evaluation of 3 types of memory. Sleep. 2006;29(4):533–544. doi: 10.1093/sleep/29.4.533. [DOI] [PubMed] [Google Scholar]
- 31.Jackson ML, Howard ME, Barnes M. Cognition and daytime functioning in sleep-related breathing disorders. Prog Brain Res. 2011;190:53–68. doi: 10.1016/B978-0-444-53817-8.00003-7. [DOI] [PubMed] [Google Scholar]
- 32.Olaithe M, Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep. 2013;36(9):1297–1305. doi: 10.5665/sleep.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engleman HM, Douglas NJ. Sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59(7):618–622. doi: 10.1136/thx.2003.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alchanatis M, Zias N, Deligiorgis N, et al. Sleep apnea-related cognitive deficits and intelligence: an implication of cognitive reserve theory. J Sleep Res. 2005;14(1):69–75. doi: 10.1111/j.1365-2869.2004.00436.x. [DOI] [PubMed] [Google Scholar]
- 35.Bartlett DJ, Rae C, Thompson CH, et al. Hippocampal area metabolites relate to severity and cognitive function in obstructive sleep apnea. Sleep Med. 2004;5(6):593–596. doi: 10.1016/j.sleep.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Morrell MJ, Jackson ML, Twigg GL, et al. Changes in brain morphology in patients with obstructive sleep apnoea. Thorax. 2010;65(10):908–914. doi: 10.1136/thx.2009.126730. [DOI] [PubMed] [Google Scholar]
- 37.Rosenzweig I, Glasser M, Polsek D, Leschziner GD, Williams SC, Morrell MJ. Sleep apnoea and the brain: a complex relationship. Lancet Respir Med. 2015;3(5):404–414. doi: 10.1016/S2213-2600(15)00090-9. [DOI] [PubMed] [Google Scholar]
- 38.Kylstra WA, Aaronson JA, Hofman WF, Schmand BA. Neuropsychological functioning after CPAP treatment in obstructive sleep apnea: a meta-analysis. Sleep Med Rev. 2013;17(5):341–347. doi: 10.1016/j.smrv.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Djavadkhani Y, Marshall NS, D'Rozario AL, et al. Ethics, consent and blinding: lessons from a placebo/sham controlled CPAP crossover trial. Thorax. 2015;70(3):265–269. doi: 10.1136/thoraxjnl-2014-206354. [DOI] [PubMed] [Google Scholar]
- 40.Borak J, Cieslicki J, Koziej M, Matuszewski A, Zieliński J. Effects of CPAP treatment on psychological status in patients with severe obstructive sleep apnoea. J Sleep Res. 1996;5(2):123–127. doi: 10.1046/j.1365-2869.1996.d01-60.x. [DOI] [PubMed] [Google Scholar]
- 41.Muñoz A, Mayoralas L, Barbe F, Pericás J, Agusti AG. Long-term effects of CPAP on daytime functioning in patients with sleep apnoea syndrome. Eur Respir J. 2000;15(4):676–681. doi: 10.1034/j.1399-3003.2000.15d09.x. [DOI] [PubMed] [Google Scholar]
- 42.Ferini-Strambi L, Baietto C, Di Gioia MR, et al. Cognitive dysfunction in patients with obstructive sleep apnea (OSA): partial reversibility after continuous positive airway pressure (CPAP). Brain. Res Bull. 2003;61(1):87–92. doi: 10.1016/s0361-9230(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 43.Kaur S, Gonzales MM, Tarumi T, et al. Serum brain-derived neurotrophic factor mediates the relationship between abdominal adiposity and executive function in middle age. J Int Neuropsychol. Soc. 2016;22(5):493–500. doi: 10.1017/S1355617716000230. [DOI] [PubMed] [Google Scholar]
- 44.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Meth Instrum Comput. 1985;17(6):652–655. [Google Scholar]
- 45.Mathieu A, Mazza S, Decary A, et al. Effects of obstructive sleep apnea on cognitive function: a comparison between younger and older OSAS patients. Sleep Med. 2008;9(2):112–120. doi: 10.1016/j.sleep.2007.03.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.