Abstract
Study Objectives:
There is a lack of data regarding adherence trajectories when switching from continuous positive airway pressure (CPAP) to adaptive servoventilation (ASV) in the context of persistent or treatment-emergent central sleep apnea (CSA). This study investigated 90-day adherence rates in patients with sleep apnea based on the type of positive airway pressure (PAP) device used and any switching of PAP modality over time.
Methods:
Telemonitoring data were obtained from a United States PAP database. Eligible patients were a 30% random sample who started PAP, plus all who started ASV, from January 1, 2015 to October 2, 2015. All received PAP and had at least one session with usage of 1 hour or more. Adherence and device usage were determined in three groups: started on CPAP and stayed on CPAP (CPAP only); started on ASV and stayed on ASV (ASV only); started on CPAP, switched to ASV (Switch). The United States Medicare definition of adherence was used.
Results:
The study included 198,890 patients; 189,724 (CPAP only), 8,957 (ASV only) and 209 (Switch). In the Switch group, average apnea-hypopnea index decreased significantly on ASV versus CPAP. At 90 days, adherence rates were 73.8% and 73.2% in the CPAP only and ASV only groups. In the Switch group, CPAP adherence was 62.7%, improving to 76.6% after the switch to ASV. Mean device usage at 90 days was 5.27, 5.31, and 5.73 h/d in the CPAP only, ASV only, and Switch groups, respectively.
Conclusions:
Treatment-emergent or persistent CSA during CPAP reduced therapy adherence, but adherence improved early after switching from CPAP to ASV.
Citation:
Pépin JL, Woehrle H, Liu D, Shao S, Armitstead JP, Cistulli PA, Benjafield AV, Malhotra A. Adherence to positive airway therapy after switching from CPAP to ASV: a big data analysis. J Clin Sleep Med. 2018;14(1):57–63.
Keywords: adaptive servoventilation, adherence, continuous positive airway pressure, sleep apnea
BRIEF SUMMARY
Current Knowledge/Study Rationale: A growing body of evidence suggests that adaptive servoventilation (ASV) might be an efficacious approach to the management of central sleep apnea (CSA) that emerges or persists during continuous positive airway pressure (CPAP) therapy. However, little is known about the effect of changes in positive airway pressure (PAP) therapy mode on adherence in patients with treatment-emergent or persistent CSA.
Study Impact: This analysis of a PAP telemonitoring database showed that a switch from CPAP to ASV in patients with lower CPAP adherence, possibly due to treatment-emergent CSA, improved adherence and device usage, and decreased the apnea-hypopnea index. This effect was greatest in patients with higher residual central apnea index in the first week of CPAP.
INTRODUCTION
Achieving good positive airway pressure (PAP) therapy adherence is a well-recognized clinical goal in sleep apnea management. The benefits of PAP therapy, especially in patients with associated comorbidities, are dependent on adequate device usage.1–5 Traditionally, interventions to improve adherence to PAP, particularly continuous positive airway pressure (CPAP), have focused on patient education, clinical support, and behavioral interventions.6–9 Other factors that might contribute to suboptimal device use are the evolution of sleep apnea during PAP therapy, particularly the emergence or persistence of central events.10
There are a variety of central sleep apnea (CSA) trajectories after initiation of CPAP to treat obstructive sleep apnea (OSA),10 including the development of CSA after the obstructive events have been successfully eliminated.11 This phenomenon was previously referred to as complex sleep apnea, but has recently been recognized in the third edition of the International Classification of Sleep Disorders, and has been called “treatment-emergent CSA.”12 The rate of treatment-emergent CSA after CPAP initiation is reportedly up to 15%,13–17 and the risk of developing treatment-emergent CSA appears to be higher in males, older patients, those with more severe sleep apnea at baseline, and patients with comorbid conditions (eg, heart failure).10,13,16,17
The results of several small studies suggest that adaptive servoventilation (ASV) might be an effective treatment option for patients with treatment-emergent CSA.18–21 However, there is relatively little information about outcomes and the effect of interventions in patients who have treatment-emergent or persistent CSA during CPAP therapy. Adherence data from those switching from one PAP modality to another are scarce.
This big data analysis investigated 90-day adherence rates in three different groups of patients with sleep apnea based on the type of PAP device used and any switching of PAP modality over time. We tested the hypothesis that ASV therapy would be associated with better adherence to PAP therapy in patients with treatment-emergent or persistent CSA.
METHODS
Data Inclusion
Data were obtained from a United States PAP telemonitoring database. A 30% random sample of patients starting therapy in 2015 was taken to identify patients using CPAP (including both fixed-level and automatic-titration PAP), plus all patients who started ASV therapy in 2015 (whether as first or subsequent PAP therapy) were eligible. Study inclusion criteria for both datasets were as follows: patients registered in the United States; use of only AirSense/AirCurve 10 device(s) (ResMed, San Diego, California, United States); therapy start date (ie, the date of first session with non-zero usage) from January 1, 2015 to October 2, 2015; at least one session with device usage of ≥ 1 hour; valid data entry (age plausible [younger than 123 years] and valid data blocks [session date and received date were synchronized]).
Each session record contained 5 types of data: (1) patient demographic data including age and sex; (2) treatment usage; (3) clinical therapy metrics including statistical summary (eg, median, 95th percentile) of leak and pressure (for automatic pressure modes); (4) respiratory events as measured by residual apnea-hypopnea index (AHI), apnea index (AI), hypopnea index (HI), obstructive apnea index (OAI), central apnea index (CAI), and unknown apnea index (UAI); and (5) pressure settings (for fixed pressure modes).
Assessments
Adherence and device usage were determined in three patient groups: CPAP only (started on CPAP and stayed on CPAP); ASV only (started on ASV and stayed on ASV); and Switch (started on CPAP, switched to ASV). Adherence was defined based on United States Medicare requirements: device usage for ≥ 4 hours per night on 70% of nights during a consecutive 30-day period anytime during the first 3 months of initial use. Average usage per day was calculated by dividing the total hours used in the period by the number of days in the period, where the period was defined as day 1 to day 30, day 60, or day 90, or to the end date of the specific therapy (defined as the date of the last available session record with non-zero usage on that therapy mode).
Statistical Analysis
Descriptive statistical data were calculated for variables including age, average AHI, CAI, AI, HI, usage, pressure, and leak in week 1 of initial treatment on PAP, and presented as mean values ± standard deviation. For reliable assessment of AHI, CAI, AI, and HI values, records with low usage (< 1 hour) were excluded from the calculations of average AHI, CAI, AI, and HI in week 1. A two-sided t test was used for between-group comparison of the normally distributed continuous variable (age). Kruskal-Wallis rank-sum test was used for between-group comparisons of non-normally distributed continuous variables (including average AHI, CAI, AI, HI, usage, pressure, and leak in week 1). A value of P ≤ .05 was considered statistically significant. For adherence rates and mean device usage, 95% confidence intervals (CIs) were calculated. All calculations were performed using R software version 3.3.1 (The R Foundation, Vienna, Austria).
RESULTS
Study Population
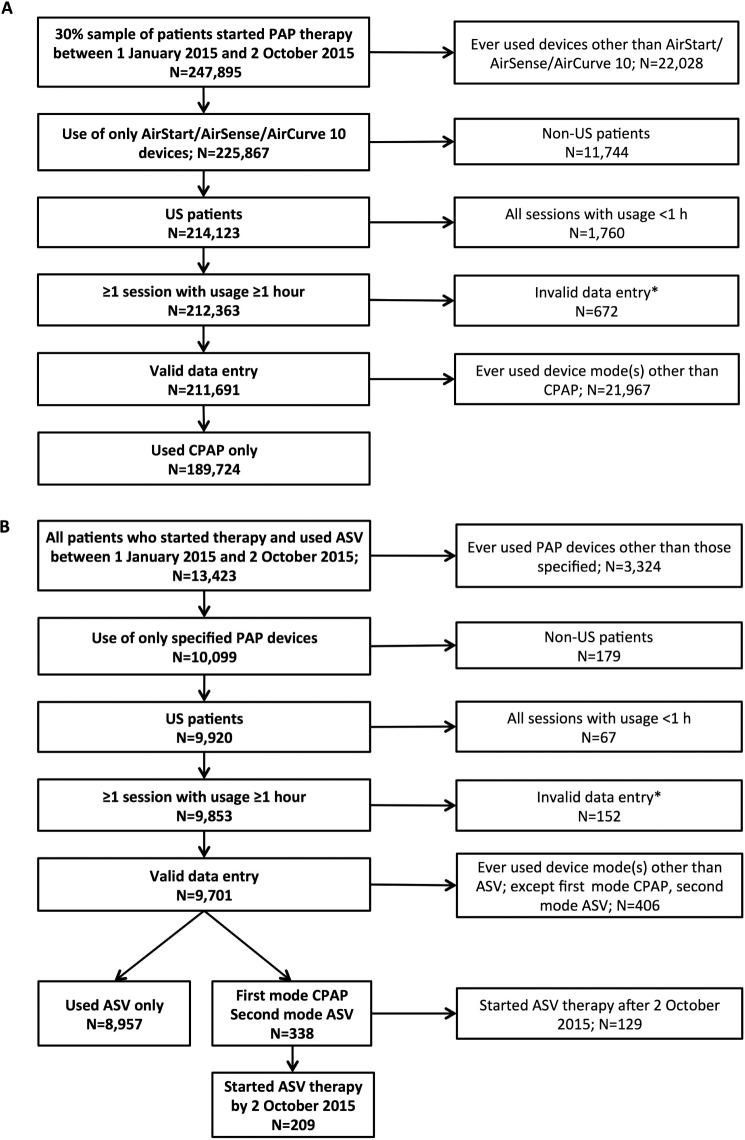
Of the 247,895 patients (who represent 30% of the database who started PAP therapy in the assessment window [January 1, 2015 to October 2, 2015]), 211,691 were registered in the United States, had used only AirSense/AirCurve 10 device(s), had valid data entry, and had at least one session with use of 1 hour or more. A total of 189,724 patients had used CPAP only (Figure 1A). ASV was started between January 1, 2015 to October 2, 2015 in 13,423 patients, 9,701 of whom were registered in the United States, had used only AirSense/AirCurve 10 device(s), and had valid data entry and at least one session with use of 1 hour or more. Of these, 8,957 had used ASV only, 338 had switched from CPAP to ASV and 209 had started ASV therapy by October 2, 2015 (Figure 1B).
Figure 1. CONSORT diagram.
(A) CPAP only patients and (B) patients who used ASV. * = invalid data entry = age implausible, or received data and session date not synchronized. ASV = adaptive servoventilation, CPAP = continuous positive airway pressure, US = United States.
Patient Characteristics and Efficacy by Device Mode
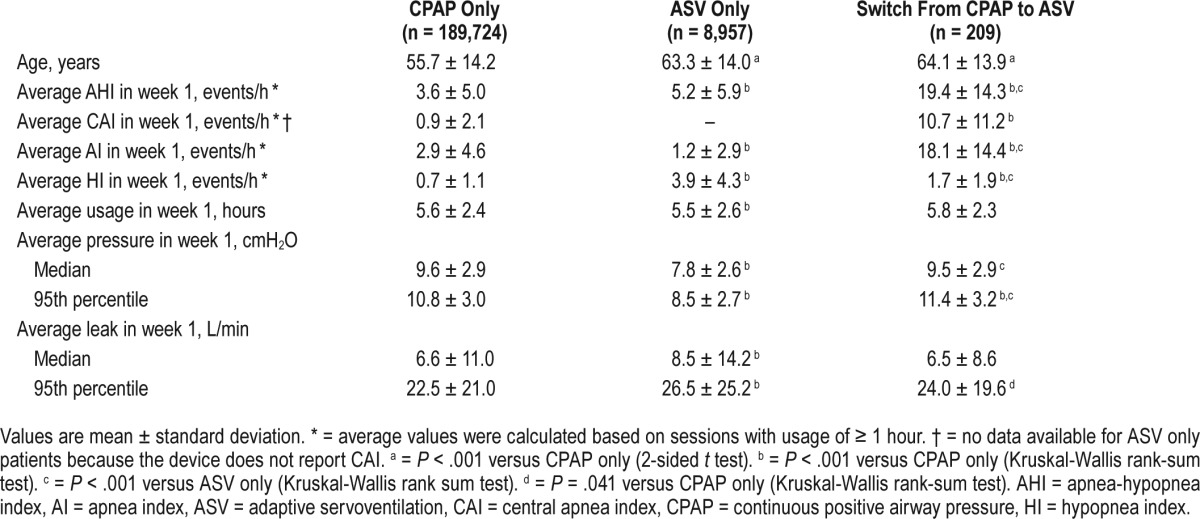
The mean age of patients in the CPAP only group was approximately 56 years and residual respiratory events, including average AHI, AI, HI, and CAI, were below 5 events/h during the first week on CPAP. Patients in the ASV only group and those who switched from CPAP to ASV were significantly older than those in the CPAP only group (P < .001; Table 1). Patients in the Switch group had significantly higher average AHI, CAI, AI, and HI in week 1 of initial CPAP treatment compared with the CPAP only group, and significantly higher AHI and AI than the ASV only group (all Ps < .001; Table 1). The significantly higher average CAI (> 10 events/h) in week 1 on CPAP therapy in the Switch group is likely due to emergence of CSA during CPAP treatment; 120 patients in the Switch group had a CAI of ≥ 5 events/h during the week 1 of CPAP therapy. In week 1 on therapy, patients in the ASV only group had significantly higher AHI and HI values, but a significantly lower AI compared with the CPAP only group (all values of P < .001; Table 1). Other statistically significant between-group differences were seen for average pressure in ASV only versus the CPAP only and Switch groups (lower with ASV), and average leak, which was higher in the ASV only versus CPAP only group (Table 1). In the Switch group, there were no statistically significant differences in patient and respiratory characteristics based on the time at which switching occurred (in the first, second, or third month of CPAP or after the third month; data not shown).
Table 1.
Patient characteristics by device mode.
Adherence and Device Usage
Ninety-day adherence rates for the different patient groups are shown in Table 2. CPAP adherence rates in patients from the Switch group were initially lower than those in patients who remained on CPAP only, but increased immediately after the switch to either fixed or variable expiratory positive airway pressure (EPAP) ASV (Table 2); this increase was similar in patients who had been using fixed or automatic CPAP. Improved adherence was also shown by better usage of ASV compared with CPAP in the Switch group (Figure 2). For adherence rate analysis, patients who did not meet adherence requirements before switch are treated as nonadherent. In the CPAP-ASV group, mean device usage at 30 and 60 days after the switch was significantly higher than that before switch (Table 3), particularly in those who switched from CPAP to fixed EPAP ASV (Table 4). In the Switch group, ASV adherence rates were highest in those who switched from CPAP to ASV in the third month (82.4% versus 67.9%, 70.9% and 80.0% in those who switched in the first, second, and after the third month, respectively).
Table 2.
Adherence rates at 90 days by patient subgroups.
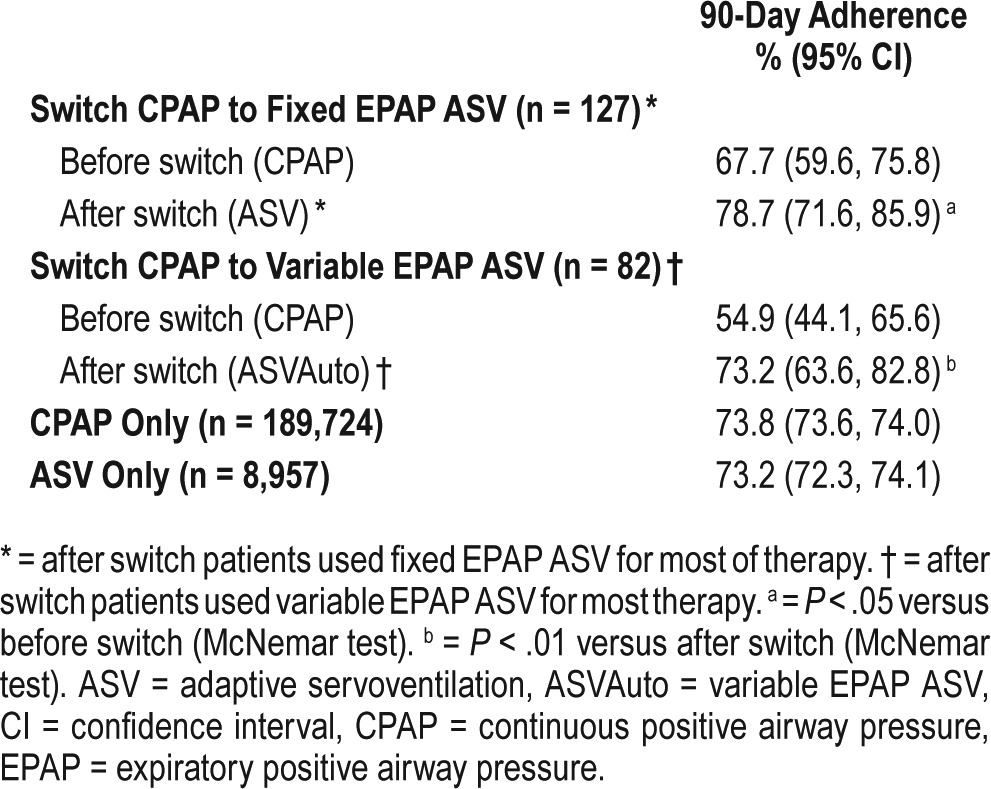
Figure 2. Trajectories of average PAP usage before versus after the switch from CPAP to ASV.
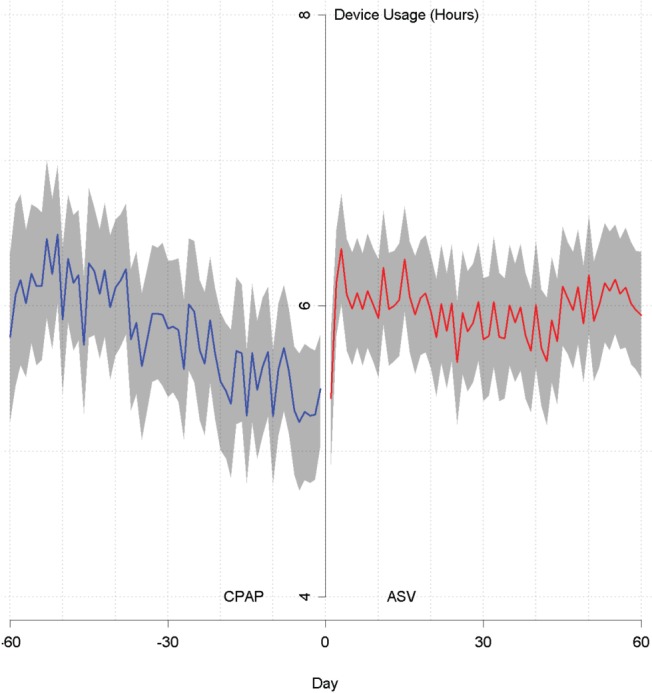
ASV = adaptive servoventilation, CPAP = continuous positive airway pressure.
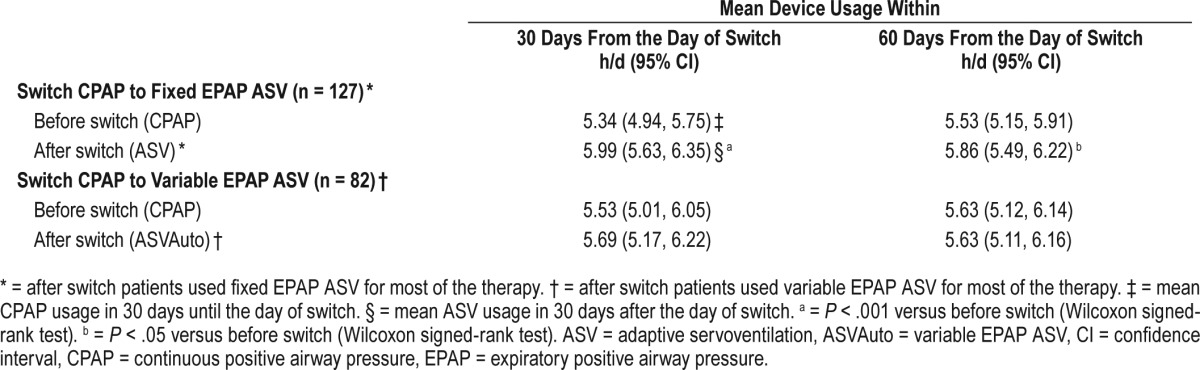
Table 3.
Mean device usage for the CPAP-ASV group in the 30 and 60 days before and after switch.
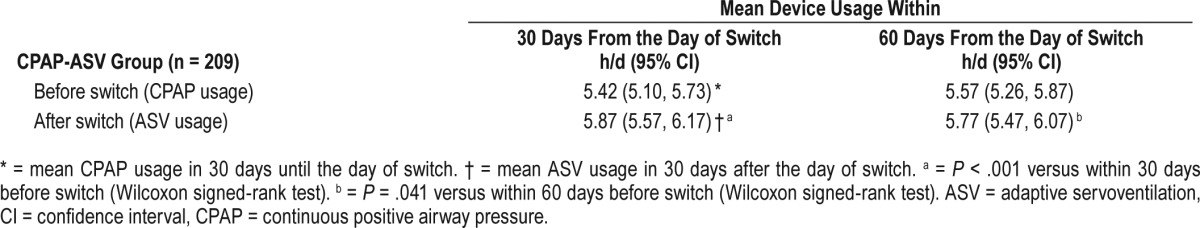
Table 4.
Mean device usage for the CPAP-ASV group in the 30 and 60 days before and after switch by type of ASV device used.
Apnea-Hypopnea Index
In the Switch group, AHI remained high during CPAP therapy, but fell immediately to just under 5 events/h after switching to ASV (Figure 3). For the subgroups of patients switched from fixed, automatic, or both fixed and automatic CPAP, residual AHI values were 4.34 (2.81, 5.88), 4.72 (3.69, 5.75), and 4.40 (2.67, 6.21) events/h, respectively. In patients who switched from CPAP to fixed EPAP ASV, mean daily AHI in the first 90 days was 17.34 (15.17, 19.41) events/h before the switch and reduced significantly to 4.10 (3.30, 4.89) events/h after the switch; corresponding values for those switched from CPAP to variable EPAP ASV were 19.76 (16.65, 22.88) events/h to 4.75 (3.56, 5.94) events/h (both P < .001, Wilcoxon signed-rank test). The decrease in AHI after switching from CPAP to ASV was greatest in patients in the Switch group who had an average CAI of ≥ 5 events/h in week 1 of CPAP (Figure 4).
Figure 3. Average AHI before versus after the switch from CPAP to ASV.
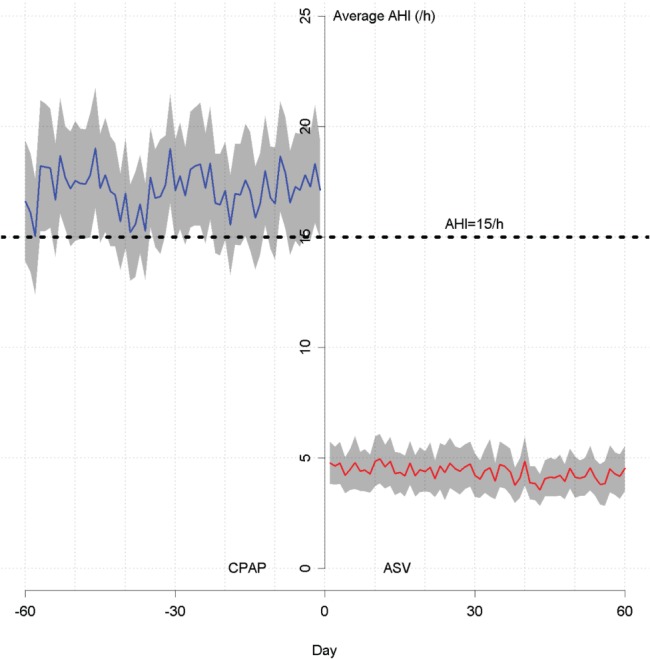
AHI = apnea-hypopnea index, ASV = adaptive servoventilation, CPAP = continuous positive airway pressure.
Figure 4. Average daily AHI in the first 90 days before and after a switch from CPAP to ASV.
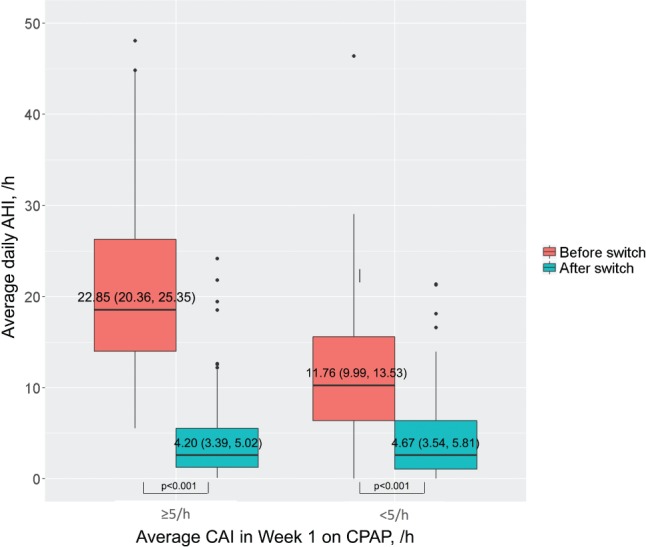
Average daily AHI in patients with an average central apnea index of ≥ 5 events/h versus < 5 events/h in the first week on CPAP. AHI = apneahypopnea index, ASV = adaptive servoventilation, CAI = central apnea index, CPAP = continuous positive airway pressure.
DISCUSSION
This big data analysis, the first of its kind to evaluate the effect of PAP modality on adherence, showed that switching from CPAP to ASV was associated with an increase in adherence and a reduction in the residual AHI, which was greatest in patients with higher residual CAI in week 1 of CPAP. Thus, poorer adherence to CPAP may be due to the presence of treatment-emergent or persistent CSA.10
Rates of adherence achieved after a switch from CPAP to ASV in our study (67.9% to 82.4%) were generally similar to those reported during use of ASV in another study that included 63 patients with treatment-emergent CSA during CPAP (84%).18 Conversely, similar adherence rates for ASV and other forms of PAP therapy were reported for patients with treatment-emergent CSA in another small study.20 The reported ASV adherence rate of 77% was, again, similar to that in our study.
In the Switch group in the current study, average AHI on CPAP was approximately 20 events/h. This reduced to an average of approximately 5 events/h after patients switched to ASV, suggesting that ASV was associated with a reduction in both obstructive and central apnea events. Other studies have also reported a reduction in the AHI after switching from CPAP to ASV. In one case series (n = 31), residual AHI during ASV was 4 ± 3 events/h compared with 9 ± 3 events/h in patients using other forms of PAP therapy (including CPAP) (P = .005).20 Similar values were reported in the only prospective, randomized clinical trial in this area, with 90-day AHI values of 4.4 events/h and 9.9 events/h in the ASV and CPAP groups, respectively (P = .0024).21 Data from a retrospective analysis showed that 80% of patients using ASV had an AHI of ≤ 5 events/h.19 Data obtained in nonrandomized trials have indicated that possible additional benefits of ASV therapy in patients with treatment-emergent CSA include less sleepiness,18,20,21 improved oxygen saturation,19 decreased respiratory-related arousals,19 a higher proportion of rapid eye movement sleep,18 and better subjective sleep quality.18 Unfortunately, such outcomes were not available in our big data analysis, and robust, randomized clinical trials are needed to better determine any potential long-term effects of ASV on clinical parameters.22 The results of another study of 21 patients who showed CSA during CPAP titration (monitored using split-night PSG) suggested that no change of therapy was necessary because treatment-emergent CSA was thought to be benign and transient.23 However, follow-up data were not available in one-third of the study sample (4 patients did not tolerate CPAP and 3 had no data).
In another study,15 a large cohort of patients was followed after CPAP initiation for OSA. Although most patients in whom treatment-emergent CSA developed eventually had spontaneous resolution of events, approximately 1.5% of patients who underwent titration showed persistence of respiratory events on follow-up. Moreover, the possibility exists that early intervention with ASV for patients with gradually resolving treatment-emergent CSA may result in improved adherence over time, as suggested by randomized trial data.21 In theory, it is possible that some patients included in our analysis were receiving opioids, which are associated with the occurrence of CSA,24 and may have been a reason for persistent CSA in some patients. Use of ASV has been shown to be effective for the treatment of CSA occurring during opioid therapy.25,26
One strength of the current study is the large number of patients included in the analysis. In addition, the fact that patients were unselected and treated as part of routine clinical practice means that the results are applicable to a range of different patients with sleep apnea. Although based on a very large dataset, this study also has a number of limitations, including a lack of demographic data for individual patients (eg, sex) and limited medical record information (eg, the type and severity of sleep apnea, and comorbidities). There was also no information on the type of apneas during diagnostic studies, meaning that we were unable to determine if some patients showed central events during initial sleep studies and therefore had persistent, rather than treatment-emergent, CSA during CPAP as shown previously.10 In addition, there are no data on the prevalence of comorbid cardiovascular disease or opioid use, factors that could influence the occurrence of CSA. Finally, the database also does not provide information on specific reasons that clinicians chose to switch their patients from CPAP to ASV. Further studies, including randomized trials, are needed to assess the effect of ASV in patients with persistent or treatment-emergent CSA during CPAP on hard clinical outcomes. In addition, we support mechanistic research to determine why treatment-emergent CSA occurs (eg, mouth and/or mask leak, washout of dead space, sleep state instability) and why it resolves in some, but not all, patients.
CONCLUSIONS
In patients with poorer CPAP adherence, possibly due to treatment-emergent CSA, PAP device usage can be improved by switching to ASV therapy. Analysis of sleep study data before and after a switch from CPAP to ASV would provide insight into the role of residual or treatment-emergent CSA in contributing to worse adherence during CPAP and improved adherence after changing to an ASV device.
DISCLOSURE STATEMENT
Work for this study was performed at ResMed Science Center Singapore in collaboration with University of Grenoble and University of San Diego. All authors have seen and approved this manuscript. This work was supported by ResMed Ltd. JLP is supported by the French National Research Agency in the framework of the “Investissements d'avenir” program (ANR-15-IDEX-02). PC holds an endowed academic Chair at the University of Sydney, created by funding from ResMed Inc. His department receives research support (equipment) from ResMed, SomnoMed, and Zephyr Sleep Technologies. He has been a consultant/advisor for Zephyr Sleep Technologies, NovoNordisk, and Fisher and Paykel Healthcare. JLP reports grants from ResMed, during the conduct of the study; grants and personal fees from ResMed, grants and personal fees from Philips, grants from Fisher and Paykel, grants and personal fees from Sefam, grants from Astra-Zeneca, grants and personal fees from Agiradom, personal fees from Elevie, grants and personal fees from Vitalaire, personal fees from Boehringer, outside the submitted work. DL, JA, AB, and SS are all employees of ResMed. HW was employed by ResMed during the conduct of the study; he has also received lecture fees from Vital Air, Boehringer Ingelheim, and ResMed, and research support from ResMed. AM is an Officer of the American Thoracic Society and has relinquished all outside personal income since 2012; ResMed provided a philanthropic donation to UC San Diego in support of a sleep center.
ACKNOWLEDGMENTS
The authors thank Gibson Gay and Yang Yan for their assistance with the analysis. Nicola Ryan, independent medical writer, supported the development of the manuscript at the direction of the authors.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AI
apnea index
- ASV
adaptive servoventilation
- CAI
central apnea index
- CI
confidence interval
- CPAP
continuous positive airway pressure
- CSA
central sleep apnea
- EPAP
expiratory positive airway pressure
- HI
hypopnea index
- OAI
obstructive apnea index
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- UAI
unknown apnea index
REFERENCES
- 1.Barbe F, Duran-Cantolla J, Sanchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307(20):2161–2168. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- 2.Bratton DJ, Stradling JR, Barbe F, Kohler M. Effect of CPAP on blood pressure in patients with minimally symptomatic obstructive sleep apnoea: a meta-analysis using individual patient data from four randomised controlled trials. Thorax. 2014;69(12):1128–1135. doi: 10.1136/thoraxjnl-2013-204993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campos-Rodriguez F, Martinez-Garcia MA, Reyes-Nunez N, Caballero-Martinez I, Catalan-Serra P, Almeida-Gonzalez CV. Role of sleep apnea and continuous positive airway pressure therapy in the incidence of stroke or coronary heart disease in women. Am J Respir Crit Care Med. 2014;189(12):1544–1550. doi: 10.1164/rccm.201311-2012OC. [DOI] [PubMed] [Google Scholar]
- 4.Craig SE, Kohler M, Nicoll D, et al. Continuous positive airway pressure improves sleepiness but not calculated vascular risk in patients with minimally symptomatic obstructive sleep apnoea: the MOSAIC randomised controlled trial. Thorax. 2012;67(12):1090–1096. doi: 10.1136/thoraxjnl-2012-202178. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Garcia MA, Campos-Rodriguez F, Catalan-Serra P, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med. 2012;186(9):909–916. doi: 10.1164/rccm.201203-0448OC. [DOI] [PubMed] [Google Scholar]
- 6.Damjanovic D, Fluck A, Bremer H, Muller-Quernheim J, Idzko M, Sorichter S. Compliance in sleep apnoea therapy: influence of home care support and pressure mode. Eur Respir J. 2009;33(4):804–811. doi: 10.1183/09031936.00023408. [DOI] [PubMed] [Google Scholar]
- 7.Richards D, Bartlett DJ, Wong K, Malouff J, Grunstein RR. Increased adherence to CPAP with a group cognitive behavioral treatment intervention: a randomized trial. Sleep. 2007;30(5):635–640. doi: 10.1093/sleep/30.5.635. [DOI] [PubMed] [Google Scholar]
- 8.Smith CE, Dauz E, Clements F, Werkowitch M, Whitman R. Patient education combined in a music and habit-forming intervention for adherence to continuous positive airway (CPAP) prescribed for sleep apnea. Patient Educ Couns. 2009;74(2):184–190. doi: 10.1016/j.pec.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith I, Nadig V, Lasserson TJ. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines for adults with obstructive sleep apnoea. Cochrane Database Syst Rev. 2009;(2):CD007736. doi: 10.1002/14651858.CD007736. [DOI] [PubMed] [Google Scholar]
- 10.Liu D, Armitstead J, Benjafield A, et al. Trajectories of emergent central sleep apnea during CPAP therapy. Chest. 2017;152(4):751–760. doi: 10.1016/j.chest.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westhoff M, Arzt M, Litterst P. Prevalence and treatment of central sleep apnoea emerging after initiation of continuous positive airway pressure in patients with obstructive sleep apnoea without evidence of heart failure. Sleep Breath. 2012;16(1):71–78. doi: 10.1007/s11325-011-0486-0. [DOI] [PubMed] [Google Scholar]
- 12.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 13.Cassel W, Canisius S, Becker HF, et al. A prospective polysomnographic study on the evolution of complex sleep apnoea. Eur Respir J. 2011;38(2):329–337. doi: 10.1183/09031936.00162009. [DOI] [PubMed] [Google Scholar]
- 14.Endo Y, Suzuki M, Inoue Y, et al. Prevalence of complex sleep apnea among Japanese patients with sleep apnea syndrome. Tohoku J Exp Med. 2008;215(4):349–354. doi: 10.1620/tjem.215.349. [DOI] [PubMed] [Google Scholar]
- 15.Javaheri S, Smith J, Chung E. The prevalence and natural history of complex sleep apnea. J Clin Sleep Med. 2009;5(3):205–211. [PMC free article] [PubMed] [Google Scholar]
- 16.Morgenthaler TI, Kagramanov V, Hanak V, Decker PA. Complex sleep apnea syndrome: is it a unique clinical syndrome? Sleep. 2006;29(9):1203–1209. doi: 10.1093/sleep/29.9.1203. [DOI] [PubMed] [Google Scholar]
- 17.Neu D, Balkissou AD, Mairesse O, Pefura-Yone EW, Noseda A. Complex sleep apnea at auto-titrating CPAP initiation: prevalence, significance and predictive factors. Clin Respir J. 2017;11(2):200–209. doi: 10.1111/crj.12325. [DOI] [PubMed] [Google Scholar]
- 18.Allam JS, Olson EJ, Gay PC, Morgenthaler TI. Efficacy of adaptive servoventilation in treatment of complex and central sleep apnea syndromes. Chest. 2007;132(6):1839–1846. doi: 10.1378/chest.07-1715. [DOI] [PubMed] [Google Scholar]
- 19.Brown SE, Mosko SS, Davis JA, Pierce RA, Godfrey-Pixton TV. A retrospective case series of adaptive servoventilation for complex sleep apnea. J Clin Sleep Med. 2011;7(2):187–195. [PMC free article] [PubMed] [Google Scholar]
- 20.Correia S, Martins V, Sousa L, Moita J, Teixeira F, Dos Santos JM. Clinical impact of adaptive servoventilation compared to other ventilatory modes in patients with treatment-emergent sleep apnea, central sleep apnea and Cheyne-Stokes respiration. Rev Port Pneumol (2006) 2015;21(3):132–137. doi: 10.1016/j.rppnen.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Morgenthaler TI, Kuzniar TJ, Wolfe LF, Willes L, McLain WC, 3rd, Goldberg R. The complex sleep apnea resolution study: a prospective randomized controlled trial of continuous positive airway pressure versus adaptive servoventilation therapy. Sleep. 2014;37(5):927–934. doi: 10.5665/sleep.3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Javaheri S, Brown LK, Randerath WJ. Clinical applications of adaptive servoventilation devices: part 2. Chest. 2014;146(3):858–868. doi: 10.1378/chest.13-1778. [DOI] [PubMed] [Google Scholar]
- 23.Dernaika T, Tawk M, Nazir S, Younis W, Kinasewitz GT. The significance and outcome of continuous positive airway pressure-related central sleep apnea during split-night sleep studies. Chest. 2007;132(1):81–87. doi: 10.1378/chest.06-2562. [DOI] [PubMed] [Google Scholar]
- 24.Webster LR, Choi Y, Desai H, Webster L, Grant BJ. Sleep-disordered breathing and chronic opioid therapy. Pain Med. 2008;9(4):425–432. doi: 10.1111/j.1526-4637.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- 25.Cao M, Cardell CY, Willes L, Mendoza J, Benjafield A, Kushida C. A novel adaptive servoventilation (ASVAuto) for the treatment of central sleep apnea associated with chronic use of opioids. J Clin Sleep Med. 2014;10(8):855–861. doi: 10.5664/jcsm.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Javaheri S, Harris N, Howard J, Chung E. Adaptive servoventilation for treatment of opioid-associated central sleep apnea. J Clin Sleep Med. 2014;10(6):637–643. doi: 10.5664/jcsm.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]