Abstract
Study Objectives:
To examine repeatability of Multiple Sleep Latency Test (MSLT) results in narcolepsy type 1 (NT1) and narcolepsy type 2 (NT2) according to the criteria of the International Classification of Sleep Disorders, Third Edition (ICSD-3).
Methods:
Repeatability of the MSLT was retrospectively evaluated in NT1 (n = 60) and NT2 (n = 54) cases, and controls (n = 15). All subjects had documented HLA-DQB1*06:02 status and/or hypocretin-1 levels from cerebrospinal fluid. All subjects had undergone 2 MSLTs (≥ 1 meeting ICSD-3 criteria for narcolepsy). Repeatability was explored in children versus adults and in those on versus not on medication(s). Subsample and multivariate analysis were performed.
Results:
Both MSLTs in unmedicated patients were positive for narcolepsy in 78%, 18%, and 7% of NT1, NT2, and controls, respectively. NT2 cases changed to idiopathic hypersomnia or to a negative MSLT 26% and 57% of the time, respectively. Although NT1 cases were 10 to 14 times more likely to demonstrate a second positive MSLT compared to NT2 cases (P < 10−5) and controls (P < 10−4), respectively, NT2 cases were not significantly different from controls (P = .64). Medication use (P = .009) but not adult versus children status (P = .85) significantly decreased the likelihood of a repeat positive MSLT.
Conclusions:
In a clinical setting, a positive MSLT for narcolepsy is a more reproducible and stable feature in NT1 than NT2. The retrospective design of this study hinders interpretation of these data, as there are many different, and possibly opposing, reasons to repeat a MSLT in NT1 versus NT2 (ie, ascertainment bias). Additional systematic MSLT repeatability studies independent of confounds are ideally needed to confirm these findings.
Citation:
Ruoff C, Pizza F, Trotti LM, Sonka K, Vandi S, Cheung J, Pinto S, Einen M, Simakajornboon N, Han F, Peppard P, Nevsimalova S, Plazzi G, Rye D, Mignot E. The MSLT is repeatable in narcolepsy type 1 but not narcolepsy type 2: a retrospective patient study. J Clin Sleep Med. 2018;14(1):65–74.
Keywords: cataplexy, HLA-DQB1*06:02, hypocretin-1, ICSD-3, idiopathic hypersomnia, MSLT, narcolepsy type 1, narcolepsy type 2
BRIEF SUMMARY
Current Knowledge/Study Rationale: Recent data have demonstrated poor repeatability of the MSLT in narcolepsy type 2 and idiopathic hypersomnia. This retrospective study compared the repeatability of the MSLT in narcolepsy type 1, narcolepsy type 2, and controls according to the criteria of the International Classification of Sleep Disorders, Third Edition.
Study Impact: We demonstrated that MSLT criterion for narcolepsy in a clinical sample is a more repeatable and stable finding in narcolepsy type 1 than narcolepsy type 2, and narcolepsy type 2 is not more repeatable than controls. Additional prospective MSLT repeatability studies are needed to confirm these findings, as there are many different, and possibly opposing, reasons to repeat an MSLT in narcolepsy type 1 versus narcolepsy type 2, which were largely unknown in this retrospective study.
INTRODUCTION
The Multiple Sleep Latency Test (MSLT) was first established to document and quantify the intensity of sleepiness in the context of sleep deprivation, and called for the immediate awakening of the subject after sleep onset in each nap (the so-called “experimental MSLT”).1 Because narcolepsy with cataplexy was shown to exhibit sleep onset rapid eye movement (REM) periods (SOREMPs) during nocturnal sleep only half of the time,2,3 the MSLT was modified, extending the recording for 15 minutes from sleep onset to increase the likelihood of documenting REM sleep during a nap (the so-called “clinical MSLT”).1 Additional studies established that a mean sleep latency (MSL) of less than 5 minutes and at least 2 SOREMPs during naps was sensitive and, therefore, by extension, diagnostic for narcolepsy with cataplexy.4,5 This criteria was subsequently modified to a MSL ≤ 8 minutes and ≥ 2 SOREMPs as this raised the sensitivity from 70% to 80% to 90% to 95% without a significant loss of specificity (∼95%).6,7 In a recent study of 479 patients with narcolepsy with cataplexy and 509 controls, specificity and sensitivity were 98.6% and 92.9%, respectively, making the MSLT a reasonable diagnostic test.8
As the field progressed, new clinical entities were described. In Japan, Matsuki et al., in 1987, coined the term “Essential Hypersomnia Syndrome” for a putatively milder form of narcolepsy (ie, sleepiness, restorative naps, and a weaker human leukocyte antigen [HLA] association), clearly differentiating it from narcolepsy with cataplexy.9 These cases were often found in relatives of individuals with narcolepsy with cataplexy.10 The MSLT was not used in Japan at the time. In the Czech Republic, Roth, in 1976, stressed the importance of unrefreshing sleep and long total sleep time to define an entity, also often familial in nature, which was called “Idiopathic Hypersomnia.”11 These cases were often diagnosed using continuous polysomnography (PSG) for 24 to 48 hours to objectively document long sleep times.12–14
In the United States, the MSLT rapidly became the gold standard for evaluating and diagnosing disorders of hyper-somnolence. Historically, as adoption of the MSLT increased, an increasing number of patients were found to satisfy MSLT criteria for narcolepsy in the absence of cataplexy. These cases were identified as “narcolepsy without cataplexy.”6 However, studies as early as 1997 by Aldrich et al.,15 and later in population-based samples,16–18 suggested that widespread administration of the MSLT was bound to generate an increased number of false positives, estimated at 2.5% to 4.7% in large case series,15–19 due, in part, to the high frequency of sleep apnea diagnoses in patients presenting to sleep clinics.19 Extended studies in large sets of population-based controls16–18 have also shown that the MSLT can also be confounded by shift work or circadian misalignment, and to a lesser extent by chronic sleep restriction.18,20 Similarly, diagnostic criteria for idiopathic hypersomnia (IH) evolved and included not only patients with prolonged sleep (eg, habitual sleep time ≥ 10 hours) but also subjects with objective sleepiness based on MSL ≤ 8 minutes alone, an a priori defined cutoff derived from results in narcolepsy with cataplexy cases.6 Using the MSL ≤ 8 minutes to define IH, according to studies in the general population, indicated that approximately 22% of the population meets this criteria,16–18 risking overdiagnosis of IH as well. In 2005, the International Classification of Sleep Disorders, Second Edition (ICSD-2) effectively captured these diagnostic categories as narcolepsy with cataplexy, narcolepsy without cataplexy, and idiopathic hypersomnia with and without long sleep time.6
Based on recent advances indicating that most cases of narcolepsy with cataplexy are caused by hypocretin/orexin deficiency, in 2014, the International Classification of Sleep Disorders, Third Edition (ICSD-3) refers to narcolepsy with and without cataplexy as narcolepsy type 1 (NT1) and narcolepsy type 2 (NT2), respectively.21 Because a nocturnal SOREMP is highly specific for narcolepsy (99%),8 the ICSD-3 narcolepsy classification was modified to include a nocturnal SOREMP in the total number of SOREMPs (ie, MSL ≤ 8 minutes, ≥ 2 SOREMPs).21 To date, studies suggest that counting a nocturnal SOREMP in the total number of SOREMPs does not lead to a significant change in diagnosis.8,22,23 Regarding IH, the ICSD-3 collapsed both with and without long sleep time into one clinical entity.21
The overreliance of the ICSD-3 on the MSLT presents the clinician with a number of diagnostic dilemmas. First, the MSLT has only been properly validated in adult patients with NT1/narcolepsy with cataplexy. The other remaining diagnostic entities subsumed under the umbrella of central disorders of hypersomnia/hypersomnolence are essentially diagnoses of exclusion that have relied upon a test prior to completion of proper validation studies. Diagnoses are therefore frequently rendered without regard to accumulating evidence that: (1) population controls without any subjective complaints of sleepiness can also demonstrate MSLT findings consistent with NT117; and (2) test-retest reliability of the MSLT outside the context of NT1 appears poor.24 A recent study, for example, found that 10 of 15 subjects in whom a diagnosis of NT2 was made on their first MSLT were reclassified as either normal (n = 7) or IH (n = 3) upon repeat testing.24 Second, MSLT requirements are especially problematic for patients with IH25,26 because: (1) false-negative MSLT results have been reported in 71% of cases with IH with long sleep times27; and (2) there are no Food and Drug Administration-approved treatments for IH.28–31 Third, the MSLT requires all treatments to be discontinued in order to be valid,32 and some patients with unequivocal NT1, who have been successfully treated sometimes for decades, have been required to undergo a valid, repeat MSLT.
In this study, we retrospectively explored the repeatability of the MSLT in NT1 cases (cataplexy and HLA-DQB1*06:02 positive, or hypocretin-1 deficient in cerebrospinal fluid [CSF]) or NT2 (no cataplexy/unclear cataplexy and HLA-DQB1*06:02 negative, or normal hypocretin level in cerebrospinal fluid). All cases had either documented HLA status or hypocretin-1 levels from CSF. All subjects had undergone 2 MSLTs (≥ 1 meeting ICSD-3 criteria for narcolepsy). The influence of medication and pediatric status was examined, and the results were compared to subjects exhibiting at least 1 positive MSLT from the Wisconsin Sleep Cohort, a population-based sample.18
METHODS
Patients and Controls
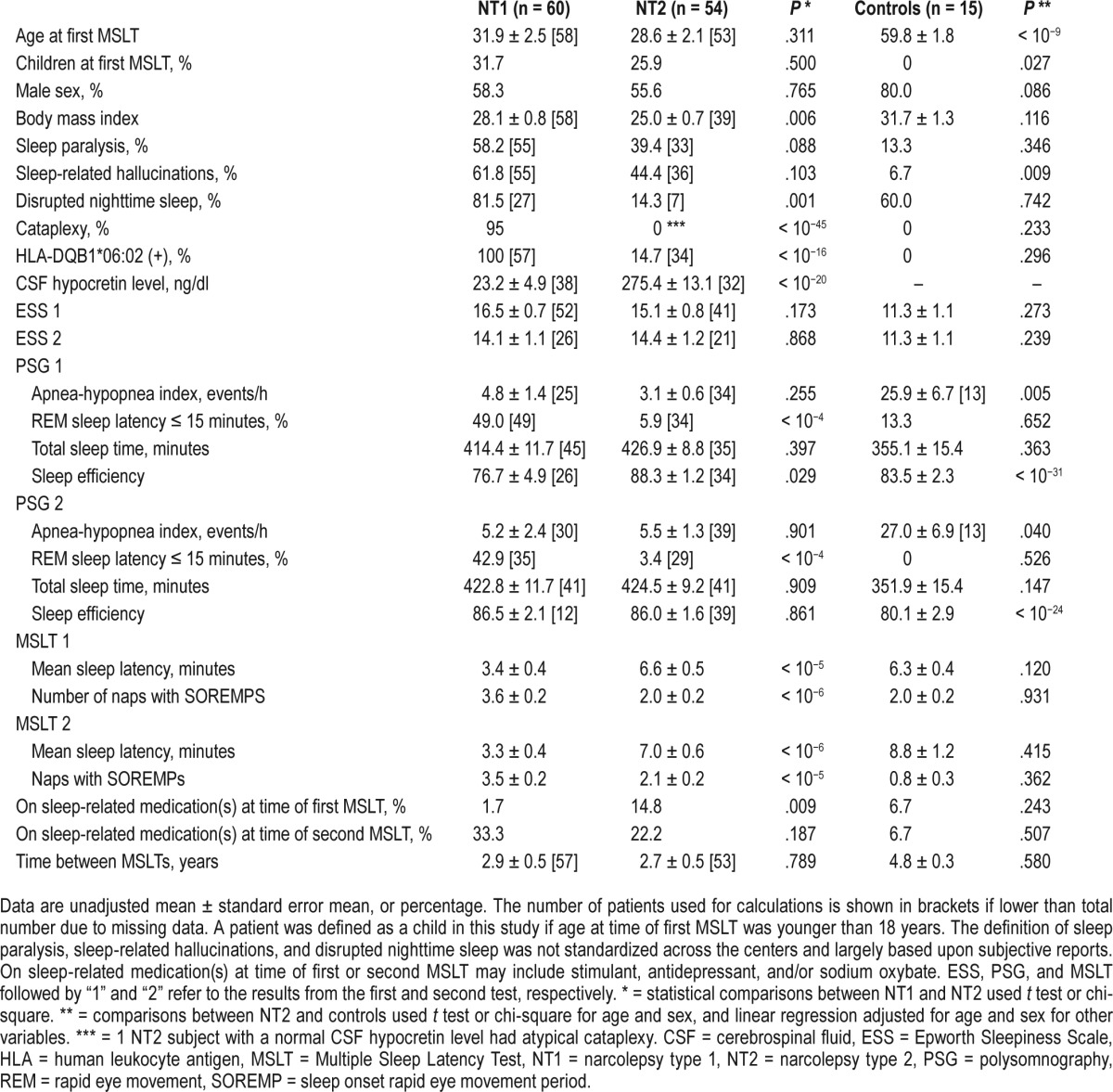
Multiple centers retrospectively identified patients with an ICSD-3 diagnosis of hypersomnia or narcolepsy who had at least 2 clinical MSLTs. Requested information included age, sex, body mass index (BMI), presence of narcolepsy symptoms, Epworth Sleepiness Scale, CSF hypocretin-1 (hypocretin-1) level, HLA-DQB1*06:02 status, PSG/MSLT results with medication status and time between the 2 MSLTs. We were not able to systematically confirm reason(s) for each repeat MSLT. Specific medications requested included antidepressants, psychostimulants or other wake-promoting agents, and sodium oxybate. When patients were on medication, antidepressant(s) were administered as prescribed the day of the PSG and MSLT; psychostimulants or other wake-promoting agents were held the day of the MSLT; and, sodium oxybate was administered the night of the PSG. Patients deemed untreated at the time of the MSLT were free of these medications for at least 2 weeks. Genetic typing of HLA-DQB1*06:02 was performed using a sequence-specific polymerase chain reaction.33 CSF hypocretin-1 concentrations were determined as previously described.34 All subjects had undergone PSG followed by a MSLT on 2 separate occasions (≥ 1 meeting ICSD-3 criteria for narcolepsy). We dichotomized these patients into definitive NT1 (ie, CSF hypocretin-1 ≤ 110 pg/mL, or clear cataplexy plus HLA-DQB1*06:02 positive status) or NT2 (no clear cataplexy and either normal CSF hypocretin-1 or HLA-DQB1*06:02 negative status). NT2 cases with HLA-DQB1*06:02 positivity but with unknown CSF hypocretin levels were excluded to avoid the possibility of pathophysiological overlap with NT1, as approximately 15% of patients with NT2 seen in sleep clinics have low CSF hypocretin when tested.21 NT2 was diagnosed after exclusion of sleep-disordered breathing defined as an apnea-hypopnea index (AHI) > 10 events/h based upon the first PSG/MSLT. We did not exclude subjects based upon AHI from the second PSG. Delayed-type circadian rhythm disorder/shift work and major depressive disorder were also excluded before diagnosing NT2 based upon clinical assessment (we did not systematically collect sleep diaries and/or actigraphy). A total of 60 subjects with NT1 and 54 with NT2 were included in our analyses (Table 1). These patients include 37 from Stanford University, 28 (including 7 from a prior publication24) from Emory University, 24 from Bologna University, 13 from Charles University, 9 from Cincinnati Children's Hospital, and 3 from Peking University. As an additional basis for comparison, we also included 15 subjects who were HLA negative, non-shift workers with at least 2 clinical MSLTs (≥ 1 meeting MSLT ICSD-3 criteria for narcolepsy) from the Wisconsin Sleep Cohort (from 273 cases with repeated MSLTs) as published in Goldbart et al.18 The reason for selecting HLA negativity was to exclude the possibility of undiagnosed NT1 cases without cataplexy, because HLA association with hypocretin deficiency is over 97%. The presence of a “control” subject with 2 positive MSLTs in the Wisconsin cohort may reflect a genuine undiagnosed and rare narcolepsy without cataplexy or the occurrence of 2 false-positive MSLTs by chance. The subject was HLA negative and could not be re-contacted for further evaluation. We did not exclude controls based upon AHI findings from either PSG.
Table 1.
Demographic and clinical data in NT1 and NT2, and controls.
Statistical Analysis
First, demographics, symptoms, and clinical presentation were first compared between NT1 and NT2, and Wisconsin Sleep Cohort volunteers who had at least 1 positive MSLT (Table 1). Demographic variables reported in the table are based on results obtained from the first PSG. The t test was used for continuous variables, and chi-square or Fisher exact tests were used for dichotomous variables. Because Wisconsin Sleep Cohort subjects were significantly older, comparisons with this group were adjusted for age, but this may not have been sufficient to eliminate group differences related to this variable. MSLT sleep latency and propensity to REM sleep decreases with age in both controls18 and individuals with narcolepsy,35 and this may explain why repeatability in controls is even lower than in NT2. Although Wisconsin Sleep Cohort subjects had a significantly elevated AHI, we thought that these controls were appropriate to include in this study given the design and goals of the study. We analyzed the repeatability of an MSLT satisfying ICSD-3 narcolepsy criterion, reported as % repeatability, before and after excluding all patients taking psychoactive medication(s) at time of first and/or second MSLT. Second, we used multivariate logistic regression analysis to probe for factors potentially influencing repeatability of the second MSLT after a first positive MSLT, exploring the effect of the following covariates: age (including children versus adults), sex, BMI, AHI, sleep paralysis, sleep hallucinations, disrupted nighttime sleep, and medication at time of the second MSLT (antidepressant, psychostimulant or other wake-promoting agent(s), sodium oxybate, or any of the aforementioned medications). Medication use (P = .009), but not adult versus children status (P = .85), significantly decreased the likelihood of a repeat positive MSLT. Other covariates did not significantly influence the repeatability of a positive MSLT. Our rationale for requiring the first MSLT be positive, not negative, for narcolepsy, was that if the first MSLT was negative, according to ICSD-3 criteria, the second MSLT would have to satisfy narcolepsy criteria (ie, a 100% chance), thereby precluding more sophisticated analyses. Third, because only medication was found to influence repeatability, we calculated % repeatability and the odds ratio (OR) for obtaining a second positive MSLT after a first positive MSLT in NT1 versus NT2, NT1 versus controls, and NT2 versus controls, all subjects untreated at the second MSLT for at least 2 weeks. As a small fraction of NT2 cases with an initial positive MSLT exhibited a second positive MSLT (n = 9), we compared demographic and PSG data between NT2 with “stable” MSLT findings versus other NT2 cases with only an initial positive MSLT (n = 21). Forth, Bland-Altman plots were created for repeatability of MSL and SOREMPs for NT1 and NT2 after excluding all patients taking medication(s) at the time of first and/or second MSLT. SPSS Statistics for Macintosh, Version 23.0 (IBM Corp., Armonk, New York, United States) was used to perform all statistical analyses. Significance level was set at 5% (P < .05).
RESULTS
Sample Characteristics
Descriptive statistics are shown in Table 1. As expected from the literature,8,36–38 subjects with NT1 had higher BMI, shorter MSL on the MSLT, and more SOREMPs on the MSLT and during nocturnal PSG in comparison with those with NT2. Similar to previous reports,8,22,23 finding a SOREMP on nocturnal PSG, and factoring it into the total number of SOREMPs as outlined by ICSD-3 criteria, did not change the results of a single MSLT in this entire sample. Because NT2 mandates exclusion of significant sleep-disordered breathing, patients with NT2 also had a lower AHI than did those with NT1. Controls from the Wisconsin Sleep Cohort were significantly older because they were recruited more than 20 years ago.18 The AHI was elevated in the Wisconsin Sleep Cohort compared to NT1 and NT2. Sleep efficiency was lowest in NT1. No significant differences were found in time elapsed between the 2 MSLTs in NT1 and NT2, although time was longer for the small sample of Wisconsin Sleep Controls. The first and second MSLT results (ie, MSL and SOREMPs) were found largely comparable within NT1, NT2, and controls but differed most across disease categories. Significantly more individuals with NT2 than NT1 were actively taking psychoactive medication(s) at time of the first MSLT but there was no significant difference found at the second MSLT. In our more sophisticated analyses, we still included medication(s) at the second MSLT as a covariate given the dearth of data on how medications affect the MSLT in NT1 and NT2.
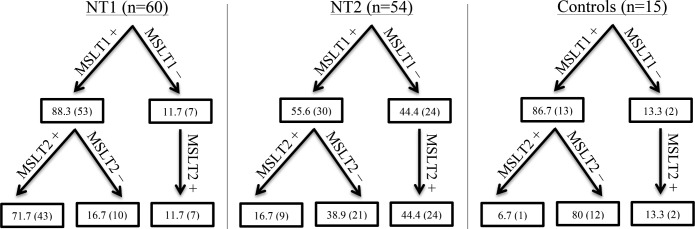
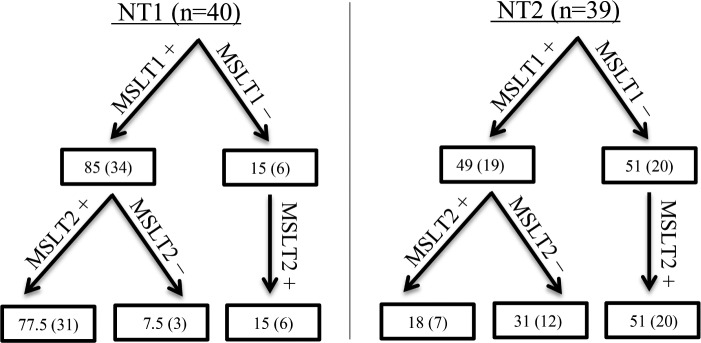
Both MSLTs satisfied narcolepsy criteria in 71.7%, 16.7%, and 6.7% in NT1 (n = 60), NT2 (n = 54), and controls (n = 15), respectively, irrespective of medication status (Figure 1). The MSLTs (n = 17) from subjects with NT1 not meeting ICSD-3 criteria for narcolepsy were due to 17.6%, 47.1%, and 35.3% not satisfying MSL, SOREMP, or both criteria, respectively. The MSLTs (n = 45) from the subjects with NT2 not meeting ICSD-3 criteria for narcolepsy were due to 16%, 31%, and 53% not satisfying MSL, SOREMP, or both criteria, respectively. As far as a change in diagnosis from the first MSLT to the second, or vice versa, NT2 cases changed from narcolepsy to IH criteria 25.9% of the time, compared to controls and NT1 results changing 26.7% and 13.3% of the time, respectively. In addition, MSLT results in NT2 cases changed from narcolepsy to not meeting IH or narcolepsy MSLT criteria (ie, a negative MSLT) 57.4% of the time, compared to controls and NT1 results changing 66.7% and 15% of the time, respectively. After excluding patients with NT1 and NT2 taking medication(s) at time of first and/or second MSLT, both MSLTs satisfied narcolepsy criteria in 77.5% (n = 40) and 18% (n = 39), respectively (Figure 2).
Figure 1. MSLT repeatability for NT1, NT2, and controls.
MSLT1 and MSLT2 refer to the first and second MSLT, respectively. Each positive and negative sign denotes whether the MSLT was positive or negative for narcolepsy criteria (MSL ≤ 8 minutes, ≥ 2 SOREMPs based on ICSD-3 criteria), respectively. Data are % (n) (of total population). ICSD-3 = International Classification of Sleep Disorders, Third Edition, MSL = mean sleep latency, MSLT = Multiple Sleep Latency Test, NT1 = narcolepsy type 1, NT2 = narcolepsy type 2, SOREMP = sleep onset rapid eye movement period.
Figure 2. MSLT repeatability for NT1 and NT2 after excluding patients taking medication(s) at time of first and/or second MSLT.
MSLT1 and MSLT2 refer to the first and second MSLT, respectively. Each positive and negative sign denotes whether the MSLT was positive or negative for narcolepsy criteria (MSL ≤ 8 minutes, ≥ 2 SOREMPs based on ICSD-3 criteria), respectively. Data are % (n) (of total population). Medication(s) may include stimulant(s), antidepressant, and/or sodium oxybate at time of second MSLT. ICSD-3 = International Classification of Sleep Disorders, Third Edition, MSL = mean sleep latency, MSLT = Multiple Sleep Latency Test, NT1 = narcolepsy type 1, NT2 = narcolepsy type 2, SOREMP = sleep onset rapid eye movement period.
Because this is retrospective study, the clinical decision to repeat a MSLT was not random. As a result, many more subjects with NT2 (44.4%) versus NT1 (11.7%) narcolepsy had an initial MSLT that was negative (OR = 6.1, P < .001) (Figure 1). In controls, subjects who had a first positive MSLT were also preferentially selected for replication,18 again, biasing the sample (subjects with multiple positive MSLTs were typically shift workers). According to ICSD-3 criteria, a diagnosis of narcolepsy cannot be made without at least 1 positive MSLT; therefore, more sophisticated analyses could not be performed on those with an initial negative MSLT (as discussed in the methods). As a consequence, additional analyses of repeatability, including all reported ORs, were performed in subjects with an initial positive MSLT, thus reducing our sample size in each category (NT1, n = 53; NT2, n = 30; controls, n = 13).
Repeatability of MSLTs Between and Within NT1 and NT2
We conducted multivariate analyses with the dependent variable being whether or not the MSLT repeats positive for narcolepsy both times and the independent variables being diagnosis (NT1 versus NT2; NT1/NT2 versus controls); age (continuous, and adults versus children < 18 years); and medication status (antidepressant, psychostimulant or other wake-promoting agent(s), or sodium oxybate). Overall, repeatability of a positive MSLT in subjects with an initial positive MSLT was 81.1%, 30%, and 7.7% in NT1 (n = 53), NT2 (n = 30), and controls (n = 13). In all models, diagnosis was highly significant but age was not. We also explored the effects of no medication versus any medication(s) on the repeatability of a positive MSLT. We found a significant effect of medication(s) (ie, untreated versus treated) in NT1 (OR = 6.03 [1.34–27.23], P = .02) but not in NT2 (OR 4.92 [0.52–47.10], P = .17). Medication status, however, was not significant when the effect of individual psychoactive drug classes (ie, antidepressant(s), stimulant(s), or sodium oxybate) were analyzed. Although we did not find a significant effect of age (ie, ≥ 18 years versus < 18 years) in NT1 nor NT2, adult subjects with NT1 not on any medications (n = 23) had a repeat positive MSLT 95.7% of the time (see also Figure S1 and Figure S2 in the supplemental material). For these reasons, all further models are presented as both unadjusted and adjusted for medication(s) at the time of the second MSLT (Table 2). Because a large age difference is present in controls, comparisons with controls were also adjusted for age as a continuous variable (Table 2).
Table 2.
Comparisons of MSLT repeatability between NT1, NT2, and controls.
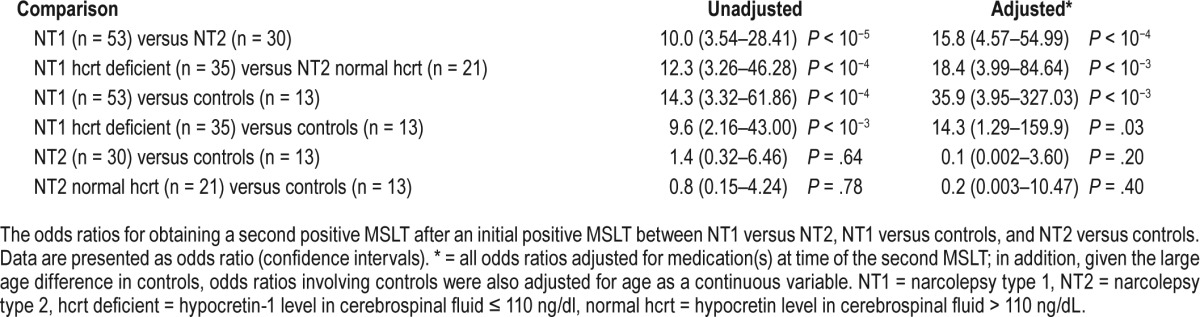
We explored the repeatability in subjects with NT1 versus NT2 after an initial positive MSLT (Table 2). As expected, repeatability was significantly higher in NT1 versus NT2 (OR = 10.0, P < 10−5) and only increased after excluding those on medication(s) at time of second MSLT (OR = 16.8 [3.84– 73.51], P < 10−4). Repeatability was significantly higher in adults with NT1 versus adults with NT2 (OR = 17 [4.70–61.24], P < 10−5) but not significantly higher in children (OR = 2.5 [0.35–18.04], P = .357). This nonsignificant finding in children is likely due, in part, to a small sample size (NT1 children, n = 13; NT2 children, n = 7). Furthermore, there was not a significant difference in repeatability in adults versus children within NT1 (P = .655) or NT2 (P = .073).
Repeatability of MSLTs in Narcolepsy Versus Controls
In comparison to controls, repeatability of an initial positive MSLT was significantly higher in NT1 but not in NT2 subjects (Table 2). A similarly high repeatability was found in the subgroup of NT1 cases whose CSF hypocretin-1 was documented to be ≤ 110 pg/mL, thereby indicating that our conservative definition of NT1 to also include subjects with HLA-DQB1*06:02 and cataplexy (ie, absent CSF determination of hypocretin-1) was justified. Repeatability of a positive MSLT was not significantly different in NT2 cases versus controls. Similar results were obtained when adjusting for age and medication (Table 2) with the caveat that age was older and age range narrower in the controls. MSLT SOREMP propensity slightly decreases with age in NT1,35 whereas the effect is unclear in controls or NT2 cases.16,18 Nonetheless, this may explain why repeatability in controls was even lower (although not statistically so) than in NT2 in this study.
Characteristics of NT2 With Concordant MSLTs
After an initial positive MSLT for narcolepsy, the second MSLT did not satisfy the ≥ 2 SOREMPs, MSL ≤ 8 minutes, and neither criteria (≥ 2 SOREMPS and MSL ≤ 8 minutes) in 13.3%, 13.3%, and 43.3% of NT2 cases (n = 30), respectively. As 30% of NT2 demonstrated a positive MSLT upon repeat testing, which exceeds the 8% observed in controls (nonsignificant), it is feasible that a portion of NT2 cases may have a unique and stable trait-like level of sleepiness as measured by MSL and increased propensity to REM sleep as measured by SOREMPs on the MSLT as part of their core phenotype. There were only 9 subjects with NT2 whose 2 MSLTs were concordant. Although these small sample sizes forbid us from performing statistical analyses, we still investigated for any potential differences in demographics and PSG characteristics between these two groups. There were no discernible differences between these two groups (data not shown).
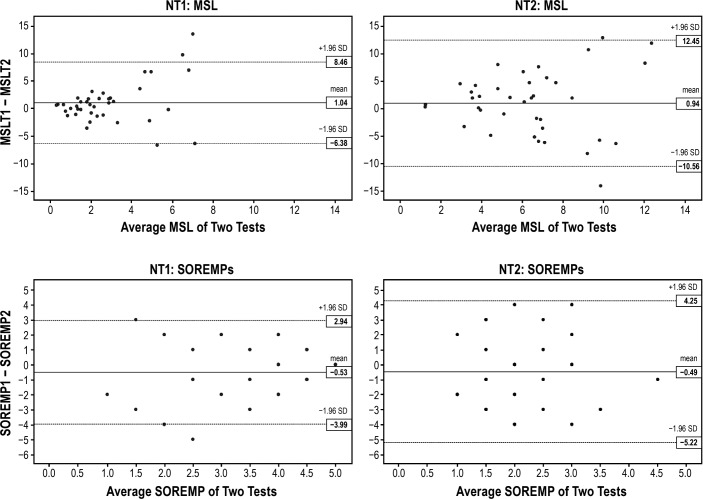
Bland-Altman Plots for MSL and SOREMPs in NT1 and NT2
After excluding patients with NT1 and NT2 taking medication(s) at time of first and/or second MSLT, Bland-Altman plots for MSL had narrower limits of agreement for NT1 than NT2 (Figure 3). Both NT1 and NT2 demonstrated less variability at the lower mean MSL values with NT1 demonstrating the least variability. Bland-Altman plots for SOREMPs had narrower limits of agreement for NT1 than NT2. Although there was a tendency for SOREMPs to vary less at the higher mean values for NT1, there was no clear trend for SOREMPs for NT2.
Figure 3. Bland-Altman plots for NT1 and NT2 after excluding patients taking medication(s) at either MSLT.
Bland Altman Plots for repeatability of MSL and SOREMPs for NT1 and NT2 after excluding patients taking medication(s) at time of first and/or second MSLT. MSL1 and MSL2 represent MSL on first and second MSLT, respectively. SOREMP1 and SOREMP2 represent number of SOREMPs on first and second MSLT, respectively. MSL = mean sleep latency, MSLT = Multiple Sleep Latency Test, NT1 = narcolepsy type 1, NT2 = narcolepsy type 2, SOREMP = sleep onset rapid eye movement period.
DISCUSSION
Our finding of a high concordance for a positive MSLT (varying from 72% to 78% depending on medication status) in NT1 supports the concept that MSLT criteria for narcolepsy as defined by the ICSD-3 is a core feature of NT1. Although this is consistent with past studies in which most of the cases were defined by the presence of cataplexy alone, all subjects in this study had biologic data (ie, documented HLA status and/or hypocretin-1 levels from CSF) to help confirm diagnosis.4,5,15,39 Although the NT1 group was shown to have a higher prevalence of nocturnal SOREMPs (approximately 50%), a nocturnal SOREMP did not change a single MSLT result (ie, either the first or second MSLT) in this study when interpreted using ICSD-3 criteria, which is consistent with previous reports.22,23,37 Our data suggest that repeating a PSG-MSLT after an initial positive MSLT (ie, documenting 2 positive MSLTs for narcolepsy) may help to confirm a diagnosis of NT1 if there is a strong suspicion of hypocretin deficiency or if the diagnosis is in question (eg, conversion disorder).40–43 Even though our data suggest that 2 positive MSLTs for narcolepsy significantly increases the odds for NT1, we are not suggesting that repeating MSLTs become routine clinical practice because performing a lumbar puncture to determine CSF hypocretin-1 concentrations is much more definitive. Not surprisingly, in NT1 cases, taking a psychoactive medication(s) significantly decreased the repeatability of a positive MSLT but this finding did not persist when we analyzed individual psychoactive drug classes (ie, antidepressants, stimulants, or sodium oxybate).
These results stand in stark contrast to data obtained in subjects with NT2. In our sample of subjects with NT2, the concordance for a positive MSLT was quite low (varying from 17% to 18% depending on medication status) and not significantly different than controls (7%). Further, 26% of the NT2 cases changed from narcolepsy to IH, and vice versa. Regarding the 30 subjects with NT2 and an initial positive MSLT, 13.3%, 13,3%, and 43.3% did not replicate due to lack of ≥ 2 SOREMPs, MSL > 8 minutes, and not meeting either criteria, respectively, leaving only 30% of subjects with NT2 with repeat positive MSLTs. Our inclusion criteria for the NT2 group was exceedingly strict in order to minimize potential confounders and type 1 (false positives) error. Inclusion criteria for this group was no clear cataplexy and either normal CSF hypo-cretin-1 or HLA-DQB1*06:02 negative status, minimizing the possibility of unrecognized NT1 (eg, HLA positive cases with no cataplexy but hypocretin deficiency36). In addition, psycho-active medication(s) did—albeit not significantly—decrease the repeatability of a positive MSLT in NT2. These results expand upon data reported by Trotti et al.,24 suggesting a single positive MSLT as defined by ICSD-3 has little diagnostic value as currently defined. This study, in contrast to that of Trotti et al., included NT1 and controls in addition to NT2 cases with biologic data available on all subjects (ie, documented HLA status and/or CSF hypocretin levels). It seems that the continued use of the MSLT as per ICSD-3 to differentiate NT2 from IH should be reevaluated.
Although the small sample sizes in the NT2 cases with versus without concordant MSLTs precluded us from performing statistical analyses, we did not find any striking differences between these two groups, arguing against the possibility of a clinically distinct subgroup of NT2 with repeatable, trait-like MSLT findings (eg, subjects with hypocretin receptor or other downstream abnormalities). Because we did not systematically collect actigraphy and/or sleep diaries, it is possible that some of these subjects with NT2 may have had undetected abnormal circadian abnormalities or increased sleep debt at the time of positive testing, associations that have been shown in controls, which could explain the poor repeatability in this group.18,20,44 Systematically following standardized MSLT procedures including the routine use of actigraphy and sleep diaries prior to testing, in addition to collecting a comprehensive history in patients with excessive daytime sleepiness, may be a critical step in routine clinical practice that has not been given enough attention to date.
This study has strengths and limitations. The strengths include a large sample size collected in a clinical setting with CSF and/or HLA testing available for all subjects with NT1 and NT2 as well as controls. Limitations include a sampling bias in that the database comprised patients from various medical facilities (threshold for re-testing and testing protocols likely vary and reason(s) for retesting were not taken into account), a retrospective design, missing data, and the difficulty in ensuring that all differential diagnoses (notably insufficient sleep and circadian phase abnormalities since actigraphy and/ or sleep diaries were not systematically collected in this study) were excluded equally across sites. Because CSF hypocretin-1 level was measured only when it is indicated based on clinical suspicion, subjects with CSF hypocretin levels available for study was nonrandom. Because at least one positive MSLT was required for inclusion in our study and diagnosis, we were unable to assess how many subjects suspected of NT1, and especially NT2 given our findings, might have failed to meet narcolepsy criteria in 2 MSLTs (ie, 2 negative MSLTs). Most of all, we were not able to confirm the reason(s) why the repeat MSLT was performed. There are many different, and possibly opposing, reasons a clinician may request a repeat MSLT in NT1 versus NT2 (eg, suspect false-negative or false-positive result; patient's condition spontaneously improves; initial test performed under medication(s); patient reported being disturbed at initial MSLT due to emotional/environmental issues; testing protocol variation(s); insufficient sleep/irregular sleep not entirely ruled out with objective data; overnight sleep testing times misaligned with habitual sleep times; and for insurance purposes), potentially placing our sample at high risk for heterogeneity. Nevertheless, NT1 and NT2 cases were retrospectively identified using the same objective selection criteria (ie, at least one positive MSLT for narcolepsy along with specific biologic data) across all centers. And, despite using the same selection criteria in this study, the NT1 and NT2 cases showed dramatically different MSLT characteristics.
The study raises important questions that mandate additional systematic studies and re-evaluation of current diagnostic criteria for NT2 and IH, as ICSD-3 criteria heavily depends on the MSLT to render these diagnoses. Although the MSLT is sensitive to sleep deprivation and a clinically useful objective tool for NT1, it may not be the best test for other pathologies of excessive daytime sleepiness according to ICSD-3 criteria. As noted by others, the MSLT measures “sleep-ability,” the ability to fall asleep in a quiet environment, rather than the ability to stay awake (‘wake-ability,” the major complaint of patients with these pathologies), a feature that may be best measured by the Maintenance of Wakefulness Test (MWT). Only a few studies have explored correlations between MSLT and MWT assessments, and in samples mostly composed of patients with NT1, demonstrating only partial correlations. This, together with the poor correlation of these measures with subjective assessments, such as the Epworth Sleepiness Scale, notably in the context of depression,45,46 mandates better characterization of “sleepiness” in individual patients before diagnostic categories can be created. Ideally, a multisite prospective study of repeatability of drug free MSLTs and MWTs, with subjective and objective measures of sleep history (sleep logs and actigraphy, respectively), circadian phase assessments (eg, dim light melatonin onset [DLMO]), and neuropsychological testing is needed to better distinguish subgroups of patients. In addition to routine overnight PSG and actigraphy, long-term (ie, 24–48-hour) PSG, which is routinely performed in many European countries to evaluate central nervous system hyper-somnias, should be collected. Analysis of daytime, nocturnal and long-term recordings should include traditional scoring methods in addition to other measures such as sleep stage sequence analysis.20,47–49
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The study was partially funded by NIH-23724 (EM); K23-NS083748 (LMT); T32 HL110952 (JC); and R01-NS089719 (DBR). Dr. Ruoff has served as an advisory board member and unpaid consultant for Jazz Pharmaceuticals. Dr. Sonka has served as a consultant for Luitpold Pharmaceutical, Boehringer Ingelheim, Bioprojet, and Eisai. Dr. Sonka has also served on speakers' bureau for Berlin-Chemie and Novartis. Mali Einen has served as a consultant for Jazz Pharmaceuticals. Dr. Simakajornboon has received funding for an investigator-initiated project from Jazz Pharmaceuticals. Dr. Plazzi has served as a consultant for UCB Pharma, Jazz Pharmaceuticals, and Bioproject. Dr. Rye has served as a consultant for Jazz Pharmaceuticals, Xenoport, UCB Pharma, Flamel Technologies, and Balance Therapeutics. Dr. Rye has also received royalties from Balance Therapeutics. Dr. Mignot has served as a consultant for and received grant support from Jazz Pharmaceuticals.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CSF
cerebrospinal fluid
- DLMO
dim light melatonin onset
- ESS
Epworth Sleepiness Scale
- HLA
human leukocyte antigen
- ICSD
International Classification of Sleep Disorders
- IH
idiopathic hypersomnia
- MSL
mean sleep latency
- MSLT
Multiple Sleep Latency Test
- MSLT1
refers to the first MSLT
- MSLT2
refers to the second MSLT
- NT1
narcolepsy type 1
- NT2
narcolepsy type 2
- PSG
polysomnography
- REM
rapid eye movement
- SOREMP
sleep onset rapid eye movement period
REFERENCES
- 1.Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9(4):519–524. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 2.Rechtschaffen A, Wolpert EA, Dement WC, Mitchell SA, Fisher C. Nocturnal sleep of narcoleptics. Electroencephalogr Clin Neurophysiol. 1963;15:599–609. doi: 10.1016/0013-4694(63)90032-4. [DOI] [PubMed] [Google Scholar]
- 3.Vogel G. Studies in psychophysiology of dreams. III. The dream of narcolepsy. Arch Gen Psychiatry. 1960;3:421–428. doi: 10.1001/archpsyc.1960.01710040091011. [DOI] [PubMed] [Google Scholar]
- 4.Mitler MM, Van den Hoed J, Carskadon MA, et al. REM sleep episodes during the Multiple Sleep Latency Test in narcoleptic patients. Electroencephalogr Clin Neurophysiol. 1979;46(4):479–481. doi: 10.1016/0013-4694(79)90149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson GS, Carskadon MA, Flagg W, Van den Hoed J, Dement WC, Mitler MM. Excessive daytime sleepiness in man: multiple sleep latency measurement in narcoleptic and control subjects. Electroencephalogr Clin Neurophysiol. 1978;45(5):621–627. doi: 10.1016/0013-4694(78)90162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 7.Moscovitch A, Partinen M, Guilleminault C. The positive diagnosis of narcolepsy and narcolepsy's borderland. Neurology. 1993;43(1):55–60. doi: 10.1212/wnl.43.1_part_1.55. [DOI] [PubMed] [Google Scholar]
- 8.Andlauer O, Moore H, Jouhier L, et al. Nocturnal rapid eye movement sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA Neurol. 2013;70(7):891–902. doi: 10.1001/jamaneurol.2013.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuki K, Honda Y, Juji T. Diagnostic criteria for narcolepsy and HLA-DR2 frequencies. Tissue Antigens. 1987;30(4):155–160. doi: 10.1111/j.1399-0039.1987.tb01614.x. [DOI] [PubMed] [Google Scholar]
- 10.Honda Y, Asaka C, Tanimura M, Furusho T. A genetic study of narcolepsy and excessive daytime sleepiness in 308 families with a narcolepsy or hypersomnia proband. In: Guilleminault C, Lugaresi E, editors. Sleep/Wake Disorders: Natural History, Epidemiology and Long-Term Evolution. New York, NY: Raven Press; 1983. pp. 187–199. [Google Scholar]
- 11.Roth B. Narcolepsy and hypersomnia: review and classification of 642 personally observed cases. Schweiz Arch Neurol Neurochir Psychiatr. 1976;119(1):31–41. [PubMed] [Google Scholar]
- 12.Billiard M. Idiopathic hypersomnia. Neurol Clin. 1996;14(3):573–582. doi: 10.1016/s0733-8619(05)70274-7. [DOI] [PubMed] [Google Scholar]
- 13.Montplaisir J, Billiard M, Takahashi S, Bell IR, Guilleminault C, Dement WC. Twenty-four-hour recording in REM-narcoleptics with special reference to nocturnal sleep disruption. Biol Psychiatry. 1978;13(1):73–89. [PubMed] [Google Scholar]
- 14.Pizza F, Moghadam KK, Vandi S, et al. Daytime continuous polysomnography predicts MSLT results in hypersomnias of central origin. J Sleep Res. 2013;22(1):32–40. doi: 10.1111/j.1365-2869.2012.01032.x. [DOI] [PubMed] [Google Scholar]
- 15.Aldrich MS, Chervin RD, Malow BA. Value of the multiple sleep latency test (MSLT) for the diagnosis of narcolepsy. Sleep. 1997;20(8):620–629. [PubMed] [Google Scholar]
- 16.Singh M, Drake CL, Roth T. The prevalence of multiple sleep-onset REM periods in a population-based sample. Sleep. 2006;29(7):890–895. doi: 10.1093/sleep/29.7.890. [DOI] [PubMed] [Google Scholar]
- 17.Mignot E, Lin L, Finn L, et al. Correlates of sleep-onset REM periods during the Multiple Sleep Latency Test in community adults. Brain. 2006;129(Pt 6):1609–1623. doi: 10.1093/brain/awl079. [DOI] [PubMed] [Google Scholar]
- 18.Goldbart A, Peppard P, Finn L, et al. Narcolepsy and predictors of positive MSLTs in the Wisconsin Sleep Cohort. Sleep. 2014;37(6):1043–1051. doi: 10.5665/sleep.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chervin RD, Aldrich MS. Sleep onset REM periods during multiple sleep latency tests in patients evaluated for sleep apnea. Am J Respir Crit Care Med. 2000;161(2 Pt 1):426–431. doi: 10.1164/ajrccm.161.2.9905071. [DOI] [PubMed] [Google Scholar]
- 20.Marti I, Valko PO, Khatami R, Bassetti CL, Baumann CR. Multiple sleep latency measures in narcolepsy and behaviourally induced insufficient sleep syndrome. Sleep Med. 2009;10(10):1146–1150. doi: 10.1016/j.sleep.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 21.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 22.Cairns A, Bogan R. Prevalence and clinical correlates of a short onset REM period (SOREMP) during routine PSG. Sleep. 2015;38(10):1575–1581. doi: 10.5665/sleep.5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reiter J, Katz E, Scammell TE, Maski K. Usefulness of a nocturnal SOREMP for diagnosing narcolepsy with cataplexy in a pediatric population. Sleep. 2015;38(6):859–865. doi: 10.5665/sleep.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trotti LM, Staab BA, Rye DB. Test-retest reliability of the multiple sleep latency test in narcolepsy without cataplexy and idiopathic hypersomnia. J Clin Sleep Med. 2013;9(8):789–795. doi: 10.5664/jcsm.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozaki A, Inoue Y, Hayashida K, et al. Quality of life in patients with narcolepsy with cataplexy, narcolepsy without cataplexy, and idiopathic hypersomnia without long sleep time: comparison between patients on psychostimulants, drug-naive patients and the general Japanese population. Sleep Med. 2012;13(2):200–206. doi: 10.1016/j.sleep.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Dauvilliers Y, Paquereau J, Bastuji H, Drouot X, Weil JS, Viot-Blanc V. Psychological health in central hypersomnias: the French Harmony study. J Neurol Neurosurg Psychiatry. 2009;80(6):636–641. doi: 10.1136/jnnp.2008.161588. [DOI] [PubMed] [Google Scholar]
- 27.Vernet C, Arnulf I. Idiopathic hypersomnia with and without long sleep time: a controlled series of 75 patients. Sleep. 2009;32(6):753–759. doi: 10.1093/sleep/32.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgenthaler TI, Kapur VK, Brown T, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1705–1711. doi: 10.1093/sleep/30.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson KN, Pilsworth S, Sharples LD, Smith IE, Shneerson JM. Idiopathic hypersomnia: a study of 77 cases. Sleep. 2007;30(10):1274–1281. doi: 10.1093/sleep/30.10.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leu-Semenescu S, Louis P, Arnulf I. Benefits and risk of sodium oxybate in idiopathic hypersomnia versus narcolepsy type 1: a chart review. Sleep Med. 2016;17:38–44. doi: 10.1016/j.sleep.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Lavault S, Dauvilliers Y, Drouot X, et al. Benefit and risk of modafinil in idiopathic hypersomnia vs. narcolepsy with cataplexy. Sleep Med. 2011;12(6):550–556. doi: 10.1016/j.sleep.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Littner MR, Kushida C, Wise M, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28(1):113–121. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 33.Hallmayer J, Faraco J, Lin L, et al. Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nat Genet. 2009;41(6):708–711. doi: 10.1038/ng.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mignot E, Lammers GJ, Ripley B, et al. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol. 2002;59(10):1553–1562. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- 35.Dauvilliers Y, Gosselin A, Paquet J, Touchon J, Billiard M, Montplaisir J. Effect of age on MSLT results in patients with narcolepsy-cataplexy. Neurology. 2004;62(1):46–50. doi: 10.1212/01.wnl.0000101725.34089.1e. [DOI] [PubMed] [Google Scholar]
- 36.Andlauer O, Moore H, 4th, Hong SC, et al. Predictors of hypocretin (orexin) deficiency in narcolepsy without cataplexy. Sleep. 2012;35(9):1247–1255. doi: 10.5665/sleep.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andlauer O, Moore H, IV, Jouhier L, et al. Nocturnal REM sleep latency for identifying patients with narcolepsy/hypocretin deficiency. JAMA Neurol. 2013;70(7):891–902. doi: 10.1001/jamaneurol.2013.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishino S, Ripley B, Overeem S, et al. Low cerebrospinal fluid hypocretin (Orexin) and altered energy homeostasis in human narcolepsy. Ann Neurol. 2001;50(3):381–388. doi: 10.1002/ana.1130. [DOI] [PubMed] [Google Scholar]
- 39.Folkerts M, Rosenthal L, Roehrs T, et al. The reliability of the diagnostic features in patients with narcolepsy. Biol Psychiatry. 1996;40(3):208–214. doi: 10.1016/0006-3223(95)00383-5. [DOI] [PubMed] [Google Scholar]
- 40.Shankar R, Jalihal V, Walker M, Zeman A. Pseudocataplexy and transient functional paralysis: a spectrum of psychogenic motor disorder. J Neuropsychiatry Clin Neurosci. 2010;22(4):445–450. doi: 10.1176/jnp.2010.22.4.445. [DOI] [PubMed] [Google Scholar]
- 41.Krahn LE. Reevaluating spells initially identified as cataplexy. Sleep Med. 2005;6(6):537–542. doi: 10.1016/j.sleep.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Simon DK, Nishino S, Scammell TE. Mistaken diagnosis of psychogenic gait disorder in a man with status cataplecticus (“limp man syndrome”) Mov Disord. 2004;19(7):838–840. doi: 10.1002/mds.20078. [DOI] [PubMed] [Google Scholar]
- 43.Pizza F, Vandi S, Poli F, et al. Narcolepsy with cataplexy mimicry: the strange case of two sisters. J Clin Sleep Med. 2013;9(6):611–612. doi: 10.5664/jcsm.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carskadon MA, Dement WC. Distribution of REM sleep on a 90 minute sleep-wake schedule. Sleep. 1980;2(3):309–317. [PubMed] [Google Scholar]
- 45.Dolenc L, Besset A, Billiard M. Hypersomnia in association with dysthymia in comparison with idiopathic hypersomnia and normal controls. Pflugers Arch. 1996;431(6 Suppl 2):R303–R304. doi: 10.1007/BF02346389. [DOI] [PubMed] [Google Scholar]
- 46.Plante DT, Finn LA, Hagen EW, Mignot E, Peppard PE. Subjective and objective measures of hypersomnolence demonstrate divergent associations with depression among participants in the Wisconsin Sleep Cohort Study. J Clin Sleep Med. 2016;12(4):571–578. doi: 10.5664/jcsm.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drakatos P, Suri A, Higgins SE, et al. Sleep stage sequence analysis of sleep onset REM periods in the hypersomnias. J Neurol Neurosurg Psychiatry. 2013;84(2):223–227. doi: 10.1136/jnnp-2012-303578. [DOI] [PubMed] [Google Scholar]
- 48.Filardi M, Pizza F, Martoni M, Vandi S, Plazzi G, Natale V. Actigraphic assessment of sleep/wake behavior in central disorders of hypersomnolence. Sleep Med. 2015;16(1):126–130. doi: 10.1016/j.sleep.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 49.Pizza F, Vandi S, Detto S, et al. Different sleep onset criteria at the multiple sleep latency test (MSLT): an additional marker to differentiate central nervous system (CNS) hypersomnias. J Sleep Res. 2011;20(1 Pt 2):250–256. doi: 10.1111/j.1365-2869.2009.00808.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.