Abstract
Study Objectives:
Night shift work is associated with increased breast cancer risk, possibly from altered sleep. Epidemiologic evidence is sparse regarding sleep disturbances and breast cancer tumor markers. We examined sleep disturbance in association with breast tumor aggressiveness and mortality following diagnosis.
Methods:
We analyzed associations of measures of sleep disturbance in a sample of 1,122 incident breast cancer cases from the Western New York Exposures and Breast Cancer (WEB) Study. Sleep disturbance was assessed using self-administered questionnaires; responses about difficulty falling asleep, waking up frequently, having trouble staying asleep, and waking up feeling tired and worn out were used to create a summary sleep disturbance score. We used general linear models to examine associations of sleep disturbance with markers of tumor aggressiveness among cases: estrogen receptor (ER) status, progesterone receptor (PR) status, and human epidermal growth factor receptor-2 (HER2) status; tumor size, stage, grade, lymph node involvement, and presence of metastasis. In addition, we examined the association between sleep disturbance and survival using Cox regression.
Results:
Among breast cancer cases, sleep disturbance was higher for women with ER− / PR− tumors compared to women with ER+ / PR+ tumors, even after adjusting for potential covariates (P for trend = .02). Results suggest that the association of sleep quality differs by menopausal status, where mild sleep disturbance is associated with higher breast cancer mortality in premenopausal women; however, we had a relatively small sample size.
Conclusions:
Sleep disturbance may be associated with aggressive subtypes of breast cancer; however, further studies are needed.
Citation:
Vaughn CB, Freudenheim JL, Nie J, Sucheston-Campbell L, Wactawski-Wende J, Marian C, Shields PG, Kallakury BV, Trevisan M, Ochs-Balcom HM. Sleep and breast cancer in the Western New York Exposures and Breast Cancer (WEB) Study. J Clin Sleep Med. 2018;14(1):81–86.
Keywords: breast cancer, sleep, tumor subtype
BRIEF SUMMARY
Current Knowledge/Study Rationale: Night shift work is considered a risk factor for breast cancer, although there is a gap in the literature regarding the effect of sleep on breast cancer risk and aggressiveness. In a large, population-based case control study in western New York, we analyzed whether self-reported sleep disturbance was associated with breast cancer aggressiveness and mortality.
Study Impact: We found that sleep disturbance was higher for women with ER− / PR− breast tumors, compared to women with ER+ and/or PR+ tumors. This study suggests that sleep disturbance may be associated with breast cancer subtype.
INTRODUCTION
Accumulating evidence suggests that night shift work is a risk factor for breast cancer.1–3 Results from two case-control studies reported higher odds of breast cancer associated with night shift work1,2; one study showed a significant trend for increased risk of breast cancer associated with increasing years of night shift work.2 In the Nurses' Health Study, more than 20 years of night shift work was associated with an almost 80% increased risk of premenopausal breast cancer.3 However, despite the known heterogeneity in breast tumors, these studies have not investigated whether this increase in risk was associated with particular breast tumor markers or tumor subtype.
Short sleep duration has been hypothesized to explain this observed association between night shift work and breast cancer risk. Although short sleep duration has been examined for associations with risk of breast cancer in several types of epidemiologic studies, only one prospective cohort study has reported such an association.4 Therefore, it may be that facets of sleep behavior other than duration play a role in breast cancer risk; to our knowledge, there have been few studies of this possible association. There is evidence suggesting that sleep may be associated with tumor aggressiveness.5 Investigators from this study found that shorter sleep duration was associated with higher genetic-based recurrence score of breast cancer. Though night shift work has been associated with increased risk of mortality,6 and studies have found that sleep disruption among breast cancer survivors is a predictor of survival,7 to our knowledge, no studies to date have evaluated the potential association between sleep disturbance prior to breast cancer diagnosis and breast cancer mortality.
Because of this gap in the literature around facets of sleep and breast tumor markers, we decided to analyze whether there were associations between sleep disturbance and breast cancer aggressiveness and mortality in a sample of breast cancer cases in the western New York region. Specifically, given the documented aggressiveness of estrogen receptor (ER)−, progesterone receptor (PR)− and human epidermal growth factor receptor-2 (HER2)− tumors,8 the antiproliferative nature of melatonin and the relationship between melatonin, and its interaction with estrogen-responsive pathways,9 we hypothesized that greater sleep disturbances are associated with more aggressive breast tumor markers as well as higher all-cause and breast cancer-specific mortality.
METHODS
Study Population
The Western New York Exposures and Breast Cancer (WEB)
Study is a population-based case-control study in the western part of New York State.10 We report here on an analysis of the cases from that study. Briefly, 1,170 cases of incident, primary, histologically confirmed breast cancer were enrolled in Erie and Niagara counties.10 Informed consent was obtained for all study participants and the study protocol was approved by the University of Buffalo's Health Sciences Institutional Review Board and the Institutional Review Board of participating hospitals.10
Data on demographics, lifestyle factors, reproductive history, smoking and other breast cancer risk factors were collected via in-person interviews.10 Physical measurements were collected by trained study staff using a standardized protocol.10
Information on tumor size, stage, grade, lymph node involvement, and metastasis was obtained from medical records.10,11 A single pathologist determined ER, PR, and HER2 status using immunohistochemistry performed on 5-μm slides using kits with the Dako Autostainer (Dako, Carpinteria, California, United States).12 For ER and PR staining, the Allred scoring system was used; values of 3 were classified as positive. HER2 status was scored using the HerceptTest (Dako); tumors with a score ≥ 3 were classified as positive.12
Sleep Disturbance Score
Information regarding sleep in the 12 to 24 months prior to the breast cancer diagnosis was collected using a self-administered questionnaire. Of the 1,170 breast cancer cases in the WEB Study, 1,122 had complete sleep data; our analysis is limited to this subgroup. In addition, there were missing data for the tumor markers of breast cancer aggressiveness for some of the cases; we therefore performed statistical analyses for each marker using cases with complete data for sleep and that particular tumor marker.
We created a sleep disturbance score composed of 4 questions from the Jenkins Sleep Questionnaire.13 The questions were as follows: in a usual month in the referenced year (1) how often did you have trouble falling asleep?; (2) how often did you wake up several times at night?; (3) how often did you have trouble staying asleep?; and (4) how often did you wake up feeling tired and worn out? For all questions, the potential responses were: 0 = not at all; 1 = 1 to 3 days; 2 = 4 to 7 days; 3 = 8 to 14 days; 4 = 15 to 21 days; and 5 = 22 to 31 days. The sleep disturbance score was calculated as a sum of these values, ranging from zero to 20, with higher numbers indicating greater sleep disturbance. This questionnaire has demonstrated reliability and internal consistency13 and is similar to the validated score constructed in the Women's Health Initiative.14–16 We analyzed the sleep disturbance score as a continuous variable, as well as a categorical variable using the following categories for the survival analysis: 0 to 3, 4 to 6, 7 to 10, and 11 to 20.
Statistical Analysis
Descriptive statistics comparing ER+ cases and ER− cases were estimated using independent-samples t tests for continuous variables and χ2 tests for categorical variables.
We built general linear models to estimate the mean sleep disturbance score according to each tumor marker. The covariates included in these models were age, body mass index, age at birth of first child, age at menarche, first-degree relative with breast cancer, previous benign breast disease, menopausal status, and smoking status. We also created combined ER/PR categories for each case as well as categories of molecular subtypes of breast tumor according to ER, PR, and HER2 status.17 Luminal A cases were positive for ER or PR and negative for HER2. Luminal B cases were positive for ER or PR and positive for HER2. HER2-expressing cases were negative for ER and PR and positive for HER2. Triple-negative cases were negative for ER and PR and HER2.
Survival Analysis
We performed a survival analysis to evaluate whether sleep disturbance was associated with all-cause and breast cancer mortality. A National Death Index search was conducted through 2013 to ascertain vital status of study participants with breast cancer. Survival time was calculated as the number of months from the date of diagnosis until date of death or the end of follow-up, December 31, 2013.
We used Cox proportional hazards regression models to estimate hazards ratios (HRs) and 95% confidence intervals (CIs) for all-cause and breast cancer-specific mortality. Two adjusted models were estimated: one adjusted for age, education, and race and the other additionally adjusted for body mass index, pack-years of smoking, stage, ER status, PR status, and menopausal status. All statistical analyses were conducted using SPSS version 20 (IBM Corp, Armonk, New York, United States) and all P values were considered statistically significant (P < .05).
RESULTS
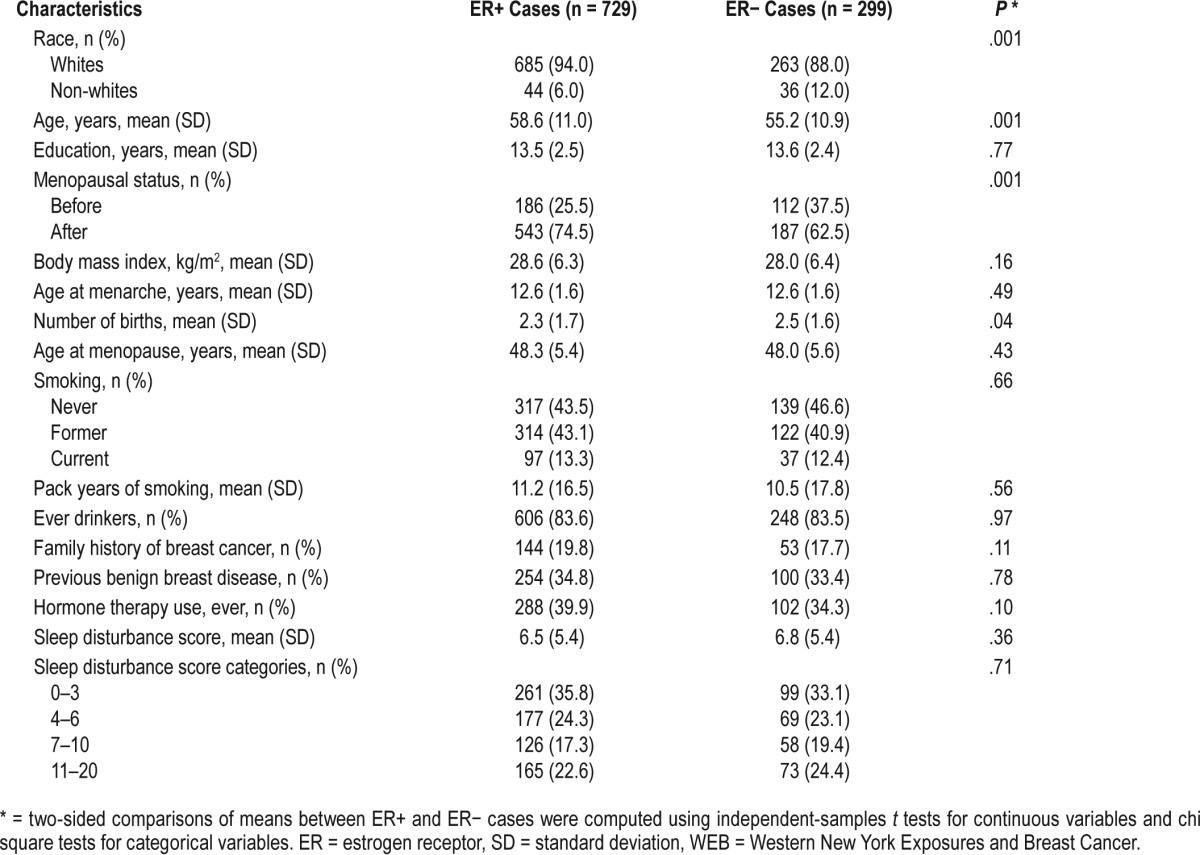
We present the characteristics of ER+ and ER− cases in Table 1. Of the 1,122 cases with sleep information in our sample, ER data were available for 1,028 participants. Approximately 71% of cases were ER+. ER+ cases were more likely to be white than ER− cases. ER+ cases were also older, on average, than ER− cases and more likely to be postmenopausal than ER− cases.
Table 1.
Characteristics of study sample by ER status, WEB Study, 1996–2001.
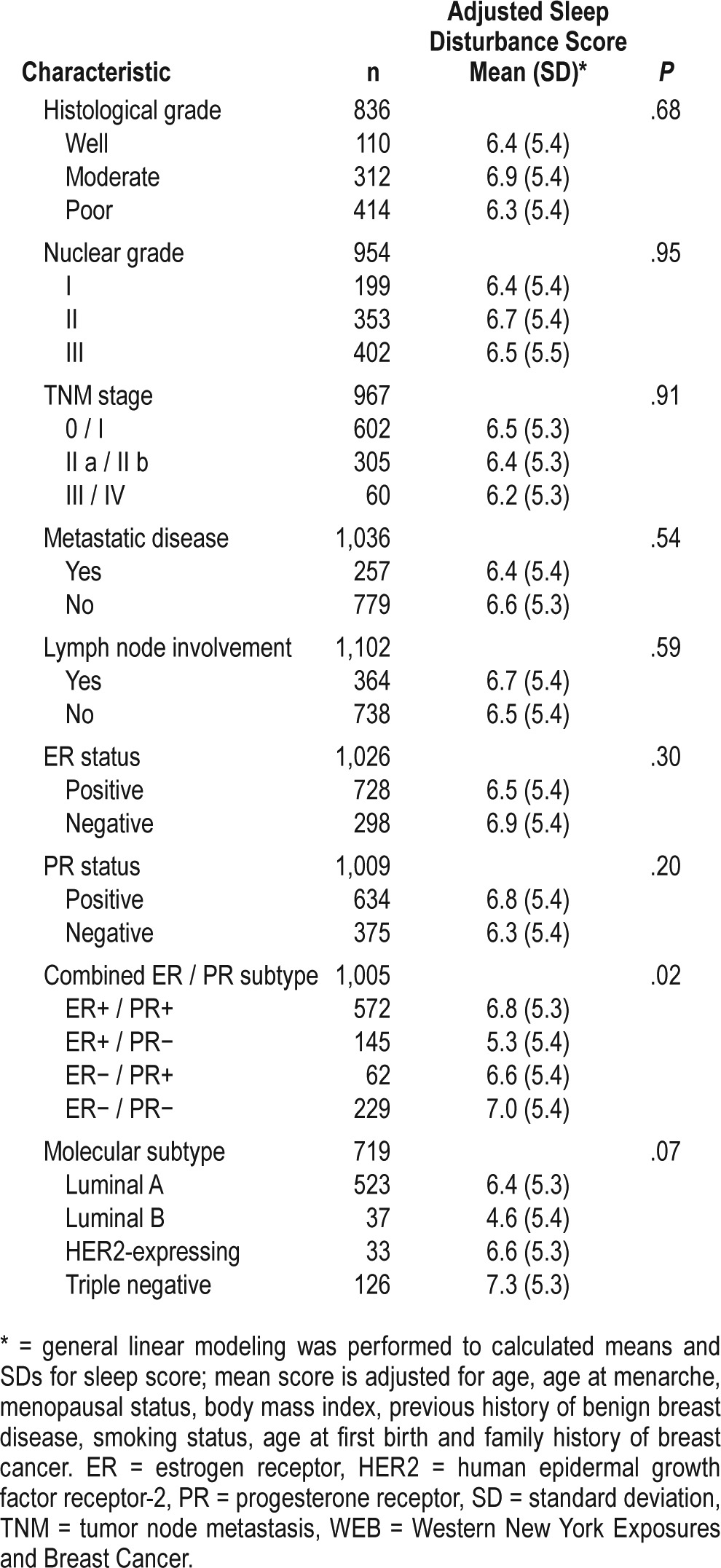
The mean sleep disturbance score according to tumor characteristics is shown in Table 2. Women with ER− / PR− tumors had higher sleep disturbance scores than those in other groups defined by combined ER/PR status (adjusted mean sleep disturbance score = 7.0, compared to mean score of 6.8 for ER+ / PR+ cases, 5.3 for ER+ / PR− cases and 6.6 for ER− / PR+ cases, P = .02). Triple negative cases had the highest sleep disturbance score compared to the other groups (adjusted mean score = 7.3, standard deviation [SD] = 5.3), although differences across the categories did not reach statistical significance (adjusted P = .07).
Table 2.
Case only analysis of sleep disturbance score according to tumor characteristics, WEB Study, 1996–2001.
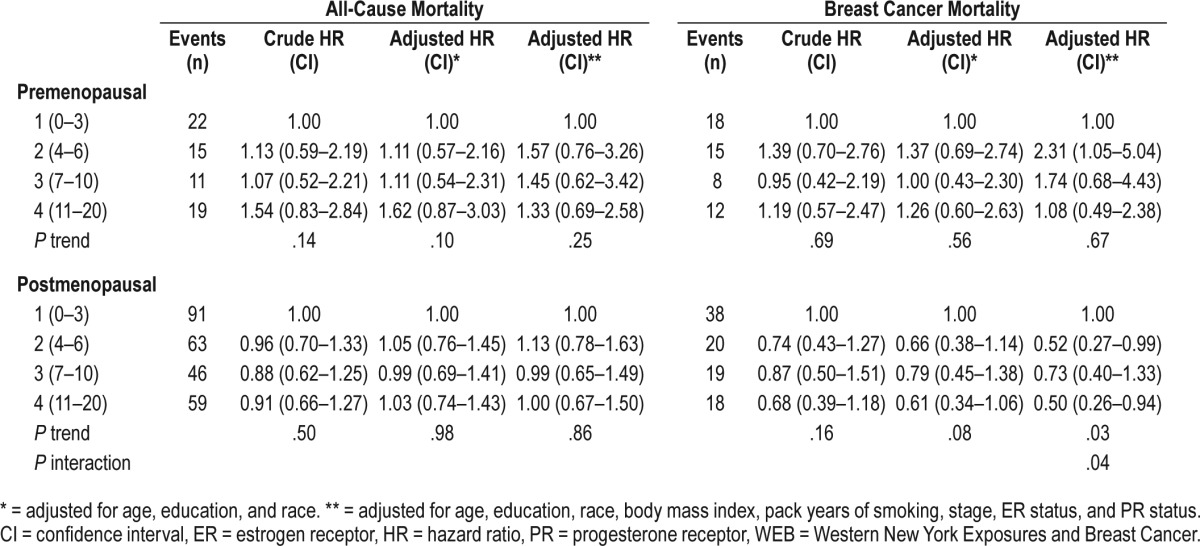
We analyzed sleep disturbance and risk of all-cause mortality and breast cancer-specific mortality (Table 3). We identified a significant interaction between menopausal status and sleep disturbance on breast cancer mortality (interaction P = .04); therefore, results are presented stratified by menopausal status. In premenopausal women, risk of breast cancer mortality was highest among those with mild sleep disturbance (WEB sleep disturbance score 4–6; HR: 2.31, 95% CI: 1.05–5.04). In postmenopausal women, we observed decreased breast cancer mortality among postmenopausal with breast cancer reporting both mild lowest (HR: 0.52, 95% CI: 0.27–0.99) and highest degree of sleep disturbance (HR: 0.50, 95% CI: 0.26–0.94). We use caution with interpretation due to the relatively small number of events, represented in the wide range of CIs.
Table 3.
Hazard ratios of mortality by sleep disturbance score among cases, WEB Study, 1996–2001.
DISCUSSION
Women with ER− and PR− tumors reported greater sleep disturbance in the 12 to 24 months preceding diagnosis. Although it did not reach significance, this pattern remained when we analyzed molecular subtypes; women with triple-negative tumors reported the most sleep disturbance. This association is consistent with our hypothesis and may suggest that sleep disturbances are associated with development of more aggressive breast tumors. Conversely, we found that sleep disturbance was associated with decreased breast cancer mortality among the postmenopausal women; among premenopausal women, there was a significant increase in breast cancer mortality with poorer sleep. In a sensitivity analysis where participants who self-reported past or current use of sedative or hypnotic medication (65 of 1,122 cases) were removed, our results were unchanged, suggesting that sleep medication use is not influencing the results of our study.
Sleep duration has been evaluated for an association with breast cancer risk; in a meta-analysis, there was no evidence of an association.18 However, most of the included studies did not include examination of breast cancer subtypes and did not assess any other facets of sleep behavior other than sleep duration. One study that examined subjective sleep quality (self-reported as very good, fairly good, fairly bad, or very bad) did not find an association with risk of breast cancer; however, their assessment of sleep quality was not particularly thorough.19
Though published studies to date do not support a link between sleep quality and breast cancer risk, there is reason to believe that risk factors for breast cancer vary by tumor sub-type.20 Recent evidence suggests that the association between sleep duration and breast cancer may differ by tumor subtype.5 Investigators performed a study on 101 patients with breast cancer and found that poorer OncotypeDX recurrence scores (a panel of the expression of 21 genes in breast tumors) were correlated with fewer hours of sleep.5 However, this study included only 101 patients with breast cancer and, as sleep behavior was queried after diagnosis, recall may have been biased.
We found that sleep disturbance was associated with increased odds of ER− / PR− and triple-negative tumor subtypes in our breast cancer cases. However, the differences that we observed in sleep disturbance between tumor subtypes were relatively small. We did not find any evidence of an association between sleep disturbance and other tumor markers, such as stage, grade, and metastasis. It is important to note the number of cases with metastasis, lymph node involvement, or tumor node metastasis stage III or IV was small.
We did not find any evidence of an association between sleep disturbance and all-cause mortality. However, we did find an association between sleep disturbance and breast cancer mortality that differed when stratified by menopausal status. We observed a stark difference in the associations of sleep disturbance and the risk of breast cancer mortality by menopausal status; in premenopausal women, risk was highest among those with mild sleep disturbance whereas in postmenopausal women risk of mortality was lowest among those with both low and high sleep disturbance (interaction, P = .04). These results suggest that estrogen and/or the influence of dynamics of age and sleep behavior may differentially influence the association of sleep and cancer. However, we caution against strong interpretation of these results because further research is needed here, with larger samples.
We were unable to assess whether perimenopausal symptoms may have influenced our results. However, we did analyze whether time since menopause influenced our results among postmenopausal women. Most of our postmenopausal sample had transitioned through menopause more than 5 years prior (mean = 14.6 years, SD = 9.5 years, median = 14.0 years). However, because we were analyzing sleep behavior in the 1 to 2 years prior to enrollment into the study, we decided to look at women who had undergone menopause in the previous 2 years and in the previous 5 years. We believe that women who were postmenopausal for longer than 5 years likely had experienced some stability of their symptoms, and their description of sleep in the 1 to 2 years previous would not be influenced. Women who had transitioned to being postmenopausal within 2 years reported slightly, though nonsignificantly, elevated sleep disturbance scores when compared to women who had become postmenopausal less recently (mean = 7.3, SD = 5.2 compared to mean = 6.6, SD = 5.4, P = .28). For women who had transitioned to being postmenopausal within 5 years, there was essentially no difference in sleep disturbance score compared to women who had become postmenopausal more than 5 years before (P = .79). Therefore, given the few breast cancer cases who had become postmenopausal within 2 years and that the difference in sleep disturbance was not statistically significant, we do not believe that this affected our results.
There is increasing plausibility of a sleep-cancer link. One possible biological mechanism is that poor sleep quality is reflective of reduced melatonin levels. Melatonin is a hormonal regulator of cell growth, and is anti-proliferative.21 It is also involved in stimulation of the immune system and has antioxidant activity.22 People who are frequently exposed to light at night experience disruptions in their circadian rhythm, suppression of melatonin levels, and sleep disturbances.21,22 Because poor sleep at night is associated with suppression of melatonin levels, and melatonin has known antineoplastic activity,21,23 it has been suggested that poor sleep may affect both cancer initiation and progression.21,22 Though there is evidence to suggest that poor sleep is associated with decreased melatonin levels,24 we did not have measured melatonin levels in our sample and are unable, therefore, to examine this particular mechanism.
Sleep may also influence cancer through circadian rhythm, as both have been demonstrated to be involved in DNA repair.25 Circadian rhythm genes are involved in the regulation of the cell cycle and apoptosis.26 Therefore, dysregulation of circa-dian rhythm may increase cancer risk.25,26 Many of the genes involved in regulation of circadian rhythm are key cell cycle regulators that control cellular proliferation and differentiation and act as transcription factors,27 and may influence these processes at different stages of breast carcinogenesis. Furthermore, a recent meta-analysis of 28 studies of circadian rhythm-associated exposures (night shift work, light at night exposure, sleep deficiency, and flight attendant work) found that circadian rhythm disruption (specifically, night shift work, light at night, and flight attendant work) was associated with an increased risk of breast cancer, though short sleep duration was not.28
Strengths of our study include that it is population based with many incident breast cancer cases, including data regarding tumor characteristics. We also had detailed reports of sleep behavior prior to breast cancer diagnosis, as well as reproductive history and demographic data that enabled us to account for potential confounding variables.
However, our study was not without limitations. Sleep data were collected after diagnosis; there may be recall bias, and/or the diagnosis may have affected recent sleep and influenced recollection of sleep before the diagnosis. However, for this case-only analysis, we do not expect differential recall according to tumor characteristics to have biased our results. An additional concern is that this sleep disturbance score was based on 4 questions about sleep disturbance in the 1 to 2 years before study enrollment; our sleep score may not adequately capture the breadth of sleep behavior. There may be other sleep characteristics that were not measured and are more strongly associated with risk. More detailed prospective assessments of sleep behavior during a woman's lifetime would be helpful in future studies to determine whether sleep behavior affects breast cancer risk.
A final limitation of our study is that we determined the molecular subtyping for our analysis based on the ER, PR, and HER2 status of breast tumors; we did not have direct measurement of gene expression.17
We found evidence that sleep behavior may be differentially associated with ER− / PR− and triple-negative breast tumors. Additional studies, particularly prospective studies with repeated sleep assessments taken prior to diagnoses, are needed.
DISCLOSURE STATEMENT
Work for this study was performed at the University at Buffalo, Buffalo, New York. This work was funded by DOD Breast Cancer Research Program (DAMD 179616202, DAMD 17030446) and United States Public Health Service (USPHS) grant numbers R25CA113951, K07CA136969 and R01CA092040 from the National Cancer Institute and P50AA09802 from the National Institute on Alcohol Abuse and Alcoholism. These studies were conducted in part at the Lombardi Comprehensive Cancer Center Histopathology & Tissue Shared resource which is supported in part by NIH/NCI grant P30CA051008. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. All authors have seen and approved of the final version of this manuscript. The authors report no conflicts of interest.
ABBREVIATIONS
- CI
confidence interval
- ER
estrogen receptor
- HER2
human epidermal growth factor receptor 2
- HR
hazard ratio
- PR
progesterone receptor
- SD
standard deviation
- TNM
tumor node metastasis
- WEB
Western New York Exposures and Breast Cancer
REFERENCES
- 1.Hansen J. Increased breast cancer risk among women who work predominantly at night. Epidemiology. 2001;12(1):74–77. doi: 10.1097/00001648-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93(20):1557–1562. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- 3.Schernhammer E, Kroenke CH, Laden F, Hankinson SE. Night work and risk of breast cancer. Epidemiology. 2006;17(1):108–111. doi: 10.1097/01.ede.0000190539.03500.c1. [DOI] [PubMed] [Google Scholar]
- 4.Kakizaki M, Kuriyama S, Sone T, Ohmori-Matsuda K, Hozawa A, Nakaya N, Fukudo S, Tsuji I. Sleep duration and the risk of breast cancer: the Ohsaki Cohort Study. Brit J Cancer. 2008;99(9):1502–1505. doi: 10.1038/sj.bjc.6604684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson C, Li L. Association of sleep duration and breast cancer OncotypeDX recurrence score. Breast Cancer Res Treat. 2012;134(3):1291–1295. doi: 10.1007/s10549-012-2144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin X, Chen W, Wei F, Ying M, Wei W, Xie X. Night-shift work increases morbidity of breast cancer and all-cause mortality: a meta-analysis of 16 prospective cohort studies. Sleep Med. 2015;16:1387–1387. doi: 10.1016/j.sleep.2015.02.543. [DOI] [PubMed] [Google Scholar]
- 7.Palesh O, Aldridge-Gerry A, Zeitzer J, et al. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep. 2014;37(5):837–842. doi: 10.5665/sleep.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. PNAS. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cos S, Gonzalez A, Guezmes A, et al. Melatonin inhibits the growth of DMBA-induced mammary tumors by decreasing the local biosynthesis of estrogens through the modulation of aromatase activity. Int J Cancer. 2006;118(2):274–278. doi: 10.1002/ijc.21401. [DOI] [PubMed] [Google Scholar]
- 10.McCann SE, Ip C, Ip MM, et al. Dietary intake of conjugated linoleid acids and risk of premenopausal and postmenopausal breast cancer, Western New York Exposures and Breast Cancer Study (WEB Study) Cancer Epidemiol Biomarkers Prev. 2004;13(9):1480–1484. [PubMed] [Google Scholar]
- 11.Tao M, Shields PG, Nie J, et al. DNA hypermethylation and clinicopathological features in breast cancer: the Western New York Exposures and Breast Cancer (WEB) Study. Breast Cancer Res Treat. 2009;114:559–568. doi: 10.1007/s10549-008-0028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han D, Nie J, Bonner MR, et al. Clustering of place of birth for women with breast cancer: differences by tumor characteristics. Cancer Causes Control. 2013;24:587–594. doi: 10.1007/s10552-012-9997-7. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins CD, Stanton B, Niemcryk SJ, Rose RM. A scale for the estimation of sleep problems in clinical research. J Clin Epidemiol. 1988;41(4):313–321. doi: 10.1016/0895-4356(88)90138-2. [DOI] [PubMed] [Google Scholar]
- 14.Levine D, Kaplan RM, Kripke DF, Bowen DJ, Naughton MJ, Shumaker SA. Factor structure and measurement invariance of the Women's Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15(2):123–136. doi: 10.1037/1040-3590.15.2.123. [DOI] [PubMed] [Google Scholar]
- 15.Levine DW, Kripke DF, Kaplan RM, et al. Reliability and validity of the Women's Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15(2):137–148. doi: 10.1037/1040-3590.15.2.137. [DOI] [PubMed] [Google Scholar]
- 16.Levine D, Dailey ME, Rockhill B, Tipping D, Naughton MJ, Shumaker SA. Validation of the Women's Health Initiative Insomnia Rating Scale in a multicenter controlled clinical trial. Psychosom Med. 2005;67(1):98–104. doi: 10.1097/01.psy.0000151743.58067.f0. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen T, Hsu F, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10(16):5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 18.Qin Y, Zhou Y, Zhang X, Wei X, He J. Sleep duration and breast cancer risk: a meta-analysis of observational studies. Int J Cancer. 2014;134(5):1166–1173. doi: 10.1002/ijc.28452. [DOI] [PubMed] [Google Scholar]
- 19.Girschik J, Heyworth J, Fritschi L. Self-reported sleep duration, sleep quality, and breast cancer risk in a population-based case-control study. Am J Epidemiol. 2013;177(4):316–327. doi: 10.1093/aje/kws422. [DOI] [PubMed] [Google Scholar]
- 20.Tamimi R, Colditz GA, Hazra A, et al. Traditional breast cancer risk factors in relation to molecular subtypes of breast cancer. Breast Cancer Res Treat. 2012;131(1):159–167. doi: 10.1007/s10549-011-1702-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blask D, Sauer LA, Dauchy RT. Melatonin as a chronobiotic/ anticancer agent: cellular, biochemical, and molecular mechanisms of action and their implications for circadian-based cancer therapy. Curr Top Med Chem. 2002;2(2):113–132. doi: 10.2174/1568026023394407. [DOI] [PubMed] [Google Scholar]
- 22.Blask D. Melatonin, sleep disturbance and cancer risk. Sleep Med Rev. 2009;13(4):257–264. doi: 10.1016/j.smrv.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Viswanathan AN, Schernhammer E. Circulating melatonin and the risk of breast and endometrial cancer in women. Cancer Lett. 2009;281(1) doi: 10.1016/j.canlet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu A, Wang R, Koh W, Stanczyk FZ, Lee H, Yu MC. Sleep duration, melatonin and breast cancer among Chinese women in Singapore. Carcinogenesis. 2008;29(6):1244–1248. doi: 10.1093/carcin/bgn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collis SJ, Boulton SJ. Emerging links between the biological clock and the DNA damage response. Chromosoma. 2007;116(4):331–339. doi: 10.1007/s00412-007-0108-6. [DOI] [PubMed] [Google Scholar]
- 26.Stevens R. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology. 2005;16(2):254–258. doi: 10.1097/01.ede.0000152525.21924.54. [DOI] [PubMed] [Google Scholar]
- 27.Wulff K, Porcheret K, Cussans E, Foster RG. Sleep and circadian rhythm disturbances: multiple genes and multiple phenotypes. Curr Opin Genet Devel. 2009;19(3):237–246. doi: 10.1016/j.gde.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 28.He C, Anand ST, Ebell MH, Vena JE, Robb SW. Circadian disrupting exposures and breast cancer risk: a meta-analysis. Int Arch Occup Environ Health. 2015;88(5):533–547. doi: 10.1007/s00420-014-0986-x. [DOI] [PubMed] [Google Scholar]


