Abstract
Study Objectives:
In children, the effect of the common phenotype of obstructive sleep apnea (OSA) on sleep architecture is not adequately documented. The aim of this study was to evaluate sleep architecture in a pediatric population with the common phenotype of OSA.
Methods:
The prospective cross-sectional study included 116 children in the age range of 3 to 8 years with suspected OSA and 51 healthy children. All children underwent standard overnight in-laboratory video polysomnography. Patients with obstructive apnea-hypopnea index ≥ 1, adenotonsillar hypertrophy, a long face, narrow palate or minor malocclusions, and no obesity were defined as a common phenotype. Polysomnographic parameters of sleep architecture and sleep clinical record were statistically analyzed according to OSA and its severity.
Results:
In total, 94 pediatric patients (59.60% male) received the diagnosis of the common phenotype of OSA (mean age of 5.25 ± 1.39 years). A lower percentage of stage N3 sleep (27.70 ± 3.76% versus 31.02 ± 4.23%; P < .05), a greater percentage of stage N1 sleep (8.40 ± 3.98% versus 2.68 ± 3.02%, P < .01), reduced deep sleep efficiency (46.01 ± 4.98% versus 50.25 ± 3.72%; P < .05) and longer sleep latency (18.40 ± 8.48 minutes versus 9.90 ± 11.55 minutes, P < .01) were found in children with the common phenotype of OSA compared with healthy controls. No significant differences were found in total sleep time, sleep efficiency, and percentage of stage R sleep and stage N2 sleep between groups and in sleep stage distribution and cyclization.
Conclusions:
These findings suggest that the most common phenotype of pediatric OSA has a negative effect on the structure of sleep, but other clinical studies are needed to confirm this result.
Citation:
Durdik P, Sujanska A, Suroviakova S, Evangelisti M, Banovcin P, Villa MP. Sleep architecture in children with common phenotype of obstructive sleep apnea. J Clin Sleep Med. 2018;14(1):9–14.
Keywords: children, common phenotype, obstructive sleep apnea, sleep architecture
BRIEF SUMMARY
Current Knowledge/Study Rationale: In children, the effect of the common phenotype of obstructive sleep apnea (OSA) on sleep architecture is not actually adequately documented. The aim of this prospective cross-sectional study was to evaluate sleep architecture in a pediatric population with the common phenotype of OSA.
Study Impact: Our findings suggest that the most common phenotype of pediatric OSA has a negative effect on the structure of sleep, but other clinical studies are needed to confirm this result.
INTRODUCTION
Obstructive sleep apnea (OSA) in children is sleep-disordered breathing characterized by a combination of repeated episodes of prolonged partial upper airway obstruction (obstructive hypopnea) and/or intermittent complete obstruction (obstructive apnea) that disturb normal sleep patterns and normal ventilation during sleep; this results in the disruption of normal gas exchange (intermittent hypoxia and hypercapnia).1,2 The common phenotype of a child with OSA is characterized by adenotonsillar hypertrophy, a long face, narrow palate, or minor malocclusions. The congenital type of OSA in children is represented by a phenotype starting from infancy and mostly related to the Pierre Robin sequence, with retrognathia and micrognathia. The adult phenotype of pediatric OSA is characterized by obesity, a short neck, and midface hypoplasia. Each phenotype may be associated with several degrees of adenoid and tonsillar enlargement, which mostly occurs in the common type.3
It is evident that adults with OSA have altered sleep architecture that will not allow adequate recovery of the whole organism, regardless of the duration of sleep.4–6 However, there is currently no available evidence of this in children, because only a small number of studies have assessed the effect of OSA on sleep architecture in children with the common phenotype. The results of previous studies are also inconsistent.4,7–11 The aim of this study was to evaluate sleep architecture in pediatric subjects with the common phenotype of OSA.
METHODS
Study Population
This is a prospective cross-sectional study. In total, 116 children in the age range of 3 to 8 years with a history of snoring and clinical features of OSA were referred to our Pediatric Sleep Centre, at the Faculty of Medicine and Psychology, “La Sapienza” University, Sant'Andrea Hospital, Rome from September 2015 to April 2016. A control group of children (n = 51) similar in age and predominantly male and with no history or parental concerns of snoring was recruited from the community. Patients with acute or chronic cardiorespiratory, neuromuscular, or neurologic diseases, congenital craniofacial abnormalities, chromosomal syndromes, or those with a history of previous treatment for OSA were excluded. All subjects underwent standard overnight in-laboratory video polysomnography (PSG). Depending on PSG result and the standard diagnostic algorithm patients with insomnia, hypersomnia, parasomnia, or periodic limb movements in sleep were also excluded. We consecutively enrolled 94 patients with the common phenotype of OSA that was defined as adenotonsillar hypertrophy and obstructive apnea-hypopnea index (OAHI) ≥ 1 events/h of total sleep time (TST) according to standard PSG, with the presence of a long face, narrow palate, or minor mal-occlusions. In the control group the absence of OSA was also confirmed by PSG, with children having an OAIH < 1 event/h of TST and no observation of snoring.
The local ethics committee approved the study protocol and the parents of all the children provided informed consent.
Study Design
After obtaining a detailed personal and family history, all subjects included in the study underwent a complete physical examination, sleep clinical record (SCR), and PSG. Body weight and height were used to calculate body mass index to exclude children with obesity defined as a body mass index over the 97th percentile according to national references. The SCR is a simple PSG test-validated screening tool for OSA that combines the patient's history with findings from a physical examination. The SCR comprises 6 parts. In the first 3 parts, the presence of upper airway obstruction, dental malocclusion, a narrow palate or pathological position of the mandible is explored. In the fourth part, the presence of a facial phenotype is noted. The fifth part describes the patient's clinical symptoms as summarized by the Brouillette OSA score. The last part incorporates symptoms of attention-deficit/hyperactivity disorder rating scale completed by the parents. The sleep clinical score (SCS) was defined as positive when the cumulative score from all items was greater than or equal to 6.5.12
Overnight PSG and Sleep Stage Scoring
All children were evaluated in our Pediatric Sleep Centre by means of full-night video PSG after an adaptation night in the hospital. Children were studied in a quiet, darkened room in the company of one of their parents. Adaptation night in the hospital was performed in the sleep laboratory without any leads placed. All the recordings were started at the patients' usual bedtime and were continued until spontaneous awakening occurred. Standard overnight PSG recordings were obtained by means of a Grass Heritage polygraph (Natus Neurology Incorporation, Middleton, Wisconsin, United States). A digital time-synchronized video recording was also performed. The variables recorded included an electroencephalogram (EEG) with at least 8 channels (F4A1, F3A2, C4A1, C3A2, O2A1, O1A2, T6A1, and T5A2), an electro-oculogram (electrodes placed 1 cm above the right outer cantus and 1 cm below the left outer cantus and referred to the mastoid electrode), a submental electromyogram (EMG) (1 electrode placed in the midline 1 cm above the inferior edge of the mandible and 2 electrodes placed 1 cm below the inferior edge of the mandible and 1 cm to the right and left of the midline of the chin), a leg electromyogram (surface electrodes were placed longitudinally and symmetrically around the middle of the anterior tibialis muscle so that they were 2 cm apart) and an electrocardiogram (1 derivation). Sleep was subdivided into 30-second epochs, and sleep stages were scored according to the standard criteria of the American Academy of Sleep Medicine (AASM).13 Chest and abdominal movements were measured by strain gauges. Oronasal airflow was recorded with a thermocouple and nasal pressure monitor when the child tolerated a nasal cannula. Arterial oxygen saturation was monitored with a pulse oximeter. End-tidal carbon dioxide was not standardly performed. Central, obstructive, mixed apnea events and respiratory event-related arousals were counted according to the criteria established by the AASM.14 OAHI was calculated as the average number of obstructive apneas and hypopneas per hour of sleep and the diagnosis of OSA was confirmed by OAHI ≥ 1 events/h of TST. Patients with the common phenotype of OSA were categorized into three groups according to severity (mild OSA, OAHI 1 to 5 events/h; moderate OSA, OAHI > 5 to 10 events/h; severe OSA, OAHI > 10 events/h). All children in the control group had OAHI < 1 event/h, with no other types of apneas, or any obesity, adenotonsillar hypertrophy, or malocclusion. Arousal was defined as a sudden change in EEG frequency consisting of alpha and theta activity or waveforms with a frequency greater than 16 Hz (but not sleep spindles) and a duration of 3 to 15 seconds. Normal sleep was recorded for at least 10 seconds before and after the event. Arousals associated with respiratory events were classified as respiratory arousals, arousals associated with limb movements were classified as movement arousals, and arousals of unknown origin were defined as spontaneous. Arousals from stage R sleep required a concurrent increase in submental EMG for a minimum of 1 second. The arousal index is the number of events that are scored as a wake reaction (arousals) per 1 hour of TST. The sleep efficiency was defined as the ratio of the TST compared to the total amount of time spent in bed and the deep sleep efficiency as the ratio of the total amount of deep sleep (stage N3 sleep and stage R sleep) compared to the TST.
Statistical Analysis
The variables were statistically analyzed by SYSTAT version 11 (Systat Software Inc., San Jose, California, United States). Data from both the control and case groups were first tested for normality and equal variance by the Shapiro-Wilk test; all data were normally distributed and recorded as mean ± standard deviation. Descriptive statistical methods were used to describe demographic and anthropometric parameters of patients and controls and the chi-square test and unpaired Student t test were used to establish differences between variables. Differences between the means of selected parameters measured during PSG recording and characterized sleep architecture were assessed using the independent nonpaired Student t test. A two-way analysis of variance was used to compare differences in arousal type (respiratory, movement and spontaneous arousals) in common phenotype of OSA. The Pearson correlation coefficient was used to correlate the association of severity of OSA in case group to parameters of sleep architecture and also arousal types. A value of P < .05 was considered statistically significant. Correlation coefficients r = 0–0.19 were considered as weak, 0.20–0.39 as mild, 0.40–0.59 as moderate, 0.60–0.79 as moderately strong, and 0.80–1.0 as a strong correlation.
RESULTS
Study Group
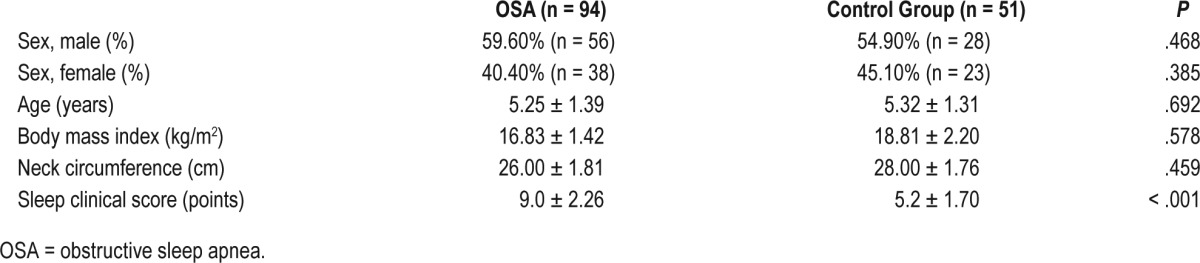
We consecutively enrolled 94 pediatric patients with the common phenotype of OSA, with a mean age of 5.25 ± 1.39 years, 56 male (59.60%) and 51 healthy children with a mean age 5.32 ± 1.31 years, 28 male (54.90%). The differences between the case group (children with common phenotype of OSA) and the control group of healthy children were not statistically significant in terms of age, sex, body mass index, and neck circumference. OAHI (6.20 ± 4.86 versus 0.21 ± 0.13; P < .001) and SCS (9.00 ± 2.26 versus 5.20 ± 1.70; P < .001) were significantly higher in the case group compared to healthy children. Average saturation during sleep was not significantly different between the two groups (97.00 ± 1.02 versus 96.00 ± 0.05; P = .246). The clinical characteristics of this prospective cohort are reported in Table 1.
Table 1.
Anthropometric parameters in children with the common phenotype of OSA and the control group.
Polysomnographic Parameters of Sleep Architecture
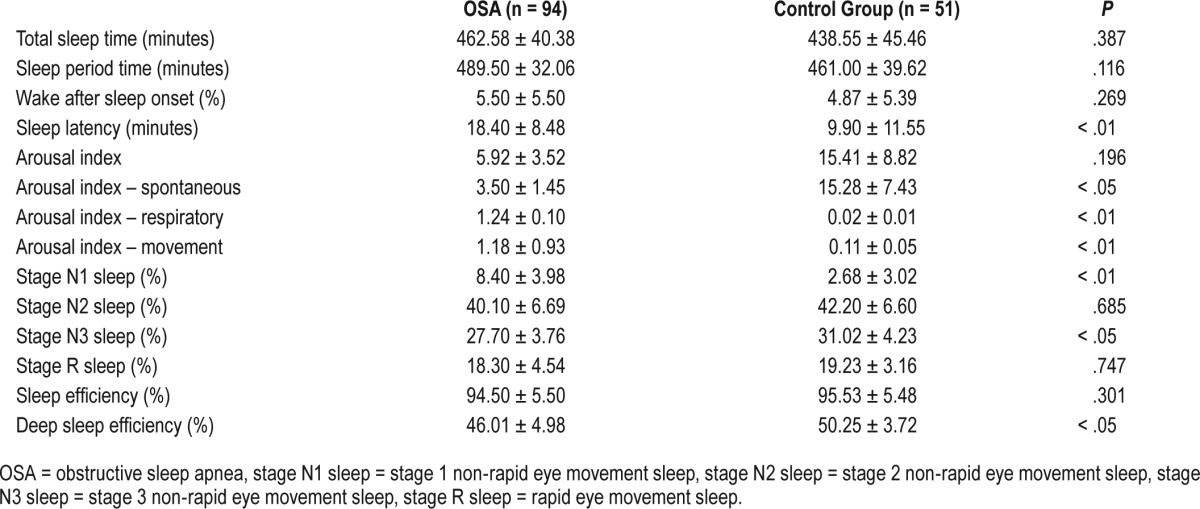
Sleep cyclization and the distribution of sleep stages were maintained in both study groups. Stage N3 sleep was significantly shorter in the case group than in the control group (27.70 ± 3.76% versus 31.02 ± 4.23%; P < .05), whereas stage N1 sleep was significantly longer (8.40 ± 3.98% versus 2.68 ± 3.02%; P < .01). Significantly reduced deep sleep efficiency (46.01 ± 4.98% versus 50.25 ± 3.72%; P < .05) and longer sleep latency (18.40 ± 8.48 minutes versus 9.90 ± 11.55 minutes; P < .01) were found in children with the common phenotype of OSA compared with healthy controls. Sleep period time (SPT) (489.50 ± 32.06 minutes versus 461.00 ± 39.62 minutes; P = .116), TST (462.58 ± 40.38 minutes versus 438.58 ± 45.46 minutes; P = .387), wake during sleep (5.50 ± 5.50 minutes versus 4.87 ± 5.39 minutes; P = .269), stage R sleep (18.30 ± 4.54% versus 19.23 ± 3.16%; P = .747), percentage of stage N2 sleep (40.10 ± 6.69% versus 42.20 ± 6.60%; P = .685) and sleep efficiency (94.50 ± 5.50% versus 95.53 ± 5.48%; P = .301) were not significantly different between the case and control groups. Table 2 shows the comparison of sleep architecture according to PSG between the two groups.
Table 2.
Polysomnographic parameters of sleep architecture in children with the common phenotype of OSA and the control group.
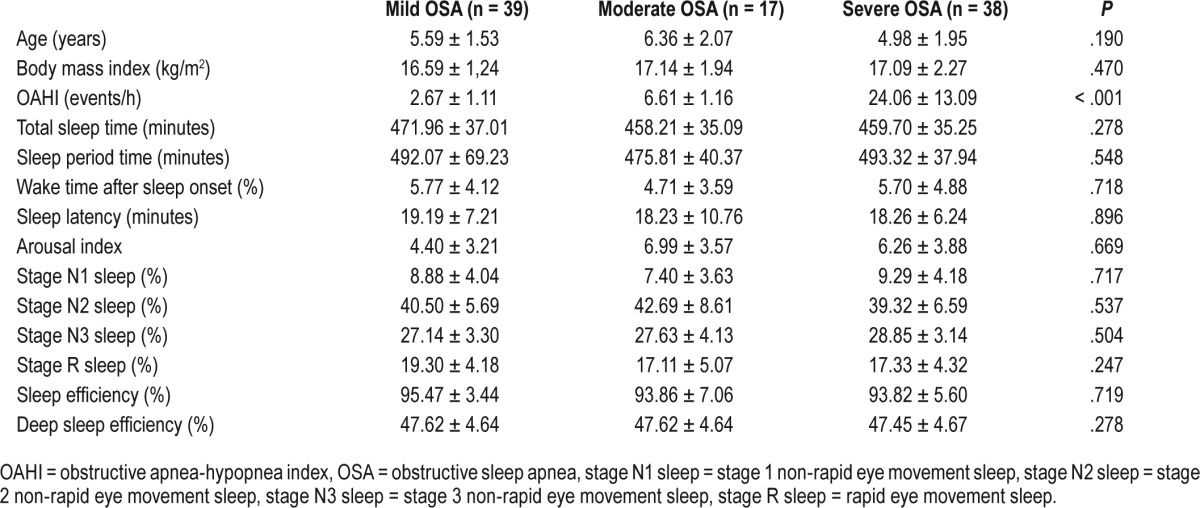
The arousal index was almost three times higher in the control group than in the case group, but the difference was not significant. As we expected, significantly more respiratory (1.24 ± 0.10 versus 0.02 ± 0.01; P < .01) and movement (1.18 ± 0.93 versus 0.11 ± 0.05; P < .01) arousals were mentioned in children with the common phenotype of OSA; however, a significantly lower number of spontaneous arousals was reported in the case group compared to healthy children (3.50 ± 1.45 versus 15.28 ± 7.43; P < .05). Although no signifi-cant changes in stage R sleep duration were observed between groups, significantly more respiratory arousals were recorded in the case group during stage R sleep (4.88 ± 0.35 in stage R sleep versus 0.36 ± 0.04 in non-stage R sleep; P < .001). The case group was divided into three groups according to the severity of OSA: 39 (41.49%) with mild OSA, 17 (18.08%) with moderate OSA, and 38 (40.43%) with severe OSA. There were no observed significant differences between the groups of pediatric patients according to the severity of OSA in the basic parameters of the structure and architecture of sleep. Table 3 shows the comparison of sleep architecture in children with the common phenotype of OSA according to the severity of OSA. A weak but nonsignificant correlation were found between OAHI and stage N3 sleep (r = 0.12; P = .346) and also stage N1 sleep (r = 0.18; P = .298). No correlation was established between OAHI and sleep latency, SPT, TST, wake during sleep, stage R sleep, stage N2 sleep, sleep efficiency, deep sleep efficiency, and arousal index in the common phenotype of OSA. There were moderate positive correlations between OAHI and respiratory arousals (r = 0.56; P < .01) and also between respiratory arousals during stage R sleep (r = 0.51; P < .01) in the case group; these correlations were statistically significant.
Table 3.
Polysomnographic parameters of sleep architecture in children with the common phenotype of OSA according to the severity of OSA.
DISCUSSION
OSA has become widely recognized as a frequent and relatively common disorder with potentially serious clinical implications in childhood with a prevalence from 1% to 5% in the pediatric population.15,16 Recently, it has been reported that delayed diagnosis of this syndrome can lead to neurobehavioral consequences and even serious cardiorespiratory morbidity and metabolic complications, as well as an increase in insulin resistance, high blood pressure, and the development of OSA in adulthood. The peak incidence of OSA in childhood occurs between the ages of 3 and 6 years due to obstruction of the nasal and oropharyngeal airway by adenotonsillar hypertrophy, resulting in increased airway resistance. Adenotonsillar hypertrophy associated with a long face, narrow palate, or minor malocclusions is characteristic of the common phenotype of pediatric OSA.1,2,15
Altered sleep architecture that will not provide adequate recovery of the organism, regardless of sleep duration, is well documented in adult patients with OSA. Adults show a disruption of sleep cyclization, higher representation of stage N1 sleep and fewer deep sleep stages, which are often lacking. Arousals that occur after respiratory events do not allow patients to get good quality, uninterrupted sleep. Adult patients suffer from excessive daytime sleepiness and fatigue.4–6,17,18
There is currently no available evidence of altered sleep architecture in children, because only a small number of studies have assessed the effect of OSA on sleep architecture in children with the common phenotype. Goh and co-workers reported that children with OSA had normal sleep architecture, but the limitation of the study was a small number of patients.4 Scholle and co-authors found a normal sleep macrostructure and increased arousal index in children with OSA compared to controls.7 The duration of sleep stages, TST, SPT, and sleep latency in children with OSA were maintained in the study by Walter et al., but reduced sleep efficiency and a significantly lower proportion of SPT in stage R sleep were documented.8 Similarly, Tauman and co-workers found that children with OSA had a significantly increased percentage of slow wave sleep and decreased stage R sleep compared with control children.9 In contrast, recently published work by Sun et al. showed that school-aged patients with OSA had a significant reduction in stage N3 sleep and prolonged stage N1 sleep compared to controls.10 Zhu and co-workers11 showed that primary snoring did not exert significant adverse influences on normal sleep architecture in prepubertal school-aged children. Nevertheless, pubertal adolescents with primary snoring had increased stage N1 sleep and wake time after sleep onset.11
Our study is the first to report on changes to selected parameters of sleep architecture in the common phenotype of pediatric OSA. Sleep cyclization and the distribution of sleep stages were maintained in the common phenotype in children with OSA in the current study. Moreover, SPT, TST, wake during sleep, stage R sleep, stage N2 sleep, and sleep efficiency showed no significant differences between the case and control groups in our work. Similar to results from Sun et al., we also report significantly shorter stage N3 sleep in children with the common phenotype of OSA than in the control group, whereas stage N1 sleep was significantly longer. OSA in children is typically predominantly reported as a stage R sleep phenomenon.17,19 Although we did not observe significant changes in stage R sleep duration in both groups, significantly more respiratory arousals were recorded during stage R sleep. Our findings support the hypothesis that the common phenotype of pediatric OSA is predominantly a stage R sleep phenomenon. According to Goh and colleagues, OSA worsens over the course of the night, independent of the changing amount of stage R sleep. Classic symptoms during the daytime in children with the common phenotype of OSA usually present as behavioral disturbances, from subtle impairments in learning, attention, and behavior to prominent neurobehavioral deficits that may mimic attention-deficit/hyperactivity disorder or learning disabilities.1,9 We hypothesize that, rather than changes in the duration of stage R sleep, altered stage R sleep due to respiratory arousals may explain the possible further consequences of neurocognitive damage in most young children with the common phenotype of OSA.
Deep sleep is important for various metabolic and endocrine processes, such as the secretion of growth hormone and other anabolic processes, especially insulin-induced glucose uptake in the peripheral organs. There is a well-documented close association between adult OSA (with typically altered or lacking deep sleep stages) and the development of type 2 diabetes mellitus.20 In our children with the common phenotype of OSA, we also recorded a significant but mild reduction in deep sleep efficiency stage N3 sleep. We hypothesize that mild alternations in deep sleep in children with OSA could lead to various minimal subclinical metabolic changes that could manifest as metabolic complications in adulthood in children with untreated OSA. Zhu and colleagues21 reported that stage N3 sleep, sleep efficiency and TST are protective factors in maintaining glucose and insulin homeostasis. However, stage N1 sleep functions in the opposite direction.8,21,22
There were no observed significant differences in the groups of pediatric patients with the common phenotype according to the severity of OSA in the basic parameters of the structure and architecture of sleep in our work. However, Jalilolghadr et al.23 showed that obese children with metabolic syndrome had increased wake after sleep onset, stage N1 sleep, and severe OSA compared with obese children without metabolic syndrome. The results regarding sleep architecture are most likely a direct result of OSA severity in obese children.23 We can assume that the obesity in children with OSA similar to adults can have negative effect on the structure and architecture of sleep according to the severity of OSA.
As we expected, more respiratory and movement arousals were observed in children with OSA. In addition, a significantly lower number of spontaneous arousals was reported in children with OSA compared to healthy children. That children with OSA should have fewer spontaneous arousals than healthy children may appear counterintuitive.8 Tauman and co-authors hypothesized that this occurred to preserve sleep homeostasis via mechanisms that may compensate for their increased respiratory arousals.9 Also, Walter and colleagues8 confirmed that children with OSA have fewer spontaneous arousals, predominantly during stage R sleep, compared with a healthy control group of children. This phenomenon likely reduces the cumulative effect of waking reactions during sleep in children with OSA and may protect children from an additive effect on cardiovascular, behavioral, and neurocognitive sequelae.8 We can also agree with the theory of the mechanism of sleep homeostasis in children with OSA as reported in previous studies. In addition, we noticed a higher spontaneous arousal index in the control group of children. Because sleep disorders had not been diagnosed in the control group, we can now only hypothesize that PSG electrodes or other factors (no conventional home stereotypes of child) may be responsible for the higher number of spontaneous arousals.
CONCLUSIONS
In summary, this study demonstrates that the sleep architecture in the common phenotype of pediatric OSA is altered. We suspected that it will have a negative effect on sleep, and even mild alterations of deep sleep could predispose to minimal subclinical metabolic changes that could manifest as metabolic complications in adulthood in children with untreated OSA. However, these results need further clinical studies to confirm our hypothesis. Finally, the important clinical implications of these findings highlight the importance of early identification and accurate management of all children with OSA.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The authors report that they have no financial interests or connections, direct or indirect, or other situations that might raise the question of bias in the work reported or the conclusions, implications, or opinions stated—including pertinent commercial or other sources of funding for the individual authors or for the associated departments or organizations, personal relationships, or direct academic competition for each author.
ACKNOWLEDGMENTS
This work was supported by the project “Centre of experimental and clinical respirology, CEKR II” (ITMS: 26220120034) co-funded by EU sources and VEGA grant 1/0252/14.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- EEG
electroencephalogram
- EMG
electromyogram
- OAHI
obstructive apnea-hypopnea index
- OSA
obstructive sleep apnea
- PSG
polysomnography
- RERA
respiratory event-related arousal
- SCR
sleep clinical record
- SCS
sleep clinical score
- SDB
sleep-disordered breathing
- SPT
sleep period time
- stage N1 sleep
stage 1 non-rapid eye movement sleep
- stage N2 sleep
stage 2 non-rapid eye movement sleep
- stage N3 sleep
stage 3 non-rapid eye movement sleep
- stage R sleep
rapid eye movement sleep
- TST
total sleep time
REFERENCES
- 1.Tauman R, Gozal D. Obstructive sleep apnea syndrome in children. Expert Rev Respir Med. 2011;5(3):425–440. doi: 10.1586/ers.11.7. [DOI] [PubMed] [Google Scholar]
- 2.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):576–584. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 3.Villa MP, Sujanska A, Vitelli O, et al. Use of the sleep clinical record in the follow-up of children with obstructive sleep apnea (OSA) after treatment. Sleep Breath. 2016;20(1):321–329. doi: 10.1007/s11325-015-1287-7. [DOI] [PubMed] [Google Scholar]
- 4.Goh DY, Galster P, Marcus CL. Sleep architecture and respiratory disturbances in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;162(2 Pt 1):682–686. doi: 10.1164/ajrccm.162.2.9908058. [DOI] [PubMed] [Google Scholar]
- 5.Ng AK, Guan C. Impact of obstructive sleep apnea on sleep-wake stage ratio. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:4660–4663. doi: 10.1109/EMBC.2012.6347006. [DOI] [PubMed] [Google Scholar]
- 6.Ratnavadivel R, Chau N, Stadler D, Yeo A, McEvoy RD, Catcheside PG. Marked reduction in obstructive sleep apnea severity in slow wave sleep. J Clin Sleep Med. 2009;5(6):519–524. [PMC free article] [PubMed] [Google Scholar]
- 7.Scholle S, Zwacka G. Arousals and obstructive sleep apnea syndrome in children. Clin Neurophysiol. 2001;112(6):984–991. doi: 10.1016/s1388-2457(01)00508-9. [DOI] [PubMed] [Google Scholar]
- 8.Walter LM, Nixon GM, Davey MJ, O'Driscoll DM, Trinder J, Horne RS. Sleep disturbance in pre-school children with obstructive sleep apnoea syndrome. Sleep Med. 2011;12(9):880–886. doi: 10.1016/j.sleep.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Tauman R, O'Brien LM, Holbrook CR, Gozal D. Sleep pressure score: a new index of sleep disruption in snoring children. Sleep. 2004;27(2):274–278. doi: 10.1093/sleep/27.2.274. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Lei F, Du L, Tang X, Yang L. [Comparison of polysomnographic characteristics in preschool and school aged children with obstructive sleep apnea hypopnea syndrome] Zhonghua Yi Xue Za Zhi. 2016;96(8):601–604. doi: 10.3760/cma.j.issn.0376-2491.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Y, Au CT, Lam HS, et al. Sleep architecture in school-aged children with primary snoring. Sleep Med. 2014;15(3):303–308. doi: 10.1016/j.sleep.2013.08.801. [DOI] [PubMed] [Google Scholar]
- 12.Villa MP, Paolino MC, Castaldo R, et al. Sleep clinical record: an aid to rapid and accurate diagnosis of paediatric sleep disordered breathing. Eur Respir J. 2013;41(6):1355–1361. doi: 10.1183/09031936.00215411. [DOI] [PubMed] [Google Scholar]
- 13.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 14.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaditis AG, Alonso Alvarez ML, Boudewyns A, et al. Obstructive sleep disordered breathing in 2- to 18-year-old children: diagnosis and management. Eur Respir J. 2016;47(1):69–94. doi: 10.1183/13993003.00385-2015. [DOI] [PubMed] [Google Scholar]
- 16.Powell S, Kubba H, O'Brien C, Tremlett M. Paediatric obstructive sleep apnoea. BMJ. 2010;340:c1918. doi: 10.1136/bmj.c1918. [DOI] [PubMed] [Google Scholar]
- 17.Loadsman JA, Wilcox I. Is obstructive sleep apnoea a rapid eye movement-predominant phenomenon? Br J Anaesth. 2000;85(3):354–358. doi: 10.1093/bja/85.3.354. [DOI] [PubMed] [Google Scholar]
- 18.Muraki M, Kitaguchi S, Ichihashi H, et al. Apnoea-hypopnoea index during rapid eye movement and non-rapid eye movement sleep in obstructive sleep apnoea. J Int Med Res. 2008;36(5):906–913. doi: 10.1177/147323000803600506. [DOI] [PubMed] [Google Scholar]
- 19.Verginis N, Jolley D, Horne RS, Davey MJ, Nixon GM. Sleep state distribution of obstructive events in children: is obstructive sleep apnoea really a rapid eye movement sleep-related condition? J Sleep Res. 2009;18(4):411–414. doi: 10.1111/j.1365-2869.2009.00760.x. [DOI] [PubMed] [Google Scholar]
- 20.Kent BD, Grote L, Ryan S, et al. Diabetes mellitus prevalence and control in sleep-disordered breathing: the European Sleep Apnea Cohort (ESADA) study. Chest. 2014;146(4):982–990. doi: 10.1378/chest.13-2403. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Y, Li AM, Au CT, et al. Association between sleep architecture and glucose tolerance in children and adolescents. J Diabetes. 2015;7(1):10–15. doi: 10.1111/1753-0407.12138. [DOI] [PubMed] [Google Scholar]
- 22.Vojtkova J, Ciljakova M, Michnova Z, Turcan T. Chronic complications of diabetes mellitus related to the respiratory system. Pediatr Endocrinol Diabetes Metab. 2012;18(3):112–115. [PubMed] [Google Scholar]
- 23.Jalilolghadr S, Yazdi Z, Mahram M, et al. Sleep architecture and obstructive sleep apnea in obese children with and without metabolic syndrome: a case control study. Sleep Breath. 2016;20(2):845–851. doi: 10.1007/s11325-015-1291-y. [DOI] [PubMed] [Google Scholar]


