Abstract
Rituximab (Rituxan®; Biogen Idec, San Diego, CA, USA) is a human-mouse chimeric monoclonal antibody specific for CD20, a surface glycoprotein expressed on B lymphocytes. Administration of rituximab as a single agent to patients with chronic lymphocytic leukemia (CLL) has limited clinical activity, but generally does not eradicate leukemia from the marrow. However, when administered in combination with chemotherapy, rituximab can improve the survival of patients relative to those treated with chemotherapy alone. As a result of this, the US Food and Drug Administration approved the use of rituximab in previously untreated and previously treated CD20-positive CLL in combination with fludarabine monophosphate and cyclophosphamide. The results of clinical studies evaluating the activity of rituximab when used alone or in combination with other antileukemia agents for the treatment of this disease are reviewed here.
Keywords: CD20, chronic lymphocytic leukemia, CLL, monoclonal antibody, Rituxan, rituximab, treatment
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is characterized by the progressive accumulation of monoclonal B cells in the blood, secondary lymphoid tissues, and marrow. Accumulation of leukemic B lymphocytes in the marrow can interfere with normal hematopoiesis. As standard therapy is not considered curative and may result in early morbidity and mortality, treatment is usually delayed until patients develop symptomatic and/or progressive disease.1 Over the past decade purine analogs, such as fludarabine administered alone or in combination with alkylating agents, have become the cornerstone of therapy for patients with CLL. However, the use of such agents alone have not been clearly demonstrated to improve overall survival.
In October 2009, the US Food and Drug Administration (FDA) approved rituximab (Rituxan®; Biogen Idec, San Diego, CA, USA) for previously untreated and previously treated CD20-positive CLL in combination with fludarabine and cyclophosphamide. This approval was based on the outcomes of two large phase 3 studies in which patients received chemotherapy alone or chemotherapy in combination with rituximab.2 The clinical trials outlined in this review highlight the studies on the safety and activity of rituximab that have provided the basis for the widespread use of the monoclonal antibody (mAb) in the treatment of patients with CLL.
Due to limitations on references and word count, those clinical studies impacting current CLL treatment approaches will be highlighted for review.
Rituximab and the Target CD20
The chimeric human-mouse mAb, rituximab, is a human immunoglobulin G1 (IgG1) kappa antibody with mouse variable regions isolated from a murine anti-CD20 mAb. The target of rituximab, CD20, is a tetraspan phosphoprotein that is expressed on B lymphocytes, but not on plasma cells or cells of other lineages. CLL cells express lower levels of CD20 than normal blood B cells or follicular lymphoma B cells. Rituximab acts via binding to CD20 expressed on the surface of B lymphocytes, which leads to the elimination of these lymphocytes through antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and by direct induction of apoptosis.3,4
Single nucleotide polymorphisms in the genes encoding Fc gamma receptors (FcGR2A and FcGR3A) can influence the binding affinity of the mAb, modulate ADCC in vitro, and alter the in vivo activity of rituximab. However, retrospective analysis of more than 400 CLL patients who received fludarabine and cyclophosphamide (FC) with or without rituximab did not reveal an association with FcGR single nucleotide polymorphisms, relative to response to therapy, progression-free survival (PFS) or overall survival (OS).5 Preclinical work on CDC suggested that treatment with this mAb induced shaving of CD20 from the leukemia-cell plasma membrane and that such shaving could contribute to diminished mAb-associated CDC.6,7 However, clinical trials using low doses of rituximab to mitigate this effect have not demonstrated clinical activity on par with that achieved by using higher amounts of rituximab.8
The pharmacokinetics of rituximab are best described by a two-compartment model with a long elimination half-life (t½), which increases with repeated weekly infusions mainly due to a progressive decrease in the antibody clearance.9,10 However, there is a marked interpatient variability in the pharmacokinetics of rituximab observed in patients with CLL.11,12 This variability may be due to the difference in total tumor volume and/or level of expression of CD20 on B-lymphoma cells among patients. Several studies have found an inverse correlation between the tumor burden and rituximab concentration in patients with B-cell lymphoma.9,13,14 Patients with CLL are often observed as having lower concentrations of rituximab than those with follicular lymphoma that are treated with the same amount of antibody.
Rituximab as Monotherapy
The high-level expression of CD20 on follicular B-cell lymphoma led to the development of rituximab for the treatment of patients with low-grade B-cell lymphomas. Rituximab as a monotherapy was examined in phase 1 studies at doses up to 400 mg/m2. These studies failed to demonstrate dose-limiting toxicities, and 375 mg/m2 was selected as the phase 2 dose.11,14 The pivotal trial evaluating the use of rituximab (375 mg/m2 once weekly for 4 weeks) in the treatment of 166 patients with low-grade lymphomas yielded an overall response rate (ORR) of 48%.15 Notably, the response rate of patients with small lymphocytic lymphoma (SLL) or CLL was lower (12%) than that noted for patients with follicular lymphoma.
Pharmacokinetics of rituximab on this study demonstrated a correlation with antibody concentration and response. Compared with follicular lymphoma patients, CLL/SLL patients achieved a significantly lower plasma concentration of the mAb and experienced a rapid clearance of the antibody.15 In addition to differences in pharmacokinetics, other explanations for the relative lack of activity in patients with CLL included the relatively low level of CD20 found on CLL cells and the relatively large intravascular tumor burden for such patients. Nevertheless, based on the results of this study, rituximab became the first mAb approved by the FDA for the treatment of cancer.
Subsequent studies evaluating the use of single-agent rituximab to treat patients with CLL used more frequent dosing and higher doses of rituximab. The Cancer and Leukemia Group B (CALGB) reported on 33 CLL/SLL patients who were treated with rituximab at 375 mg/m2 three times a week for 4 weeks.7 All patients received the first dose of rituximab split over two infusions, with 100 mg on the first infusion day over 4 hours and the remainder of the 375 mg/m2 dose administered on day 3. This stepped-up dosing was well tolerated with only 6% of the patients experiencing severe infusion reactions, and a decreased incidence of infusion reactions with each subsequent infusion. Clinically the only factor associated with an increased risk of infusion toxicity was age >70 years. Infusion reactions were noted to be associated with release of inflammatory cytokines. The ORR to therapy was 45% with one patient achieving a complete remission (CR). The responses observed in CLL/SLL patients treated with the additional doses and more frequent administration of the antibody compared favorably to the responses observed with a once weekly dosing regimen.6,7 Those patients that responded to therapy were noted to have a median time to progression of 11 months.
O’Brien and investigators at the MD Anderson Cancer Center (MDACC) administered escalated doses of rituximab (500–2250 mg/m2) for 4 weekly infusions.16 Infusion reactions to the first dose of rituximab were noted in 94% of patients with 12% experiencing severe first-dose toxicity. Thirty-six percent of CLL patients responded well to therapy and all achieved some perceived clinical benefit. However, the median time to progression for responding patients was only 8 months. Higher doses of rituximab were associated with improved responses to therapy with 22%, 43%, and 75% of patients responding to 500–835 mg/m2, 1000–1500 mg/m2, and 2250 mg/m2 doses, respectively. Although no dose-limiting toxicity was seen with this antibody at higher doses, more-frequent moderate toxicities were observed at the dose of 2250 mg/m2 that precluded further increases in dose.
These and other17 single-agent studies in CLL patients indicated that rituximab had clinical activity in the treatment of patients with CLL. However, treatment using single-agent rituximab achieved primarily partial responses and following the completion of therapy these responses were not particularly durable. As such, subsequent studies have largely focused on the use of rituximab in combination with other agents.
RITUXIMAB IN COMBINATION WITH CHEMOTHERAPY FOR THE FRONTLINE TREATMENT OF CLL
The following studies discussing rituximab in combination with other drugs for the initial treatment of CLL are summarized within Table 1.18–29
Table 1.
Select clinical trials evaluating rituximab containing chemoimmunotherapy combinations for the initial treatment of chronic lymphocytic leukemia.18–29
| Number of patients |
Median age (years) |
Chemotherapy* (mg/m2) |
Rituximab schedule (mg/m2) |
Cumulative rituximab dose (mg/m2) |
Overall response rate† (%) |
Complete response rate† (%) |
Progression free survival (median) |
Reference |
|---|---|---|---|---|---|---|---|---|
| 104 Sequential arm: n = 53 | 63 | Fludarabine 25 d 1–5×6 cycles | 2 months following initial 6 cycles, Rituximab 375 weekly for 4 weeks | 1500 | 77 | 28 | 40 mo | Byrd18–19 |
| Concurrent arm: n = 51 | 63 | Fludarabine 25 d 1–5×6 cycles | Rituximab 375 d 1 & 4 cycle 1 and d | 4125 | 90 | 47 | 32 mo | |
| 1 cycle 2–6 then | ||||||||
| 2 months following initial 6 cycles, Rituximab 375 weekly for 4 weeks | ||||||||
| 300 | 58 | Fludarabine 25 d 2–4 cycle 1, d 1 −3 cycles 2–6 | Rituximab 375 d 1 cycle 1 and 500 d 1 cycle 2–6 | 2875 | 95 | 70 | 57% at 6 years | Keating20–21 |
| Cyclophosphamide 250 d 2–4 cycle 1, d 1–3 cycles 2–6 | ||||||||
| 817 FC arm: n = 409 | 61 | Fludarabine 25 d 1–3×6 cycles | None | 0 | 85 | 23 | 32 mo | Hallek22 |
| Cyclophosphamide 250 d 1–3°—6 cycles | ||||||||
| FCR arm: n = 408 | 61 | FCR (same as FC arm) | Rituximab 375 cycle 1 d 0 and 500 on d 1 cycle 2–6 | 2875 | 93 | 45 | 43 mo | |
| 64 | 63 | Pentostatin 2 d 1 q 3 weeks × 6 cycles | Rituximab 100 d 1, 375 d 3 and 5 cycle 1 and 375 d 1 cycle 2–6 | 2725 | 91 | 41 | 33 mo | Kay23 |
| Cyclophosphamide 600 d 1 q 3 weeks × 6 cycles (cycles administered every 21 days) | ||||||||
| 50 | 58 | Fludarabine 20 d 2–4 cycle 1, d 1 −3 cycles 2–6 | Rituximab 375 d 1 cycle 1 and 500 d 14 cycle 1, d 1 and 14 cycle 2–6 & Rituximab 500 every 3 mo until relapse | 5875+500 every 3 mo until relapse | 100 | 79 | Response duration 22 mo | Foon24 |
| Cyclophosphamide 150 d 2–4 cycle 1, d 1–3 cycles 2–6 | ||||||||
| 36 | 59 | Fludarabine 25 d 1–5×6 cycles then Cyclophosphamide 3000 every 3 wks × 3 cycles | Following 9 cycles of sequential FC, Rituximab consolidation 375 weekly for 4 weeks | 1500 | 89 | 61 | 43 mo | Lamanna25 |
| 30 | 57 | Fludarabine 25 d 2–4 cycle 1, d 1 −3 cycles 2–6 | Rituximab 375 d 1 cycle 1 and 500 d 1 cycle 2–6 | 2875 | 96 | 83 | Not reached at 39 mo | Faded27 |
| Cyclophosphamide 250 d 2–4 cycle 1, d 1–3 cycles 2–6 | ||||||||
| Mitoxantrone 6 d 2 cycle 1, d 1 cycles 2–6 | ||||||||
| 72 | 60 | Fludarabine 25 d 1–3×6 cycles | Rituximab 375 d 1 cycle 1 and 500 d | 5875 | 93 | 82 | Not reported | Bosch26 |
| Cyclophosphamide 250 d 1–3×6 | 1 cycle 2–6, responders receive 375 q | |||||||
| cycles Mitoxantrone 6 d 1 × 6 cycles | 3 months for 2 years | |||||||
| 117 | 64 | Bendamustine 90 d 1–2×6 cycles | Rituximab 375 d 1 cycle 1 and 500 d 1 cycle 2–6 | 2875 | 91 | 33 | 76% at 18 mo, median not reached | Fischer28 |
| 60 | 59 | Cyclophosphamide 200 d 3–5×6 cycles | Rituximab 375 d 2 cycle 1 and 500 d 2 cycle 2- 6 | 2875 | 92 | 72 | 38 mo | Parikh29 |
| Fludarabine 20 d 3–5×6 cycles | ||||||||
| Alemtuzumab 30 mg flat dose d 1, 3, 5×6 cycles |
Cycles are 28 days unless otherwise noted.
All responses were assessed by National Cancer Institute-working group (NCI-WG) 1996 criteria.
CFAR=FCR and alemtuzumab; CR=complete response; d=days; FC=fludarabine and cyclophosphamide; FR=fludarabine and rituximab; FCR=FC and rituximab; FCM-R=FCR and mitoxantrone; mo=months; PFS=progression-free survival; PCR=pentostatin, cyclophosphamide, and rituxumab; pt(s)=patients; RD=response duration.
Fludarabine and Rituximab
Preclinical studies suggested that fludarabine and rituximab (FR) might be more active than fludarabine alone in killing leukemia/lymphoma B cells.30,31 This led to phase 2 studies evaluating the use of FR to treat patients with CLL. The German CLL Study Group (GCLLSG) performed a phase 2 evaluation of rituximab in combinaton with fludarabine in both treatment-naive patients and those who were previously treated. Treatment with FR was associated with a high ORR of 87% with a fraction of these patients achieving CR to therapy.32 In a randomized phase 2 study, the CALGB treated 104 previously untreated CLL patients with six cycles of fludarabine administered concomitantly or preceding treatment with rituximab (CALGB 9712).18 Fludarabine was administered for 5 days in each cycle either alone (in the sequential arm) or concurrently with rituximab for a total of six cycles. The protocol was amended in an effort to minimize severe infusion reactions by using step-up doses of rituximab (50 mg/m2 on day 1 and 325 mg/m2 on day 3) similar to the previously described single-agent study.33
During the initial 6 months of therapy, infusion reactions and grade 3/4 neutropenia were observed more frequently in the concurrent regimen then in the sequential arm. Responses assessed following the six cycles of induction therapy favored the concurrent regimen with OR and CR rate of 90% and 33%, respectively, compared with 77% and 15% in the sequential regimen.18 All patients with at least stable disease following 6 months of induction therapy were then treated with rituximab 375 mg/m2 weekly for 4 weeks. Two additional patients in the concurrent regimen and seven in the sequential regimen improved from partial remission (PR) to CRs. The comprehensive CR rate to all treatment was significantly higher in the concurrent arm (47%) than in the sequential arm (28%).18 However, patients treated in the concurrent arm received significantly greater amounts of rituximab administered over a longer period of time prior to response assessment compared to patients treated in the sequential arm. This difference in amount of rituximab administered to this group makes it difficult to conclude that the superior response rates of patients treated in the concurrent arm were due solely to the use of both antileukemia drugs together. In fact, when reporting on subsequent PFS and OS,19,34 the data from both arms of treated patients are often combined because the differences in these parameters between the two treatment groups did not maintain statistical significance. Median PFS for all patients was 37 months.
A retrospective comparison of patients treated with single-agent fludarabine (CALGB 9011) revealed improved 2-year PFS and OS in those patients treated with FR (CALGB 9712) compared with fludarabine alone.19 Those patients with immunoglobulin heavy chain genes that lacked mutation, or those that had high-risk cytogenetic abnormalities of 17p or 11q deletions had significantly shorter treatment-free survival and OS when compared with those patients that did not have these characteristics.34
Fludarabine, Cyclophosphamide, and Rituximab (FCR)
The combination of FC was compared with fludarabine alone in two randomized multicenter studies.35,36 Treatment with the FC combination was associated with superior clinical activity, albeit with higher rates of toxicity, than treatment with fludarabine alone.35,36 Keating and colleagues at MDACC added rituximab to the active FC combination and developed the FCR regimen.20
This regimen incorporates higher doses of rituximab at 500 mg/m2 than used in patients with non-Hodgkin lymphoma (NHL) based on phase 2 data,16 demonstrating superior activity in CLL patients treated with higher doses of rituximab as monotherapy. The FCR regimen in treatment-naive CLL patients was associated with a 95% ORR and a 70% CR rate. The most frequent adverse events (AEs) were myelosuppression, infection, and rituximab-associated infusion reactions. Thirty-eight percent of patients could not receive the full six cycles of FCR therapy without dose reduction or treatment discontinuation with 58 patients who discontinued treatment prematurely and an additional 26 patients required FC dose reductions, most often due to hematologic toxicity. Older patients (>65 years), those with advanced-stage disease, elevated serum beta-2 microglobulin (β2-M), or an elevated serum creatinine were more likely not to tolerate treatment with the full six courses of therapy. Long-term follow-up of 300 treatment-naive CLL patients receiving FCR as initial therapy at MDACC, demonstrated a 6-year overall and failure-free survival of 77% and 51%, respectively.37 Eradication of minimal residual disease (MRD) at the end of treatment correlated with improved survival and was associated with superior time to progression (median 85 months vs. 49 months) and survival (84% vs. 65% at 6 years). Approximately 3% of patients developed secondary myelodysplasia during more than 5 years of follow-up. The risk of late infection (≥ grade 3, opportunistic or viral) was 10% during the first year after therapy and declined to 1.5% during the third year of follow-up.37 Pretreatment characteristics independently associated with inferior response were age ≥70 years, a serum β2-M greater than or equal to twice the upper limit of normal, white cell count >150,000, loss of the short arm of chromosome 17 (del [17p]), and a serum lactate dehydrogenase greater than twice the upper limit of normal.
Analysis of the impact of immunoglobulin heavy-chain variable-region gene (IGHV) mutational status on clinical outcomes revealed that patients with CLL cells that used unmutated IGHV achieved responses similarly to patients with mutated IGHV; however, remission duration was significantly shorter in patients with CLL that used unmutated IGHV than those with CLL cells with mutated IGHV (time to progression 47% vs. 82% at 6 years, P<0.001).38 Patients with leukemia cells that use IGHV 3–21 genes were at an increased risk for early relapse regardless of mutational status.38 Although the majority of the 300 patients treated on this frontline study experienced durable treatment-free survival, 79 (26%) required salvage therapy at a median of 31 months.21 Of these patients only 25% achieved a meaningful response to their first salvage regimen, most often an alemtuzumab-containing regimen.21 Most of the long-term survivors had allogeneic hematopoietic stem cell transplantation.39 As such, despite the effectiveness of FCR in the frontline treatment of patients, an effective salvage regimen for these patients who fail FCR has not yet been identified.
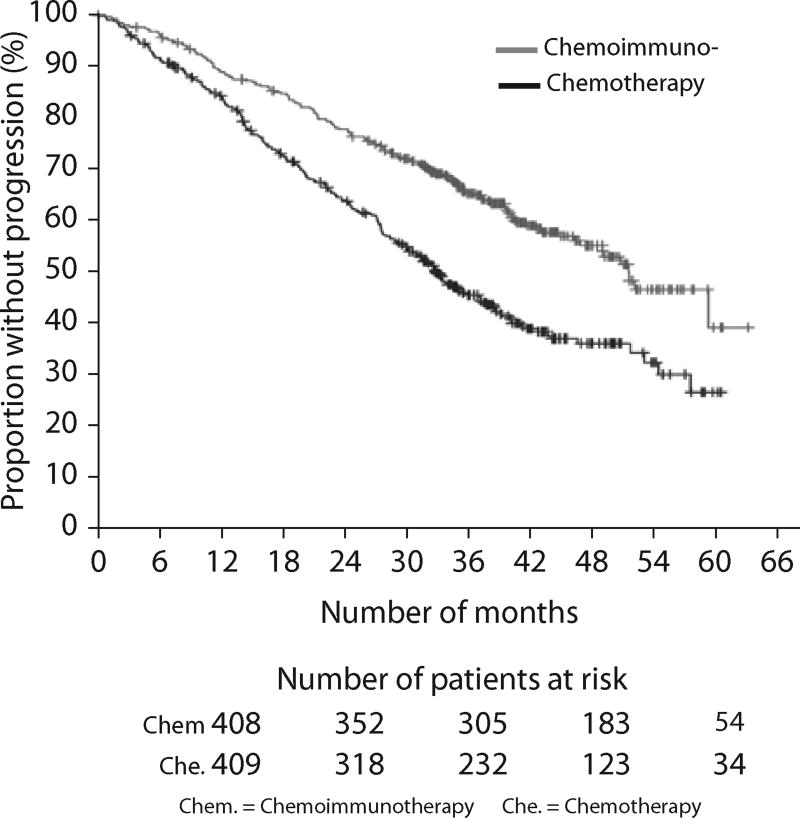
The efficacy of rituximab in the FCR regimen was confirmed in a large, randomized phase 3 study (CLL8) conducted by the GCLLSG22 (phase 3 studies summarized in Table 2). A total of 408 patients were randomly assigned to the FCR arm and 409 patients to the FC arm. FCR were administered in the same doses and fashion as pioneered by the MDACC investigators. All patients who enrolled were considered physically fit and those individuals who had a cumulative illness rating score >6, creatinine clearance <70 mL/min, or Eastern Cooperative Oncology Group (ECOG) performance status >1 were excluded. Patients were assessed for response following three cycles of therapy and only those who were assessed as responding to therapy were continued for a maximum of six cycles. FCR induced a higher ORR (95% vs. 88%, respectively) and more CRs (44% vs. 22%, respectively) when compared with FC alone. Similarly, the proportion of patients who did not respond was lower in the chemoimmunotherapy arm (10%) compared with the chemotherapy arm (20%). The primary endpoint of the study, PFS, was superior in those treated with FCR with 65% not progressing at 3 years, compared with 45% in the FC arm (hazard ratio 0.56; P<0.0001), as demonstrated in Figure 1.22
Table 2.
Randomized phase 3 clinical trials evaluating chemotherapy with or without rituximab for the treatment of chronic lymphocytic leukemia.5–22–44
| Study | Patient population | Arm (number of patients) |
Treatment* (mg/m2) |
Response†: ORR (CR) (%) |
Progression free survival (months) |
Overall survival |
|---|---|---|---|---|---|---|
| Hallek22 | Treatment naive CLL and an indication for therapy physically fit patients of any age with normal kidney function were enrolled | Chemotherapy arm-FC (409) | Fludarabine 25 d 1–3×6 cycles | 80 (22) | Median 32.8 | 83% at 3 yrs |
| CLL08 | Cyclophosphamide 250 d 1–3×6 cycles | (95% CI 29.6–36) 45 % at 3 yrs | ||||
| Chemoimmunotherapy arm-FCR (408) | FC same as chemotherapy arm | 90 (44) | Median 51.8 | 87% at 3 yrs | ||
| Rituximab 375 d 0 cycle 1, 500 mgd 1cycle 2–6 | (95% CI 46.2- 57.6) 65% at 3 yrs | |||||
| Reynolds44 | 80% previously untreated and 20% relapsed-”minimally treated” patients | PCR (92) | Pentostatin 4 d 1 × 8 cycles Cyclophosphamide 600 d 1 × 8 cycles | 45(7) | Not reported | Not reported |
| US oncology | Rituximab 375 (split dose d 8 and 9 of cycle 1, d 1 cycles 2–8) Cycles administered every 21 d | |||||
| FCR (92) | Fludarabine 20 d 1–5×6 cycles | 58(17) | Not reported | Not reported | ||
| Cyclophosphamide 600 d 1 × 6 cycles | ||||||
| Rituximab 375 (split dose d 8 & 9 cycle 1, d 1 cycles 2–6) | ||||||
| Robak5 | Previously treated patients with an indication for therapy | Chemotherapy arm-FC (276) | 58(13) | 20.6 | 52 mo | |
| REACH | Chemoimmunotherapy arm-FCR (276) | 70 (24) | 30.6 | Not reached |
Cycles are 28 days unless otherwise noted.
All responses were assessed by National Cancer Institute-working group (NCI-WG) criteria.
CI=confidence interval; CR=complete response; d=day(s); FC=fludarabine and cyclophosphamide; FCR=FC and rituximab; mo=month(s); ORR=overall response rate; pt(s)=patients; PCR=pentostatin, cyclophosphamide, and rituximab.
Figure 1.
Progression-free survival in all patients treated in the randomized phase 3 study evaluating the addition of rituximab to chemotherapy with fludarabine and cyclophosphamide. Chemoimmunotherapy=fludarabine, cyclophosphamide, and rituximab.
Chemotherapy=fludarabine and cyclophosphamide. Reproduced from “Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial.” Lancet. 2010;376:1164–1174. With permission from Elsevier.
The median PFS was estimated to be 52 months for chemoimmunotherapy group compared with 33 months in those treated with FC. Treatment with chemotherapy without rituximab, leukemia cell use of unmutated IGHV genes, β2-M of at least 3.5 mg/L, white blood cell count >50,000/L, and chromosome 17p abnormalities were independent predictors of an inferior duration of response to therapy. For the first time in a prospective, randomized study of combination therapy for initial treatment of CLL, a statistically significant benefit was observed for OS for those patients treated with FCR compared with FC with 87% and 83% alive at 3 years, respectively (hazard ratio 0.67; P<0.012). In addition, an ECOG performance status >0, β2-M of at least 3.5 mg/L, an elevated serum thymidine kinase level, and chromosome 17p abnormalities were independent predictors of OS. The largest benefit in PFS and OS observed with treatment of FCR compared with FC was observed in those patients with earlier-stage disease (Binet A and B). Forty-seven percent of patients treated with FCR required dose reductions of fludarabine monophosphate and/or cyclophosphamide of more than 10% for one or more cycles. Twenty-six percent of patients treated in the chemoimmunotherapy arm received less than the planned six cycles of therapy. The rituximab-containing combination was more frequently associated with infusion reactions and severe neutropenia. However, other AEs including infection were not significantly increased in the FCR-treated patients. Physically-fit patients, who enrolled on this study, 65 years or older benefited similarly when compared with younger patients in combination chemoimmunotherapy with rituximab in terms of response and response duration; however, they experienced a significantly higher frequency of grade 3/4 hematologic toxicity and bacterial infections.22
Previous studies have demonstrated that patients with leukemia cells characterized by trisomy 12 also have high-density CD20 expression and suggest that these patients might be particularly sensitive to treatment with rituximab-based chemotherapy.40 The combination of FCR was associated with improved outcomes over FC in most subgroups of patients including those with trisomy 12; however, FCR did not significantly improve the PFS or OS patients who had no genetic abnormalities as classified by the Döhner hierarchical model and did not improve the OS of those patients with a loss of the short arm of chromosome 17 (del [17p]).41 The ongoing US intergroup randomized, control trial of FR with or without cyclophosphamide will evaluate the benefit of the alkylating agent in FR-treated patients and will investigate the role of lenalidomide maintenance. In this study patients who have an 11q deletion are not randomized but assigned to FCR, as previous studies have demonstrated a clear benefit from the addition of alkylating agent to a purine analog-based regimen in these patients.35,36
As with previous phase 2 studies with rituximab-containing chemoimmunotherapy regimens, CLL8 patients with IGHV unmutated compared with mutated had a significantly shorter PFS with 55% and 80% remaining in remission at 3 years, respectively. Improving outcomes for those patients who are noted to have the shortest remission durations to chemoimmunotherapy, in particular patients with leukemia cells that use an IGHV unmutated or VH3-21 gene, is an active area of research. Clinical studies designed to test the activity of alternative first-line regimens or strategies that incorporate the use of postremission therapy, such as consolidation or maintenance therapy hold promise for improving the outcomes of such patients.
PENTOSTATIN, CYCLOPHOSPHAMIDE, AND RITUXIMAB (PCR)
Investigators at the Mayo clinic and Ohio State University evaluated PCR as the initial treatment of 65 CLL patients.23 The PCR regimen was associated with a high ORR of 91% and 41% of patients achieved a CR. Those who achieved a CR enjoyed a median response duration of 36 months compared with 20 months for those who achieved a PR. Similarly, those who experienced erradication of residual disease to <1% leukemia cells enjoyed a longer remission duration than those who were MRD-positive after therapy. Analysis of the 18 patients over the age of 70 suggested that these older individuals tolerated the regimen and achieved complete responses at a rate similar to the younger patients.42 A subsequent study investigating higher doses of pentostatin (4 mg/m2) in combination with rituximab, was associated with lower response rates and shorter PFS when compared with previous studies of PCR, suggesting that increasing the dose of the purine analog does not overcome the benefit of cyclophosphamide.43
Investigators of the US Oncology Group conducted a randomized phase 3 study that compared a modified dose FCR with a modified PCR regimen in a heterogeneous population of treatment-naive and minimally treated relapsed CLL patients and presented early data at the 50th Annual Meeting of the American Society of Hematology (Table 2). The primary endpoint was infectious complications, which appeared in similar rates for both regimens.44 In this community-based effort, primarily focused on treatment-related toxicity, 33% of FCR-treated patients required hospitalization during therapy, which was comparable with 41% of those treated on the PCR arm. More than 25% of patients on either arm discontinued therapy due to AEs. The abstract detailed response rates lower than that observed for FCR or PCR in previous phase 2 studies or the CLL8 study.44 Inferior clinical outcomes in this study might be partially attributed to the differences in the chemoimmunotherapy regimens and that this study was undertaken in a community-based setting. In addition, differences in toxicity may be present due to modified dose and schedules on this study,44 for example, the PCR regimen evaluated in this study, which incorporated pentostatin doses of 4 mg/m2, was apparently associated with greater toxicity than the 2 mg/m2 regimen23 reported by Kay and colleagues.
Variations on FCR and Bendamustine Rituximab
Foon and colleagues reported on a dose-modified FCR regimen, called “FCR-Lite” with the goal to maintain efficacy but decrease the toxicity of the full-dose regimen in 50 untreated patients with CLL of which most (84%) had early-stage disease.24 The FCR-Lite regimen incorporated lower doses of both fludarabine and cyclophosphamide and more frequent and increased amounts of rituximab (eg, 500 mg/m2 on days 1 and 14 of each cycle). This treatment was followed by subsequent treatment with rituximab administered every 3 months until disease progression (the so-called “rituximab maintenance”). The authors estimate the additional cost of rituximab in this regimen was US$57,000 more than standard FCR. All patients were given neutrophil growth factors the day after chemotherapy and antimicrobial prophylaxis throughout treatment and following therapy. All patients responded to FCR-Lite, with 37 achieving a CR to therapy. Despite reduced doses of fludarabine and cyclophosphamide, the small number of patients over the age of 70 often could not receive the full six cycles of therapy. FCR-Lite administered with growth factors was associated with a lower incidence of severe neutropenia when compared with standard FCR without growth factors. Investigators at Memorial Sloan-Kettering Cancer Center evaluated a sequential regimen of FCR (using high-dose cyclophosphamide) administered over approximately 12 months to 36 treatment-naive patients.25 The sequential therapy was associated with high response rates and durable remissions. Early results from a phase 2 study of the combination of bendamustine and rituximab (BR) were reported at the 2009 American Society of Hematology meeting45 by the GCLLSG. BR administered to 117 treatment-naïve CLL patients was associated with a 91% response rate and 33% achieving a CR. Based on these results, an ongoing phase 3 study of the GCLLSG (CLL10) is randomizing treatment-naive patients to FCR and BR to compare the clinical activity and toxicity of these two regimens.
To improve upon the activity of FCR, combination chemoimmunotherapy incorporating FCR with mitozantrone has been investigated predominately in younger patients.26,27 MDACC data comparing the outcomes of patients treated with FCR and mitozantrone were compared with those of patients treated with FCR and this analysis did not favor the addition of the anthracycline to standard FCR.27 It is also unclear whether additional cytotoxic agents added to FCR might increase the incidence of treatment-related myeloid neoplasia.46 At this point, there does not appear to be a significant benefit in the front-line setting to using mitozantrone together with FCR compared with using FCR alone. Similarly, Parikh and colleagues reported on 48 previously untreated patients with high-risk CLL including del [17p] who were treated with FCR plus alemtuzumab (CFAR).29 CFAR was effective in MRD eradication compared with FCR; however, this double-antibody chemotherapy combination was associated with greater myelosuppression.
RITUXIMAB IN COMBINATION WITH CHEMOTHERAPY FOR THE TREATMENT OF RELAPSED OR REFRACTORY CLL
Investigators at MDACC reported their results of a single-center phase 2 study of and FCR in treating 177 previously treated CLL patients.47 Seventy-three percent of these patients responded to therapy with CR achieved in 25% of the patients. When these results were compared historically with previous studies of fludarabine and FC in a similar population, combination chemoimmunotherapy with FCR was superior to chemotherapy alone. The REACH (Rituximab in the Study of Relapsed Chronic Lymphocytic Leukemia) study,5 an international, phase 3, randomized, open-label study confirmed the benefit of rituximab when administered in combination with FC (FCR) compared with FC alone in CLL patients who received one prior therapy (Table 25). Five hundred and fifty-two patients were stratified by previous treatment, time from diagnosis, and serum β2-M and then randomized to either treatment arm. Patients in either arm received similar doses of FC during therapy. PFS, the primary endpoint of the study improved significantly in those who received FCR (median 30.6 months) compared with those who received FC alone (20.6 months). Overall the incidence of grade 3 or 4 AEs (74% FC vs. 80% FCR), serious AEs (48% vs. 50%), and fatal AEs (10% vs. 14%) were higher in the FCR group compared with FC. However, more patients were able to complete six cycles of the FCR regimen (68%) than FC (61%) with more patients in the FC arm stopping therapy early due to lack of response (5% vs. 1%). Despite a higher rate of grade 3/4 neutropenia in FCR-treated patients, the incidence of infections was similar between both arms. ORR and CR rate was superior for FCR-treated patients compared with FC, respectively.5
Again, in efforts to improve the clinical activity of FCR, lumiliximab, an anti-CD23 mAb, was evaluated in combination with the FCR regimen in a phase 1/2 study of 31 previously treated CLL patients.48 Toxicity was not increased when compared with reported FCR data. The ORR was 65%, with 52% of patients achieving a complete response that compares favorably with the CR rate reported for FCR in relapsed CLL. Responders enjoyed a median PFS estimated to be approximately 28.7 months.48 A randomized study of patients treated with FCR with or without lumiliximab (LUCID trial [evaluation of LUmiliximab In Combination with FCR in PatIents with relapseD CLL]) was terminated and results have not yet been reported.
Rituximab has been administered in combination with other purine analogs, including pentostatin and cladribine, with or without cyclophosphamide, to patients with relapsed CLL. Treatment of relapsed patients with pentostatin at a dose of 4 mg/m2 in combination with cyclophosphamide and rituximab (PCR) has yielded high response rates, even in patients considered refractory to treatment with fludarabine.49 Cladribine has been administered subcutaneously or intravenously for 5 days together with rituximab for treatment of naive or relapsed CLL patients. This yielded a response in most patients, including CRs.50,51 Rituximab in combination with cladribine could be repeated every 4 weeks. However, most patients received less than four cycles of therapy. The toxicities with the cladribine- and pentostatin-based rituximab regimens in CLL were similar to those noted with fludarabine-based rituximab combinations, consisting of primarily hematologic, immunologic, and infectious toxicities.
Other chemotherapy combinations with rituximab, such as BR29 and CFAR,52 have been evaluated in patients with relapsed or refractory disease in phase 2 studies. These chemoimmunotherapy combinations are associated with promising response rates and have been without unexpected toxicity as reported in abstract form. For patients with fludarabine-refractory disease or Richter’s transformation, the regimen oxaliplatin, fludarabine, cytarabine, and rituximab can yield responses in 33% and 50% of patients, respectively.53
RITUXIMAB IMMUNOTHERAPY COMBINATIONS
High-Dose Methylprednisolone (HDMP) and Rituximab
As combination chemoimmunotherapy is often poorly tolerated in older individuals, those with comorbidities or pretreatment cytopenias, and those that have been previously exposed to cytotoxic agents, regimens combining rituximab with agents that have less myelossuppresion have been evaluated in a number of phase 2 studies. HDMP and rituximab administered over three 4-week cycles has been investigated at the University of California San Diego in both fludarabine refractory and treatment-naive CLL patients.54,55 This regimen is associated with a high ORR (93%–96%) with CR achieved in approximately one-third of patients in both frontline and refractory settings. CLL patients refractory to fludarabine who were treated with HDMP-rituximab experienced a median PFS of 15 months while treatment-naive patients experienced a median PFS of 30.5 months.54,55 The HDMP-rituximab regimen was associated with minimal hematologic toxicity and actually ameliorated impaired marrow function during therapy. Subset analysis demonstrated that treatment-naive patients over the age of 70 responded as well as the younger patients in the study, with 38% of these patients achieving CR.54
Etanercept and Rituximab
In an effort to improve the activity of rituximab and decrease cytokine-mediated infusion reactions investigators at the Ohio State University evaluated the combination of the tumor necrosis factor (TNF)-alpha inhibitor, etanercept, in addition to rituximab in 36 previously treated CLL patients, half of which were refractory to fludarabine.56 Thirty-four patients completed the 5 week protocol and 29% achieved a response to therapy with one CR observed. Historical comparison to the three-times-weekly rituximab regimen33 suggested similar response rates with a trend towards more durable responses in those who received entanercept along with less frequent infusion reactions (39%) compared with the single-agent study (61%). Although fludarabine refractory patients were noted to respond similarly to chemosensitive patients, no patient with a del [17p] achieved a response.56
Rituximab and Alemtuzumab
Several phase 2 studies have investigated the combination of alemtuzumab and rituximab in varying doses and schedules for the treatment of CLL.57–59 Alemtuzumab is a humanized mAb to CD52, which is very effective in erradicating blood and marrow CLL B cells, but is less active in bulky lymph nodes. CD52 is expressed on all lymphocytes and for this reason treatment with alemtuzumab is associated with a profound T lymphopenia. As such, patients treated with rituximab in combination with alemtuzumab receive prophylaxis for Pneumocystis jiroveci pnemonia and herpes viruses. In 40 relapsed patients (64% fludarabine refractory) the combination of alemtuzumab, administered first by continuous infusion and then subcutaneously, and weekly rituximab, induced a response in 53% of patients with 18% achieving CR.58
The ORR was similar to what Faderl and colleagues observed in intravenous alemtuzumab and rituximab combination,59 with a trend towards a higher CR rate in those treated with the continuous infusion – subcutaneous alemtuzumab protocol.58 Most patients achieved their maximum response to therapy following the first 5 weeks of treatment. Asymptomatic cytomegalovirus reactivation was observed, but there was no unexpected toxicity. Similarly, early treatment with the combination of alemtuzumab and rituximab administered over 4 weeks was evaluated in high-risk CLL patients without a standard indication for therapy.57 Thirty early-stage patients were treated with the combination and experienced a high ORR with five patients achieving an MRD-negative CR. The median response duration for these patients treated with the antibody combination was 14.4 months. Together the double antibody combination is associated with high ORR and CR rates; however, responses lack the durability that has been observed with rituximab in combination with other agents.
Rituximab and Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF)
Additional efforts to improve the activity of single-agent rituximab strategies incorporating GM-CSF or lenalidomide to improve on rituximab-dependent ADCC have been pursued in phase 2 studies.60 The combination of GM-CSF and rituximab was evaluated in a phase 2 study conducted by Ferrajoli and colleagues within the CLL Research Consortium as initial treatment of elderly patients, those with high-risk disease, and for those patients with recurrent CLL.60 The ORR in 118 patients was 65%, including 9% CRs. The combination was well tolerated with the most common toxicity consisting in mild GM-CSF injectionsite erythema.
Lenalidomide and Rituximab
Lenalidomide, an immunomodulatory agent similar to thalidomide, has been shown in preclinical models of NHL to enhance the activity of rituximab.61 This has prompted the evaluation of the combination of lenalidomide and rituximab, which is currently undergoing evaluation in phase 2 clinical studies of treatment-naive patients through the CLL Research Consortium (CRC)62 and in relapsed or refractory CLL patients at the MDACC and within the CRC. Early reports suggest that the addition of the CD20 mAb to lenalidomide treatment reduces the lenalidomide-associated tumor-flare reaction and leads to higher responses rates, including CRs when compared with previous studies with either agent alone.33,62–64
RITUXIMAB AS CONSOLIDATION OR MAINTENANCE
The use of rituximab maintenance has been shown to decrease the rate of relapse in follicular lymphoma patients; however, the efficacy of rituximab maintenance in CLL has not been clearly demonstrated. Nonetheless, with progress in defining those CLL patients with the highest risk of relapse (eg, those with unmutated IGHV or with certain cytogenetic abnormalities) and the clinical benefit observed in patients who are able to achieve longer remission, the use of consolidation and maintenance treatments for CLL is of great interest. Studies including CALGB 971218 and the Memorial Sloan-Kettering Cancer Centre evaluation of sequential FCR25 report increasing numbers of individuals who are able to achieve complete responses following a rituximab consolidation phase. Additional single-arm phase 2 studies17 have suggested a potential benefit to a rituximab maintenance phase. Italian investigators administered FR in a sequential fashion to 60 previously untreated patients and reported high CR rates (78%).65
Following initial treatment with fludarabine, rituximab was administered to patients who had detectable residual disease as a form of consolidation therapy.66 Twenty-eight patients who were in a CR or PR with MRD after therapy received four doses of rituximab 375 mg/m2 each month, followed by treatment with rituximab at 150 mg/m2 each month for 12 months. Response duration was significantly longer in those that received such consolidation and maintenance therapy compared with those who did not receive additional rituximab (87% vs. 32% at 5 years). Although promising, the efficacy of rituximab as maintenance or consolidation requires confirmation in a randomized study and, currently, is not recommended for the routine management of CLL patients outside of clinical studies.
RITUXIMAB-RELATED TOXICITY
Infusion Reactions
The most frequently observed AEs associated with rituximab treatment in CLL are infusion related. Infusion-related reactions are typically mild (grade 1–2 in severity) and frequently include fever, chills, or rashes. Hypotension (often asymptomatic), transient hypoxemia, and/or dyspnea can also accompany the first dose of rituximab and is associated with a cytokine release of interleukins 6 and 8, TNF, and interferon-gamma.33 Infusion-related toxicity is most frequently observed during the first infusion(s). Premedication with acetaminophen and an antihistamine along with a slow rate of antibody infusion can mitigate some infusion toxicity. In addition, CLL patients might benefit from the first dose of rituximab administered as a split dose, typically 100 mg (or 50 mg/m2) on day 1 with the remainder of the first dose on a following day, particularly if they have higher circulating leukemia cell numbers.
Hematologic Toxicity
Transient hematologic toxicity, in particular neutropenia is also observed with rituximab therapy.5 Combination randomized studies comparing chemotherapy with or without rituximab have reported more frequent neutropenia when rituximab is administered concurrently with chemotherapy; however, in the same studies concurrent administration of rituximab has not been associated with a significant rise in the infectious complications.5,18 Delayed neutropenia has also been reported in rituximab-treated CLL patients. Rituximab administration can also be associated with a transient thrombocytopenia, particularly in those patients with pretreatment thrombocytopenia and those who experience infusion reactions.33
Immune Deficiency
B lymphopenia is common with depletion of normal B lymphocytes in addition to the malignant B cells following the first infusions of rituximab with recovery of normal B cells usually occurring more than 6 months after therapy.15 Rituximab treatment can be associated with hypogammaglobulinemia of IgG in treated patients. However, infections, particularly opportunistic infections, including fungal infections, are rarely observed in CLL patients treated with rituximab monotherapy.17 The most common observed infections in patients treated with rituximab are bacterial and are typically grade 1–2 in severity. Prior or concurrent treatment with purine analogs and/or alemtuzumab is associated with deficits in cell-mediated immunity resulting from quantitative defects in T lymphocytes that can be observed for 1–2 years following therapy and as such these patients may benefit from antimicrobial prophylaxis for herpes viruses and/or Pneumocystis jiroveci pneumonia.
Viral Complications
Progressive multifocal leukoencephalopathy (PML), a severe and often fatal central nervous system demyelinating disease, has been reported in patients with lymphoproliferative disorders treated with rituximab.67 PML is associated with defects in cellular immunity and resultant active infection of the John Cunningham (JC) virus. As such, the rare reports of PML in CLL appear to be often associated with previous or concurrent treatments with agents or modalities that impact T lymphocytes in addition to treatment with rituximab.68 Hepatitis B reactivation, including fulminate hepatitis and hepatic failure, is a potential complication in patients with inactive chronic hepatitis B receiving chemotherapy and anti-CD20 mAb treatment.69 Screening for hepatitis B virus and pre-emptive therapy for patients who are antigen- or DNA-positive is recommended.
NEWER CD20 MONOCLONAL ANTIBODIES
The success of rituximab has fueled the development of the next generation of CD20 mAbs. Newer-generation CD20 can be divided into two types based on the mAb activity profile: type 1 mAbs, such as rituximab, where the primary mechanism of action is CDC and/or ADCC; and type 2 mAbs in which the primary mechanism is direct induction of apoptosis. The next generation of CD20 mAbs are humanized to decrease potential immunogenicity, have properties that may convey improved clinical activity in CLL, and that may be successfully employed in those patients who are deemed refractory to rituximab.
Ofatumumab
Ofatumumab is a humanized type 1 CD20 mAb recently approved for the treatment of CLL patients refractory to both fludarabine and alemtuzumab. Ofatumumab appears to bind to a unique epitope of CD20.70 Preclinical studies demonstrated enhanced complement-mediated cytotoxicity of ofatumumab even in CLL cells with characteristically low-density CD20 expression and activity in rituximab-resistant NHL cell lines. The pivotal study leading to the approval of ofatumumab enrolled both fludarabine and alemtuzumab-refractory patients and a second group of patients that were refractory to fludarabine and had bulky lymph nodes and so were thought not to be appropriate candidates for alemtuzumab.58 Patients received ofatumumab 300 mg during week 1, doses two to twelve were 2000 mg administered weekly. Treatment was associated with 58% of double-refractory patients responding and 47% of those patients in the bulky fludarabine-refractory group achieving a response. Median PFS for both groups was just under 6 months suggesting patients frequently progressed prior to completing the planned 24 weeks of therapy. AEs appeared similar to those observed in rituximab-treated patients with grade 1–2 infusion reactions and infections reported most frequently. Early reports of a phase 2 study of ofatumumab at two dose levels, 500 mg and 1000 mg, administered in combination with FC suggests the chemoimmunotherapy regimen is active with response rates similar to what was reported in the CLL8 study.71 Higher CR rates were noted in patients who received the 1000 mg dose (50%) compared with the 500 mg arm (32%). Additional type 1 humanized CD20 mAbs in earlier stages of development for CLL include ocrelizumab and veltuzumab.
Afutuzumab
Afutuzumab (GA101) is a type 2 humanized mAb that binds with high affinity to a unique epitope of CD20. Unlike type 1 anti-CD20 mAbs (rituximab and ofatumumab) that are able to modulate CD20 expression and are more potent in CDC-mediated cytotoxicity, the type 2 mAbs (GA101) do not modulate CD20 expression and are able to induce direct apoptosis while being less effective at mediating CDC. Preclinical data demonstrate that GA101 is more potent at inducing ADCC, is more effective in inducing cytotoxicity in CLL samples, and can induce a higher degree of B-cell depletion in whole blood samples than rituximab.72 Early phase 1 data in treating patients with CD20+ lymphomas suggest that GA101 treatment is tolerable at doses of 400–2000 mg (administered for nine doses) without dose-limiting toxicity.73 ORR was 62% including one patient achieving CR with persistent cytopenias. The most common AEs were infusion reactions and transient grade 3/4 neutropenia that were observed in the majority of patients.
Newer mAbs and alternative approaches to target CD20, including small modular immunopharmaceutical agents, are also in development.
CONCLUSION
In October 2009, the FDA approved rituximab for the treatment of CLL. This approval was based on two large phase 3 randomized studies that demonstrated the superiority of chemoimmunotherapy with rituximab compared with chemotherapy alone for both the initial and subsequent treatment of patients with CLL. Additional phase 2 studies have evaluated alternate rituximab-based chemoimmunotherapy regimens, and reported varying remission rates, toxicities, and PFS. However, these combinations have not been directly compared with each other. Moreover, the optimal treatment for older individuals, those with high-risk genetic abnormalities, and how patients should be treated on relapse has yet to be defined. In addition, patients, such as those with CLL cells that use unmutated IGHV genes, appear to have responses that are shorter in duration than those with CLL cells that use mutated IGHV. Such patients may benefit from postremission therapies or alternative frontline therapy. As such, the best treatment for an individual is based on patient characteristics and goals of therapy and may often include enrollment into a clinical trial.
Acknowledgments
D.F.J. is supported by a Lymphoma Research Foundation Fellowship. D.F.J. is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Footnotes
The authors declare that they have no conflicts of interest.
References
- 1.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration. [Accessed May 18, 2011];FDA approves rituxan to treat chronic lymphocytic leukemia. Available at: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm201069.htm.
- 3.Maloney DG, Smith B, Rose A. Rituximab: mechanism of action and resistance. Semin Oncol. 2002;29(Suppl. 2):2–9. [PubMed] [Google Scholar]
- 4.Smith MR. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. 2003;22:7359–7368. doi: 10.1038/sj.onc.1206939. [DOI] [PubMed] [Google Scholar]
- 5.Robak T, Dmoszynska A, Solal-Celigny P, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1756–1765. doi: 10.1200/JCO.2009.26.4556. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Williams ME, Cousar JB, Pawluczkowycz AW, Lindorfer MA, Talory RP. Rituximab-CD20 complexes are shaved from Z138 mantle cell lymphoma cells in intravenous and subcutaneous SCID mouse models. J Immunol. 2007;179:4263–4271. doi: 10.4049/jimmunol.179.6.4263. [DOI] [PubMed] [Google Scholar]
- 7.Williams ME, Densmore JJ, Pawluczkowycz AW, et al. Thrice-weekly low-dose rituximab decreases CD20 loss via shaving and promotes enhanced targeting in chronic lymphocytic leukemia. J Immunol. 2006;177:7435–7443. doi: 10.4049/jimmunol.177.10.7435. [DOI] [PubMed] [Google Scholar]
- 8.Aue G, Lindorfer MA, Beum PV, et al. Fractionated subcutaneous rituximab is well-tolerated and preserves CD20 expression on tumor cells in patients with chronic lymphocytic leukemia. Haematologica. 2010;95:329–332. doi: 10.3324/haematol.2009.012484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berinstein NL, Grillo-Lopez AJ, White CA, Bence, et al. Association of serum rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin’s lymphoma. Ann Oncol. 1998;9:995–1001. doi: 10.1023/A:1008416911099. [DOI] [PubMed] [Google Scholar]
- 10.Regazzi MB, Iacona I, Avanzini MA, et al. Pharmacokinetic behavior of rituximab: a study of different schedules of administration for heterogeneous clinical settings. Ther Drug Monit. 2005;27:785–792. doi: 10.1097/01.ftd.0000184162.60197.c1. [DOI] [PubMed] [Google Scholar]
- 11.Maloney DG, Liles TM, Czerwinski DK, et al. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood. 1994;84:2457–2466. [PubMed] [Google Scholar]
- 12.Tobinai K, Kobayashi Y, Narabayashi M, et al. Feasibility and pharmacokinetic study of a chimeric anti-CD20 monoclonal antibody (IDEC-C2B8, rituximab) in relapsed B-cell lymphoma. The IDEC-C2B8 Study Group. Ann Oncol. 1998;9:527–534. doi: 10.1023/a:1008265313133. [DOI] [PubMed] [Google Scholar]
- 13.Tobinai K, Igarashi T, Itoh K, et al. Japanese multicenter phase II and pharmacokinetic study of rituximab in relapsed or refractory patients with aggressive B-cell lymphoma. Ann Oncol. 2004;15:821–830. doi: 10.1093/annonc/mdh176. [DOI] [PubMed] [Google Scholar]
- 14.Maloney DG, Grillo-Lopez AJ, Bodkin DJ, et al. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin’s lymphoma. J Clin Oncol. 1997;15:3266–3274. doi: 10.1200/JCO.1997.15.10.3266. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien SM, Kantarjian H, Thomas DA, et al. Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol. 2001;19:2165–2170. doi: 10.1200/JCO.2001.19.8.2165. [DOI] [PubMed] [Google Scholar]
- 17.Hainsworth JD, Litchy S, Barton JH, et al. Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2003;21:1746–1751. doi: 10.1200/JCO.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 18.Byrd JC, Peterson BL, Morrison VA, et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712) Blood. 2003;101:6–14. doi: 10.1182/blood-2002-04-1258. [DOI] [PubMed] [Google Scholar]
- 19.Byrd JC, Rai K, Peterson BL, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood. 2005;105:49–53. doi: 10.1182/blood-2004-03-0796. [DOI] [PubMed] [Google Scholar]
- 20.Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 21.Keating MJ, O’Brien S, Tam C, Lerner S, Kantarjian H. Salvage therapy following failure or relapse after FCR chemo-immunotherapy as initial treatment for chronic lymphocytic leukemia (CLL) J Clin Oncol. 2007;25(Suppl. 18) Abstract 7009. [Google Scholar]
- 22.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 23.Kay NE, Geyer SM, Call TG, et al. Combination chemoimmunotherapy with pentostatin, cyclophosphamide, and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B chronic lymphocytic leukemia. Blood. 2007;109:405–411. doi: 10.1182/blood-2006-07-033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foon KA, Boyiadzis M, Land SR, et al. Chemoimmunotherapy with low-dose fludarabine and cyclophosphamide and high dose rituximab in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27:498–503. doi: 10.1200/JCO.2008.17.2619. [DOI] [PubMed] [Google Scholar]
- 25.Lamanna N, Jurcic JG, Noy A, et al. Sequential therapy with fludarabine, high-dose cyclophosphamide, and rituximab in previously untreated patients with chronic lymphocytic leukemia produces high-quality responses: molecular remissions predict for durable complete responses. J Clin Oncol. 2009;27:491–497. doi: 10.1200/JCO.2008.16.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosch F, Abrisqueta P, Villamor N, et al. Rituximab, fludarabine, cyclophosphamide, and mitoxantrone: a new, highly active chemoimmunotherapy regimen for chronic lymphocytic leukemia. J Clin Oncol. 2009;27:4578–4584. doi: 10.1200/JCO.2009.22.0442. [DOI] [PubMed] [Google Scholar]
- 27.Faderl S, Wierda W, O’Brien S, Ferrajoli A, Lerner S, Keating MJ. Fludarabine, cyclophosphamide, mitoxantrone plus rituximab (FCM-R) in frontline CLL <70 years. Leuk Res. 2010;34:284–288. doi: 10.1016/j.leukres.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischer K, Stilgenbauer S, Schweighofer CD, et al. Bendamustine in combination with rituximab (BR) for patients with relapsed chronic lymphocytic leukemia (CLL): a multicentre phase II trial of the German CLL Study Group (GCLLSG); American Society of Hematology Annual Meeting; December 6–9, 2008; San Fransisco, CA. Abstract 330. [Google Scholar]
- 29.Parikh SA, O’Brien S, Ferrajoli A. Frontline combined chemoimmunotherapy with fludarabine, cyclophosphamide, alemtuzumab and rituximab (CFAR) in high-risk chronic lymphocytic leukemia; American Society of Hematology Annual Meeting; December 5–8, 2009; New Orleans, LA. Abstract 208. [Google Scholar]
- 30.Di Gaetano N, Xiao Y, Erba E, et al. Synergism between fludarabine and rituximab revealed in a follicular lymphoma cell line resistant to the cytotoxic activity of either drug alone. Br J Haematol. 2001;114:800–809. doi: 10.1046/j.1365-2141.2001.03014.x. [DOI] [PubMed] [Google Scholar]
- 31.Alas S, Bonavida B, Emmanouilides C. Potentiation of fludarabine cytotoxicity on non-Hodgkin’s lymphoma by pentoxifylline and rituximab. Anticancer Res. 2000;20:2961–2966. [PubMed] [Google Scholar]
- 32.Schulz H, Klein SK, Rehwald U, et al. Phase 2 study of a combined immunochemotherapy using rituximab and fludarabine in patients with chronic lymphocytic leukemia. Blood. 2002;100:3115–3120. doi: 10.1182/blood-2002-03-0972. [DOI] [PubMed] [Google Scholar]
- 33.Byrd JC, Murphy T, Howard RS, et al. Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J Clin Oncol. 2001;19:2153–2164. doi: 10.1200/JCO.2001.19.8.2153. [DOI] [PubMed] [Google Scholar]
- 34.Byrd JC, Gribben JG, Peterson BL, et al. Select high-risk genetic features predict earlier progression following chemoimmunotherapy with fludarabine and rituximab in chronic lymphocytic leukemia: justification for risk-adapted therapy. J Clin Oncol. 2006;24:437–443. doi: 10.1200/JCO.2005.03.1021. [DOI] [PubMed] [Google Scholar]
- 35.Flinn IW, Neuberg DS, Grever MR, et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E2997. J Clin Oncol. 2007;25:793–798. doi: 10.1200/JCO.2006.08.0762. [DOI] [PubMed] [Google Scholar]
- 36.Eichhorst BF, Busch R, Hopfinger G, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood. 2006;107:885–891. doi: 10.1182/blood-2005-06-2395. [DOI] [PubMed] [Google Scholar]
- 37.Tam CS, O’Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin KI, Tam CS, Keating MJ, et al. Relevance of the immunoglobulin VH somatic mutation status in patients with chronic lymphocytic leukemia treated with fludarabine, cyclophosphamide, and rituximab (FCR) or related chemoimmunotherapy regimens. Blood. 2009;113:3168–3171. doi: 10.1182/blood-2008-10-184853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Constantine ST, Wierda GW, O’Brien S, et al. Life after fludarabine, cyclophosphamide, & rituximab (FCR) – the clinical outcome of patients with chronic lymphocytic leukemia who receive salvage treatment after frontline FCR; American Society of Hematology Annual Meeting; December 6–9, 2008; San Fransisco, CA. Abstract 2090. [Google Scholar]
- 40.Tam CS, Otero-Palacios J, Abruzzo LV, et al. Chronic lymphocytic leukaemia CD20 expression is dependent on the genetic subtype: a study of quantitative flow cytometry and fluorescent in-situ hybridization in 510 patients. Br J Haematol. 2008;141:36–40. doi: 10.1111/j.1365-2141.2008.07012.x. [DOI] [PubMed] [Google Scholar]
- 41.Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 42.Shanafelt TD, Lin T, Geyer SM, et al. Pentostatin, cyclophosphamide, and rituximab regimen in older patients with chronic lymphocytic leukemia. Cancer. 2007;109:2291–2298. doi: 10.1002/cncr.22662. [DOI] [PubMed] [Google Scholar]
- 43.Kay NE, Wu W, Kabat B, et al. Pentostatin and rituximab therapy for previously untreated patients with B-cell chronic lymphocytic leukemia. Cancer. 2010;116:2180–2187. doi: 10.1002/cncr.25028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reynolds CM, Di Bella N, Lyons RM, et al. Phase III trial of fludarabine, cyclophosphamide, and rituximab vs. pentostatin, cyclophosphamide, and rituximab in B-cell chronic lymphocytic leukemia; American Society of Hematology Annual Meeting; December 6–9, 2008; San Fransisco, CA. Abstract 327. [DOI] [PubMed] [Google Scholar]
- 45.Fischer KPC, Stilgenbauer S, Busch R, et al. Bendamustine combined with rituximab (BR) in first-line therapy of advanced CLL: a multicenter phase II trial of the German CLL Study Group (GCLLSG); American Society of Hematology Annual Meeting; December 5–8, 2009; New Orleans, LA. Abstract 205. [Google Scholar]
- 46.Carney DA, Westerman DA, Tam CS, et al. Therapy-related myelodysplastic syndrome and acute myeloid leukemia following fludarabine combination chemotherapy. Leukemia. 2010;24:2056–2062. doi: 10.1038/leu.2010.218. [DOI] [PubMed] [Google Scholar]
- 47.Wierda W, O’Brien S, Wen S, et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4070–4078. doi: 10.1200/JCO.2005.12.516. [DOI] [PubMed] [Google Scholar]
- 48.Byrd JC, Kipps TJ, Flinn IW, et al. Phase 1/2 study of lumiliximab combined with fludarabine, cyclophosphamide, and rituximab in patients with relapsed or refractory chronic lymphocytic leukemia. Blood. 2010;115:489–495. doi: 10.1182/blood-2009-08-237727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lamanna N, Kalaycio M, Maslak P, et al. Pentostatin, cyclophosphamide, and rituximab is an active, well-tolerated regimen for patients with previously treated chronic lymphocytic leukemia. J Clin Oncol. 2006;24:1575–1581. doi: 10.1200/JCO.2005.04.3836. [DOI] [PubMed] [Google Scholar]
- 50.Bertazzoni P, Rabascio C, Gigli F, et al. Rituximab and subcutaneous cladribine in chronic lymphocytic leukemia for newly diagnosed and relapsed patients. Leuk Lymphoma. 2010;51:1485–1493. doi: 10.3109/10428194.2010.495799. [DOI] [PubMed] [Google Scholar]
- 51.Robak T, Smolewski P, Cebula B, Grzybowska-Izydorczyk O, Blonski JZ. Rituximab plus cladribine with or without cyclophosphamide in patients with relapsed or refractory chronic lymphocytic leukemia. Eur J Haematol. 2007;79:107–113. doi: 10.1111/j.1600-0609.2007.00889.x. [DOI] [PubMed] [Google Scholar]
- 52.Badoux Xavier C, Susan O’Brien MK, et al. Chemoimmunotherapy with cyclophosphomide, fludarabine, alemtuzumab and rituximab (CFAR) is effective in relapsed patients with chronic lymphocytic leukemia (CLL); American Society of Hematology Annual Meeting; December 5–8, 2009; New Orleans, LA. Abstract 3431. [Google Scholar]
- 53.Tsimberidou AM, Wierda WG, Plunkett W, et al. Phase I-II study of oxaliplatin, fludarabine, cytarabine, and rituximab combination therapy in patients with Richter’s syndrome or fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2008;26:196–203. doi: 10.1200/JCO.2007.11.8513. [DOI] [PubMed] [Google Scholar]
- 54.Castro JE, James DF, Sandoval-Sus JD, et al. Rituximab in combination with high-dose methylprednisolone for the treatment of chronic lymphocytic leukemia. Leukemia. 2009;23:1779–1789. doi: 10.1038/leu.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Castro JE, Sandoval-Sus JD, Bole J, Rassenti L, Kipps TJ. Rituximab in combination with high-dose methylprednisolone for the treatment of fludarabine refractory high-risk chronic lymphocytic leukemia. Leukemia. 2008;22:2048–2053. doi: 10.1038/leu.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woyach JA, Lin TS, Lucas MS, et al. A phase I/II study of rituximab and etanercept in patients with chronic lymphocytic leukemia and small lymphocytic lymphoma. Leukemia. 2009;23:912–918. doi: 10.1038/leu.2008.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zent CS, Call TG, Shanafelt TD, et al. Early treatment of high-risk chronic lymphocytic leukemia with alemtuzumab and rituximab. Cancer. 2008;113:2110–2118. doi: 10.1002/cncr.23824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Faderl S, Ferrajoli A, Wierda W, O’Brien S, Lerner S, Keating MJ. Alemtuzumab by continuous intravenous infusion followed by subcutaneous injection plus rituximab in the treatment of patients with chronic lymphocytic leukemia recurrence. Cancer. 2010;116:2360–2365. doi: 10.1002/cncr.24958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Faderl S, Thomas DA, O’Brien S, et al. Experience with alemtuzumab plus rituximab in patients with relapsed and refractory lymphoid malignancies. Blood. 2003;101:3413–3415. doi: 10.1182/blood-2002-07-1952. [DOI] [PubMed] [Google Scholar]
- 60.Ferrajoli A. Incorporating the use of GM-CSF in the treatment of chronic lymphocytic leukemia. Leuk Lymphoma. 2009;50:514–516. doi: 10.1080/10428190902763541. [DOI] [PubMed] [Google Scholar]
- 61.Reddy N, Hernandez-Ilizaliturri FJ, Deeb G, et al. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br J Haematol. 2008;140:36–45. doi: 10.1111/j.1365-2141.2007.06841.x. [DOI] [PubMed] [Google Scholar]
- 62.James DF, Brown JR, Werner L, et al. Lenalidomide and rituximab for the initial treatment of chronic lymphocytic leukemia: report of an ongoing study. J Clin Oncol. 2010;28(Suppl. 15) doi: 10.1200/JCO.2013.51.5890. Abstract 6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferrajoli AXCB, O’Brien S, Wierda WG, et al. Combination therapy with lenalidomide and rituximab in patients with relapsed chronic lymphocytic leukemia (CLL); American Society of Hematology Annual Meeting; December 5–8, 2009; New Orleans, LA. Abstract 206. [Google Scholar]
- 64.Ferrajoli A, Lee BN, Schlette EJ, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111:5291–5297. doi: 10.1182/blood-2007-12-130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Del Poeta G, Del Principe MI, Consalvo MA, et al. The addition of rituximab to fludarabine improves clinical outcome in untreated patients with ZAP-70-negative chronic lymphocytic leukemia. Cancer. 2005;104:2743–2752. doi: 10.1002/cncr.21535. [DOI] [PubMed] [Google Scholar]
- 66.Del Poeta G, Del Principe MI, Buccisano F, et al. Consolidation and maintenance immunotherapy with rituximab improve clinical outcome in patients with B-cell chronic lymphocytic leukemia. Cancer. 2008;112:119–128. doi: 10.1002/cncr.23144. [DOI] [PubMed] [Google Scholar]
- 67.Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113:4834–4840. doi: 10.1182/blood-2008-10-186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.D’Souza A, Wilson J, Mukherjee S, Jaiyesimi I. Progressive multifocal leukoencephalopathy in chronic lymphocytic leukemia: a report of three cases and review of the literature. Clin Lymphoma Myeloma Leukemia. 2010;10:E1–9. doi: 10.3816/CLML.2010.n.009. [DOI] [PubMed] [Google Scholar]
- 69.Niscola P, Del Principe MI, Maurillo L, et al. Fulminant B hepatitis in a surface antigen-negative patient with B-cell chronic lymphocytic leukaemia after rituximab therapy. Leukemia. 2005;19:1840–1841. doi: 10.1038/sj.leu.2403914. [DOI] [PubMed] [Google Scholar]
- 70.Teeling JL, French RR, Cragg MS, et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood. 2004;104:1793–1800. doi: 10.1182/blood-2004-01-0039. [DOI] [PubMed] [Google Scholar]
- 71.Wierda WG, Kipps TJ, Dürig J, Griskevicius L, et al. Chemoimmunotherapy with ofatumumab, fludarabine, and cyclophosphamide (O-FC) in previously untreated patients with chronic lymphocytic leukemia (CLL) J Clin Oncol. 2010;28(Suppl. 15) Abstract 6520. [Google Scholar]
- 72.Patz M, Forcob N, Müller B, et al. Depletion of chronic lymphocytic leukemia cells from whole blood samples mediated by the anti-CD20 antibodies rituximab and GA101 [abstract]; American Society of Hematology Annual Meeting; December 5–8, 2009; New Orleans, LA. Abstract 2365. [Google Scholar]
- 73.Morschhauser FGC, Lamy T, Milpied NJ, et al. Phase I study of RO5072759 (GA101) in relapsed/ refractory chronic lymphocytic leukemia; American Society of Hematology Annual Meeting; December 5–8, 2009; New Orleans, LA. Abstract 884. [Google Scholar]