Abstract
Introduction
Modern anti-cancer strategies have distinctly increased survival rates; nevertheless, often accompanied by sterility. Currently, the only option for preserving fertility in prepubertal females is to cryopreserve ovarian tissue and re-transplant frozen-thawed tissue to restore fertility after treatment. Our aim was to report the occurrence of repetitive antral follicle formation and oocyte maturation in a prepubescent ovarian tissue xenograft without exogenous hormone stimulation.
Material and Methods
Frozen-thawed ovarian tissue from a 6-year-old patient suffering from nephroblastoma was xenotransplanted in oophorectomized severe combined immunodeficiency (SCID) mice to evaluate follicle development.
Ergebnisse
Repetitive follicle development to the antral stage occurred in the same xenograft of prepubertal ovarian tissue without exogenous hormone administration; 37 days after retrieving a maturing oocyte (this first retrieval has been previously published), another, completely mature oocyte was harvested from the xenograft. Subsequent histological evaluation of the grafted tissue showed primordial follicles, nearly all stages of developing follicles, as well as large atretic ones. Many clusters with dormant primordial follicles were also present.
Conclusion
Xenotransplanted prepubertal ovarian tissue has the potential for repetitive oocyte retrieval cycles without administering exogenous hormones. The results indicate that the human ovarian tissue might be able to synchronize the hypothalamus-hypophysis-axes of the mouse to the physiological human cycle; this should be investigated in future studies.
Key words: fertility preservation, ovarian tissue cryopreservation, prepubertal ovarian tissue, xenotransplantation, metaphase II oocyte
Zusammenfassung
Einleitung Moderne Krebsbekämpfungsstrategien haben zu einer deutlichen Steigerung der Überlebensraten geführt, was jedoch oft mit Sterilität einhergeht. Um die Fertilität präpubertärer Mädchen zu erhalten, besteht die einzige Option zurzeit aus der Kryokonservierung von Ovargewebe, gefolgt von einer Retransplantation des gefrorenen Eierstockgewebes nach Abschluss der onkologischen Behandlung, um die Fertilität wiederherzustellen. Unser Ziel war es, einen Nachweis zu führen über eine wiederholte Bildung antraler Follikel und Oozytenreifung in einem Xenotransplantat von präpubertärem Eierstockgewebe ohne exogene hormonelle Stimulation.
Material und Methoden Eingefrorenes Eierstockgewebe, das einem an einem Nephroblastom erkrankten 6-jährigen Mädchen entnommen wurde, wurde in oophorektomierten Mäusen mit SCID (schwerem kombinierten Immundefekt) xenotransplantiert, die anschließende Follikelreifung wurde evaluiert.
Ergebnisse Es folgte eine wiederholte Follikelbildung bis zum antralen Stadium in ebendiesem Xenotransplantat von präpubertärem Ovargewebe ohne exogene hormonelle Stimulation; 37 Tage nach der Entnahme einer heranreifenden Eizelle (weitergehende Daten zu dieser ersten Oozytenentnahme wurden bereits veröffentlicht), konnte eine weitere ausgereifte Eizelle dem Xenotransplantat entnommen werden. Eine anschließende histologische Untersuchung des transplantierten Gewebes zeigte sowohl Primordialfollikel und Follikel fast aller Entwicklungsstufen wie auch große atretische Follikel. Es waren also mehrere Ansammlungen ruhender Primordialfollikel vorhanden.
Schlussfolgerung Xenotransplantiertes präpubertäres Ovargewebe hat das Potenzial, wiederholt zyklisch Eizellen zur Entnahme zu generieren, auch ohne die zusätzliche Gabe exogener Hormone. Dieses Ergebnis zeigt, dass menschliches Eierstockgewebe möglicherweise in der Lage sein könnte, die Hypothalamus-Hypophysen-Achse der Maus mit den menschlichen physiologischen Zyklen zu synchronisieren. Dieser Frage sollte in zukünftigen Studien nachgegangen werden.
Schlüsselwörter: Fertilitätserhalt, Kryokonservierung von Eierstockgewebe, präpubertäres Ovargewebe, Xenotransplantation, Metaphase-II-Oozyte
Introduction
Advances in diagnosis and treatment of oncological diseases have increased the survival prognosis in recent years, particularly in children and juvenile cancer patients. Unfortunately, treatment strategies are frequently gonadotoxic and may lead to a depletion of the ovarian reserve accompanied by premature ovarian failure. Consequently, various strategies have been developed for fertility preservation; the most advantageous option usually depends on the patientʼs particular situation, including age as well as potential arising risk in case of postponing anti-cancer treatment. The only possibility in preserving fertility in prepubertal females is ovarian tissue cryoconservation, while there are multiple additional options for adult females. Thawing and re-transplantation of frozen tissue can be performed to restore fertility after anti-cancer treatment 1 , 2 , 3 , 4 , 5 . This preservation method profits from two main advantages; ovarian cortex usually incorporates a huge amount of follicles 6 , 7 and provides a high developmental potential, especially in prepubertal females 8 , 9 . Two transplantation studies have been performed, reporting that frozen-thawed prepubertal ovarian cortex could induce puberty 10 , 11 ; potentially, even restore fertility. Demeestere et al. 12 reported a case of ovarian tissue auto-transplantation with fertility restoration, resulting in a live birth; the tissue had been collected at the age of 13 years and 11 months, before the first menstrual bleeding. These results and worldwide more than 85 reported live births after re-transplantation of autologous frozen-thawed ovarian tissue to date 13 emphasize the potential of this technique; according to the criteria of the European Society of Human Reproduction and Embryology (ESHRE) special interests groups “Ethics and Law” as well as “Safety and Quality in Assisted Reproductive Technology” and the American Society for Reproductive Medicine (ASRM), this method should be graded as innovative 14 , 15 , on the way to routine clinical practice 16 .
We could demonstrate that a maturing oocyte can be obtained from prepubertal human ovarian tissue; achieved via xenotransplantation in oophorectomized severe combined immunodeficiency (SCID) mice without any exogenous hormone stimulation 17 . Our following aim was to investigate if spontaneous antral follicle formation as well as oocyte maturation would occur again from the same xenograft, still without administering any hormones. Herein, we report repetitive collection of oocytes from the same xenotransplant; to our knowledge, it has not been published before.
Material and Methods
Patient
A detailed description has been given by Lotz et al. 17 . In brief, ovarian tissue from a 6-year-old patient affected by nephroblastoma was removed in the course of laparotomy for nephroblastoma excision and cryopreserved for fertility preservation in 2009; informed consent as well as approval of the local ethical committee (University Womenʼs Hospital, Bonn, Germany) had been given prior to the intervention. About half of the ovarian cortex was removed from both ovaries during the surgery for nephroblastoma removal. Histological examination of the ovarian cortex revealed a sufficient amount of primordial follicles and excluded the presence of malignant cells. Less than 5% of the frozen tissue was used for this experiment.
Ovarian tissue freezing and thawing
Cryopreservation and thawing procedures for ovarian cortex tissue are identical to our first report 17 and were previously described by Isaschenko et al. in 2008 18 . In summary, biopsies from ovarian tissue were cut into small pieces of approximately 1 × 2 × 1 mm and equilibrated in a freezing solution including 1.5 mol/L dimethylsulfoxyde (DMSO) as cryoprotectant in Leibovitz medium. The tissue fragments were frozen in 1.8 mL Nunc cryovials via slow freezing protocol in a closed freezing system (Icecube 14S; Synlab) with auto-seeding. Cryopreserved tissue pieces were shipped to the Erlangen University Hospital (Erlangen, Germany) for thawing as well as transplantation experiments. Rapid warming was performed in a 37 °C water bath, subsequently exposing the tissue fragments to stepwise decreasing sucrose concentrations (release from cryoprotectants).
Animals
Female SCID mice (6 weeks old, Harlan-Winkelmann, Borchen, Germany) were housed for a study comparing prepubertal ovarian tissue from several females in vivo versus in vitro; the herein reported findings were part of this larger study. One mouse out of 7 which had been xenografted with tissue from the 6-year-old nephroblastoma patient showed the herein reported findings; 2 mice died after transplantation and 4 were sacrificed after a much shorter grafting time for the main study (before discovery of the first lump). Animals lived in groups of five mice per cage with water and food (Altromin 1314, Altromin, Lage, Germany) ad libitum; a daily rhythm of 12 h light and 12 hours dark. All tests and procedures were carried out under laminar flow conditions in a positive pressure room. Approval for the study was obtained from the local ethical committee on animal experimentation (Erlangen University Hospital, Erlangen, Germany) and animals were maintained in accordance with Animal Care and Use Committee regulations. A detailed description of the housing conditions is available in 17 .
Transplantation procedure, xenograft treatment and oocyte retrieval
The whole approach was already described after the first oocyte retrieval 17 . Surgery for xenotransplantation was performed under isoflurane (Isoflo ® ad us. vet., Abott AG, Baar, Germany) anesthesia without regard to the estrous cycle stage. Study animals were oophorectomized following xenotransplantation by placing one frozen-thawed piece of human ovarian tissue in an intramuscular pocket of the neck. Exogenous hormones were not administered during the whole experiment; neither in the first 122 days between transplantation and puncture of the first oocyte, nor during the following period of 37 days until the second oocyte retrieval. All mice were checked daily for health and behavior; follicle growth was monitored through palpation of the neck. Each time a lump could be distinctly identified at the graft site, the animal was anesthetized (Isoflo ® ad us. vet., Abott AG, Baar, Germany) for subsequent follicle aspiration by using a modified sterile needle (20 G Supra, Karl Lettenbauer GmbH, Erlangen, Germany); needles had been specially beveled as well as sharpened before, to minimize injury during intervention. Collected fluid was then inspected for the presence of cumulus oocyte complexes (COC); all complexes were harvested and placed into warmed, pre-equilibrated culture medium without exogenous gonadotropins (Universal IVF Medium, Origio, Malov, Denmark). After a rest period of one hour in a CO 2 incubator (5% CO 2 in air), COC were enzymatically denudated (Hyaluronidase, Origio, Malov, Germany) to examine meiotic stage and then placed back in the incubator for further in vitro culture. The animal was returned to its cage after first follicle puncture for continued monitoring of another follicle growth.
Graft recovery and histological assessment
The mouse was sacrificed by cervical dislocation immediately after the second oocyte retrieval. The xenograft was recovered and fixed in 4% paraformaldehyde (Sigma-Aldrich Chemistry Ltd., Munich, Germany). After routine paraffin embedding, the entire sample was cut in serial sections and stained with hematoxylin and eosin for histological examination. Classification of follicles based on their morphological appearance was as follows:
primordial follicles; oocyte surrounded by a single layer of flattened granulosa cells,
primary stage; oocyte surrounded by one layer of cuboidal granulosa cells,
preantral follicles; the granulosa cells are cuboidal with a minimum of two cell layers around the oocyte,
antral stage; an antrum is visible within multilayered cuboidal granulosa cells.
Results
Retrieval of a mature oocyte
Another lump started to grow at the graft site during ongoing observation following our previously reported retrieval of a metaphase I oocyte from a 7-mm lump (oocyte diameter 150 µm; Zona pellucida width 14.6 µm); this first oocyte reached the mature metaphase II stage within 22 h of additional in vitro culture ( Fig. 1 a to c ). This time, the lump again exhibited a diameter of approximately 7 mm, 37 days after the first puncture; the follicle fluid was aspirated and one COC, containing one metaphase II oocyte (oocyte diameter and Zona pellucida width similar to the first oocyte), was harvested ( Fig. 2 a to c ).
Fig. 1.
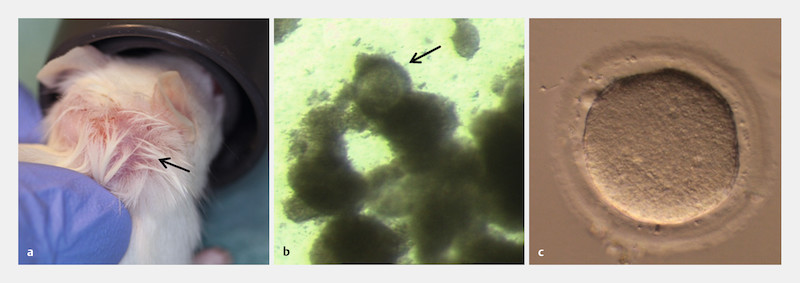
First oocyte retrieval from an unstimulated prepubertal human ovarian tissue xenograft in a SCID mouse. This data has been published previously: One frozen-thawed fragment of prepubertal human ovarian tissue was transplanted in the neck of an oophorectomized SCID mouse. A lump of approximately 7 mm in diameter was visible ( a ; lump indicated by an arrow) after 122 days grafting time. This lump was punctured and a cumulus oocyte complex (COC) could be identified under a stereo-microscope in the aspirated fluid ( b ; magnification at 35 ×, arrow pointing to the COC). The COC contained one maturing metaphase I oocyte ( c ; magnification at 320 ×), which matured to metaphase II within 22 hours of additional in vitro culture (oocyte diameter 150 µm; Zona pellucida width 14.6 µm).
Fig. 2.
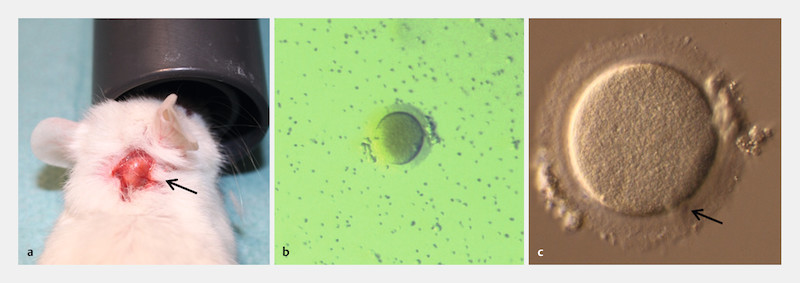
Second oocyte retrieval from the same unstimulated xenograft. Another lump grew at the graft site during ongoing observation ( a ; lump indicated by an arrow); we aspirated the follicle fluid 37 days after the first oocyte retrieval and examined the aspirate under a stereo-microscope. One oocyte could be harvested, COC already dropped out ( b ; 35 × magnification). Further inspection under an inverted microscope identified one mature metaphase II oocyte ( c ; arrow indicating the first polar body, magnification at 320 ×).
Identification of follicles in all developmental stages during histological evaluation of the graft
All developmental stages of follicles could be identified during investigation of histological serial sections; primordial, primary, preantral as well as antral follicles with intact morphology ( Fig. 3 a to f ) and very occasionally with multinucleated oocytes ( Fig. 4 a ). In addition, some large atretic follicles were present in the ovarian tissue part as well as multinucleated oocytes ( Fig. 4 b ). Furthermore, several clusters with dormant primordial follicles could be identified ( Fig. 5 a and b ).
Fig. 3.
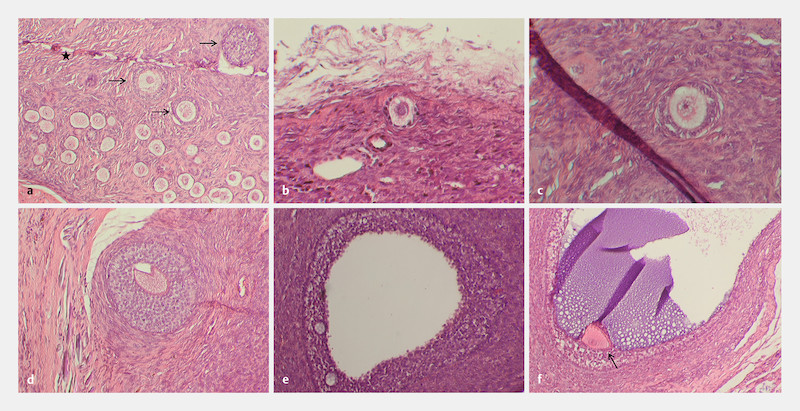
Histological sections of the xenograft showing different developmental stages of follicles. All developmental stages of follicles could be identified during investigation of histological serial sections of the prepubertal ovarian tissue xenograft. Representative sections showing primordial, primary and preantral follicles ( a – d ; developing follicles in [ a ] are indicated with an arrow, star in [ a ] marks tissue injury caused by the follicle puncture) as well as antral follicles ( e, f ; Cumulus oophorus is indicated with an arrow in f ). Sections were stained with hematoxylin–eosin; magnification at 100 × in a and d – f ; 200 × in b, c ).
Fig. 4.
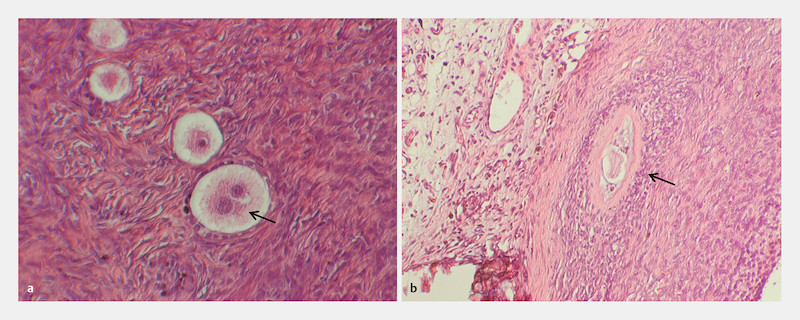
Histological sections of the xenograft depicting a multinucleated oocyte as well as an atretic follicle. Extremely rare, multinucleated oocytes were present ( a ; 200 × magnification) inside the xenograft. In addition, some large atretic follicles could be identified ( b ; 100 × magnification).
Fig. 5.
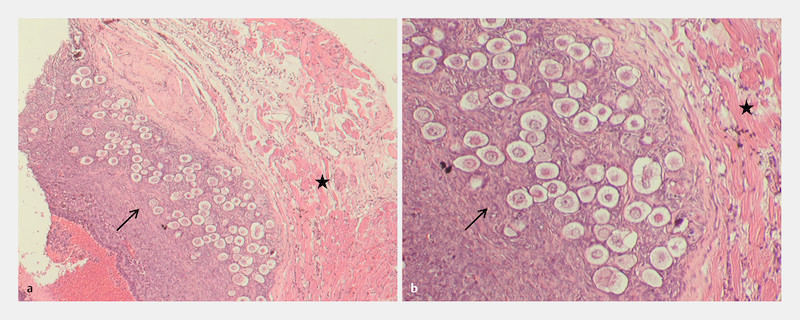
Histological sections illustrating a cluster of dormant follicles. Several clusters with dormant primordial follicles could be identified. A representative example is given in ( a ; magnification at 50 ×). Parts of the same site are given in ( b ) with a higher magnification (100 ×). In both cases, arrow indicates the primordial follicles and the graftʼs muscle tissue is marked with a star.
Discussion
Ovarian tissue cryopreservation in combination with later re-transplantation for fertility preservation in prepubertal females is currently the only realistic preservation option for these patients 1 , 2 , 3 . Nevertheless, only few autografting cases can be found for prepubertal tissue in the literature 10 , 11 . As ovarian function and developmental potential in frozen thawed tissue can be tested very well in vivo via xenotransplantation into immunodeficient mice 20 or in vitro by using the LIFE/DEAD assay 21 , this reluctant usage of frozen thawed tissue might be caused by safety concerns. In terms of safety, previous studies show contrasting results: Lotz et al. 22 as well as Greve et al. 23 , for instance, could not detect any malignant cell contamination and/or reintroduction of malignancy, whereas Dolmans et al. 24 reported leukemia induction after xenotransplantation. Hence, more studies would be necessary to clarify if ovarian tissue re-transplantation after cancer treatment is safe or not; for each malignancy entity separately.
Using the xenografting method to restore fertility would be a potential capability to circumvent the risk of reintroducing cancer through reimplantation of tissue which contains malignant cells; in short, xenografting potentially contaminated tissue with the objective of receiving mature oocytes for in vitro fertilization and later embryo replacement. From the methodical point of view, it would be a convenient technique, as cancer cells are not able to pass the Zona pellucida. Nevertheless, there are ethical and potential safety issues, which are not answered yet and have to be addressed in further research 20 .
A further approach for safety reasons is in vitro maturation (IVM) of oocytes; different options are imaginable and already subject of intensive research: For instance, in vitro culturing of whole human ovarian tissue or isolated follicles alone with the aim to activate follicle development and receive mature oocytes for cryopreservation or subsequent in vitro fertilization; an additional IVM period might be necessary in case collected oocytes are not completely mature. This method has successfully been used in some animal models, but not with human tissue so far (among others, reviewed in 25 , 26 , 27 , 28 ). Another possibility is oocyte aspiration from removed ovarian tissue prior to cryopreservation, combined with IVM culturing of oocytes either before freezing or after warming; this has already been done with prepubertal tissue 29 .
Another, very promising alternative to autografting of frozen-thawed ovarian tissue is transplanting an artificial ovary. During this method, isolated follicles from ovarian tissue attached to or encapsulated in an artificial matrix are provided for transplantation instead of whole tissue; the main idea is to get rid of potential malignant cells during this processing and thus avoid reintroducing cancer. Altogether, there are many different basics approaches, most of them reviewed in 30 and a recent report by Laronda et al. including 3D printed scaffold as artificial ovary matrix 31 .
Our histological evaluation showed almost all developmental stages of follicles (primordial, primary, preantral as well as antral follicles) and several primordial follicle clusters. This is in accordance with the findings by Luyckx et al. 9 who investigated xenografting of prepubertal ovarian tissue for 21 weeks, partly administering follicle stimulating hormone (FSH); they showed that a high number of follicles survived the freezing-thawing as well as the transplantation process and retained developmental capacity. Interestingly, we could observe a conformable follicular development without any exogenous hormone stimulation. To date, this study by Luyckx et al. is the only one showing such results for prepubertal human ovarian tissue.
The described repetitive retrieval of a maturing as well as a mature oocyte from the same xenograft of prepubertal human ovarian tissue without exogenous hormone stimulation is, to our best knowledge, the first report of that kind. Retrieving mature oocytes from unstimulated xenografts corresponds with similar results from Lotz et al. 32 ; nevertheless, they received these oocytes from frozen-thawed ovarian fragments from adult women and not from prepubertal tissue. In summary, Lotz et al. compared two stimulation protocols (hMG alone and hMG + GnRHa, both groups with additional hCG administration) as well as the non-stimulated control in xenografted SCID mice and received one maturing and two metaphase II oocytes. One of the metaphase II oocytes originated in the control without exogenous stimulation and in absence of hCG supply 32 . Receiving mature oocytes without any stimulation and/or hCG administration is in conflict with parts of the discussion in Gook et al. 33 . Gook et al. also worked with frozen-thawed ovarian tissue from adult women, xenografted in SCID mice. They reported 32 antral follicles with COC via histological evaluation, five of them containing metaphase II oocytes; gonadotropin stimulation as well as hCG administration were performed during grafting time. With this, Gook et al. described the first metaphase II oocyte development from frozen-thawed human ovarian tissue in 2003. In their discussion, they attribute the fail to gain mature oocytes in previous studies to lacking exogenous hormone administration. Further xenografting studies also reported the development of maturating or mature oocytes in frozen-thawed human ovarian tissue; all of these studies worked with tissue from adult females and supplied exogenous hormones 34 , 35 , 36 . Meanwhile, the present finding as well as those reported in 17 and 32 show that full oocyte maturation in cryopreserved human ovarian tissue without exogenous hormones is possible. Nevertheless, the follicle maturation process in human ovarian tissue takes a couple of months 37 ; this makes xenografting studies with the aim to retrieve metaphase II oocytes from human tissue difficult and therefore rare. Obtaining mature oocytes from ovarian tissue grafts without hormonal stimulation might contribute to clinical benefit since prolonged stimulation with FSH or luteinizing hormone (LH) can cause primordial follicle loss in xenografts 38 or in vitro 39 .
In the present study, two metaphase II oocytes could be obtained at a distance of 37 days without any administration of exogenous hormones. A full human cycle has a length of 23 – 35 days; although, 5% of all women go beyond 35 days 40 . In comparison, the spontaneous cycle length in mice is 4 – 5 days; 2 days follicular phase and 2 – 3 days luteal phase 41 . Accordingly, spontaneous follicle and oocyte maturation in this prepubertal xenograft are more comparable to the human reproductive system. This indicates that the human ovarian tissue might be able to synchronize the hypothalamus-hypophysis-axes of the mouse to the physiological human cycle; however, the essence from the present report is limited since a single observation is described. Future studies would be necessary to examine such a hypothesis.
In the present report, follicular diameter was approximately 7 mm both times, which is comparable to the findings from Solaimani et al. 34 ; they reported antral follicles of 7, 10 and 12 mm in diameter for xenograft position in the back muscle of SCID mice. Both harvested oocytes from our prepubertal ovarian tissue xenograft seemed normal with regard to their morphological appearance and also with regard to oocyte diameter and Zona pellucida width. Nevertheless, their fertilization as well as their developmental capacity cannot be assessed by morphological criteria alone.
Conclusion
Repetitive oocyte retrieval cycles can be achieved with non-stimulated xenografts of prepubertal ovarian tissue; this highlights the potential of prepubertal ovarian tissue. The results further indicate that the human ovarian tissue might be able to synchronize the hypothalamus-hypophysis-axes of the mouse to the physiological human cycle; future studies are necessary to investigate this hypothesis.
Funding.
This work in part supported by Wilhelm Sander Foundation (reference no. 2012.127/1), Munich, Germany.
Acknowledgements
The present work was performed by Nathalie Raffel in partial fulfillment of the requirements of the Friedrich-Alexander University of Erlangen–Nürnberg for obtaining the degree “Dr. rer. biol. hum.”.
Conflict of Interest The authors declare that they have no conflict of interest.
These authors contributed equally to this work.
References
- 1.Jadoul P, Dolmans M-M, Donnez J. Fertility preservation in girls during childhood: is it feasible, efficient and safe and to whom should it be proposed? Hum Reprod Update. 2010;16:617–630. doi: 10.1093/humupd/dmq010. [DOI] [PubMed] [Google Scholar]
- 2.Song Y, Sharp R, Lu F. The future potential of cryopreservation for assisted reproduction. Cryobiology. 2010;60:S60–S65. doi: 10.1016/j.cryobiol.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson R A, Wallace W H. Fertility preservation in girls and young women. Clin Endocrinol (Oxf) 2011;75:409–419. doi: 10.1111/j.1365-2265.2011.04100.x. [DOI] [PubMed] [Google Scholar]
- 4.Dittrich R, Lotz L, Keck G. Live birth after ovarian tissue autotransplantation following overnight transportation before cryopreservation. Fertil Steril. 2012;97:387–390. doi: 10.1016/j.fertnstert.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 5.Findeklee S, Lotz L, Heusinger K. Fertility protection in female oncology patients: how should patients be counseled? Geburtsh Frauenheilk. 2015;75:1243–1249. doi: 10.1055/s-0035-1558184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lintern-Moore S, Peters H, Moore G P. Follicular development in the infant human ovary. J Reprod Fertil. 1974;39:53–64. doi: 10.1530/jrf.0.0390053. [DOI] [PubMed] [Google Scholar]
- 7.Peters H, Byskov A G, Grinsted J. Follicular growth in fetal and prepubertal ovaries of humans and other primates. Clin Endocrinol Metab. 1978;7:469–485. doi: 10.1016/s0300-595x(78)80005-x. [DOI] [PubMed] [Google Scholar]
- 8.Fabbri R, Vicenti R, Macciocca M. Cryopreservation of ovarian tissue in pediatric patients. Obstet Gynecol Int. 2012;2012:910698. doi: 10.1155/2012/910698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luyckx V, Scalercio S, Jadoul P. Evaluation of cryopreserved ovarian tissue from prepubertal patients after long-term xenografting and exogenous stimulation. Fertil Steril. 2013;100:1350–1357. doi: 10.1016/j.fertnstert.2013.07.202. [DOI] [PubMed] [Google Scholar]
- 10.Poirot C, Abirached F, Prades M. Induction of puberty by autograft of cryopreserved ovarian tissue. Lancet. 2012;379:588. doi: 10.1016/S0140-6736(11)61781-9. [DOI] [PubMed] [Google Scholar]
- 11.Ernst E, Kjaersgaard M, Birkebaek N H. Case report: stimulation of puberty in a girl with chemo- and radiation therapy induced ovarian failure by transplantation of a small part of her frozen/thawed ovarian tissue. Eur J Cancer. 2013;49:911–914. doi: 10.1016/j.ejca.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 12.Demeestere I, Simon P, Dedeken L. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum Reprod. 2015;30:2107–2109. doi: 10.1093/humrep/dev128. [DOI] [PubMed] [Google Scholar]
- 13.Jadoul P, Guilmain A, Squifflet J. Efficacy of ovarian tissue cryopreservation for fertility preservation: lessons learned from 545 cases. Hum Reprod. 2017;32:1046–1054. doi: 10.1093/humrep/dex040. [DOI] [PubMed] [Google Scholar]
- 14.Donnez J, Dolmans M M, Pellicer A. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99:1503–1513. doi: 10.1016/j.fertnstert.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 15.Provoost V, Tilleman K, DʼAngelo A. Beyond the dichotomy: a tool for distinguishing between experimental, innovative and established treatment. Hum Reprod. 2014;29:413–417. doi: 10.1093/humrep/det463. [DOI] [PubMed] [Google Scholar]
- 16.Beckmann M W, Dittrich R, Findeklee S. Surgical aspects of ovarian tissue removal and ovarian tissue transplantation for fertility preservation. Geburtsh Frauenheilk. 2016;76:1057–1064. doi: 10.1055/s-0042-115017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lotz L, Liebenthron J, Nichols-Burns S M. Spontaneous antral follicle formation and metaphase II oocyte from a non-stimulated prepubertal ovarian tissue xenotransplant. Reprod Biol Endocrinol. 2014;12:41. doi: 10.1186/1477-7827-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isachenko V, Isachenko E, Reinsberg J. Cryopreservation of human ovarian tissue: effect of spontaneous and initiated ice formation. Reprod Biomed Online. 2008;16:336–345. doi: 10.1016/s1472-6483(10)60593-7. [DOI] [PubMed] [Google Scholar]
- 19.Weissman A, Gotlieb L, Colgan T. Preliminary experience with subcutaneous human ovarian cortex transplantation in the NOD-SCID mouse. Biol Reprod. 1999;60:1462–1467. doi: 10.1095/biolreprod60.6.1462. [DOI] [PubMed] [Google Scholar]
- 20.Dittrich R, Lotz L, Fehm T. Xenotransplantation of cryopreserved human ovarian tissue–a systematic review of MII oocyte maturation and discussion of it as a realistic option for restoring fertility after cancer treatment. Fertil Steril. 2015;103:1557–1565. doi: 10.1016/j.fertnstert.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Maltaris T, Kaya H, Hoffmann I. Comparison of xenografting in SCID mice and LIVE/DEAD assay as a predictor of the developmental potential of cryopreserved ovarian tissue. In Vivo. 2006;20:11–16. [PubMed] [Google Scholar]
- 22.Lotz L, Montag M, van der Ven H. Xenotransplantation of cryopreserved ovarian tissue from patients with ovarian tumors into SCID mice–no evidence of malignant cell contamination. Fertil Steril. 2011;95:2612–26140. doi: 10.1016/j.fertnstert.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Greve T, Clasen-Linde E, Andersen M T. Cryopreserved ovarian cortex from patients with leukemia in complete remission contains no apparent viable malignant cells. Blood. 2012;120:4311–4316. doi: 10.1182/blood-2012-01-403022. [DOI] [PubMed] [Google Scholar]
- 24.Dolmans M M, Marinescu C, Saussoy P. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood. 2010;116:2908–2914. doi: 10.1182/blood-2010-01-265751. [DOI] [PubMed] [Google Scholar]
- 25.Picton H M, Harris S E, Muruvi W. The in vitro growth and maturation of follicles. Reprod Suppl. 2008;136:703–715. doi: 10.1530/REP-08-0290. [DOI] [PubMed] [Google Scholar]
- 26.Telfer E E, McLaughlin M. In vitro development of ovarian follicles. Semin Reprod Med. 2011;29:15–23. doi: 10.1055/s-0030-1268700. [DOI] [PubMed] [Google Scholar]
- 27.Telfer E E, McLaughlin M. Strategies to support human oocyte development in vitro. Int J Dev Biol. 2012;56:901–907. doi: 10.1387/ijdb.130001et. [DOI] [PubMed] [Google Scholar]
- 28.Telfer E E, Zelinski M B. Ovarian follicle culture: advances and challenges for human and nonhuman primates. Fertil Steril. 2013;99:1523–1533. doi: 10.1016/j.fertnstert.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Revel A, Revel-Vilk S, Aizenman E. At what age can human oocytes be obtained? Fertil Steril. 2009;92:458–463. doi: 10.1016/j.fertnstert.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Amorim C A, Shikanov A. The artificial ovary: current status and future perspectives. Future Oncol. 2016;12:2323–2332. doi: 10.2217/fon-2016-0202. [DOI] [PubMed] [Google Scholar]
- 31.Laronda M M, Rutz A L, Xiao S. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun. 2017;8:15261. doi: 10.1038/ncomms15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lotz L, Schneider H, Hackl J. Does stimulation with human gonadotropins and gonadotropin-releasing hormone agonist enhance and accelerate the developmental capacity of oocytes in human ovarian tissue xenografted into severe combined immunodeficient mice? Fertil Steril. 2014;101:1477–1484. doi: 10.1016/j.fertnstert.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 33.Gook D A, Edgar D H, Borg J. Oocyte maturation, follicle rupture and luteinization in human cryopreserved ovarian tissue following xenografting. Hum Reprod. 2003;18:1772–1781. doi: 10.1093/humrep/deg365. [DOI] [PubMed] [Google Scholar]
- 34.Soleimani R, Heytens E, Van den Broecke R. Xenotransplantation of cryopreserved human ovarian tissue into murine back muscle. Hum Reprod. 2010;25:1458–1470. doi: 10.1093/humrep/deq055. [DOI] [PubMed] [Google Scholar]
- 35.Kim S S, Kang H G, Kim N H. Assessment of the integrity of human oocytes retrieved from cryopreserved ovarian tissue after xenotransplantation. Hum Reprod. 2005;20:2502–2508. doi: 10.1093/humrep/dei099. [DOI] [PubMed] [Google Scholar]
- 36.Gook D A, Edgar D H, Borg J. Diagnostic assessment of the developmental potential of human cryopreserved ovarian tissue from multiple patients using xenografting. Hum Reprod. 2005;20:72–78. doi: 10.1093/humrep/deh550. [DOI] [PubMed] [Google Scholar]
- 37.Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod. 1986;1:81–87. doi: 10.1093/oxfordjournals.humrep.a136365. [DOI] [PubMed] [Google Scholar]
- 38.Maltaris T, Beckmann M W, Mueller A. Significant loss of primordial follicles after prolonged gonadotropin stimulation in xenografts of cryopreserved human ovarian tissue in severe combined immunodeficient mice. Fertil Steril. 2007;87:195–197. doi: 10.1016/j.fertnstert.2006.05.058. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Chang Q, Sun J. Effects of HMG on revascularization and follicular survival in heterotopic autotransplants of mouse ovarian tissue. Reprod Biomed Online. 2012;24:646–653. doi: 10.1016/j.rbmo.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 40.Raith-Paula E, Frank-Hermann P, Freundl G. 4. Aufl. Heidelberg: Springer Verlag; 2008. Natürliche Familienplanung heute: Modernes Zykluswissen für Beratung und Anwendung. [Google Scholar]
- 41.Hau J, Svendsen P, Schapiro S. Boca Raton, Florida: CRC Press; 1994. Handbook of laboratory Animal Science, Animal Models. [Google Scholar]


