Abstract
Bleeding disorders, including hemophilia, can be seen in every ethnic population in the world. Among various bleeding disorders, reduced bone density has been addressed in hemophilia A. In recent years, there has been an increasing interest in addressing osteopenia and osteoporosis in hemophilia A. There is little or no study about the possible susceptibility of other individuals with bleeding disorders to reduced bone density. Questions have been raised about the role of blood coagulation factors in bone mineralization. This review provides new insight and ideas for further survey in the field of bleeding disorders and reduced bone density.
Keywords: Hemophilia A, Hemophilia B, Osteoporosis, Reduced bone density, von Willebrand diseases
INTRODUCTION
Hemophilia is a male bleeding disorder with worldwide distribution. Affected individuals have no or reduced levels of coagulation factor VIII and IX in plasma (Hemophilia A and Hemophilia B respectively). Hence, they encounter unusual bleeding pictures spontaneously or after trauma and surgery. The spectrum of bleeding manifestations varies from superficial ecchymosis to lethal hemorrhage in central nervous system.[1,2]
Extensive research over the past two decades have provided important information on tendency of hemophilia to reduced bone density in comparison with the control group. Hemarthrosis, infection with hepatitis C virus, low physical activity, etc. are common reported causes of susceptibility of hemophilia to reduced bone density.
To date, there are few studies that have investigated the status of bone mineral density (BMD) in rare bleeding disorders (RBD). However, much uncertainty still exists and need to be investigated. This review provides new insights into further surveys in this field.
COMMON BLEEDING DISORDERS: HEMOPHILIA
Hereditary deficiency of each coagulation factor in hemostatic cascade has been explained. Among them, hemophilia A, von Willebrand's disease (vWD) and hemophilia B are the most common coagulation factor deficiencies around the world. They can be found in all ethnic population.[1] While, the prevalence of hemophilia A varies among different countries, its prevalence has been estimated to be about 3 to 20 per 100,000 population. Hemophilia B is seen as 1 case among 5,000 male births in the European countries and United State.[3] It is estimated that the total number of hemophilia to be about 500,000 in the universe.[4] Among 77 countries in the world, the total number of individuals with hemophilia has been estimated 100,000.[5] Hemophilia A or classic hemophilia comprises about 80% of all cases with hemophilia. Individuals with hemophilia A and B have no or reduced levels of coagulation factor VIII and IX in circulation respectively. Lack or reduced levels of coagulation factors VIII and IX results in impaired clot formation and tendency to bleeding episodes. These lifelong disorders are categorized as severe type (<1%), medium type (1–5%), and mild type (5–30%) of a relevant coagulation factor in blood circulation.[6] Hemarthrosis is regarded as a hallmark of severe hemophilia that is associated with long immobility period.[7] Recurrent hemarthrosis are considered as the main cause of joint deterioration in hemophilia (arthropathy).[8] Replacement therapy using plasma derived or recombinant coagulation factor concentrates are base of treatment of bleeding episodes and prophylaxis regimens.[9]
vWD
It is the most common inherited bleeding disorder worldwide that is associated with defect in adhesion and aggregation platelets.[10] After vascular injury, von Willebrand's factor (vWF) mediates attachment of platelets to the subendothelium. Also, it acts as carriers of coagulation factor VIII in plasma.[11]
The prevalence of vWD varies based on the definition of disease and on the studied population, nevertheless is about 1 in 10,000 in symptomatic patients who has visited in a hemophilia treatment center.[10] This bleeding disorder characterized by bleeding after trauma and surgery and also mucosal tissues, hemorrhage. The history of bleeding tendency in the patient and his/her family with a laboratory pattern of abnormalities in vWF is necessary for diagnosis and categorizing the disease.[12] On the whole, if we consider the estimated numbers of individuals with common and RBD, there are a noticeable number of patients with bleeding disorders in the world.[13]
RBD
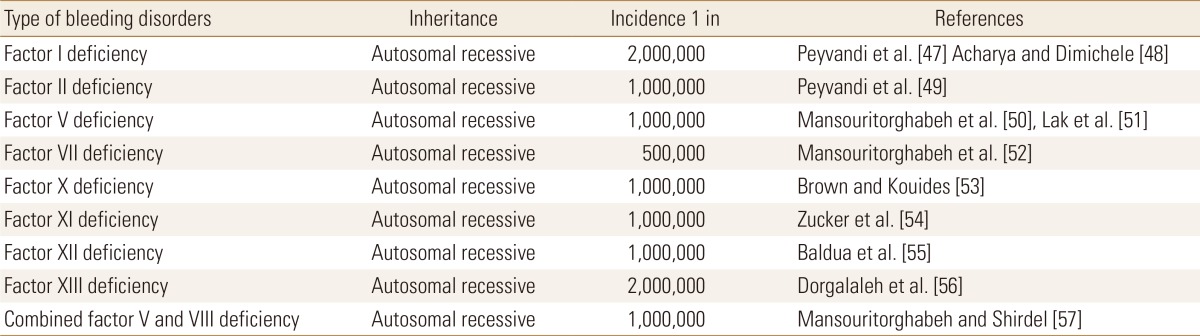
RBD comprise an important cluster of coagulation disorders.[14] They are usually inherited as an autosomal recessive genetic pattern with an estimated prevalence of about 1 to 2 cases per 1,000,000 population (Table 1).[15] They include factor I deficiency, factor II deficiency, factor V deficiency, factor VII deficiency, factor X deficiency, factor XI deficiency, factor XIII deficiency, and combined factor V and VIII deficiency. Affected individuals with RBD have no detectable level or reduced level of relevant coagulation factor in circulation that usually pay the way toward hemorrhage. One of the most important events in the two current decades in hemostasis field was remarkable findings in understanding the molecular basis that leads to each type of RBD.[16] One of the greatest challenges in working on RBD is the rarity of cases. This can be overcome by participating in mulicentral studies and also make use of the international database of RBD (http://www.rbdd.org), European Network of Rare Bleeding Disorders project (EN-RBD, http://www.rbdd.eu), or World Federation of Haemophilia database (www.wfh.org) that can facilitate the relevant studies.[17]
Table 1. Frequency and types of rare bleeding disorders in population.
QUALITY OF LIFE (QOL) IN HEMOPHILIA
In the recent decade, the overall QOL in individuals with hemophilia has drawn more attention.[18,19,20] While, participation of individuals with hemophilia in the sport, activities is considered a beneficial and helpful factor for improving QOL, reduced physical activities in hopes of reducing the risk of hemorrhage, is considered as a destructive agent in the quality of everyday life in individuals with hemophilia and their families.[21,22] On the other side of the coin, reduced bone density is a silent disease without any sign and symptom until a bone fracture occurs. In the current decade, with the improvement of treatments in hemophilia, life expectancy has been increased in individuals with hemophilia.[23] Accordingly, femoral neck fracture in hemophilia due to impaired bone stability may occur subsequent trivial trauma in young patients, what is rarely seen in normal population.[24]
REDUCED BONE DENSITY IN HEMOPHILIA
A large and growing body of literature has investigated osteopenia and osteoporosis both in children and adults with hemophilia.[25,26,27,28,29,30,31,32] Reduced bone density in hemophilia can be explained by recurrent hemarthrosis, and lower physical activities that may have an influence on bone mineralization.[33] While, physical activity is considered as a central indicator of bone resorption, patients with bleeding disorders are less likely to engage in high impact activities and also weight-bearing exercises.[34] The exercises with the possibility of physical damages are not recommended for patients with hemophilia, due to increasing risk of bleeding episodes. Individuals with severe hemophilia are usually recommended being engaged in soft exercises such as tennis, swimming, golf, cycling and ping pong.[35] In developing countries due to limited sources of coagulation factor concentrates, children with hemophilia rarely engage in high impact sports to avoid risk of hemorrhage.[21] Those patients with hemophilia and arthropathy, characterized by swelling, pain, limited immobility and joint instability, may have a bigger risk of reduced bone density due to less likely to precipitate in exercise activities.[26,27,28]
Recently, a survey in hemophilia A, hemophilia B and vWD showed higher risk of bone health outcome in comparison with health control and has suggested a possible role of endogenous coagulation factors in maintaining bone mass.[36] A study on a mouse model of hemophilia B showed factor IX deficient mice have bone disease and coagulation factors have a direct impact on bone health.[37] On the other side of coin, the results of a study on the effect of prophylaxis in hemophilia on bone health indicate that replacement therapy as prophylaxis regimen since early childhood may reserve normal BMD in severe hemophilia.[38]
THE NEW HORIZON FOR FURTHER SURVEYS
For the first time Barnes et al.[28] reported reduced bone density in 10 severe hemophiliacs A in 2004. This confirmed by further surveys on individuals with hemophilia.[3,39,40,41,42,43,44] After that reduced bone density has been shown in two other bleeding disorders (hemophilia B and combined factor V and VIII deficiency).[3,44,45] There are gaps in status of bone density in vWD, factor VII deficiency, factor V deficiency, factor X deficiency, factor XIII deficiency, fibrinogen abnormalities, and platelet disorders. Also, our finding and understanding about the prevalence of osteopenia and osteoporosis in various types of bleeding disorders (severe, moderate, and mild), effective risk factors, pathogenesis of reduced bone density, influences of various treatments of osteopenia and osteoporosis in bleeding patients and possible influence of physical therapy and sports are limited and need to be investigated.
Furthermore, the effect of hemoglobin levels on susceptibility to reduce bone density has been reported by Oh et al.[46] recently. Hence, measuring of hemoglobin level and other indexes of red blood cells in patients with hemophilia and other bleeding disorders is another new horizon needs to be paid attention by further multinational collaborative efforts. Similarly, vitamin D levels in patients with bleeding disorders and detection of haplotype of vitamin D need to be addressed by further surveys.
However, much uncertainty still exists about the relation between the lower level of each coagulation factor in blood circulation and reduced bone density.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Mansouritorghabeh H. Clinical and laboratory approaches to hemophilia A. Iran J Med Sci. 2015;40:194–205. [PMC free article] [PubMed] [Google Scholar]
- 2.Mansouritorghabeh H, Rahimi H, Mohades ST, et al. Causes of death among 379 patients with hemophilia: a developing country's report. Clin Appl Thromb Hemost. 2017 doi: 10.1177/1076029617713873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mansouritorghabeh H, Rezaieyazdi Z, Saadati N, et al. Reduced bone density in individuals with severe hemophilia B. Int J Rheum Dis. 2009;12:125–129. doi: 10.1111/j.1756-185X.2009.01394.x. [DOI] [PubMed] [Google Scholar]
- 4.Stonebraker JS, Bolton-Maggs PH, Soucie JM, et al. A study of variations in the reported haemophilia A prevalence around the world. Haemophilia. 2010;16:20–32. doi: 10.1111/j.1365-2516.2009.02127.x. [DOI] [PubMed] [Google Scholar]
- 5.Teitel JM, Barnard D, Israels S, et al. Home management of haemophilia. Haemophilia. 2004;10:118–133. doi: 10.1046/j.1365-2516.2003.00853.x. [DOI] [PubMed] [Google Scholar]
- 6.Dorgalaleh A, Dadashizadeh G, Bamedi T. Hemophilia in Iran. Hematology. 2016;21:300–310. doi: 10.1080/10245332.2015.1125080. [DOI] [PubMed] [Google Scholar]
- 7.De la Corte-Rodríguez H, Rodríguez-Merchán EC, De la Corte-García H. Rehabilitation of joint surgery in hemophiliacs. In: Rodríguez-Merchán E, editor. Joint surgery in the adult patient with hemophilia. Cham, CH: Springer; 2014. pp. 89–99. [Google Scholar]
- 8.van Galen KPM, Timmer M, de Kleijn P, et al. Clinical joint outcome after joint bleeds in patients with von Willebrand disease is comparable to moderate and severe hemophilia A despite fewer joint bleeds. Blood. 2016;128:3789. [Google Scholar]
- 9.Oldenburg J. Optimal treatment strategies for hemophilia: achievements and limitations of current prophylactic regimens. Blood. 2015;125:2038–2044. doi: 10.1182/blood-2015-01-528414. [DOI] [PubMed] [Google Scholar]
- 10.James AH. Von Willebrand: An underdiagnosed disorder: Several forms of the disease exist, and women with excessive reproductive tract bleeding should be tested for it. Contemp OB. GYN. 2017;62:20–26. [Google Scholar]
- 11.Leebeek FW, Eikenboom JC. Von Willebrand's disease. N Engl J Med. 2016;375:2067–2080. doi: 10.1056/NEJMra1601561. [DOI] [PubMed] [Google Scholar]
- 12.Laffan MA, Lester W, O'Donnell JS, et al. The diagnosis and management of von Willebrand disease: a United Kingdom Haemophilia Centre Doctors Organization guideline approved by the British Committee for Standards in Haematology. Br J Haematol. 2014;167:453–465. doi: 10.1111/bjh.13064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bullinger M, Von Mackensen S. Quality of life in children and families with bleeding disorders. J Pediatr Hematol Oncol. 2003;25(Suppl 1):S64–S67. doi: 10.1097/00043426-200312001-00015. [DOI] [PubMed] [Google Scholar]
- 14.Acharya SS, Coughlin A, Dimichele DM. Rare bleeding disorder registry: deficiencies of factors II, V, VII, X, XIII, fibrinogen and dysfibrinogenemias. J Thromb Haemost. 2004;2:248–256. doi: 10.1111/j.1538-7836.2003.t01-1-00553.x. [DOI] [PubMed] [Google Scholar]
- 15.Girolami A, De Marco L, Dal Bo, et al. Rarer quantitative and qualitative abnormalities of coagulation. Clin Haematol. 1985;14:385–411. [PubMed] [Google Scholar]
- 16.Peyvandi F, Bolton-Maggs PH, Batorova A, et al. Rare bleeding disorders. Haemophilia. 2012;18(Suppl 4):148–153. doi: 10.1111/j.1365-2516.2012.02841.x. [DOI] [PubMed] [Google Scholar]
- 17.Peyvandi F, Spreafico M. National and international registries of rare bleeding disorders. Blood Transfus. 2008;6(Suppl 2):S45–S48. doi: 10.2450/2008.0037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer K, de Kleijn P, Negrier C, et al. The association of haemophilic arthropathy with health-related quality of life: a post hoc analysis. Haemophilia. 2016;22:833–840. doi: 10.1111/hae.13120. [DOI] [PubMed] [Google Scholar]
- 19.Howell C, Scott K, Patel DR. Sports participation recommendations for patients with bleeding disorders. Transl Pediatr. 2017;6:174–180. doi: 10.21037/tp.2017.04.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salomon T, Chaves DG, Brener S, et al. Determining the health-related quality of life in individuals with haemophilia in developing economies: results from the Brazilian population. Haemophilia. 2017;23:42–49. doi: 10.1111/hae.13130. [DOI] [PubMed] [Google Scholar]
- 21.Mansouritorghabeh H, Rezaieyazdi Z. Bleeding disorders and reduced bone density. Rheumatol Int. 2011;31:283–287. doi: 10.1007/s00296-010-1534-y. [DOI] [PubMed] [Google Scholar]
- 22.Paschou SA, Anagnostis P, Karras S, et al. Bone mineral density in men and children with haemophilia A and B: a systematic review and meta-analysis. Osteoporos Int. 2014;25:2399–2407. doi: 10.1007/s00198-014-2773-7. [DOI] [PubMed] [Google Scholar]
- 23.Konkle BA, Huston H, Nakaya Fletcher S, Hemophilia A. In: GeneReviews(R) Adam MP, Ardinger HH, Pagon RA, et al., editors. Seattle, WA: University of Washington, Seattle; 1993-2017. [Google Scholar]
- 24.Miljić P, Bodrožić J, Tulic G, et al. Femoral neck fracture in hemophilia patients: a single center experience. Haemophilia. 2016;22:88. [Google Scholar]
- 25.Gallacher SJ, Deighan C, Wallace AM, et al. Association of severe haemophilia A with osteoporosis: a densitometric and biochemical study. Q J Med. 1994;87:181–186. [PubMed] [Google Scholar]
- 26.Wallny TA, Scholz DT, Oldenburg J, et al. Osteoporosis in haemophilia - an underestimated comorbidity? Haemophilia. 2007;13:79–84. doi: 10.1111/j.1365-2516.2006.01405.x. [DOI] [PubMed] [Google Scholar]
- 27.Nair AP, Jijina F, Ghosh K, et al. Osteoporosis in young haemophiliacs from western India. Am J Hematol. 2007;82:453–457. doi: 10.1002/ajh.20877. [DOI] [PubMed] [Google Scholar]
- 28.Barnes C, Wong P, Egan B, et al. Reduced bone density among children with severe hemophilia. Pediatrics. 2004;114:e177–e181. doi: 10.1542/peds.114.2.e177. [DOI] [PubMed] [Google Scholar]
- 29.Abdelrazik N, Reda M, El-Ziny M, et al. Evaluation of bone mineral density in children with hemophilia: Mansoura University children hospital (MUCH) experience, Mansoura, Egypt. Hematology. 2007;12:431–437. doi: 10.1080/10245330701383700. [DOI] [PubMed] [Google Scholar]
- 30.Gerstner G, Damiano ML, Tom A, et al. Prevalence and risk factors associated with decreased bone mineral density in patients with haemophilia. Haemophilia. 2009;15:559–565. doi: 10.1111/j.1365-2516.2008.01963.x. [DOI] [PubMed] [Google Scholar]
- 31.Iorio A, Fabbriciani G, Marcucci M, et al. Bone mineral density in haemophilia patients. A meta-analysis. Thromb Haemost. 2010;103:596–603. doi: 10.1160/TH09-09-0629. [DOI] [PubMed] [Google Scholar]
- 32.Tlacuilo-Parra A, Morales-Zambrano R, Tostado-Rabago N, et al. Inactivity is a risk factor for low bone mineral density among haemophilic children. Br J Haematol. 2008;140:562–567. doi: 10.1111/j.1365-2141.2007.06972.x. [DOI] [PubMed] [Google Scholar]
- 33.Mansouritorghabeh H, Rezaieyazdi Z, Badiei Z. Are individuals with severe haemophilia A prone to reduced bone density? Rheumatol Int. 2008;28:1079–1083. doi: 10.1007/s00296-008-0591-y. [DOI] [PubMed] [Google Scholar]
- 34.Lanyon LE. Using functional loading to influence bone mass and architecture: objectives, mechanisms, and relationship with estrogen of the mechanically adaptive process in bone. Bone. 1996;18:37s–43s. doi: 10.1016/8756-3282(95)00378-9. [DOI] [PubMed] [Google Scholar]
- 35.Heijnen L, Mauser-Bunschoten EP, Roosendaal G. Participation in sports by Dutch persons with haemophilia. Haemophilia. 2000;6:537–546. doi: 10.1046/j.1365-2516.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- 36.Booth J, Lu M, Gallo D, et al. Increased risk of adverse bone health outcomes in people with bleeding disorders. Blood. 2016;128:250. [Google Scholar]
- 37.Larson EA, Taylor JA. Factor IX-deficient mice have decreased skeletal health. Blood. 2016;128:559. [Google Scholar]
- 38.Khawaji M, Akesson K, Berntorp E. Long-term prophylaxis in severe haemophilia seems to preserve bone mineral density. Haemophilia. 2009;15:261–266. doi: 10.1111/j.1365-2516.2008.01912.x. [DOI] [PubMed] [Google Scholar]
- 39.Boban A, Zupancic Salek S, Kastelan D, et al. Quantitative ultrasound and dual energy X-ray absorptiometry in the assessment of osteoporosis in patients with haemophilia. Haemophilia. 2014;20:e420–e422. doi: 10.1111/hae.12529. [DOI] [PubMed] [Google Scholar]
- 40.Kempton CL, Antun A, Antoniucci DM, et al. Bone density in haemophilia: a single institutional cross-sectional study. Haemophilia. 2014;20:121–128. doi: 10.1111/hae.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee A, Boyd SK, Kline G, et al. Premature changes in trabecular and cortical microarchitecture result in decreased bone strength in hemophilia. Blood. 2015;125:2160–2163. doi: 10.1182/blood-2014-10-602060. [DOI] [PubMed] [Google Scholar]
- 42.Roushan N, Meysamie A, Managhchi M, et al. Bone mineral density in hemophilia patients. Indian J Hematol Blood Transfus. 2014;30:351–355. doi: 10.1007/s12288-013-0318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wells AJ, McLaughlin P, Simmonds JV, et al. A case-control study assessing bone mineral density in severe haemophilia A in the UK. Haemophilia. 2015;21:109–115. doi: 10.1111/hae.12565. [DOI] [PubMed] [Google Scholar]
- 44.Anagnostis P, Karras S, Paschou SA, et al. Haemophilia A and B as a cause for secondary osteoporosis and increased fracture risk. Blood Coagul Fibrinolysis. 2015;26:599–603. doi: 10.1097/MBC.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 45.Mansouritorghabeh H, Rezaieyazdi Z, Rezai J. Reduced bone density in individuals with combined factor V and VIII deficiency. Haemophilia. 2007;13:340–343. doi: 10.1111/j.1365-2516.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- 46.Oh YH, Moon JH, Cho B. Association between hemoglobin level and bone mineral density in Korean adults. J Bone Metab. 2017;24:161–173. doi: 10.11005/jbm.2017.24.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peyvandi F, Duga S, Akhavan S, et al. Rare coagulation deficiencies. Haemophilia. 2002;8:308–321. doi: 10.1046/j.1365-2516.2002.00633.x. [DOI] [PubMed] [Google Scholar]
- 48.Acharya SS, Dimichele DM. Rare inherited disorders of fibrinogen. Haemophilia. 2008;14:1151–1158. doi: 10.1111/j.1365-2516.2008.01831.x. [DOI] [PubMed] [Google Scholar]
- 49.Peyvandi F, Kaufman RJ, Seligsohn U, et al. Rare bleeding disorders. Haemophilia. 2006;12(Suppl 3):137–142. doi: 10.1111/j.1365-2516.2006.01271.x. [DOI] [PubMed] [Google Scholar]
- 50.Mansouritorghabeh H, Manavifar L, Mobalegh A, et al. Haemorrhagic manifestations and prevalence of factor V deficiency in north-eastern Iran. Haemophilia. 2010;16:376–380. doi: 10.1111/j.1365-2516.2009.02139.x. [DOI] [PubMed] [Google Scholar]
- 51.Lak M, Sharifian R, Peyvandi F, et al. Symptoms of inherited factor V deficiency in 35 Iranian patients. Br J Haematol. 1998;103:1067–1069. doi: 10.1046/j.1365-2141.1998.01077.x. [DOI] [PubMed] [Google Scholar]
- 52.Mansouritorghabeh H, Badiei Z, Noori F. Clinical pictures and prevalence of factor VII deficiency in Northeastern of Iran. Haemophilia. 2008;14:157–159. doi: 10.1111/j.1365-2516.2007.01568.x. [DOI] [PubMed] [Google Scholar]
- 53.Brown DL, Kouides PA. Diagnosis and treatment of inherited factor X deficiency. Haemophilia. 2008;14:1176–1182. doi: 10.1111/j.1365-2516.2008.01856.x. [DOI] [PubMed] [Google Scholar]
- 54.Zucker M, Seligsohn U, Salomon O, et al. Abnormal plasma clot structure and stability distinguish bleeding risk in patients with severe factor XI deficiency. J Thromb Haemost. 2014;12:1121–1130. doi: 10.1111/jth.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baldua V, Kadam P, Castellino N, et al. Factor XII deficiency. RGUHS J Med Sci. 2017;7:81–82. [Google Scholar]
- 56.Dorgalaleh A, Naderi M, Hosseini MS, et al. Factor XIII deficiency in Iran: a comprehensive review of the literature. Semin Thromb Hemost. 2015;41:323–329. doi: 10.1055/s-0034-1395350. [DOI] [PubMed] [Google Scholar]
- 57.Mansouritorghabeh H, Shirdel A. Desmopressin acetate as a haemostatic elevator in individuals with combined deficiency of factors V and VIII: a clinical trial. J Thromb Haemost. 2016;14:336–339. doi: 10.1111/jth.13207. [DOI] [PubMed] [Google Scholar]