Abstract
Background
The aim of this study is to determine the proportion of cancers presenting with parathyroid hormone (PTH) related protein (PTHrP)-mediated hypercalcemia, examine the clinical and biochemical characteristics, identify predictive factors for survival. And we also compared those characteristics between solid organ and hematologic malignancy groups.
Methods
Cancer patients with PTHrP-mediated hypercalcemia who were treated at Chonnam National University Hospital in Korea from January 2005 to January 2015 were retrospectively reviewed.
Results
Of all 115 patients, solid organ malignancies were the most common etiology (98 cases, 85.2%), with squamous cell carcinoma (50 cases, 43.4%), adenocarcinoma (27 cases, 23.4%). Interestingly, hepatocellular carcinoma (HCC; 18 cases, 15.7%) and cholangiocarcinoma (11 cases, 9.6%) were much more common causes than other previous reports. Hematologic malignancy was less common (17 cases, 14.8%), with multiple myeloma (9 cases, 7.8%) and non-Hodgkin's lymphoma (5 cases, 4.3%). Overall median survival was only 37 days. There was significant difference in median survival between two groups (35 days for solid organ malignancy and 72 days for hematologic malignancy; P=0.015). Cox regression analysis identified age, the type of malignancy and the time interval of developing hypercalcemia after cancer diagnosis as independent predictive factors for survival time.
Conclusions
PTHrP-mediated hypercalcemia was most frequently caused by solid organ malignancy. However, HCC and cholangiocarcinoma were important causes of PTHrP-mediated hypercalcemia may be due to geographic differences in cancer incidence in Korean population. Age, the type of malignancy and the time interval of developing hypercalcemia after cancer diagnosis were independent poor predictive factors for survival time.
Keywords: Hypercalcemia, Neoplasms, Parathyroid hormone-related protein
INTRODUCTION
Hypercalcemia is a common complication of cancer and can occur in 20% to 30% of patients during the course of their malignant disease.[1] There are two major mechanisms for malignancy-associated hypercalcemia (MAH). The first is humoral hypercalcemia of malignancy (HHM), and the second is local osteolytic hypercalcemia. HHM is most commonly mediated by parathyroid hormone (PTH) related protein (PTHrP), representing 75% to 80% of patients with MAH.[2,3] In 1941, Albright [4] postulated that tumors might produce a PTH-like factor. Intensive work in the 1980s led to the biochemical identification of the syndrome of HHM, which in turn led to the characterization of PTHrP. PTHrP is found in normal keratinocytes, lactating mammary tissue, placenta, parathyroid glands, the central nervous system, and a number of other sites, suggesting that it may have a widespread physiologic role.[3] PTHrP is often a normal product of the cells of origin tumor, but in the setting of malignancy, PTHrP production is upregulated by tumor cell. As a result, PTHrP enters the systemic circulation and stimulates type I PTH/PTHrP receptor (PTHR-1) and leads to hypercalcemia by increasing calcium resorption from bone and reabsorption in the kidneys. Classically, it is reported to be produced in various malignant tumors such as squamous, renal, and genitourinary malignancies, breast cancers, and human T-cell leukemia virus type 1–associated adult T-cell leukemia/lymphomas.[5] Of these, it is reported that squamous cell carcinoma is the most common cause of PTHrP-mediated hypercalcemia, although many studies have identified other tumors and nonmalignant causes.[1,6,7] However, little is known about the proportion of cancers caused by PTHrP-mediated hypercalcemia. In addition, although it is known that the presence of malignancy-MAH indicates the poor prognosis,[8] there are few data about the clinical course of cancer patients presenting with PTHrP-mediated hypercalcemia.
In this study, we aimed to determine the proportion of cancer patients presenting with PTHrP-mediated hypercalcemia, examine the clinical and biochemical characteristics, and analyze predictive factors for survival including comparison between solid organ and hematologic malignancy groups.
METHODS
1. Subjects
The data of hypercalcemic patients with simultaneously elevated plasma PTHrP who were treated at Chonnam National University Hospital from January 2005 to January 2015 in Korea were retrospectively reviewed. Inclusion criteria for the study subjects were elevated serum calcium (albumin-corrected calcium of >10.2 mg/dL, the upper limit of normal for the assay) on at least 2 consecutive assessments, associated simultaneously with plasma PTHrP level >1.1 pmol/L. A total of 128 patients were identified over the study period. Of them, 13 patients were excluded because 5 cancer patients were not confirmed by tissue biopsy and 8 patients had non-malignant conditions associated with hypercalcemia (specifically, pneumonia, ischemic heart disease, vitamin D intoxication and liver cirrhosis). Finally, 115 cancer patients with PTHrP-mediated hypercalcemia were included in our study.
2. Laboratory assays
PTHrP assay was performed with a 2-site immunoradiometric assay from Mitsubishi with a reference range of ≤1.1 pmol/L (Mitsubishi Kagaku Iatron Inc., Tokyo, Japan). This assay recognizes the N and C termini of PTHrP and cross-react with PTHrP (1-87), PTHrP (1-95), PTHrP (1-108) and PTHrP (1-141). This assay was reported to be not cross-react with serum PTH.
3. Statistical analysis
We obtained biochemical and demographic descriptive statistics with subgroup analysis of differences between solid organ and hematologic malignancy groups. For survival analysis, we used the following variables such as age, sex, severity of hypercalcemia, presence or absence of metastasis (bone, liver, lung, kidney), the number of metastasis site, treatment with or without bisphosphonate, degree of plasma PTHrP levels, the type of malignancy, the time interval of developing hypercalcemia after cancer diagnosis.
Data were expressed as means and standard deviation for continuous variables and as numbers (percentages) for categorical variables. Mann-Whitney U-test and the χ2 test were performed to compare difference between two groups. Pearson correlation analysis was performed to assess whether there was a linear correlation between two parameters. Patients' survival was analyzed by Cox-regression analysis and the Kaplan-Meier method. We determined survival status of the patients using patient's medical records or Korean National Health Insurance data. The survival period was calculated from the day of hypercalcemia development to death or the last hospital follow-up day. All statistical analysis was carried out with SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). A P-value of <0.05 was considered statistically significant.
RESULTS
The underlying identified causes are shown in Table 1. Solid organ malignancies represented the vast majority of causes identified (98 cases, 85.2%), with squamous cell carcinoma (50 cases, 43.4%) and adenocarcinoma (27 cases, 23.4%). Hematologic malignancy was less common (17 cases, 14.8%), with myeloma (9 cases, 7.8%) and non-Hodgkin's lymphoma (5 cases, 4.3%).
Table 1. Identified causes of PTHrP-mediated hypercalcemia (n=115).
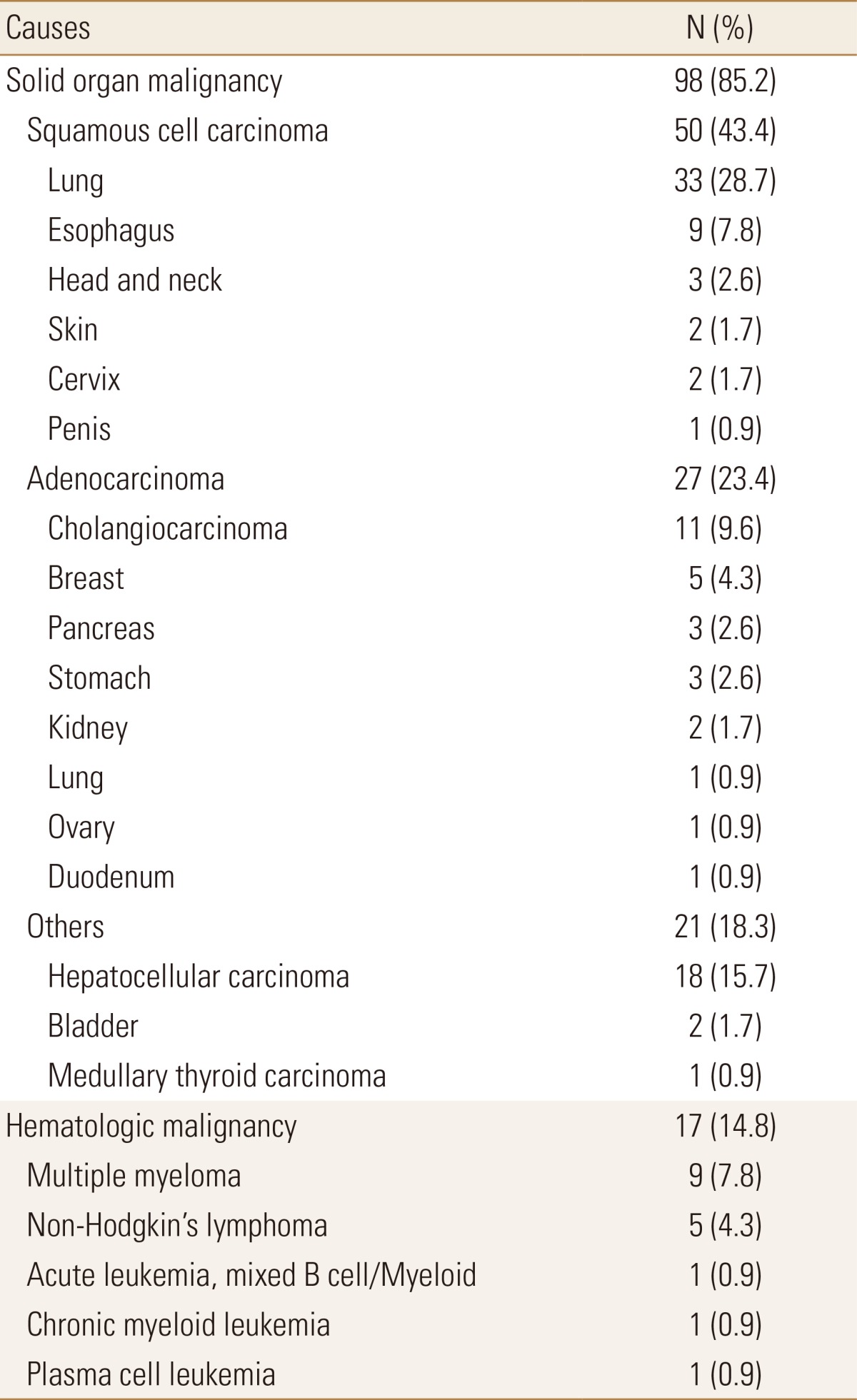
PTHrP, parathyroid hormone related protein.
The clinical and biochemical characteristics of the 115 cancer patients and the subgroups of solid organ and hematologic malignancy are shown in Tables 2 and 3. The mean age of the patients was 65.1 years (range, 31–92), with 92 males (80.0%) and 23 females (20.0%). Albumin-corrected serum calcium were 14.0±1.7 mg/dL. Severe hypercalcemia as determined by albumin-corrected serum calcium level of >14 mg/dL was observed in 59 cases (51.3%). Serum phosphorus levels were 3.3±1.3 mg/dL. Hypophosphatemia was observed in 37 cases (32.1%). Serum PTH were 6.6±9.9 pg/mL. Ninety-two cases (80.0%) had suppressed serum PTH levels of less than the lower normal limit of 9 pg/mL. Plasma PTHrP levels were 9.4±12.3 pmol/L. Pearson correlation analysis showed that albumin corrected serum calcium correlated to plasma PTHrP levels in solid organ malignancy (r=0.41, P=0.000), while there was no linear correlation between albumin corrected serum calcium and plasma PTHrP in hematologic malignancy. There were significant difference in sex (male, 83.6 vs. 58.8%), serum phosphorus (3.1±1.1 vs. 4.6±1.4 mg/dL), serum creatinine (1.3±0.7 vs. 2.7±1.8 mg/dL), serum PTH (5.6±7.9 vs. 12.2±16.5 pg/mL) between patients with solid organ and hematologic malignancy. The patients with solid organ malignancy had higher levels of plasma PTHrP (10.6±12.9 vs. 2.4±1.3 pmol/L).
Table 2. Characteristics of 115 patients with PTHrP-mediated hypercalcemia.
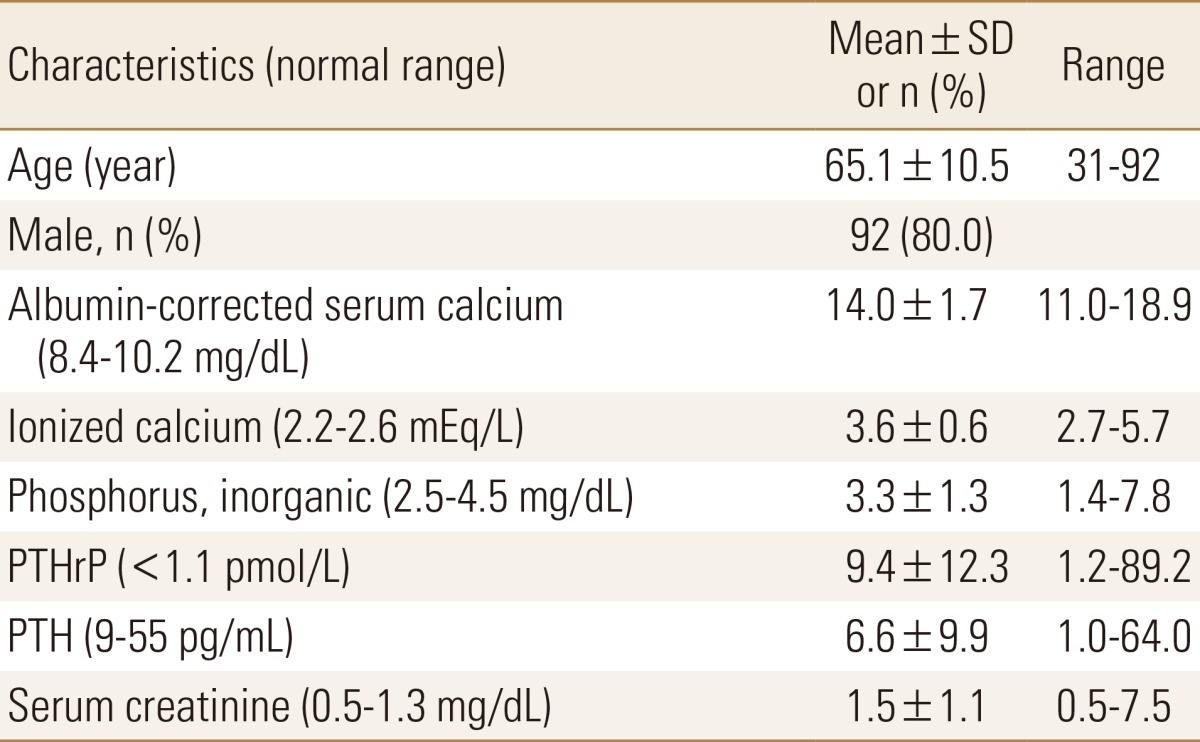
PTHrP, parathyroid hormone related protein; PTH, parathyroid hormone; SD, standard deviation.
Table 3. Characteristics of solid organ and hematologic malignancy.
The data is presented as mean±standard deviation or number (%).
PTHrP, parathyroid hormone related protein; PTH, parathyroid hormone.
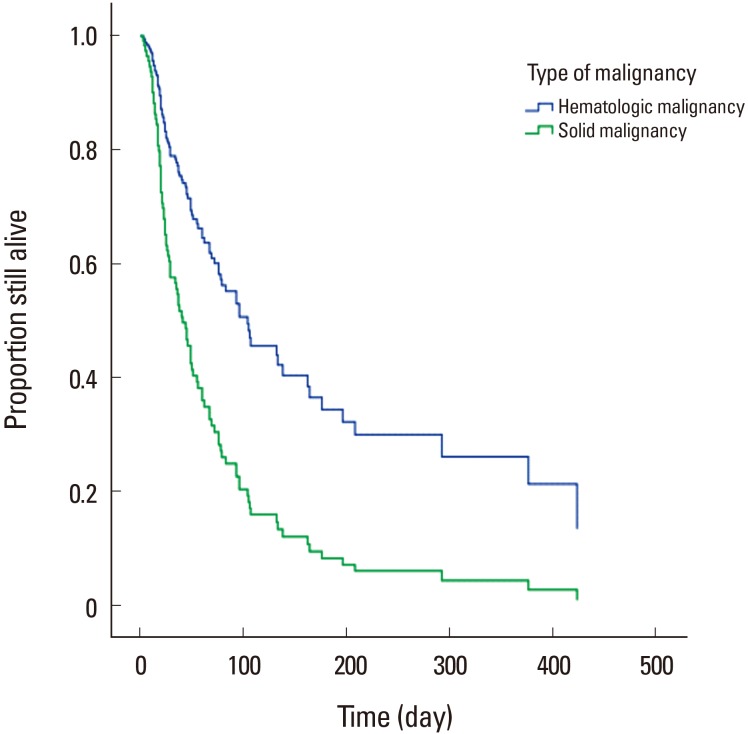
Only seven cases (6.0%) were still alive at the end of the follow-up period. Median survival from the time of developing PTHrP-mediated hypercalcemia for all 115 patients was only 37 days (interquartile range, 19–93 days). There was significant difference in median survival between the solid organ and hematologic malignancy. The solid organ malignancy group had a median survival of 35 days (interquartile range, 18–78 days), whereas the hematologic malignancy group had a median survival of 72 days (interquartile range, 19–318 days) (P=0.015) (Fig. 1). Cox regression analysis identified the type of malignancy, age, and the time interval of developing hypercalcemia after cancer diagnosis as independent predictive factors in survival time, as shown in Table 4.
Fig. 1. Survival of cancer patients with parathyroid hormone related protein-mediated hypercalcemia according to type of malignancy. There is significant difference in median survival between the solid organ and hematologic malignancy (P=0.015).
Table 4. Cox regression analysis for predictive factors of survival.

HR, hazard ratio; CI, confidence interval.
DISCUSSION
In this study, we retrospectively reviewed the clinical and biochemical characteristics of cancer patients with PTHrP-mediated hypercalcemia. Our study represents the relatively large scale of causes of this condition and identified rare causes, including plasma cell leukemia, acute leukemia mixed B cell/myeloid, duodenal adenocarcinoma and medullary thyroid carcinoma in Korea. Solid organ malignancy was the most common causes and squamous cell carcinoma of various site accounted for 43.4% of all subjects. As we expected, squamous cell carcinoma of the lung was the most commonly identified cause.
Interestingly, our study showed high incidence of a hepatocellular carcinoma (HCC; 18 cases, 15.7%) and cholangiocarcinoma (11 cases, 9.6%). On the other hand, a recent study in 138 patients with PTHrP-mediated hypercalcemia by Donovan et al.[9] showed very low incidence of HCC (1 case, 0.7%) and cholangiocarcinoma (1 case, 0.7%). This finding might be explained by different prevalence of primary liver cancer (HCC and intrahepatic bile duct carcinoma) worldwide. Approximately 75% to 80% of cases of HCC occur in Asia and Africa, although there is considerable variation within continents. For example, the incidence of liver cancer is highest in Mongolia, 116.6 per 105 for men; in Korea, 38.4 per 105 for men; in China, 37.4 per 105 for men; in Middle Africa 18.9 per 105 for men. By contrast, the incidence of liver cancer is much lower in North America, 6.8 per 105 for men; in Western Europe 7.2 per 105 for men; in Australia/New Zealand 5.0 per 105 for men.[10,11] For another reason, because the PTHrP assay is available in clinical practice since 1987 and it takes a lot of time to identify the results of the test, it may not been requested by clinicians, these might result in low incidence of PTHrP-mediated hypercalcemia with primary liver cancer.
There were significant differences in biochemical parameters between the solid organ and hematologic malignancy. Serum phosphorus and serum creatinine were higher in hematologic malignancy than those in solid organ malignancy. Although plasma PTHrP was higher in solid organ malignancy, albumin-corrected serum calcium levels were similar in both groups. These findings are partially explained by decreased glomerular filtration rate. In our study, multiple myeloma producing monoclonal proteins of varying type make up half of the hematologic malignancies. Because multiple myeloma patients often have irreversible impairment in renal function and increased renal tubular calcium reabsorption, the capacity of the kidneys to clear excess calcium load from the circulation effectively is overwhelmed, resulting in elevated serum calcium levels, may lead to severe hypercalcemia and renal failure. Many studies have identified cytokines produced by myeloma cell that is able to cause osteolysis, such as interleukin (IL)-6, IL-1α, IL-1β, IL-3, transforming growth factor-α, tumor necrosis factor-α (TNF-α), TNF-β.[12] Many of these cytokines in bone cell microenvironment might synergistically enhance the action of PTHrP in bone resorption, despite low levels of plasma PTHrP.[13,14]
Our study showed a weak but significant positive correlation between albumin-corrected serum calcium and plasma PTHrP in solid organ malignancy (r=0.41, P=0.000) which was consistent with other studies.[15,16] Whereas other study found no correlation between albumin-corrected serum calcium and plasma PTHrP.[9] As PTHrP induces hypercalcemia via stimulation of osteoclastic resorption, the severity of hypercalcemia is theoretically expected to strongly correlated with plasma PTHrP levels. These inconsistent results of the correlation between albumin-corrected serum calcium and plasma PTHrP among these studies might be explained by the difference in the method used for PTHrP assay in each study. For instance, patients with renal insufficiency may have high PTHrP levels when it is measured by assays that detect a carboxyl-terminal epitopes of PTHrP, because of accumulation of carboxyl-terminal fragments of PTHrP. For another reason, although PTH and PTHrP act on the same receptor, several biochemical aspect of HHM differ from changes in primary hyperparathyroidism. First, renal calcium clearance is higher in HHM than it is in hyperparathyroidism. Second, although PTHrP levels are elevated, 1,25-dihydroxyvitamin D (1,25 [OH]2D) levels are suppressed in HHM, whereas the elevations in PTH in primary hyperparathyroidism result in increased 1,25(OH)2D levels. Finally, bone histomorphometric studies in patients with HHM demonstrate very elevated rates of bone resorption but suppressed bone formation rates.[17] However, the mechanism for theses difference are unknown but may be due to another factors produced in conjunction with PTHrP in HHM. We assume that the underlying pathophysiological mechanism of MAH might be more complex and many factors could be involved.
PTHrP was known to be a useful prognostic factor in MAH. [18] In our study, the prognosis of PTHrP-mediated hypercalcemia was surprisingly poor: 50% of the patients died within 1 to 2 month and nearly 75% died within 3 months. By comparison, one study [19] reported that most patients with MAH survived from 3 to 6 months from presentation and other studies [8,20,21] reported a median survival of 1 to 3 months. Our finding implies that PTHrP production predicts a more aggressive clinical course. However, there is conflicting clinical evidence as to whether PTHrP production by the primary tumor, especially in breast cancer, is predictor of poor survival.[22,23,24] Nevertheless, the very short survival (median survival, 37 days) compared to previous studies suggests that PTHrP production is a very poor prognostic marker. Regrettably, we were not able to obtain data from an appropriately matched control group of normal levels of PTHrP with hypercalcemic cancer patients because of the wide variety of tumor types included in our study.
Although prognosis may be poor in cancer patients with PTHrP-mediated hypercalcemia, there were significant difference in median survival between the solid organ and hematologic malignancy. The solid organ malignancy group had a median survival of 35 days (interquartile range, 18–78 days), whereas the hematologic malignancy group had a median survival of 72 days (interquartile range, 19–318 days) (P=0.015). This difference might be likely due to the late stage of the disease at the time of developing hypercalcemia and difference of treatment modalities of disease. Sixty-five patients (66.3%) of the solid organ malignancy had already received extensive anticancer treatment by the time they presented with hypercalcemia and had one or more distant metastasis, whereas 9 patients (52.9%) (7 with multiple myeloma, 2 with Non-Hodgkin's lymphoma) of the hematologic malignancy had hypercalcemia at the time of cancer diagnosis and were promptly treated by intravenous hydration, diuretics and bisphosphonates followed by specific cancer treatment, such as chemotherapy including biological agents, bone marrow transplantation. Six patients of hematologic malignancy are still alive. This finding suggests that although prognosis may be poor in cancer patients with PTHrP-mediated hypercalcemia, prompt treatment for hypercalcemia and specific cancer treatment can partly improve survival outcome when the tumor is controlled.
Against our expectations, albumin-corrected serum calcium >14 mg/dL, high levels of plasma PTHrP, the number of metastasis site ≥2 were not poor predictive factors in Cox regression analysis. The most potent predictive factor was the type of malignancy (hazard ratio [HR]=2.33, P=0.015) showing that the solid organ malignancy group had shorter survival than hematologic malignancy group. Because the bone metastasis is known to be important mechanism for hypercalcemia in malignancy, we analyzed whether the bone metastasis was significant prognostic factor for survival. Although the median survival was longer in the absence of bone metastasis (37 vs. 17 days), there was no statistical significance. As we mentioned above, the survival prolongation observed in hematologic malignancy group suggests that poor prognosis in PTHrP-mediated hypercalcemia can be partly overcome when the tumor is controlled by definitive treatment.
Our study has several limitations. First, this was a retrospective study and a relatively small sample size due to the rarity of the condition. In addition, we could not compare survival in our sample patients with those in a control group of hypercalcemic cancer patients without elevated plasma PTHrP because of failure of complete clinical follow-up and the various tumor type. Second, because our study population was cancer patients cared for in a single center, our results might have been affected by selection bias. Third, plasma PTHrP levels were not measured in all patients presented with cancerous cause of hypercalcemia because of limitation on commercial use and also it is required considerable time to check the results of PTHrP assay. So, the incidence of malignancy with PTHrP-medicated hypercalcemia may have been underestimated. Finally, because this is a retrospective study and patients were managed in various departments, we could not obtain several important laboratory data for bone metabolism such as 24-hr urine calcium, serum 25(OH)D, calcitriol, and bone turnover markers. Nevertheless, our study is the first relatively large scale analysis in cancer patients with PTHrP-mediated hypercalcemia in Korea providing valuable information on proportion of causes, survival and poor predictive factors.
CONCLUSION
In conclusion, PTHrP-mediated hypercalcemia was most frequently caused by solid organ malignancy. However, the proportion of cancers caused by PTHrP-mediated hypercalcemia can be variable according to geographic difference in cancer incidence. Survival time was very short in cancer patients with PTHrP-mediated hypercalcemia. Age, type of malignancy and the time interval of developing hypercalcemia after cancer diagnosis were independent poor predictive factors of survival time.
ACKNOWLEDGMENT
This study was supported by a grant (CRI13905-23.5) Chonnam National University Hospital Biomedical Research Institute.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Stewart AF. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med. 2005;352:373–379. doi: 10.1056/NEJMcp042806. [DOI] [PubMed] [Google Scholar]
- 2.Stewart AF, Horst R, Deftos LJ, et al. Biochemical evaluation of patients with cancer-associated hypercalcemia: evidence for humoral and nonhumoral groups. N Engl J Med. 1980;303:1377–1383. doi: 10.1056/NEJM198012113032401. [DOI] [PubMed] [Google Scholar]
- 3.Burtis WJ, Brady TG, Orloff JJ, et al. Immunochemical characterization of circulating parathyroid hormone-related protein in patients with humoral hypercalcemia of cancer. N Engl J Med. 1990;322:1106–1112. doi: 10.1056/NEJM199004193221603. [DOI] [PubMed] [Google Scholar]
- 4.Mallory TB. Case records of the Massachusetts general hospital: case 27461. N Engl J Med. 1941;225:789–791. [Google Scholar]
- 5.Watanabe T, Yamaguchi K, Takatsuki K, et al. Constitutive expression of parathyroid hormone-related protein gene in human T cell leukemia virus type 1 (HTLV-1) carriers and adult T cell leukemia patients that can be trans-activated by HTLV-1 tax gene. J Exp Med. 1990;172:759–765. doi: 10.1084/jem.172.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobs TP, Bilezikian JP. Clinical review: rare causes of hypercalcemia. J Clin Endocrinol Metab. 2005;90:6316–6322. doi: 10.1210/jc.2005-0675. [DOI] [PubMed] [Google Scholar]
- 7.Savvari P, Peitsidis P, Alevizaki M, et al. Paraneoplastic humorally mediated hypercalcemia induced by parathyroid hormone-related protein in gynecologic malignancies: a systematic review. Onkologie. 2009;32:517–523. doi: 10.1159/000226209. [DOI] [PubMed] [Google Scholar]
- 8.Ralston SH, Gallacher SJ, Patel U, et al. Cancer-associated hypercalcemia: morbidity and mortality. Clinical experience in 126 treated patients. Ann Intern Med. 1990;112:499–504. doi: 10.7326/0003-4819-112-7-499. [DOI] [PubMed] [Google Scholar]
- 9.Donovan PJ, Achong N, Griffin K, et al. PTHrP-mediated hypercalcemia: causes and survival in 138 patients. J Clin Endocrinol Metab. 2015;100:2024–2029. doi: 10.1210/jc.2014-4250. [DOI] [PubMed] [Google Scholar]
- 10.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 11.Yang JD, Roberts LR. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 2010;7:448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mundy GR. Hypercalcemic factors other than parathyroid hormone-related protein. Endocrinol Metab Clin North Am. 1989;18:795–806. [PubMed] [Google Scholar]
- 13.de la Mata J, Uy HL, Guise TA, et al. Interleukin-6 enhances hypercalcemia and bone resorption mediated by parathyroid hormone-related protein in vivo. J Clin Invest. 1995;95:2846–2852. doi: 10.1172/JCI117990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guise TA, Yoneda T, Yates AJ, et al. The combined effect of tumor-produced parathyroid hormone-related protein and transforming growth factor-alpha enhance hypercalcemia in vivo and bone resorption in vitro. J Clin Endocrinol Metab. 1993;77:40–45. doi: 10.1210/jcem.77.1.8325957. [DOI] [PubMed] [Google Scholar]
- 15.Ratcliffe WA, Hutchesson AC, Bundred NJ, et al. Role of assays for parathyroid-hormone-related protein in investigation of hypercalcaemia. Lancet. 1992;339:164–167. doi: 10.1016/0140-6736(92)90220-w. [DOI] [PubMed] [Google Scholar]
- 16.Lee JK, Chuang MJ, Lu CC, et al. Parathyroid hormone and parathyroid hormone related protein assays in the investigation of hypercalcemic patients in hospital in a Chinese population. J Endocrinol Invest. 1997;20:404–409. doi: 10.1007/BF03347992. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama K, Fukumoto S, Takeda S, et al. Differences in bone and vitamin D metabolism between primary hyperparathyroidism and malignancy-associated hypercalcemia. J Clin Endocrinol Metab. 1996;81:607–611. doi: 10.1210/jcem.81.2.8636276. [DOI] [PubMed] [Google Scholar]
- 18.Truong NU, deB Edwardes MD, Papavasiliou V, et al. Parathyroid hormone-related peptide and survival of patients with cancer and hypercalcemia. Am J Med. 2003;115:115–121. doi: 10.1016/s0002-9343(03)00310-3. [DOI] [PubMed] [Google Scholar]
- 19.Mundy GR, Martin TJ. The hypercalcemia of malignancy: pathogenesis and management. Metabolism. 1982;31:1247–1277. doi: 10.1016/0026-0495(82)90012-9. [DOI] [PubMed] [Google Scholar]
- 20.Le Tinier F, Vanhuyse M, Penel N, et al. Cancer-associated hypercalcaemia in squamous-cell malignancies: a survival and prognostic factor analysis. Int J Oral Maxillofac Surg. 2011;40:938–942. doi: 10.1016/j.ijom.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 21.Penel N, Dewas S, Doutrelant P, et al. Cancer-associated hypercalcemia treated with intravenous diphosphonates: a survival and prognostic factor analysis. Support Care Cancer. 2008;16:387–392. doi: 10.1007/s00520-007-0322-z. [DOI] [PubMed] [Google Scholar]
- 22.Henderson MA, Danks JA, Slavin JL, et al. Parathyroid hormone-related protein localization in breast cancers predict improved prognosis. Cancer Res. 2006;66:2250–2256. doi: 10.1158/0008-5472.CAN-05-2814. [DOI] [PubMed] [Google Scholar]
- 23.Bundred NJ, Walls J, Ratcliffe WA. Parathyroid hormone-related protein, bone metastases and hypercalcaemia of malignancy. Ann R Coll Surg Engl. 1996;78:354–358. [PMC free article] [PubMed] [Google Scholar]
- 24.Takagaki K, Takashima T, Onoda N, et al. Parathyroid hormone-related protein expression, in combination with nodal status, predicts bone metastasis and prognosis of breast cancer patients. Exp Ther Med. 2012;3:963–968. doi: 10.3892/etm.2012.521. [DOI] [PMC free article] [PubMed] [Google Scholar]