Abstract
OBJECTIVE
Plasma interleukin-1 beta (IL1β) may influence sepsis mortality, yet recombinant human interleukin-1 receptor antagonist (rhIL1RA) did not reduce mortality in randomized trials. We tested for heterogeneity in the treatment effect of rhIL1RA by baseline plasma IL1β or IL1RA concentration.
DESIGN
Retrospective subgroup analysis of randomized controlled trial
SETTING
Multicenter North American and European clinical trial
PATIENTS
529 subjects with sepsis and hypotension or hypoperfusion, representing 59% of the original trial population
INTERVENTIONS
Random assignment of placebo or rhIL1RA × 72 hours
MEASUREMENTS AND MAIN RESULTS
We measured pre-randomization plasma IL1β and IL1RA and tested for statistical interaction between rhIL1RA treatment and baseline plasma IL1RA or IL1β concentration on 28-day mortality. There was significant heterogeneity in the effect of rhIL1RA treatment by plasma IL1RA concentration whether plasma IL1RA was divided into deciles (interaction p=0.046) or dichotomized (interaction p=0.028). Interaction remained present across different predicted mortality levels. Among subjects with baseline plasma IL1RA above 2071 pg/ml (n=283), rhIL1RA therapy reduced adjusted mortality from 45.4% to 34.3% (adjusted risk difference, ARD, −0.12, 95% CI −0.23 to −0.01), p=0.044. Mortality in subjects with plasma IL1RA below 2071 pg/ml was not reduced by rhIL1RA (ARD +0.07, 95% CI −0.04 to +0.17), p=0.230. Interaction between plasma IL1β concentration and rhIL1RA treatment was not statistically significant.
CONCLUSIONS
We report a heterogeneous effect of rhIL1RA on 28-day sepsis mortality that is potentially predictable by plasma IL1RA in one trial. A precision clinical trial of rhIL1RA targeted to septic patients with high plasma IL1RA may be worthy of consideration.
Keywords: Sepsis, Interleukin-1 receptor antagonist, heterogeneous treatment effect, predictive enrichment
INTRODUCTION
Sepsis and septic shock are common causes of death in intensive care units and are estimated to affect 19 million people annually worldwide (1). The mortality rate for sepsis exceeds 25% (2). Numerous pharmacologic agents have failed to decrease sepsis mortality, leading many to believe that a precision medicine option may be a more effective approach (3). Ideally, a precision medicine approach would rely on a predictive enrichment tool such as a clinical risk factor, plasma biomarker, or gene expression pattern to identify patients most likely to benefit from the therapy in question (4, 5). This approach has been highly successful in cancer therapy, leading to widespread appreciation that therapies may act differently among distinct endotypes of patients (6–8).
One sepsis therapy that showed promise was recombinant interleukin-1 receptor antagonist (rhIL1RA), a synthetic form of the naturally occurring anti-inflammatory cytokine IL1RA. IL1RA competes with interleukin-1 alpha and beta (IL1α, IL1β) to bind the interleukin-1 receptor without triggering receptor signaling (9, 10). There was enthusiasm for rhIL1RA in sepsis because IL1β incites permeability and activates inflammatory cytokine production in vitro (11–13), and in animals, IL1RA reverses IL1β-mediated febrile vasodilatory shock (14–16). Our group has shown improved sepsis survival associated with a high-functioning variant in the gene encoding IL1RA and, in a Mendelian randomization analysis, that genetically determined variation in plasma IL1β associates with sepsis mortality (17, 18), potential evidence that plasma IL1β may have a causal role in sepsis outcomes. Three randomized placebo controlled trials of rhIL1RA in sepsis were conducted in the 1990s (19–21). Although these trials demonstrated a potential effect for reduced mortality, the effect was small (2 – 5% absolute risk reduction), not statistically significant, and predictors of response were lacking.
We undertook the current study to test for potential heterogeneity in rhIL1RA treatment effect by plasma IL1RA or IL1β concentration. We hypothesized that treatment may interact with baseline plasma status to influence mortality, and that patients with an activated IL-1 axis would benefit from rhIL1RA.
MATERIALS AND METHODS
Patients enrolled in the Phase III rhIL1RA Sepsis Syndrome Study, a multicenter trial enrolling during 1992, were eligible for inclusion in this study if baseline plasma was available (20). For the original trial, eligible adult patients had a strongly suspected infection, SIRS criteria, and hypotension or hypoperfusion attributed to sepsis within 24 hours of enrollment (22). Major exclusion criteria included pregnancy, obesity, prior transplant, immunosuppression, or morbid status. At each participating center, the Institutional Review Board or Ethical Review Committee approved the trial, and informed consent for participation was obtained from patient or family before study participation (20). Of 893 subjects in the original trial, banked plasma drawn pre-randomization and clinical data were available for 529 (59%). Patients were randomized 1:1:1 in a blinded fashion to bolus rhIL1RA 100 mg and 72-hour infusion of 1.0 mg/kg/hr rhIL1RA or 2.0 mg/kg/hr rhIL1RA; or to bolus placebo (vehicle) and 72-hour infusion of placebo. Antibiotic, fluid, and ventilator care were managed by the treating physician. The primary outcome of the trial was 28 day survival (20); we analyzed 28-day mortality (23). To adjust for severity of illness, the predicted risk of mortality (PRM) was calculated from APACHE III data (24, 25). Plasma collected at screening pre-randomization was frozen at −70 °C. Plasma IL1RA and IL1β level were measured by ELISA (R&D Systems) in duplicate for 20% of the population and singlet for the remainder due to low sample volume. The standard range for IL1RA was 39 – 5000 pg/ml and for IL1β was 1.9 – 250 pg/ml. Laboratory personnel were blinded to clinical data including treatment status and survival.
Statistical analysis: Subjects who received either dose of rhIL1RA were considered to have received rhIL1RA and were compared to those receiving placebo. Continuous variables were compared by the Wilcoxon rank sum test. Categorical variables were compared by chi square testing. Correlation was assessed by Spearman statistics.
Our primary analysis was to test for heterogeneity in rhIL1RA treatment effect by baseline plasma biomarker concentrations, which would be indicated by a statistically significant interaction term. We followed a recommended framework to detect and report potential heterogeneity (26, 27). We tested for rhIL1RA treatment effect heterogeneity by assessing the p-value of the interaction terms [rhIL1RA*biomarker decile] and [rhIL1RA*biomarker cut point dichotomization] in logistic regression of mortality upon APACHE III score, rhIL1RA treatment, and interaction defined above (26, 28). We reasoned that treating plasma biomarker concentration by deciles would simulate continuous data and maximize information content, whereas dichotomizing the data would be easier to operationalize in a clinical setting as “biomarker positive” or “marker negative.” The same approach was applied for IL1RA and IL1β concentration. We used a data-driven approach, the Youden method, to select the cut point in plasma concentration that best optimized the area under the mortality receiver operating characteristics curve (29, 30). We also dichotomized the population according to median IL1RA and IL1β concentrations. We report the results of the Mantel-Haenszel test for inhomogeneity between stratum-specific odds ratios (31). To ensure that the heterogeneity observed was a function of plasma level rather than potentially collinear severity of illness measures like the APACHE III score, we tested the interaction between rhIL1RA treatment and biomarker level across tertiles of predicted mortality, selecting tertiles given published examples (26) and because 3 levels of illness severity reconciled with clinical “high, low, intermediate” risk judgments. To display the interaction, we plotted Kaplan-Meier estimated survival for groups defined by baseline plasma IL1RA and rhIL1RA treatment.
When interaction was statistically significant (p<0.05), we categorized patients into strata by biomarker cut point and used logistic regression accounting for APACHE III score to determine the association between rhIL1RA treatment with mortality. Following each stratum-specific logistic regression model, we used post-estimation marginal analysis to convert odds ratios to risk differences by plasma marker concentration (32), as this approach allows an estimation of the average treatment effect of rhIL1RA across all observations while holding other covariates at their original values. We used Stata Release 12 (College Station, TX) and considered a 2-sided p-value < 0.05 significant. We assumed a value of 0.1 pg/ml for subjects with undetectable plasma IL1β; plasma IL1RA was almost uniformly detectable. In sensitivity analyses, we excluded subjects with undetectable IL1β. As exploratory analyses, we tested for statistical interaction for the ratio of IL1RA to IL1β and the product IL1RA*IL1β. The online supplement presents additional analyses and further detail.
RESULTS
Characteristics of eligible patients who survived 28 days compared to those who died are shown in Table 1 and patient flow is depicted in Figure 1. Plasma was available for 59% of the original trial population. We had no information to explain why some subjects had available plasma and some did not. Subjects with plasma were similar to those who did not have plasma available (Table E1, Supplement). The distribution of baseline characteristics among the 529 subjects with plasma was similar in the randomly assigned treatment groups (Table E2, Supplement). Plasma IL1β concentration was detectable in 76% of subjects. Mean intra-individual coefficients of variation were 6.08% for IL1β and 7.30% for IL1RA. Correlation between plasma IL1RA and IL1β was moderate, Spearman rho 0.42, p<0.001, and remained moderate (rho 0.40) if low IL1β samples were excluded. Non-survivors had significantly higher plasma IL1RA (Table 1). In the overall population with plasma tested, similar to the reported trial results (20), treatment with rhIL1RA was not significantly associated with mortality: adjusted risk difference (ARD) of −0.03 (95% CI −0.09 to 0.04).
Table 1. Characteristics of the study population by 28-day vital status.
Survivors and non-survivors were compared by the Wilcoxon rank sum test for continuous data and by chi square test for categorical data. Data are presented as number (percentage) or as median (interquartile range). Organ dysfunctions were defined as in the original trial (20) and are defined in the online supplement. IL1RA: plasma interleukin-1 receptor antagonist; IL1β: plasma interleukin-1 beta; pg/ml: picogram/milliliter.
| Vital Status at 28 Days | |||
|---|---|---|---|
|
| |||
| Dead (n=167) | Alive (n=362) | p-value | |
|
| |||
| Female Gender | 69 (41%) | 142 (39%) | 0.648 |
|
| |||
| APACHE III-predicted risk of mortality | 0.47 (0.27, 0.66) | 0.27 (0.17, 0.41) | < 0.001 |
|
| |||
| Infection source: | 0.940 | ||
| Gram negative | 40 (24%) | 96 (27%) | |
| Gram positive | 31 (19%) | 69 (19%) | |
| Mixed bacterial | 49 (29%) | 107 (30%) | |
| Other | 12 (7%) | 23 (6%) | |
| Unknown | 35 (21%) | 67 (19%) | |
|
| |||
| Septic Shock | 141 (84%) | 287 (79%) | 0.161 |
|
| |||
| Acute respiratory distress syndrome | 46 (27%) | 81 (22%) | 0.189 |
|
| |||
| Disseminated intravascular coagulopathy | 37 (22%) | 32 (9%) | < 0.001 |
|
| |||
| Biliary dysfunction | 59 (35%) | 73 (20%) | < 0.001 |
|
| |||
| Macrophage activation syndrome (32) | 11 (7%) | 15 (4%) | 0.227 |
|
| |||
| Acute tubular necrosis | 73 (44%) | 93 (26%) | < 0.001 |
|
| |||
| rhIL1RA treatment | 113 (68%) | 252 (70%) | 0.652 |
|
| |||
| Plasma IL1RA (pg/ml) | 3687 (909, 14859) | 2041 (502, 8036) | 0.009 |
|
| |||
| Plasma IL1β (pg/ml) | 3.4 (0.39, 24.1) | 2.4 (0.1, 15.9) | 0.065 |
|
| |||
| IL1RA/IL1β ratio | 781 (143, 4112) | 1001 (99, 5588) | 0.978 |
|
| |||
| IL1RA*IL1β | 12748 (377, 260375) | 3402 (165, 99691) | 0.012 |
Figure 1.
Study population.
Our primary analysis was to test for potential heterogeneity in rhIL1RA treatment effect by plasma marker concentration. We performed logistic regression of 28-day mortality accounting for rhIL1RA treatment, APACHE III score, plasma IL1RA concentration (in deciles), and an interaction term [rhIL1RA treatment*plasma IL1RA decile]. Both the APACHE III score (p<0.001) and the interaction term between IL1RA decile and rhIL1RA treatment (p=0.046) were associated with mortality. We used the Youden method to determine an empiric threshold of plasma IL1RA level associated with mortality and dichotomized the population by this value, a plasma IL1RA level of 2071 pg/ml. We repeated the logistic regression including the interaction term [rhIL1RA treatment*plasma IL1RA cut point] and the interaction term remained significantly associated with mortality, p=0.028. If we changed the cut point to the median plasma IL1RA concentration, the interaction term was also significant: p=0.036. The significance of interaction terms was similar in models that excluded APACHE III score, and for log(IL1RA) treated continuously (p=0.055).
Because there was potential collinearity between plasma IL1RA and severity of illness (Table 1 and Table E3, Supplement), we tested the interaction effect of plasma IL1RA level across tertiles (26) or deciles of APACHE III-predicted risk of mortality, and the interaction term remained statistically associated with mortality (p=0.037 or p=0.047, respectively). Having established a statistically significant interaction between plasma IL1RA concentration and rhIL1RA treatment effect, we undertook a stratified analysis of trial results by Youden-determined plasma IL1RA cut point. Applying the cut point, 286 subjects (54%) were characterized as being ‘plasma IL1RA high’ and 243 (46%) as ‘plasma IL1RA low.’
We performed stratum-specific logistic regression of mortality accounting for APACHE III score and rhIL1RA treatment (Table 2). Among subjects with plasma IL1RA below 2071 pg/ml, rhIL1RA resulted in a nonsignificant increase in mortality, ARD 0.07 (95% CI −0.04 to 0.17). In contrast, subjects with baseline IL1RA above 2071 pg/ml demonstrated significantly reduced mortality with rhIL1RA, ARD – 0.12 (95% CI −0.23 to −0.01). A Mantel-Haenszel test determined the effect of rhIL1RA to be significantly inhomogeneous between these strata (p=0.026). As shown in Table 3, the treatment effect of rhIL1RA remained inhomogeneous by baseline plasma IL1RA concentration across different levels of predicted mortality, whereas no interaction was detected between rhIL1RA treatment and APACHE tertiles themselves. Figure 2 displays the interaction between rhIL1RA treatment and baseline plasma IL1RA, with a different direction of treatment effect depending on plasma IL1RA status. Results were unchanged when we used the median plasma IL1RA concentration to divide the population (Table E4, Supplement). When we further stratified the analysis by rhIL1RA dose, there was no evidence for a dose-responsive aspect to the interaction, as shown in Table E5, Supplement.
Table 2. Effect Modification of rhIL1RA Treatment by Baseline Plasma IL1RA Level on the Risk for Sepsis Mortality.
Subjects with high plasma IL1RA had significantly reduced mortality when treated with rhIL1RA, whereas those with low baseline plasma IL1RA did not. Baseline plasma IL1RA was dichotomized at 2071 pg/ml, the point at which the area under the receiver operator curve is maximized. Mantel-Haenszel test for inhomogeneity: p=0.026. In a multivariable logistic regression model including APACHE III score, rhIL1RA treatment and the interaction term, the p-value for the interaction term [rhIL1RA*plasma IL1RA cut point] was 0.028. rhIL1RA: recombinant human interleukin-1 receptor antagonist. pg/ml: picograms per milliliter. CI: confidence intervals
| Plasma IL1RA by Cut point | Mortality Rate | Adjusted Risk Difference (95% CI) | Adjusted Relative Risk of Mortality (95% CI) | p-value | |
|---|---|---|---|---|---|
| Placebo | rhIL1RA | ||||
| Low (n=243) < 2071 pg/ml | 14 / 76 (18.4%) | 45 / 167 (26.9%) | +0.07 (−0.04, 0.17) | 1.34 (0.81, 2.21) | p=0.230 |
| High (n=286) ≥ 2071 pg/ml | 40 / 88 (45.4%) | 68 / 198 (34.3%) | −0.12 (−0.23, −0.01) | 0.74 (0.56, 0.98) | p=0.044 |
Table 3. Treatment effect heterogeneity is apparent across multiple levels of illness severity.
The raw mortality and adjusted risk difference (ARD) of rhIL1RA treatment versus placebo is shown, stratified by APACHE III predicted mortality tertile and plasma IL1RA concentration. A consistent direction of effect was observed for rhIL1RA treatment for subjects with plasma IL1RA above 2071 pg/ml across predicted mortality tertiles, whereas patients with low plasma IL1RA did not benefit. Maximal benefit was observed for patients with high APACHE III scores and elevated plasma IL1RA. The proportion of subjects with plasma IL1RA above the cut point 2071 pg/ml increases from 45% among the lowest APACHE III tertile, to 66% among the highest APACHE III tertile, as shown by the italicized number of subjects (n) in each cell. Whereas the interaction term [rhIL1RA*IL1RA cut point] remains statistically associated with adjusted mortality with a p-value = 0.047, the interaction term [rhIL1RA*APACHE III tertile] was not significantly associated with adjusted mortality (p=0.469). IL1RA: Interleukin-1 receptor antagonist. ARD: adjusted risk difference
| APACHE III Mortality Risk < 24% | APACHE III Mortality Risk 24 – 41% | APACHE III Mortality Risk > 41% | ||||
|---|---|---|---|---|---|---|
| Low plasma IL1RA | Placebo 3 /36 (8%) | rhIL1RA 10/65 (15%) | Placebo 6/24 (25%) | rhIL1RA 13/57 (23%) | Placebo 5/16 (31%) | rhIL1RA 22/45 (49%) |
| ARD 0.08 (−0.05, +0.20) | ARD −0.03 (−0.24, +0.17) | ARD 0.18 (−0.08, + 0.44) | ||||
| High plasma IL1RA | Placebo 7/25 (28%) | rhIL1RA 12/55 (22%) | Placebo 7/27 (26%) | rhIL1RA 15/59 (24%) | Placebo 26/36 (72%) | rhIL1RA 41/84 (49%) |
| ARD −0.05 (−0.24, +0.14) | ARD −0.02 (−0.22, +0.18) | −0.24 (−0.41, −0.06) | ||||
Figure 2.
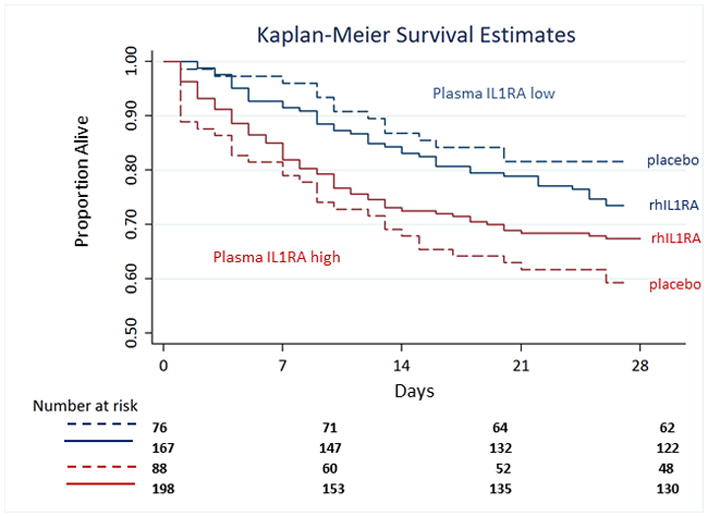
Kaplan Meier 28-day survival estimates for subjects stratified by rhIL1RA treatment status and baseline plasma IL1RA concentrations. Subjects with baseline plasma IL1RA below 2071 pg/ml are shown in blue, and those with IL1RA above 2071 pg/ml are shown in red. Placebo treatment is indicated by dashed lines and rhIL1RA treatment by solid lines. The interaction term IL1RA cutpoint*rhIL1RA remains significantly associated with survival in Cox regression, p=0.044.
Analyses by IL1β level yielded no statistically significant interaction between plasma IL1β level and rhIL1RA treatment by IL1β decile (p=0.24), Youden-determined IL1β cut point for mortality (p=0.26), or by the median IL1β concentration (p=0.49). Results were similar whether patients with undetectable plasma IL1β were included or excluded from the analyses (Table E6, Supplement). Similarly, neither IL1RA/IL1β ratio nor IL1RA*IL1β product displayed significant interaction with rhIL1RA treatment (Table E6, Supplement). Because rhIL1RA was demonstrated to reduce mortality in subjects with macrophage activation syndrome (MAS) subtype as defined by simultaneous coagulopathy and hepatobiliary dysfunction (33), we conducted a sensitivity analysis excluding subjects with MAS from our analysis and the statistical interaction between rhIL1RA and IL1RA level remained significant (p=0.048).
DISCUSSION
We have demonstrated significant heterogeneity in the effect of rhIL1RA on sepsis mortality according to baseline plasma IL1RA concentration in a retrospective subgroup analysis of one randomized clinical trial. Subjects with low plasma IL1RA did not seem to benefit from rhIL1RA and may have incurred increased mortality, whereas subjects with higher baseline plasma IL1RA had approximately 12% mortality reduction when treated with rhIL1RA. Early plasma IL1RA level may act as an enrichment factor to select septic patients who may benefit from rhIL1RA therapy (4).
It has long been recognized that a hyperimmune response to infection occurs in some patients with sepsis (34, 35) and that persistently elevated plasma levels of inflammatory cytokines strongly associate with death (36). The plasma concentration of IL1β may be in the causal pathway towards septic shock and mortality (14, 17). However, attempts to dramatically improve sepsis survival by blocking IL1β, although effective in animal models (37, 38), repeatedly failed in human trials (19–21). We hypothesized that subjects with an activated interleukin-1 axis would exhibit the largest beneficial treatment effect from rhIL1RA, yet were uncertain whether plasma IL1β or IL1RA would be the optimal marker (17, 18). Although we did not detect a statistically significant interaction between rhIL1RA treatment and plasma IL1β, it is possible that, with a larger study, a more sensitive assay, and improved power, plasma IL1β would also function as an enrichment factor (39) (Table E5). An important but unanswered question is whether it may be harmful to suppress IL1β signaling in septic patients whose IL-1 axis is not activated. The interaction detected here suggests that rhIL1RA treatment had either no mortality effect or that the treatment worsened outcomes in the subgroup with low plasma IL1RA. The ambulatory use of rhIL1RA for non-septic conditions is usually well tolerated, although severe infection and liver toxicity are rare but described complications (40). Although the drug appeared safe in 3 trials (19, 21, 23), rhIL1RA treatment may contribute to sepsis-induced immune suppression (41, 42). A hyperimmune response may occur concurrently with suppressed adaptive immunity during sepsis (43, 44), and a hyperinflammatory state may contribute to subsequent T cell exhaustion or immunoparalysis. Overexpression of IL-1 pathway genes in whole blood during sepsis was associated with a higher likelihood of secondary delayed infection (45), potentially linking IL-1 dysfunction and hypoimmunity. Future attempts to replicate a benefit of rhIL1RA should interrogate markers of adaptive immune exhaustion at baseline and with treatment (46).
During sepsis, plasma IL1RA exists in quantities often in excess of 1000-fold higher than plasma IL1β (47), and it is counterintuitive that subjects with high plasma IL1RA were those who benefitted from exogenous rhIL1RA (48). Our findings are somewhat contrary to our prior report that subjects with a genetic variant showing more efficient IL1RN expression had a lower sepsis mortality (18). We hypothesize that plasma IL1RA and IL1β are more strongly correlated as sepsis persists (18, 47), and since IL1β induces gene expression of both itself and the gene encoding IL1RA (15, 49), early abundant IL1RA expression may dampen IL1β, stopping the cycle of IL1β-IL1RA amplification. We posit that a high circulating plasma IL1RA concentration is an indicator that IL1B is transcriptionally active. Conversely, when septic patients do not mount a plasma IL1RA response, this may indicate that IL-1 pathway activation is not a major contributor to the patient’s condition. However, prospective clinical trials with carefully timed plasma collection are necessary to answer this question directly.
The significant interaction we detected between rhIL1RA treatment and baseline plasma IL1RA concentration was present across multiple levels of predicted mortality (26). Because patients with higher severity of illness have a higher mortality event rate, this group will be better powered to detect a treatment effect. The APACHE III score thus functions as a prognostic biomarker for rhIL1RA response (4, 23). The value of high plasma IL1RA as a biomarker may likewise select a more sick population, for prognostic enrichment, or it may be that high plasma IL1RA provides information about a patient’s likely response to rhIL1RA as a predictive biomarker (4). Biomarker-enriched clinical trials can benefit from both prognostic and predictive enrichment (4), and as evident in Table 3, it may be that the optimal design for a future trial of rhIL1RA in sepsis would require both a high APACHE III and an elevated plasma IL1RA for eligibility.
Our study had important limitations. We were limited in the number of subjects available with plasma, the plasma quantity available, and the clinical information stored in the permanently de-identified database. We evaluated slightly less than 60% of the original trial population, which risks selection bias; the behavior of plasma IL1RA in subjects without stored plasma is unknown. Although neither Phase III trial of rhIL1RA in sepsis detected a significant beneficial effect (20, 21), the subpopulation analyzed here was drawn from the 1994 trial which had more signal for benefit, and it is thus possible that subgroup analyses would favor benefit in this trial but not the 1997 trial. Unfortunately, samples from the 1997 trial (21) do not exist. We assayed plasma proteins on plasma stored for over 20 years. There is precedent for reporting plasma IL1RA and IL1β on samples stored > 15 years (50) and these proteins are stable through multiple freeze/thaw cycles (51), however many questions remain regarding the kinetics of these proteins during sepsis. However, these limitations apply equally to all samples and any degradation would be expected to bias our findings toward the null hypothesis.
We derived a plasma IL1RA threshold with optimal operating characteristics (≥ 2071 pg/ml), however this threshold may not have inherent value because a cut point is always best fit to its discovery population. Furthermore, although the observed coefficient of variation for these assays was not excessive (7%), our lack of duplicates for a majority of samples may decrease the precision of the threshold. In addition, temporal changes in sepsis and supportive care, including ventilation, are likely to have impacted the observed range of plasma IL1β and IL1RA (52). The values observed here were between 3- and 10-fold higher than those reported in a 2008 sepsis trial (17, 18). For these multiple reasons, we advocate an independent trial to validate thresholds of IL1RA in a modern sample as a necessary precursor to a new precision trial.
We tested 2 primary and 2 exploratory biomarkers (IL1RA, IL1β, IL1RA/IL1β, and IL1RA*IL1β) and did not adjust for multiple comparisons, because having demonstrated moderate to strong correlation between IL1RA and IL1β during sepsis (17, 18, 53), we did not consider these tests independent. With an alpha level of 0.05 and 4 tests, no marker would be expected to show significant interaction by chance, however our interaction testing would not be robust to a Bonferroni adjustment (27). In our secondary analysis, we report significance testing for subgroups (Table 2) without multiple comparison adjustment. Subgroup analyses have reduced statistical power, increased variance, and a high rate of both false positive and false negative statistical significance testing (54). Our results, similar to other subgroup reports, are not a basis for clinical decisions but may be grounds for justifying a prospective clinical trial (27, 54). Finally, we acknowledge that the ELISA kits used, although optimized for human plasma in the research setting, have not been tested to the standard for clinical laboratory testing, and are not readily available as rapid point-of-care testing. Prior to advancing to a precision trial reassessing the utility of rhIL1RA in biomarker-defined subgroups with sepsis, these key concerns should be answered.
CONCLUSIONS
We report statistically significant heterogeneity in the mortality treatment effect of rhIL1RA during sepsis that may be predictable by plasma IL1RA concentration. Our findings prompt the reconsideration of a precision clinical trial of rhIL1RA targeted to high-acuity septic patients with high plasma IL1RA.
Supplementary Material
Acknowledgments
Funding: This study was funded by NHLBI R56HL122474 (NJM), the American Thoracic Society Foundation (NJM), and the University of Pennsylvania Center for Pharmacoepidemiology Research and Training (NJM). Additional support for personnel was provided by NIH HL125723 (JPR), DK097307 (MGS), and HL115354 (JDC).
Footnotes
Author contributions: Dr. Meyer had access to all data and takes responsibility for the integrity of the data and the accuracy of the data analysis. NJM, JPR, JDC, and SMO conceived of and designed the study. NJM obtained funding. NJM, TGD, and SMO acquired data. NJM, JPR, BJA, TKJ, JAP, MGS, and RF analyzed and interpreted the data. NJM drafted the manuscript. All authors critically reviewed and edited the manuscript and approve its submission.
Conflict of Interest / Financial disclosures:
The authors declare that they have no conflict of interest. Dr. Meyer and Dr. Christie receive research funding from GlaxoSmithKline unrelated to this work. Dr. Meyer and Dr. Opal have received consultant fees as members of an advisory board to SOBI, Inc. SOBI, Inc. had no role in the design, analysis, or reporting of this manuscript.
Copyright form disclosure: Drs. Meyer, Reilly, Anderson, and Shashaty received support for article research from the National Institutes of Health (NIH). Dr. Meyer’s institution received funding from National Heart, Lung, and Blood Institute, the American Thoracic Society, the University of Pennsylvania Center for Pharmacoepidemiology Research and Training, and GlaxoSmithKline; and she received funding from SOBI, Inc. Dr. Reilly’s institution received funding from the NIH. Dr. Anderson’s institution received funding from the NIH, and he received funding from National Institutes of Health Loan Repayment Program. Dr. Shashaty’s institution received funding from NIH (NIDDK) K23 career development award. Dr. Christie’s institution received funding from GSK and BMS, and he disclosed that he has several NIH grants (not related to current work). The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med. 2016;193(3):259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour C, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Opal SM, Dellinger RP, Vincent JL, et al. The next generation of sepsis clinical trial designs: what is next after the demise of recombinant human activated protein C?*. Crit Care Med. 2014;42(7):1714–1721. doi: 10.1097/CCM.0000000000000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freidlin B, Korn EL. Biomarker enrichment strategies: matching trial design to biomarker credentials. Nat Rev Clin Oncol. 2014;11(2):81–90. doi: 10.1038/nrclinonc.2013.218. [DOI] [PubMed] [Google Scholar]
- 5.Mehta C, Schafer H, Daniel H, et al. Biomarker driven population enrichment for adaptive oncology trials with time to event endpoints. Stat Med. 2014;33(26):4515–4531. doi: 10.1002/sim.6272. [DOI] [PubMed] [Google Scholar]
- 6.Flaherty KT, Robert C, Hersey P, et al. Improved Survival with MEK Inhibition in BRAF-Mutated Melanoma. New England Journal of Medicine. 2012;367(2):107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 7.Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. The Lancet Oncology. 2011;12(11):1004–1012. doi: 10.1016/S1470-2045(11)70232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kellum JA, Pike F, Yealy DM, et al. Relationship Between Alternative Resuscitation Strategies, Host Response and Injury Biomarkers, and Outcome in Septic Shock: Analysis of the Protocol-Based Care for Early Septic Shock Study. Critical Care Medicine. 2017;45(3):438–445. doi: 10.1097/CCM.0000000000002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granowitz EV, Porat R, Mier JW, et al. Pharmacokinetics, safety and immunomodulatory effects of human recombinant interleukin-1 receptor antagonist in healthy humans. Cytokine. 1992;4(5):353–360. doi: 10.1016/1043-4666(92)90078-6. [DOI] [PubMed] [Google Scholar]
- 10.Granowitz EV, Clark BD, Mancilla J, et al. Interleukin-1 receptor antagonist competitively inhibits the binding of interleukin-1 to the type II interleukin-1 receptor. J Biol Chem. 1991;266(22):14147–14150. [PubMed] [Google Scholar]
- 11.Herold S, Tabar TS, Janssen H, et al. Exudate Macrophages Attenuate Lung Injury by the Release of IL-1 Receptor Antagonist in Gram-negative Pneumonia. Am J Respir Crit Care Med. 2011;183(10):1380–1390. doi: 10.1164/rccm.201009-1431OC. [DOI] [PubMed] [Google Scholar]
- 12.Ganter MT, Roux J, Miyazawa B, et al. Interleukin-1beta causes acute lung injury via alphavbeta5 and alphavbeta6 integrin-dependent mechanisms. Circ Res. 2008;102(7):804–812. doi: 10.1161/CIRCRESAHA.107.161067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinarello C. Biologic basis for interleukin-1 in disease. Blood. 1996;87(6):2095–2147. [PubMed] [Google Scholar]
- 14.Okusawa S, Gelfand JA, Ikejima T, et al. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 1988;81(4):1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinarello CA, Ikejima T, Warner SJ, et al. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987;139(6):1902–1910. [PubMed] [Google Scholar]
- 16.Leff JA, Bodman ME, Cho OJ, et al. Post-insult treatment with interleukin-1 receptor antagonist decreases oxidative lung injury in rats given intratracheal interleukin-1. American Journal of Respiratory and Critical Care Medicine. 1994;150(1):109–112. doi: 10.1164/ajrccm.150.1.8025734. [DOI] [PubMed] [Google Scholar]
- 17.Wang F, Meyer NJ, Walley KR, et al. Causal genetic inference using haplotypes as instrumental variables. Genet Epidemiol. 2015;40(1):35–44. doi: 10.1002/gepi.21940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer NJ, Ferguson JF, Feng R, et al. A functional synonymous coding variant in the IL1RN gene associates with survival in septic shock. Am J Respir Crit Care Med. 2014;190(6):656–664. doi: 10.1164/rccm.201403-0586OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher CJ, Jr, Slotman GJ, Opal SM, et al. Initial evaluation of human recombinant interleukin-1 receptor antagonist in the treatment of sepsis syndrome: a randomized, open-label, placebo-controlled multicenter trial. Crit Care Med. 1994;22(1):12–21. doi: 10.1097/00003246-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Fisher CJ, Jr, Dhainaut JF, Opal SM, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA. 1994;271(23):1836–1843. [PubMed] [Google Scholar]
- 21.Opal SM, Fisher CJ, Dhainaut JF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: A phase III, randomized, doubleblind, placebo-controlled, multicenter trial. Critical Care Medicine. 1997;25(7):1115–1124. doi: 10.1097/00003246-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Bone RC, Balk RA, Cerra FB, et al. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–874. [PubMed] [Google Scholar]
- 23.Rubenfeld GD, Angus DC, Pinsky MR, et al. Outcomes Research in Critical Care. American Journal of Respiratory and Critical Care Medicine. 1999;160(1):358–367. doi: 10.1164/ajrccm.160.1.9807118. [DOI] [PubMed] [Google Scholar]
- 24.Knaus WA, Harrell FE, Fisher CJ, Jr, et al. The clinical evaluation of new drugs for sepsis. A prospective study design based on survival analysis. JAMA. 1993;270(10):1233–1241. [PubMed] [Google Scholar]
- 25.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 26.Kent DM, Rothwell PM, Ioannidis JP, et al. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials. 2010;11(1):1–11. doi: 10.1186/1745-6215-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang R, Lagakos SW, Ware JH, et al. Statistics in medicine--reporting of subgroup analyses in clinical trials. N Engl J Med. 2007:357. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 28.Brookes ST, Whitely E, Egger M, et al. Subgroup analyses in randomized trials: risks of subgroup-specific analyses; power and sample size for the interaction test. J Clin Epidemiol. 2004:57. doi: 10.1016/j.jclinepi.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biometrical journal Biometrische Zeitschrift. 2005;47(4):458–472. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 30.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 32.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55(2):652–659. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 33.Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial*. Critical Care Medicine. 2016;44(2):275–281. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaukonen KM, Bailey M, Pilcher D, et al. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372(17):1629–1638. doi: 10.1056/NEJMoa1415236. [DOI] [PubMed] [Google Scholar]
- 35.Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: Results of the genetic and inflammatory markers of sepsis (genims) study. Archives of Internal Medicine. 2007;167(15):1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119(8):771–778. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- 37.Wakabayashi G, Gelfand JA, Burke JF, et al. A specific receptor antagonist for interleukin 1 prevents Escherichia coli-induced shock in rabbits. The FASEB Journal. 1991;5(3):338–343. doi: 10.1096/fasebj.5.3.1825816. [DOI] [PubMed] [Google Scholar]
- 38.Ohlsson K, Bjork P, Bergenfeldt M, et al. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature. 1990;348(6301):550–552. doi: 10.1038/348550a0. [DOI] [PubMed] [Google Scholar]
- 39.Marshall SW. Power for tests of interaction: effect of raising the Type I error rate. Epidemiologic Perspectives & Innovations. 2007;4(1):4. doi: 10.1186/1742-5573-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossi-Semerano L, Fautrel B, Wendling D, et al. Tolerance and efficacy of off-label anti-interleukin-1 treatments in France: a nationwide survey. Orphanet Journal of Rare Diseases. 2015;10(1):19. doi: 10.1186/s13023-015-0228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306(23):2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monneret G, Lepape A, Voirin N, et al. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006;32(8):1175–1183. doi: 10.1007/s00134-006-0204-8. [DOI] [PubMed] [Google Scholar]
- 43.Pachot A, Monneret G, Voirin N, et al. Longitudinal study of cytokine and immune transcription factor mRNA expression in septic shock. Clinical Immunology. 2005;114(1):61–69. doi: 10.1016/j.clim.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 44.Tschaikowsky K, Hedwig-Geissing M, Schiele A, et al. Coincidence of pro- and anti-inflammatory responses in the early phase of severe sepsis: Longitudinal study of mononuclear histocompatibility leukocyte antigen-DR expression, procalcitonin, C-reactive protein, and changes in T-cell subsets in septic and postoperative patients. Crit Care Med. 2002;30(5):1015–1023. doi: 10.1097/00003246-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 45.van Vught LA, Klein Klouwenberg PC, Spitoni C, et al. Incidence, risk factors, and attributable mortality of secondary infections in the intensive care unit after admission for sepsis. JAMA. 2016 doi: 10.1001/jama.2016.2691. [DOI] [PubMed] [Google Scholar]
- 46.Chang K, Svabek C, Vazquez-Guillamet C, et al. Targeting the programmed cell death 1: programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis. Critical Care. 2014;18(1):R3. doi: 10.1186/cc13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldie AS, Fearon KC, Ross JA, et al. Natural cytokine antagonists and endogenous antiendotoxin core antibodies in sepsis syndrome. The Sepsis Intervention Group. JAMA. 1995;274(2):172–177. [PubMed] [Google Scholar]
- 48.Dinarello CA, Cannon JG. Cytokine Measurements in Septic Shock. Annals of Internal Medicine. 1993;119(8):853–854. doi: 10.7326/0003-4819-119-8-199310150-00013. [DOI] [PubMed] [Google Scholar]
- 49.Granowitz EV, Clark BD, Vannier E, et al. Effect of interleukin-1 (IL-1) blockade on cytokine synthesis: I. IL-1 receptor antagonist inhibits IL-1-induced cytokine synthesis and blocks the binding of IL-1 to its type II receptor on human monocytes. Blood. 1992;79(9):2356–2363. [PubMed] [Google Scholar]
- 50.Crasto CL, Semba RD, Sun K, et al. Endogenous secretory receptor for advanced glycation end products is associated with low serum interleukin-1 receptor antagonist and elevated IL-6 in older community-dwelling adults. The journals of gerontology Series A, Biological sciences and medical sciences. 2011;66(4):437–443. doi: 10.1093/gerona/glq225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thavasu PW, Longhurst S, Joel SP, et al. Measuring cytokine levels in blood. Journal of Immunological Methods. 1992;153(1):115–124. doi: 10.1016/0022-1759(92)90313-i. [DOI] [PubMed] [Google Scholar]
- 52.Ranieri VM, Suter PM, Tortorella C, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282(1):54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 53.Meyer NJ, Feng R, Li M, et al. IL1RN Coding Variant Is Associated with Lower Risk of Acute Respiratory Distress Syndrome and Increased Plasma IL-1 Receptor Antagonist. Am J Respir Crit Care Med. 2013;187(9):950–959. doi: 10.1164/rccm.201208-1501OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rothwell PM. Treating individuals 2. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005:365. doi: 10.1016/S0140-6736(05)17709-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


