Abstract
Context
Recent analyses of Medicare data show decreases over time in intensity of end-of-life care. Few studies exist regarding trends in intensity of end-of-life care for those under 65.
Objectives
To examine recent temporal trends in place of death, and both hospital and ICU utilization, for age-stratified decedents with chronic, life-limiting diagnoses (<65 versus ≥65 years) who received care in a large healthcare system.
Methods
Retrospective cohort using death certificates and electronic health records for 22,068 patients with chronic illnesses who died between 2010 and 2015. We examined utilization overall and stratified by age using multiple regression.
Results
The proportion of deaths at home did not change, but hospital admissions in the last 30 days of life decreased significantly from 2010 to 2015 (hospital b=-0.026; CI=-0.041,-0.012). ICU admissions in the last 30 days also declined over time for the full sample and for patients 65 or older (overall b=-0.023, CI=-0.039,-0.007) but was not significant for younger decedents. Length of stay did not decrease for those using the hospital or ICU.
Conclusion
From 2010 to 2015, we observed a decrease in hospital admissions for all age groups and in ICU admissions for those over 65. As there were no changes in the proportion of patients with chronic illness who died at home nor in hospital or ICU length-of-stay in the last 30 days, hospital and ICU admissions in the last 30 days may be a more responsive quality metric than site of death or length of stay for palliative care interventions.
Background
Health care expenditures in the US exceeded $3.2 trillion in 20151 and are expected to rise further due to factors such as the aging population and advances in healthcare technology.2 A disproportionate amount of spending occurs at the end of life for Medicare beneficiaries, with 80% of people who die each year in the US using 30% percent of Medicare expenditures.2,3 Intensity of care at the end of life, including the number and length of hospitalizations and use of the ICU in the last 30 days of life, account for 78% of costs in the final year of life.2,4 Thus, end-of-life care is an important focus for providers, researchers, and policymakers interested in reducing costs.
Recent Medicare analyses report a 4% decline in costs per decedent in the last six months of life (2010: $36,392 vs. 2014: $34,837 per person).5 Similarly, hospital admissions in the last 30 days decreased by 35 admissions per 1,000 decedents (2010: 629 vs. 2014: 594 admissions per 1,000 decedents).5 Although these trends have been demonstrated for Medicare beneficiaries, few data address utilization at the end of life for decedents younger than 65. Studies comparing Medicare vs. non-Medicare costs and utilization have focused on geographical rather than temporal trends, and report almost no correlation between Medicare vs. commercial spending by region.6-8
In this study, we used death certificate and electronic health record (EHR) data to examine temporal trends in location of death and hospital and ICU utilization (2010-2015) for decedents with chronic illness receiving care in a single healthcare system. We compared patients age <65 vs. ≥65 across the full range of payers. We hypothesized that there would be decreasing trends in intensity of end-of-life care for both age groups.
Methods
Setting and Study Population
This analysis used data from Washington State Death Certificates that includes all deaths in Washington State from 2010-2015 and from the UW Medicine data warehouse that includes clinical and administrative information from a university medical center, a county safety-net hospital, a community hospital, a large clinic network, and an outpatient cancer center. Annual patient volume exceeded 64,000 hospital admissions and 1.6 million outpatient and emergency department visits.9,10
Decedents included in the study were 18 years or older at the time of death with at least one of the nine chronic conditions used by the Dartmouth Atlas to study end-of-life care in the US: malignant cancer/leukemia, chronic pulmonary disease, coronary artery disease (CAD), congestive heart failure (CHF), chronic liver disease, chronic renal disease, dementia, diabetes with end-organ damage, and peripheral vascular disease (PVD).5 Affiliation with UW Medicine was defined as at least one non-surgical inpatient visit at a UW Medicine hospital in the 24 months prior to death; or two outpatient visits at the same site in the last 32 months of life, with at least one visit in the last 24 months of life. This method excluded patients referred for elective surgery or to obtain a second opinion.
The University of Washington Institutional Review Board determined that this project did not involve human subjects because all patients were deceased. A waiver of HIPAA consent was obtained as required by Washington State law.
Outcomes
Site of death
Place of death (hospital, home or other location [e.g., nursing home, inpatient hospice facility]) was determined by death certificate or the EHR if omitted from the death certificate.
Utilization
The following events, occurring in the last 30 days of life at one of the two largest hospitals, were assessed from the EHR: 1) hospitalizations: any admission and length of stay (LOS); and 2) ICU stays: any admission and LOS.
Confounders
Age at death, gender, race/ethnicity (white non-Hispanic vs. minority), education, specific Dartmouth Atlas conditions, and the number of outpatient visits in the year prior to the last month of life (as a marker of contact with the healthcare system) were considered as possible confounders. Age, gender, type of diagnoses and number of outpatient visits were obtained from the EHR. Race/ethnicity and level of education were obtained from death certificates.
Statistical Analysis
Descriptive statistics were used to characterize the sample (overall and by age group). Tests for trends over time were run for the full sample, and then for samples stratified by age. All associations were tested with regression models: probit regression estimated with weighted mean- and variance-adjusted least squares for binary outcomes (any hospital care, any ICU care); multinomial regression estimated with restricted maximum likelihood for the unordered categorical outcome (place of death); and negative binomial regression estimated with restricted maximum likelihood for count outcomes (inpatient and ICU LOS in days). Year of death was modeled as an ordinal predictor. All models were tested for confounding: we included variables that changed the year-of-death coefficient by at least 10% when added to the bivariate model. Analyses were conducted with Mplus (https://www.statmodel.com/) with p-value ≤0.01 connoting statistical significance and taking into account multiple comparisons.
Results
Patient Demographics and Site of Death
Our sample included 22,068 individuals who died in Washington State from 2010-2015 and met our eligibility criteria. The mean age of the patients was 65.8 years, and 46% were younger than age 65. Forty-three percent were female, and 18% were non-white or Hispanic (Table 1). The most common chronic illness was cancer (53%), followed by chronic pulmonary disease (26%) and CAD (25%). Overall, 25% received care in the hospital during the last 30 days of life and 18% had an ICU stay. Of the 20,479 patients with known place of death, 41% died in a hospital, 36% died at home, and 23% died in other locations. More patients under age 65 died in the hospital than older patients (46% vs. 38%; p<0.001; Table 1). Compared with deaths in the hospital, deaths at home and in other locations did not vary significantly over time for the total sample or either age group (Table 2).
Table 1. Characteristics of Decedentsa.
| Characteristic | Total Sample | Under Age 65 | Age 65 and Older | |||
|---|---|---|---|---|---|---|
| Valid n | Statisticb | Valid n | Statisticb | Valid n | Statisticb | |
| Female | 22,068 | 9,455 (42.8) | 10,178 | 4,292 (42.2) | 11,890 | 5,163 (43.4) |
| Racial/Ethnic Minority | 19,834 | 3,516 (17.7) | 8,812 | 1,835 (20.8) | 11,022 | 1,681 (15.3) |
| Diagnoses | 22,068 | 10,178 | 11,890 | |||
| Cancer | 11,786 (53.4) | 6,181 (60.7) | 5,605 (47.1) | |||
| COPD | 5,808 (26.3) | 2,386 (23.4) | 3,422 (28.8) | |||
| CAD | 5,458 (24.7) | 1,541 (15.1) | 3,917 (32.9) | |||
| CHF | 4,860 (22.0) | 1,666 (16.4) | 3,194 (26.9) | |||
| PVD | 2,776 (12.6) | 758 (7.4) | 2,018 (17.0) | |||
| Liver disease | 2,670 (12.1) | 1,921 (18.9) | 749 (6.3) | |||
| Diabetes | 1,932 (8.8) | 891 (8.8) | 1,041 (8.8) | |||
| Renal disease | 4,234 (19.2) | 1,675 (16.5) | 2,559 (21.5) | |||
| Dementia | 1,984 (9.0) | 307 (3.0) | 1,677 (14.1) | |||
| Age at Death | 22,068 | 10,178 | 11,890 | |||
| Range | 18-104 | 18-64 | 65-104 | |||
| Mean (SD) | 65.8 (14.8) | 53.1 (9.7) | 76.7 (8.5) | |||
| Median (IQR) | 66 (19) | 56 (11) | 75 (14) | |||
| Year of Death | 22,068 | 10,178 | 11,890 | |||
| 2010 | 2,846 (12.9) | 1,431 (14.1) | 1,415 (11.9) | |||
| 2011 | 3,415 (15.5) | 1,646 (16.2) | 1,769 (14.9) | |||
| 2012 | 3,666 (16.6) | 1,743 (17.1) | 1,923 (16.2) | |||
| 2013 | 3,947 (17.9) | 1,790 (17.6) | 2,157 (18.1) | |||
| 2014 | 3,982 (18.0) | 1,790 (17.6) | 2,192 (18.4) | |||
| 2015 | 4,212 (19.1) | 1,778 (17.5) | 2,434 (20.5) | |||
| Outpatient Visits, Year Prior to Last Month of Life | 22,068 | 10,178 | 11,890 | |||
| Range | 0-158 | 0-158 | 0-122 | |||
| Mean (SD) | 8.5 (12.8) | 10.4 (14.6) | 7.0 (10.7) | |||
| Median (IQR) | 3 (12) | 4 (14) | 2 (9) | |||
| Place of Death | 20,479 | 9,194 | 11,285 | |||
| Hospital | 8,461 (41.3) | 4,229 (46.0) | 4,232 (37.5) | |||
| Home | 7,279 (35.5) | 3,379 (36.8) | 3,900 (34.6) | |||
| Other Locationc | 4,739 (23.1) | 1,586 (17.3) | 3,153 (27.9) | |||
| Intensity of End-of-Life Care | ||||||
| Inpatient Care, Last 30 Days of Life | ||||||
| Any Care | 22,068 | 5,590 (25.3) | 10,178 | 2,866 (28.2) | 11,890 | 2,724 (22.9) |
| Days of Care, if hospitalized | 5,590 | 2,866 | 2,724 | |||
| Range | 2-30 | 2-30 | 2-30 | |||
| Mean (SD) | 10.8 (7.0) | 11.2 (7.3) | 10.2 (6.6) | |||
| Median (IQR) | 9 (10) | 9 (10) | 8 (9) | |||
| ICU Care, Last 30 Days of Life | ||||||
| Any Care | 22,068 | 3,945 (17.9) | 10,178 | 2,119 (20.8) | 11,890 | 1,826 (15.4) |
| Days of Care, if any ICU care | 3,945 | 2,119 | 1,826 | |||
| Range | 2-30 | 2-30 | 2-30 | |||
| Mean (SD) | 8.8 (6.7) | 9.3 (7.1) | 8.2 (6.3) | |||
| Median (IQR) | 6 (8) | 7 (9) | 6 (6) | |||
Table is based on 22,068 persons who died in 2010-2015
Unless otherwise noted, the statistics presented are n (% of valid cases).
Other places of death included nursing homes (n=3,121), inpatient hospice facilities (n=1,251), other unspecified types of medical facility (n=18), and other unspecified places (n=349).
Table 2. Linear Effects of Year of Death on End-of-Life Outcomes, by Age at Deatha.
| Total Sample | Age 18-64 at Death | Age 65+ at Death | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Valid n | b | p | 99% CI | Valid n | b | p | 99% CI | Valid n | b | p | 99% CI |
| Place of Deathb | 20,749 | 9,194 | 11,285 | |||||||||
| Hospital (reference category) | ||||||||||||
| Home | 0.008 | 0.450 | -0.018, 0.033 | -0.013 | 0.345 | -0.048, 0.022 | 0.025 | 0.073 | -0.011, 0.061 | |||
| Other Location | -0.018 | 0.111 | -0.047, 0.011 | -0.037 | 0.038 | -0.083, 0.009 | 0.000 | 0.974 | -0.038, 0.037 | |||
| Intensity of End-of-Life Care | ||||||||||||
| Inpatient Care, Last 30 Days | ||||||||||||
| Any Carec | 22,068 | -0.026 | <0.001 | -0.041, -0.012 | 10,178 | -0.023 | 0.004 | -0.044, -0.003 | 11,890 | -0.030 | <0.001 | -0.050, -0.010 |
| If Any Care, Days of Cared | 5,590 | 0.012 | 0.016 | -0.001, 0.026 | 2,866 | 0.013 | 0.076 | -0.006, 0.031 | 2,724 | 0.011 | 0.137 | -0.008, 0.030 |
| ICU Care, Last 30 Days | ||||||||||||
| Any Caree | 22,068 | -0.023 | <0.001 | -0.039, -0.007 | 10,178 | -0.020 | 0.019 | -0.043, 0.002 | 11,890 | -0.026 | 0.003 | -0.048, -0.003 |
| If Any Care, Days of Caref | 3,143 | -0.015 | 0.064 | -0.035, 0.006 | 2,119 | -0.005 | 0.635 | -0.030, 0.021 | 1,826 | -0.016 | 0.142 | -0.043, 0.005 |
All associations were tested with regression models: probit regression estimated with weighted mean- and variance-adjusted least squares (WLSMV) for binary outcomes, multinomial regression estimated with restricted maximum likelihood for place of death, and negative binomial regression estimated with restricted maximum likelihood for count outcomes (days of inpatient and ICU care). Year of death was modeled as an ordinal predictor ranging from 0 to 5.
The models for the total sample and age 65+ were adjusted for age at death, the specific Dartmouth Atlas conditions with which the decedent had been diagnosed, and the number of outpatient visits in the year prior to the last month of life; the model for age 18-64 was adjusted only for age at death.
The model for the total sample was adjusted for age at death and the specific Dartmouth Atlas conditions with which the decedent had been diagnosed; the models for age 18-64 and age 65+ were adjusted for Dartmouth Atlas conditions.
The models for the total sample and age 18-64 were adjusted for the specific Dartmouth Atlas conditions with which the decedent had been diagnosed; the model for age 65+ was adjusted for Dartmouth Atlas conditions and age at death.
The model for the total sample was adjusted for age at death and the specific Dartmouth Atlas conditions with which the decedent had been diagnosed; the model for age 18-64 was adjusted for Dartmouth Atlas conditions; the model for age 65+ was adjusted for Dartmouth Atlas conditions and the number of outpatient visits in the year prior to the last month of life.
The model for the total sample was adjusted for age at death, education, the specific Dartmouth Atlas conditions with which the decedent had been diagnosed, and the number of outpatient visits in the year prior to the last month of life; the high p-value for the model for ages 18-64 precluded the need to test for confounders; values for that group are for the bivariate model. For ages 65+, adjustment was made for age at death and Dartmouth Atlas conditions.
Utilization Outcomes
Hospital utilization
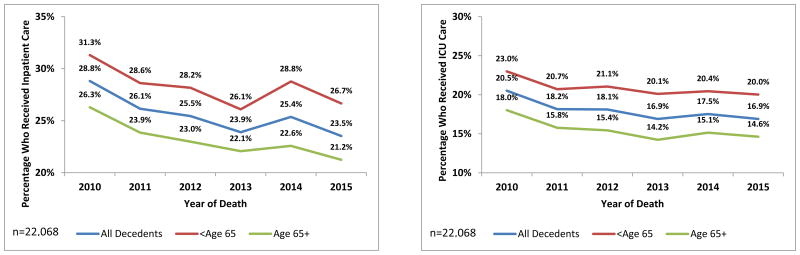
As shown in Figure 1, hospitalization in the last 30 days of life decreased over time (b=-0.026; CI=-0.041, -0.012) with similar findings for each age group (<65: b=-0.023, CI=-0.044,-0.003; ≥65: b=-0.030, CI=-0.050,-0.010). For the full sample, these findings can be interpreted as suggesting that with each succeeding year the “average patient” (based on the values for mean age and proportion with each disease) would have had an approximately 3.1% decrease in the probability of having inpatient care. For those admitted to the hospital, hospital LOS did not change significantly over time for either group (Table 2).
Figure 1. Percentage of Decedents with High-Intensity Care in the Last 30 Days of Life, by Age and Year of Death, from 2010 to 2015.
ICU utilization
There was a significant decrease over time in ICU admissions in the last 30 days of life for the overall sample and for patients older than 65 (overall: b=-0.023, CI=-0.039,-0.007; ≥65: b=-0.026, CI=-0.048,-0.003; Figure 1). There was a trend toward reduced ICU admission for decedents under age 65, but this did not achieve statistical significance (b=-0.020, CI=-0.043,0.002). For those admitted to the ICU, ICU LOS did not vary over time (Table 2).
Discussion
Despite the growth of advance care planning, palliative care, and hospice programs during the time-period of this study,11,12 we found no significant changes in the proportion of patients dying at home, in the hospital, or in another setting.10 Although studies suggest that as many as 60-80% of adults would prefer to die at home,13-15 only about one-third of patients (all with chronic life-limiting illness) died at home and over one-third died in an acute care hospital. Prior analyses from our healthcare system show that about 40% of these patients died in the hospital with no significant change between 2010 and 2015.10 In contrast to a stable pattern in site of death, we found that the proportion of chronically ill patients who were hospitalized during the last 30 days of life decreased from 2010 to 2015. This finding is similar to recent trends seen among Medicare beneficiaries,5 and our study demonstrates similar trends among patients under 65 years. Our findings suggest that hospital admission in the last 30 days may be a more responsive palliative care quality metric than site of death, since site of death may be more dependent on more difficult to control for healthcare systems such as social support.4,16-18 These consistent declines in hospital admissions in the last 30 days of life across all age groups may be due to increased penetration of advance care planning and primary and specialty palliative care programs.19-22 UW Medicine has made advance care planning and palliative care a priority since 2012, which may have contributed to our findings.23 However, it is difficult to attribute these changes over time to any specific program, provider, or group of providers in an observational study. This is an important challenge for healthcare systems interested in attributing credit or accountability for changes in the intensity of care at the end of life.
Admission to the ICU in the last 30 days of life also decreased amongst all patients in our sample and for those over 65. Others have reported a similar decrease in ICU admission among Medicare beneficiaries.5 For patients 18 to 65, we saw a trend toward reduction in ICU admission in the last 30 days of life (although not statistically significant by our definition of p<0.01). It may be that ICU use for younger patients follows a different pattern, even in the context of a chronic illness.24,25 For example, the course of illness for younger patients may be less predictable than for older patients and younger patients may favor more aggressive care, making reductions in the use of intensive care more difficult to achieve.17,26 It is important to note that although the chronic conditions that patients experienced differed between age groups, our analyses controlled for these differences and, therefore, this is unlikely to explain our findings.
Our study has several important limitations. First, we used data from a single healthcare system in a single state, and our findings may not generalize to other healthcare systems or states. However, the academic healthcare system we examined is a large, diverse system that may be similar to other academic healthcare systems. Second, we used data from the EHR that were collected for clinical and billing purposes rather than research, and our findings may be limited by misclassification associated with inaccurate or incomplete documentation. Third, we were unable to determine patient preferences for intensity of care at the end of life in this study; future studies should use such preferences to assess goal-concordance of care. Fourth, although we describe the proportion of patients that died at home, we do not know the proportion who died at home with hospice since this information is not accurately captured in our EHR or on death certificates. Finally, although our analyses are limited to decedents and may not generalize to non-decedents, our focus is on end-of-life care in the context of chronic illness and therefore this is less of a concern.27
In summary, although the proportion of deaths at home did not change for patients with chronic illness between 2010 and 2015, we found a significant decrease in the proportion of these patients admitted to the hospital and to the ICU in the last 30 days of life. Our findings can provide direction for healthcare systems striving to document changes in intensity of end-of-life care for patients with chronic illness suggesting that hospital and ICU admissions in the last 30 days of life may be more responsive quality metrics than site of death or hospital or ICU LOS for interventions such as enhanced advance care planning and palliative care. Our findings also suggest that modifying site of death and hospital or ICU LOS may require more robust interventions. Our study demonstrates a reduction in the use of the acute care hospital in the last 30 days of life among patients both under and over 65 years of age, but less prominent reduction in ICU use for the younger group, suggesting the presence of additional barriers to reducing ICU use in the last 30 days of life for patients with chronic illness who are under 65 years of age.
Acknowledgments
Funding: Funded by the Cambia Health Foundation and UW Medicine
Footnotes
Author's Contributions: All authors made substantial contributions to the design of the work or the acquisition, analysis, or interpretation of the data; AND participated in revising it critically; AND provided final approval of the version to be published; AND agree to be accountable for the work.
Conflict of interest statement: The authors have no financial conflicts of interests
References
- 1. [Accessed June 6, 2017];Center for Medicare and Medicaid Services: National Health Expenditures 2015 Highlights. 2016 at https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet.html.
- 2.Zhang B, Wright AA, Huskamp HA, et al. Health care costs in the last week of life: Associations with end-of-life conversations. Arch Intern Med. 2009;169:480–8. doi: 10.1001/archinternmed.2008.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medicare spending at the end of life: A snapshot of beneficiaries who died in 2014 and the cost of their care. [Accessed August 25, 2017];Kaiser Family Foundation, 2016. at http://www.kff.org/medicare/issue-brief/medicare-spending-at-the-end-of-life/
- 4.Aldridge MD, Bradley EH. Epidemiology And Patterns Of Care At The End Of Life: Rising Complexity, Shifts In Care Patterns And Sites Of Death. Health Aff (Millwood) 2017;36:1175–83. doi: 10.1377/hlthaff.2017.0182. [DOI] [PubMed] [Google Scholar]
- 5. [Accessed August 25, 2017];The Dartmouth Atlas of Health Care: Understanding of the Efficiency and Effectiveness of the Health Care System. 2014 at http://www.dartmouthatlas.org/
- 6.Institute of Medicine. Variation in health care spending: Target decision making, not geography. Washington, DC: The National Academies Press; 2013. [PubMed] [Google Scholar]
- 7.Newhouse JP, Garber AM. Geographic variation in health care spending in the United States: Insights from an Institute of Medicine report. JAMA. 2013;310:1227–8. doi: 10.1001/jama.2013.278139. [DOI] [PubMed] [Google Scholar]
- 8.Chernew ME, Sabik LM, Chandra A, Gibson TB, Newhouse JP. Geographic correlation between large-firm commercial spending and Medicare spending. Am J Manag Care. 2010;16:131–8. PMC3322373. [PMC free article] [PubMed] [Google Scholar]
- 9.Lavin K, Davydow DS, Downey L, et al. Effect of Psychiatric Illness on Acute Care Utilization at End of Life From Serious Medical Illness. J Pain Symptom Manage. 2017;54:176–85. doi: 10.1016/j.jpainsymman.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Hicks K, Downey L, Engelberg RA, et al. Predictors of death in the hospital for patients with chronic serious illness. J Palliat Med. 2017 doi: 10.1089/jpm.2017.0127. In press. [DOI] [PubMed] [Google Scholar]
- 11.Dumanovsky T, Augustin R, Rogers M, Lettang K, Meier DE, Morrison RS. The growth of palliative care in U.S. hospitals: A status report. J Palliat Med. 2016;19:8–15. doi: 10.1089/jpm.2015.0351. PMC4692111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tolle SW, Teno JM. Lessons from Oregon in embracing complexity in end-of-life care. N Engl J Med. 2017;376:1078–82. doi: 10.1056/NEJMsb1612511. [DOI] [PubMed] [Google Scholar]
- 13.Burge F, Lawson B, Johnston G, Asada Y, McIntyre PF, Flowerdew G. Preferred and actual location of death: What factors enable a preferred home death? J Palliat Med. 2015;18:1054–9. doi: 10.1089/jpm.2015.0177. [DOI] [PubMed] [Google Scholar]
- 14.Higginson IJ, Sen-Gupta G. Place of care in advanced cancer: A qualitative systematic literature review of patient preferences. J Palliat Med. 2000;3:287–300. doi: 10.1089/jpm.2000.3.287. [DOI] [PubMed] [Google Scholar]
- 15.Views and experiences with end-of-life medical care in the U.S. [Accessed August 25, 2017];Kaiser Family Foundation, 2017. at http://www.kff.org/other/report/views-and-experiences-with-end-of-life-medical-care-in-the-u-s/
- 16.Muni S, Engelberg RA, Treece PD, Dotolo D, Curtis JR. The influence of race/ethnicity and socioeconomic status on end-of-life care in the ICU. Chest. 2011;139:1025–33. doi: 10.1378/chest.10-3011. PMC3198381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shugarman LR, Decker SL, Bercovitz A. Demographic and social characteristics and spending at the end of life. J Pain Sympt Manage. 2009;38:15–26. doi: 10.1016/j.jpainsymman.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Teno JM, Gozalo PL, Bynum JP, et al. Change in end-of-life care for Medicare beneficiaries: Site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309:470–7. doi: 10.1001/jama.2012.207624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bischoff KE, Sudore R, Miao Y, Boscardin WJ, Smith AK. Advance care planning and the quality of end-of-life care in older adults. J Am Geriat Soc. 2013;61:209–14. doi: 10.1111/jgs.12105. PMC3760679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dumanovsky T, Augustin R, Rogers M, Lettang K, Meier DE, Morrison RS. The Growth of Palliative Care in U.S. Hospitals: A Status Report. J Palliat Med. 2016;19:8–15. doi: 10.1089/jpm.2015.0351. PMC4692111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinkman-Stoppelenburg A, Rietjens JA, van der Heide A. The effects of advance care planning on end-of-life care: a systematic review. Palliat Med. 2014;28:1000–25. doi: 10.1177/0269216314526272. [DOI] [PubMed] [Google Scholar]
- 22.Lakin JR, Koritsanszky LA, Cunningham R, et al. A Systematic Intervention To Improve Serious Illness Communication In Primary Care. Health Aff (Millwood) 2017;36:1258–64. doi: 10.1377/hlthaff.2017.0219. [DOI] [PubMed] [Google Scholar]
- 23.Cambia Palliative Care Center of Excellence at UW. [Accessed July 01, 2017];2017 at https://depts.washington.edu/pallcntr/
- 24.Voogt E, van der Heide A, Rietjens JA, et al. Attitudes of patients with incurable cancer toward medical treatment in the last phase of life. J Clin Oncol. 2005;23:2012–9. doi: 10.1200/JCO.2005.07.104. [DOI] [PubMed] [Google Scholar]
- 25.Parr JD, Zhang B, Nilsson ME, et al. The influence of age on the likelihood of receiving end-of-life care consistent with patient treatment preferences. J Palliat Med. 2010;13:719–26. doi: 10.1089/jpm.2009.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weeks JC, Cook EF, O'day SJ, et al. Relationship between cancer patients' predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709–14. doi: 10.1001/jama.279.21.1709. [DOI] [PubMed] [Google Scholar]
- 27.Bach PB, Schrag D, Begg CB. Resurrecting treatment histories of dead patients: a study design that should be laid to rest. JAMA. 2004;292:2765–70. doi: 10.1001/jama.292.22.2765. [DOI] [PubMed] [Google Scholar]