Abstract
Objective
The original Pediatric Sepsis Biomarker Risk Model (PERSEVERE) and revised (PERSEVERE-II) biomarker-based risk prediction models have demonstrated utility for estimating baseline 28-day mortality risk in pediatric sepsis. Given the paucity of prediction tools in pediatric acute respiratory distress syndrome (ARDS), and given the overlapping pathophysiology between sepsis and ARDS, we tested the utility of PERSEVERE and PERSEVERE-II for mortality prediction in a cohort of pediatric ARDS, with an a priori plan to revise the model if these existing models performed poorly.
Design
Prospective observational cohort study.
Setting
University affiliated pediatric intensive care unit.
Patients
Mechanically ventilated children with ARDS.
Interventions
Blood collection within 24 hours of ARDS onset and biomarker measurements.
Measurements and Main Results
In 152 children with ARDS, PERSEVERE performed poorly and PERSEVERE-II performed modestly (areas under receiver operating characteristic curve of 0.61 and 0.76, respectively). Therefore, we randomly selected 80% of the cohort (n = 122) to re-derive a risk prediction model for pediatric ARDS (PARDSEVERE). We used classification and regression tree methodology, considering the PERSEVERE biomarkers in addition to variables relevant to ARDS. The final model was comprised of 3 biomarkers and age, and more accurately estimated baseline mortality risk (area under receiver operating characteristic curve 0.85, p < 0.001 and p = 0.053 compared with PERSEVERE and PERSEVERE-II, respectively). The model was tested in the remaining 20% of subjects (n = 30), and demonstrated similar test characteristics.
Conclusion
A validated, biomarker-based risk stratification tool designed for pediatric sepsis was adapted for use in pediatric ARDS. The newly derived PARDSEVERE demonstrates good test characteristics internally, and requires external validation in a larger cohort. Tools such as PARDSEVERE have the potential to provide improved risk stratification and prognostic enrichment for future trials in pediatric ARDS.
Keywords: Acute respiratory distress syndrome, pediatric, biomarker, mortality, interleukin-8
INTRODUCTION
The heterogeneity of acute respiratory distress syndrome (ARDS) translates to complex decision-making for both clinicians and researchers. Prognostic enrichment, in which a study population is enriched for subjects more likely to have the primary outcome of interest, may improve future trial selection. Enrichment tools offer the potential for reliable identification of patients most likely to benefit from higher risk or aggressive interventions, as well as stratify risk in trials to assess whether efficacy of an intervention varies with the variable mortality risk of the population. For instance, the ACURASYS (ARDS et Curarisation Systematique) trial testing cisatracurium (1) and PROne Positioning in SEVere ARDS (PROSEVA) trial (2) used prognostic enrichment by limiting enrollment to patients with PaO2/FIO2 < 150 (higher risk of death), with positive results. However, oxygenation is inconsistently associated with outcome in ARDS (3), with minimal prognostic utility (4), making it an imperfect stratification tool. For children, this is compounded by the fact that neither the 1994 American-European Consensus Conference (5) nor the 2012 Berlin (4) definitions of ARDS addressed the pediatric population.
The Pediatric Sepsis Biomarker Risk Model (PERSEVERE) has demonstrated utility for estimating baseline 28-day mortality risk in septic shock (6, 7). PERSEVERE was developed using transcriptomics and quantification of circulating proteins, resulting in a classification and regression tree (CART)-based model incorporating 5 biomarkers plus age. Testing within multisystem organ failure (MSOF) phenotypes prompted a revised model (PERSEVERE-II) incorporating platelet levels (8). Given the overlapping pathophysiology of sepsis and ARDS (9, 10), and since infection accounts for most pediatric ARDS triggers (11–13), we tested the utility of PERSEVERE and PERSEVERE-II for mortality prediction in a cohort of pediatric ARDS, with an a priori plan to revise the model if these existing models performed poorly.
METHODS
Study Design and Patient Selection
This ongoing prospective cohort study was approved by the Children’s Hospital of Philadelphia’s (CHOP) Institutional Review Board, and informed consent was obtained from caregivers prior to enrollment. Clinical data were collected prospectively. Consecutive patients in the pediatric intensive care unit (PICU) were screened for Berlin-defined ARDS between July 1, 2014 and December 30, 2016. Inclusion criteria were 1) acute (≤ 7 days of known risk factor) respiratory failure requiring invasive (endotracheal) mechanical ventilation, 2) invasive arterial access, 3) age > 1 month (to avoid confounding by neonatal physiology) and < 18 years, 4) PaO2/FIO2 ≤ 300 on 2 consecutive arterial blood gases separated by ≥ 1 hour on positive end-expiratory pressure (PEEP) ≥ 5 cmH2O, and 5) bilateral infiltrates on radiograph. Exclusion criteria were 1) respiratory failure from cardiac failure (by echocardiography), 2) exacerbation of underlying chronic lung disease, 3) chronic ventilator dependence, 4) cyanotic heart disease, 5) ventilation for > 7 days before PaO2/FIO2 ≤ 300, 6) ARDS established outside of the CHOP PICU, 7) inability to obtain consent, or 8) prior enrollment.
Absent a standardized ventilator protocol, institutional practice is to initiate conventional ventilation with PEEP ≥ 5 cmH2O, and attempt to wean FIO2 to ≤ 0.60, keeping PaO2 ≥ 60 mmHg. Inability to wean FIO2 prompts PEEP escalation and subsequent efforts to wean FIO2. We exclusively utilize decelerating flow (either pressure control or pressure-regulated volume control). Persistently elevated peak pressures (≥ 35 cmH2O), hypercarbia (PaCO2 ≥ 80), or oxygenation difficulties (inability to wean FIO2 ≤ 0.60 despite increasing PEEP) prompted consideration for changing mode of ventilation, or escalating to extracorporeal support. Actual transition was left to the discretion of the attending physician. There was no standardization of ancillary therapies (inhaled nitric oxide, neuromuscular blockade, corticosteroids).
Plasma Collection and Measurements
Blood was collected within 24 hours of ARDS onset (defined as time of meeting all Berlin criteria) in citrated tubes (Becton, Dickinson and Company; Franklin Lakes, NJ), centrifuged within 30 minutes of collection (2000 g, 20 minutes, 20C) to generate platelet-poor plasma, and stored at −80C. Samples were shipped on dry ice to Cincinnati Children’s Hospital Medical Center for measurement of PERSEVERE biomarkers.
PERSEVERE Biomarkers
PERSEVERE includes C-C chemokine ligand 3 (CCL3), interleukin 8 (IL8), heat shock protein 70 kda 1B (HSPA1B), granzyme B (GMZB), and matrix metallopeptidase 8 (MMP8) (6). Biomarker concentrations were measured using a multiplex magnetic bead platform (MILLIPLEX™ MAP) designed for PERSEVERE by EMD Millipore (Billerica, MA), and a Luminex® 100/200 system (Luminex, Austin, TX), according to manufacturers’ specifications. Assay performance data have been previously published (6). Platelets measured at the time of blood collection for biomarkers were included for modeling of PERSEVERE-II (8).
Equations and Definitions
Oxygenation was measured using PaO2/FIO2 and oxygenation index (OI, [mean airway pressure [mPaw] × FIO2 × 100]/PaO2). The vasopressor score (14) was: dopamine (μg/kg/min) × 1 + dobutamine (μg/kg/min) × 1 + epinephrine (μg/kg/min) × 100 + norepinephrine (μg/kg/min) × 100 + phenylephrine (μg/kg/min) × 100 + milrinone (μg/kg/min) × 10 + vasopressin (U/kg/min) × 10,000. Severity of illness was scored using the Pediatric Risk of Mortality (PRISM) III at 12 hours. Non-pulmonary organ failures at ARDS diagnosis were identified using accepted pediatric definitions (15). The designation of “immunocompromised” required presence of an immunocompromising diagnosis (oncologic, immunologic, rheumatologic, transplant) and active immunosuppressive therapy, or congenital immunodeficiency (16). Etiology of ARDS was dichotomized to “infectious” or non-infectious.” The primary outcome was PICU mortality. We also report ventilator-free days (VFD) at 28 days, defined by subtracting total ventilator days from 28 in survivors. All patients with ventilator days ≥ 28 days and all PICU non-survivors were assigned VFD = 0.
CART and Other Statistical Analyses
Data are reported as median [interquartile range, IQR], and differences between groups compared using Wilcoxon rank-sum test. Categorical data were compared using Fisher exact test. Descriptive analyses were performed with Stata/SE 14.2 (College Station, TX).
Initially, subjects were classified according to PERSEVERE and PERSEVERE-II, and assigned a baseline probability of mortality. Performance was assessed using diagnostic test statistics, including computing area under the receiver operating characteristic (AUROC) curve. A priori, we determined that if PERSEVERE and PERSEVERE-II did not perform well in this cohort, we would re-derive an ARDS-specific model using the PERSEVERE biomarkers in addition to variables relevant to ARDS. Additional variables considered for the Pediatric ARDS Biomarker Risk Model (PARDSEVERE) included: age, platelets, infectious versus non-infectious ARDS etiology, presence or absence of an immunocompromising condition, and initial and 24-hour PaO2/FIO2 and OI. Age and platelets were modeled because they were part of PERSEVERE and PERSEVERE II, respectively; infectious ARDS etiology was modeled because PERSEVERE was developed in a septic cohort, and we reasoned this may affect model performance; immunocompromised status is a known strong baseline predictor of mortality in pediatric ARDS (13, 17–19); oxygenation at onset and 24 hours was modeled to account for accurate stratification for ARDS severity (13). We used CART methodology (Salford Predictive Modeler v8.0; Salford Systems, San Diego, CA). Terminal nodes that did not improve classification based on the class probability method were pruned. Weighting of cases and costs for misclassification were not used. The PARDSEVERE model was developed in a random 80% sampling of the ARDS cohort, and tested in the remaining 20%. Diagnostic statistics were computed separately for derivation and test cohorts.
Additionally, we tested if VFD decreased across worsening PARDSEVERE categories using non-parametric test of trend. Finally, to assess impact on duration of ventilation, Fine and Gray competing risk regression (20) was used to test association of PARDSEVERE risk categories with probability of extubation, with extubation as primary outcome and death as competing risk.
RESULTS
Description of the Cohort
Over the study period, 152 children with ARDS were included, with biomarkers measured at median 11 [IQR 6, 19] hours after ARDS onset. Subjects were intubated for a median of 4 [0, 10] hours prior to meeting ARDS criteria. Demographics are provided in Table 1. The 24 (16%) non-survivors had worse PRISM III scores, more organ failures, higher vasopressor scores, and were more likely to be immunocompromised. Of the 24 deaths, 12 had an infectious ARDS etiology (6 pneumonia, 6 non-pulmonary sepsis), and 12 had non-infectious (6 aspiration, 4 drowning, 1 smoke inhalation, 1 trauma). Twelve patients died after withdrawal for poor neurologic prognosis, 9 of MSOF, and 3 of refractory hypoxemia.
Table 1.
Demographics of the ARDS cohort
| Variables | All (n = 152) | Survivors (n = 128) | Non-survivors (n = 24) | p valuea |
|---|---|---|---|---|
| Age (years) | 4.2 [1.5, 10.4] | 4.2 [1.5, 10.6] | 5.2 [1.6, 9.4] | 0.762 |
| Female/male (%/%) | 75/77 (49/51) | 65/63 (51/49) | 10/14 (42/58) | 0.506 |
| Severity of illness | ||||
| PRISM III at 12 hours | 11 [5, 18] | 10 [4, 15] | 20 [14, 32] | < 0.001 |
| Organ failures | 2 [1, 3] | 1 [1, 2] | 3 [2, 4] | < 0.001 |
| Immunocompromised (%) | 28 (18) | 19 (15) | 9 (38) | 0.018 |
| Vasopressor score | 10 [3, 16] | 8 [0, 15] | 25 [9, 58] | < 0.001 |
| ARDS etiology (%) | ||||
| Infectious | 110 (72) | 116 (91) | 12 (50) | < 0.001 |
| Non-infectious | 42 (28) | 12 (9) | 12 (50) | |
| ARDS onset | ||||
| PaO2/FIO2 | 170 [106, 235] | 175 [119, 234] | 152 [90, 244] | 0.465 |
| OI | 10 [6.8, 18.2] | 9.6 [6.4, 16.9] | 13.4 [7.6, 24.5] | 0.134 |
| PIP (cmH2O) | 31 [27, 35] | 31 [27, 35] | 31 [28, 34] | 0.864 |
| PEEP (cmH2O) | 10 [8, 12] | 10 [8, 12] | 12 [9, 13] | 0.068 |
| mPaw (cmH2O) | 17 [15, 21] | 17 [15, 21] | 19 [17, 23] | 0.080 |
| VT (mL/kg) | 7.3 [6.4, 8.2] | 7.2 [6.3, 8] | 7.7 [7.1, 8.3] | 0.151 |
| 24 hours after onset | ||||
| PaO2/FIO2 | 240 [158, 316] | 235 [162, 312] | 270 [126, 366] | 0.701 |
| OI | 6.4 [4.3, 12.3] | 6.3 [4.3, 11.7] | 7.6 [4.7, 14.4] | 0.202 |
| PIP (cmH2O) | 27 [23, 30] | 27 [22, 30] | 27 [25, 34] | 0.065 |
| PEEP (cmH2O) | 10 [8, 12] | 10 [8, 10] | 11 [10, 13] | 0.001 |
| mPaw (cmH2O) | 16 [13, 20] | 15 [13, 19] | 19 [15, 22] | 0.004 |
| VT (mL/kg) | 7.1 [6.3, 8] | 7.1 [6.3, 7.9] | 7.1 [6.2, 8.1 | 0.826 |
| Ancillary therapies (%) | ||||
| Inhaled nitric oxide | 53 (35) | 40 (31) | 13 (54) | 0.037 |
| Neuromuscular blockade | 71 (47) | 60 (47) | 11 (46) | > 0.999 |
| Corticosteroids | 68 (45) | 57 (45) | 11 (46) | > 0.999 |
| Alternative ventilator | 45 (30) | 37 (29) | 8 (33) | 0.635 |
| ECMO | 6 (4) | 6 (5) | 0 | 0.590 |
P values are results of Wilcoxon rank-sum. Bold lettering reflects significant differences (p < 0.05).
Utility of PERSEVERE and PERSEVERE-II in pediatric ARDS
We initially applied the PERSEVERE predictive model to the ARDS cohort (Supplementary Figure 1, Supplementary Table 1). Discriminative ability for mortality was poor, with an AUROC curve of 0.61 (95% CI 0.49 to 0.73). PERSEVERE-II, which incorporates platelet levels, performed better (Supplementary Figure 2, Supplementary Table 1), with an AUROC of 0.76 (95% CI 0.65 to 0.86; p = 0.029 compared with PERSEVERE).
Development of PARDSEVERE
Because of the modest predictive ability of PERSEVERE-II, we developed a mortality prediction model specifically for the ARDS cohort, using the PERSEVERE biomarkers and additional variables as described in Methods. The final PARDSEVERE model retained CCL3, HSPA1B, IL8, and age (Figure 1). In the derivation cohort, terminal nodes 1 and 4 were low-risk, with mortality rates of 0%. Terminal node 2 was intermediate risk, with 20% mortality. Terminal nodes 3 and 5 were high-risk, with mortality rates > 33%. Discriminative ability was good for the derivation, with AUROC of 0.85 (Table 2). The test cohort displayed similar characteristics (Figure 2), with low-risk node mortality of 0 and 5.6%, the intermediate node with 20% mortality, and high-risk node mortality of > 33%. PARDSEVERE demonstrated improved mortality discrimination over PERSEVERE (p < 0.001) and PERSEVERE-II (p = 0.053) when comparing the respective AUROC. PARDSEVERE performed comparably to PRISM III (AUROC 0.80, 95% CI 0.60 to 0.91) and number of non-pulmonary organ failures (AUROC 0.85, 95% CI 0.73 to 0.92; both p > 0.05 relative to PARDSEVERE). When restricted to immunocompetent patients, PARDSEVERE also demonstrated excellent discrimination (n = 124, AUROC 0.89, 95% CI 0.83 to 0.94). Finally, worsening PARDSEVERE risk categories demonstrated decreasing VFD and decreased probability of extubation given the competing risk of death (Supplementary Figure 3).
Figure 1.
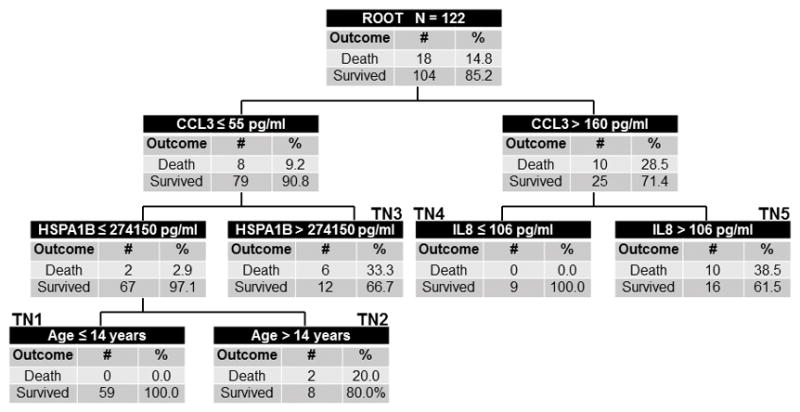
The derived PARDSEVERE decision tree from the derivation cohort (n = 122, 18 non-survivors). The tree contains the mortality probability, C-C chemokine ligand 3 (CCL3), interleukin 8 (IL8), heat shock protein 70 kda 1B (HSPA1B), and age in years. Biomarker concentrations are expressed in pg/mL. Terminal nodes 1 and 4 were low-risk (0% risk of death), terminal node 2 was intermediate-risk (20% risk), and terminal nodes 3 and 5 high risk (≥ 33% risk).
Table 2.
Test characteristics of PARDSEVERE
| Variable | Value | 95% CI |
|---|---|---|
| Derivation (n = 122, 18 non-survivors) | ||
| True negatives | 68 | - |
| False positives | 36 | - |
| True positives | 18 | - |
| False negatives | 0 | - |
| Sensitivity | 1 | 0.78 to 1 |
| Specificity | 0.65 | 0.55 to 0.74 |
| Positive predictive value | 0.33 | 0.21 to 0.48 |
| Negative predictive value | 1 | 0.93 to 1 |
| Positive likelihood ratio | 2.9 | 2.2 to 3.8 |
| Negative likelihood ratio | - | - |
| Area under ROC curve | 0.85 | 0.78 to 0.92 |
| Test (n = 30, 6 non-survivors) | ||
| True negatives | 17 | - |
| False positives | 7 | - |
| True positives | 5 | - |
| False negatives | 1 | - |
| Sensitivity | 0.83 | 0.36 to 0.99 |
| Specificity | 0.71 | 0.49 to 0.87 |
| Positive predictive value | 0.42 | 0.16 to 0.71 |
| Negative predictive value | 0.94 | 0.71 to 1 |
| Positive likelihood ratio | 2.9 | 1.4 to 5.9 |
| Negative likelihood ratio | 0.2 | 0 to 1.4 |
| Area under ROC curve | 0.82 | 0.62 to 1 |
Figure 2.
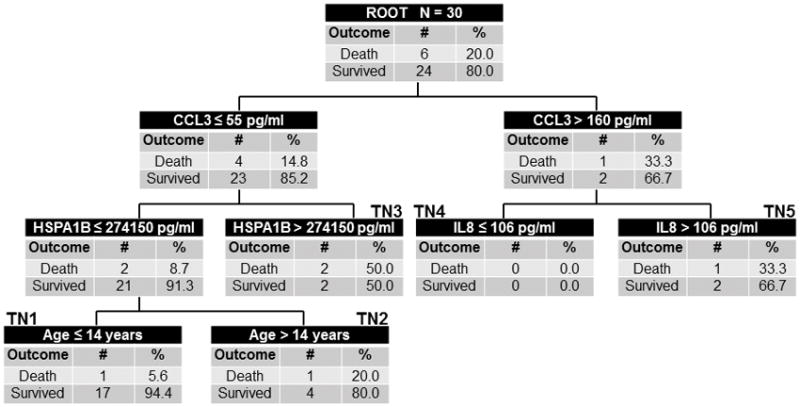
The derived PARDSEVERE decision tree from the test cohort (n = 30, 6 non-survivors). The tree contains the mortality probability, C-C chemokine ligand 3 (CCL3), interleukin 8 (IL8), heat shock protein 70 kda 1B (HSPA1B), and age in years. Biomarker concentrations are expressed in pg/mL. Terminal nodes 1 and 4 were low-risk (≤ 6% risk of death), terminal node 2 was intermediate-risk (20% risk), and terminal nodes 3 and 5 high-risk (≥ 33% risk).
Biological plausibility of the model was demonstrated by examination of false positives (i.e., those predicted to die by PARDSEVERE but survived). False positives were more ill than true negatives (Table 3), with higher PRISM III, more non-pulmonary organ failures, higher vasopressor scores, and higher proportion of immunocompromised. This suggests that the biomarker-based PARDSEVERE accurately identified increasing illness severity, and that false positives were at higher risk of death, which was potentially mitigated by clinical management.
Table 3.
Comparison of True Negatives and False Positives based on PARDSEVERE
| Variables | True Negatives (n = 85) | False Positives (n = 43) | p valuea |
|---|---|---|---|
| Age (years) | 3. 4 [1.3, 7.1] | 6.8 [3.8, 16.3] | < 0.001 |
| Female/male (%/%) | 40/45 (47/53) | 25/18 (58/42) | 0.159 |
| Severity of illness | |||
| PRISM III at 12 hours | 8 [3, 14] | 13 [8, 18] | 0.016 |
| Organ failures | 1 [0, 2] | 2 [1, 3] | < 0.001 |
| Immunocompromised (%) | 6 (7) | 13 (30) | 0.001 |
| Vasopressor score | 6 [0, 12] | 13 [5, 24] | < 0.001 |
| ARDS etiology (%) | |||
| Infectious | 64 (75) | 34 (79) | 0.826 |
| Non-infectious | 21 (25) | 9 (21) | |
| ARDS onset | |||
| PaO2/FIO2 | 188 [120, 237] | 147 [95, 210] | 0.170 |
| OI | 9.2 [6.4, 15.9] | 11.3 [6.2, 19.3] | 0.431 |
| PIP (cmH2O) | 31 [26, 36] | 31 [28, 35] | 0.840 |
| PEEP (cmH2O) | 10 [8, 12] | 10 [8, 12] | 0.616 |
| mPaw (cmH2O) | 17 [15, 21] | 17 [14, 20] | 0.701 |
| VT (mL/kg) | 7.2 [6.3, 7.8] | 7.3 [6.4, 8.5] | 0.374 |
| 24 hours after onset | |||
| PaO2/FIO2 | 226 [162, 290] | 246 [153, 361] | 0.169 |
| OI | 6.3 [4.4, 11.5] | 6.3 [3.3, 12.4] | 0.475 |
| PIP (cmH2O) | 26 [22, 30] | 27 [22, 30] | 0.871 |
| PEEP (cmH2O) | 10 [8, 10] | 8 [8, 10] | 0.879 |
| mPaw (cmH2O) | 15 [12, 19] | 16 [13, 20] | 0.538 |
| VT (mL/kg) | 7.1 [6.2, 8] | 7.2 [6.5, 7.9] | 0.996 |
| Ancillary therapies (%) | |||
| Inhaled nitric oxide | 28 (33) | 12 (28) | 0.687 |
| Neuromuscular blockade | 45 (53) | 15 (35) | 0.062 |
| Corticosteroids | 40 (47) | 17 (40) | 0.456 |
| Alternative ventilator | 27 (32) | 10 (23) | 0.410 |
| ECMO | 4 (5) | 2 (5) | > 0.999 |
P values are results of Wilcoxon rank-sum. Bold lettering reflects significant differences (p < 0.05).
DISCUSSION
PERSEVERE and PERSEVERE-II, risk prediction tools developed for pediatric sepsis, did not perform well in pediatric ARDS. Therefore, we derived PARDSEVERE using the PERSEVERE biomarkers, in addition to select clinical variables, using CART methodology, similar to development of PERSEVERE. PARDSEVERE demonstrated good test characteristics within the derivation and test subsets sampled from the entire ARDS cohort, and suggested biological plausibility, with higher severity of illness in false positives (predicted to die) relative to true negatives. PARDSEVERE separated the cohort into low-, intermediate-, and high-probability of death, and may have utility for risk stratification or prognostic enrichment in future pediatric ARDS trials.
The biomarkers CCL3, HSPA1B, IL8 were retained in the PARDSEVERE predictive model. These biomarkers constitute the primary predictors in PERSEVERE for pediatric sepsis (6). IL8 is associated with increased mortality in both pediatric and adult sepsis and ARDS (21–25). Half of the deaths in this cohort were non-infectious, and the retention of these 3 biomarkers in a mortality prediction model suggests that sepsis shares a circulating cytokine profile with other inflammatory conditions (ARDS in this study), some of which are typically considered sterile. This raises the intriguing possibility of testing these 3 biomarkers for stratifying mortality risk in non-infectious (yet inflammatory) etiologies, such trauma, cardiac arrest, and post-cardiopulmonary bypass. This is especially true if the shared mortality risk from these conditions is predominantly manifested through MSOF, with which these biomarkers appear to be correlated.
It is notable that previously described predictors of mortality, such as immunocompromised status and oxygenation (11, 13, 19, 26), were not retained in PARDSEVERE. A recently proposed simple model (19) for predicting mortality in pediatric ARDS using only day 1 OI and cancer/stem-cell transplant status resulted in modest predictive ability in our cohort (AUROC 0.71, 95% CI 0.60 to 0.83). PARDSEVERE outperformed this model when comparing AUROC (p = 0.047).
One potential explanation is that the mortality risk from being immunocompromised is better explained by circulating biomarkers, rather than a binary clinical designation reflecting immune function. Four of the 5 PERSEVERE biomarkers (other than MMP8) and platelets were elevated in immunocompromised patients (Supplementary Table 2). The retention of the biomarkers, but not the clinical condition, suggests that mortality risk was better stratified by the biomarker profile. OI was only associated with a single biomarker (HSPA1B, rho = 0.29, p < 0.001), and the lack of inclusion in PARDSEVERE may be due to the low sample size, infrequency of death, or the over-representation of deaths for neurologic (rather than hypoxemia or MSOF) reasons. Despite these limitations, PARDSEVERE demonstrated biologic plausibility, with higher illness severity in false positives relative to true negatives, and retention of the same 3 biomarkers comprising the upper level decision rules for PERSEVERE. External validation of PARDSEVERE in a larger population will be necessary to determine whether oxygenation would retain predictive utility in the presence of these markers, and as with PERSEVERE, we expect this tool to be updated with additional data.
PARDSEVERE performs comparably to PRISM III and number of non-pulmonary organ failures. A biomarker-based risk stratification tool offers advantages over PRISM III, which is typically neither available nor calculated at presentation. Furthermore, experts in the field have argued strongly against using illness severity scores (such as PRISM) for individual patient stratification, entry criteria for trials, or treatment decisions, as such scores were not designed for use in individual patients or specific interventions (27). The similar performance of organ failures suggests that PARDSEVERE is reproducing the mortality risk associated with MSOF in this ARDS cohort. PARDSEVERE may offer advantages over an organ failure score in its objectivity and the association of biomarkers with underlying inflammatory pathophysiology, thus potentially identifying targetable pathways. Calfee et al demonstrated the presence of different endotypes of adult ARDS defined by circulating biomarkers (10); PARDSEVERE may similarly be identifying endotypes of pediatric ARDS with varying degrees of inflammatory insult defined, in part, by the 3 biomarkers.
Our study has limitations. The study was conducted at a single center, and while demographics and severity are comparable to other cohorts, mortality rate, ventilator practices, and utilization of ancillary therapies or alternative ventilator modes may not allow translation to other institutions. However, a lack of protocolization should improve generalizability. We measured plasma biomarkers, which may not be the compartment most reflective of ARDS biology. However, given the infrequency of bronchoalveolar lavage in our population, it was not feasible to include biomarkers measured from the alveolar space. Patient heterogeneity, low number of total deaths, high proportion of neurologic deaths, and modest proportion of deaths from patients with an infectious etiology limits the model. However, the rarity of deaths in this cohort is typical for modern pediatric ARDS cohorts (13, 19). While the etiology of death in pediatric ARDS is unknown, adult ARDS data suggests that primary neurologic failure is a common phenomenon (28). In that study, 29% of adult ARDS patients had neurologic dysfunction as the organ failure precipitating death, and 67% had active withdrawal of support. Finally, clinical application of PARDSEVERE requires an efficient platform that can generate actionable data within about 1 hour and is amenable to measuring individual samples. The technologies to achieve these capabilities are available, and a rapid assay platform for the PERSEVERE biomarkers is currently in development.
Despite limitations, PARDSEVERE demonstrated good predictive characteristics, was internally validated, and had biologic plausibility. Samples were collected prospectively in a well-characterized, contemporary pediatric ARDS cohort early in the disease course, and demonstrated utility for stratifying the outcomes VFD at 28 days and probability of extubation given competing risk of death. The ability to stratify outcomes other than mortality is necessary for pediatric ARDS, as mortality is low and only partly related to severity of lung injury.
CONCLUSIONS
A validated, biomarker-based risk stratification tool designed for pediatric sepsis was adapted for use in pediatric ARDS. The newly derived PARDSEVERE demonstrates good test characteristics internally, and requires external validation in a larger cohort. Tools such as PARDSEVERE have the potential to provide improved risk stratification and prognostic enrichment for future trials in pediatric ARDS.
Supplementary Material
The PERSEVERE decision tree applied to the ARDS cohort. The tree contains C-C chemokine ligand 3 (CCL3), interleukin 8 (IL8), heat shock protein 70 kda 1B (HSPA1B), granzyme B (GMZB), and matrix metallopeptidase 8 (MMP8), and age in years. Biomarker concentrations are expressed in pg/mL. Red labeling indicates terminal nodes and mortality probabilities according to PERSEVERE. Actual mortality is provided in the boxes.
The PERSEVERE-II decision tree applied to the ARDS cohort. The tree contains C-C chemokine ligand 3 (CCL3), interleukin 8 (IL8), heat shock protein 70 kda 1B (HSPA1B), granzyme B (GMZB), and matrix metallopeptidase 8 (MMP8), platelet count (number × 103/μL), and age in years. Biomarker concentrations are expressed in pg/mL. Red labeling indicates terminal nodes and mortality probabilities according to PERSEVERE-II. Actual mortality is provided in the boxes.
(A) VFD at 28 days and (B) probability of extubation given competing risk of death, stratified by PARDSEVERE risk categories. Low-risk are terminal nodes 1 and 4, intermediate is terminal node 2, and high are terminal nodes 3 and 5. P value represents a non-parametric test of trend.
Acknowledgments
Financial Support: K12 HL-109009 (NY); RO1 GM-099773, RO1 GM-108025 (HRW)
Footnotes
Institution: Children’s Hospital of Philadelphia
Reprints Planned: No
Copyright form disclosure: Dr. Yehya’s institution received funding from National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute. Dr. Wong’s institution received funding from NIH grants. The authors received support for article research from the NIH, and disclosed that they have applied for a patent for the risk prediction tool described in the manuscript (PERSEVERE).
References
- 1.Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 2.Guerin C, Reignier J, Richard JC, et al. Prone Positioning in Severe Acute Respiratory Distress Syndrome. N Engl J Med. 2013 doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 3.Acute Respiratory Distress Syndrome N. Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 4.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 5.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 6.Wong HR, Salisbury S, Xiao Q, et al. The pediatric sepsis biomarker risk model. Crit Care. 2012;16(5):R174. doi: 10.1186/cc11652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong HR, Weiss SL, Giuliano JS, Jr, et al. Testing the prognostic accuracy of the updated pediatric sepsis biomarker risk model. PloS one. 2014;9(1):e86242. doi: 10.1371/journal.pone.0086242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong HR, Cvijanovich NZ, Anas N, et al. Pediatric Sepsis Biomarker Risk Model-II: Redefining the Pediatric Sepsis Biomarker Risk Model With Septic Shock Phenotype. Crit Care Med. 2016;44(11):2010–2017. doi: 10.1097/CCM.0000000000001852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 10.Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. The Lancet Respiratory medicine. 2014;2(8):611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flori HR, Glidden DV, Rutherford GW, et al. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171(9):995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Fernandez Y, Azagra AM, de la Oliva P, et al. Pediatric Acute Lung Injury Epidemiology and Natural History study: Incidence and outcome of the acute respiratory distress syndrome in children. Crit Care Med. 2012;40(12):3238–3245. doi: 10.1097/CCM.0b013e318260caa3. [DOI] [PubMed] [Google Scholar]
- 13.Yehya N, Servaes S, Thomas NJ. Characterizing degree of lung injury in pediatric acute respiratory distress syndrome. Crit Care Med. 2015;43(5):937–946. doi: 10.1097/CCM.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 14.Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11(2):234–238. doi: 10.1097/PCC.0b013e3181b806fc. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein B, Giroir B, Randolph A, et al. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 16.Yehya N, Topjian AA, Thomas NJ, et al. Improved oxygenation 24 hours after transition to airway pressure release ventilation or high-frequency oscillatory ventilation accurately discriminates survival in immunocompromised pediatric patients with acute respiratory distress syndrome. Pediatr Crit Care Med. 2014;15(4):e147–156. doi: 10.1097/PCC.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yehya N, Bhalla AK, Thomas NJ, et al. Alveolar Dead Space Fraction Discriminates Mortality in Pediatric Acute Respiratory Distress Syndrome. Pediatr Crit Care Med. 2016;17(2):101–109. doi: 10.1097/PCC.0000000000000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yehya N, Thomas NJ. Disassociating Lung Mechanics and Oxygenation in Pediatric Acute Respiratory Distress Syndrome. Crit Care Med. 2017;45(7):1232–1239. doi: 10.1097/CCM.0000000000002406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spicer AC, Calfee CS, Zinter MS, et al. A Simple and Robust Bedside Model for Mortality Risk in Pediatric Patients With Acute Respiratory Distress Syndrome. Pediatr Crit Care Med. 2016;17(10):907–916. doi: 10.1097/PCC.0000000000000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 21.Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33(1):1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. discussion 230–232. [DOI] [PubMed] [Google Scholar]
- 22.Wong HR, Cvijanovich N, Wheeler DS, et al. Interleukin-8 as a stratification tool for interventional trials involving pediatric septic shock. Am J Respir Crit Care Med. 2008;178(3):276–282. doi: 10.1164/rccm.200801-131OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calfee CS, Thompson BT, Parsons PE, et al. Plasma interleukin-8 is not an effective risk stratification tool for adults with vasopressor-dependent septic shock. Crit Care Med. 2010;38(6):1436–1441. doi: 10.1097/CCM.0b013e3181de42ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zinter MS, Orwoll BE, Spicer AC, et al. Incorporating Inflammation into Mortality Risk in Pediatric Acute Respiratory Distress Syndrome. Crit Care Med. 2017;45(5):858–866. doi: 10.1097/CCM.0000000000002370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikacenic C, Price BL, Harju-Baker S, et al. A Two Biomarker Model Predicts Mortality in the Critically Ill with Sepsis. Am J Respir Crit Care Med. 2017 doi: 10.1164/rccm.201611-2307OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trachsel D, McCrindle BW, Nakagawa S, et al. Oxygenation index predicts outcome in children with acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2005;172(2):206–211. doi: 10.1164/rccm.200405-625OC. [DOI] [PubMed] [Google Scholar]
- 27.Vincent JL, Opal SM, Marshall JC. Ten reasons why we should NOT use severity scores as entry criteria for clinical trials or in our treatment decisions. Crit Care Med. 2010;38(1):283–287. doi: 10.1097/CCM.0b013e3181b785a2. [DOI] [PubMed] [Google Scholar]
- 28.Stapleton RD, Wang BM, Hudson LD, et al. Causes and timing of death in patients with ARDS. Chest. 2005;128(2):525–532. doi: 10.1378/chest.128.2.525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The PERSEVERE decision tree applied to the ARDS cohort. The tree contains C-C chemokine ligand 3 (CCL3), interleukin 8 (IL8), heat shock protein 70 kda 1B (HSPA1B), granzyme B (GMZB), and matrix metallopeptidase 8 (MMP8), and age in years. Biomarker concentrations are expressed in pg/mL. Red labeling indicates terminal nodes and mortality probabilities according to PERSEVERE. Actual mortality is provided in the boxes.
The PERSEVERE-II decision tree applied to the ARDS cohort. The tree contains C-C chemokine ligand 3 (CCL3), interleukin 8 (IL8), heat shock protein 70 kda 1B (HSPA1B), granzyme B (GMZB), and matrix metallopeptidase 8 (MMP8), platelet count (number × 103/μL), and age in years. Biomarker concentrations are expressed in pg/mL. Red labeling indicates terminal nodes and mortality probabilities according to PERSEVERE-II. Actual mortality is provided in the boxes.
(A) VFD at 28 days and (B) probability of extubation given competing risk of death, stratified by PARDSEVERE risk categories. Low-risk are terminal nodes 1 and 4, intermediate is terminal node 2, and high are terminal nodes 3 and 5. P value represents a non-parametric test of trend.


