Abstract
Objective
Primary cytoreduction for ovarian cancer often requires extended radical procedures and is associated with significant morbidity. In 2010, neoadjuvant chemotherapy was shown to have similar survival to primary cytoreduction but with less need for radical surgery. We hypothesized that the increased use of neoadjuvant chemotherapy would decrease the use of radical cytoreductive procedures and thus examined trends in the performance of radical cytoreductive procedures.
Methods
We used the Nationwide Inpatient Sample (NIS) to determine the annual number of extended procedures (colon, small intestine, liver, diaphragm, spleen, and gastric resection, ileostomy, colostomy) performed in women undergoing surgery for ovarian cancer from 1998–2013. Estimates were weighted to provide national averages. To account for changes in incidence over time, we used national incidence rates and report procedures performed per 1000 new cases of ovarian cancer. Trends were assessed using Cochrane-Armitage tests.
Results
We identified 274,639 ovarian cancer patients who underwent surgery, ranging from 15,720 to 18,714 procedures performed each year. We identified significant increase in the use of extended procedures over this time period. These differences were significant for absolute numbers of procedures, rate per 1000 new ovarian cancer cases, and percent per hysterectomy/BSO for rectosigmoid resection, diaphragm resection, splenectomy, ileostomy, and liver resection. Specifically, the use of these procedures rose from 1998 to 2010, declined in 2011, and rose again in 2012 and 2013.
Conclusions
While there was a transient decrease in the use of extended cytoreductive procedures from 2010 to 2011 after the publication randomized neoadjuvant trial data, use of these procedures again rose in 2012 and 2013.
Introduction
It is well established that complete surgical cytoreduction to microscopic disease is the best predictor of progression-free and overall survival in patients with advanced stage ovarian cancer.1–4 However, extensive procedures required for optimal cytoreduction are often associated with significant morbidity and mortality.5,6 Following cytoreductive surgery, many patients do not receive chemotherapy or they experience a delay in the initiation of chemotherapy, which adversely affects survival.7 Because of the morbidity associated with cytoreductive surgery, neoadjuvant chemotherapy (NACT) has been utilized in patients who are poor surgical candidates, have high perioperative risk profiles, or have a low likelihood of achieving cytoreduction to <1cm of residual disease.8 Advocates of NACT argue that it may also allow for an assessment of chemotherapy response, which may identify platinum resistant disease that confers poor prognosis and high risk of recurrence regardless of surgical intervention.9
To date, two phase III clinical trials have evaluated the use of NACT in advanced stage ovarian cancer. The European Organization for Research and Treatment of Cancer (EORTC) and the Chemotherapy OR Upfront Surgery (CHORUS) studies suggested that NACT increases the likelihood of complete cytoreduction, decreases the need for extensive procedures, decreases perioperative morbidity, and has similar progression-free and overall survival outcomes compared to primary cytoreductive surgery.10–12 The use of NACT appears to be increasing, as recently published data utilizing the National Cancer Database demonstrated that the use of NACT in advanced-stage ovarian cancer increased from 8.6% in 2004 to 22.6% in 2013 (p<0.001). The increase in NACT was most pronounced in older women.13 Given these results, the Society for Gynecologic Oncology and the American Society for Clinical Oncology released a joint statement recommending that NACT be considered in patients with advanced stage disease who have a low likelihood of achieving optimal cytoreduction (to <1 cm of residual disease, ideally no gross residual disease) or who are poor surgical candidates.8
Although the use of NACT is increasing, little is known about how this has impacted the surgical patterns of care for women with ovarian cancer. We hypothesized that increased use of NACT would reduce the need to perform extended radical procedures on the gastrointestinal and genitourinary tracts in women with ovarian cancer. We performed a population-based analysis to examine the use of extended cytroredctive procedures among women with ovarian cancer before and after the release of phase III NACT data.
Methods
Data from the Nationwide Inpatient Sample (NIS) were used for this analysis. The NIS is an inpatient care database developed for the Healthcare Cost and Utilization Project through the Agency for Healthcare Research and Quality. It is the largest publicly available all-payer inpatient database in the United States, containing a random sample of up to 20% of all hospital discharges throughout the U.S. It provides data from approximately 7 million hospital stays annually, yielding more than 35 million national weighted estimates.14
We examined the number of concomitant extended procedures performed in women who underwent inpatient hysterectomy, bilateral salpingoophorectomy (BSO), or debulking procedure for a diagnosis of ovarian, fallopian tube, or peritoneal cancer from 1998–2013. Diagnoses and procedures were categorized by their International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) coding (Supplemental table). The study population included women with ovarian, fallopian tube, or peritoneal cancer, who underwent hysterectomy, salpingoophorectomy or cytoreduction. The extended procedures analyzed included small bowel resection, colon resection, rectosigmoid resection, liver resection, bladder resection, diaphragm resection, splenectomy, gastric resection, ileostomy, and colostomy.
Clinical and demographic characteristics included age (<40, 40–49, 50–59, 60–69, 70–79, ≥80 years), race/ethnicity (white, black, Hispanic, other/unknown), year (1998–2013), median household income of residents in a patient’s zip code (low, medium low, medium high, high, unknown), and insurance status (Medicare, Medicaid, private, self-pay, other/unknown). Comorbidity was measured using the Elixhauser comorbidity score 15 and classified as 0, 1, or ≥2. Hospital characteristics included hospital bed size (small, medium, large, unknown), location, and teaching status (rural, urban non-teaching, urban teaching, unknown), and region (northeast, midwest, south, west). Cutpoints for the classification of hospital bed size as small, medium or large vary for each region of the country as well as based on urban/rural location and hospital teaching status.
The primary objective of this study was to examine trends in the performance of extended procedures over time as the use of neoadjuvant chemotherapy increased in the U.S. The EORTC’s trial of neoadjuvant chemotherapy was published in 2010.10 We examined trends in the use of each procedure from 1998 to 2010 and from 2011 to 2013 after these data were available.
National estimates of the number of extended procedures performed were derived from NIS-provided weighted estimates.14 Starting with 2012, discharges were sampled from all hospitals representative of the population on unidentified hospital, hospital Census division, urban/rural location, bed size, ownership, teaching status, beds, diagnosis-related group and admission month of the hospital stay. The discharge weights were used for weighted estimates for 2012 and 2013. Prior to 2012, hospitals within the NIS were stratified based on hospital Census division, urban/rural location, bed size, ownership, and teaching status. The number of discharges within a given stratum were then compared with data from the American Hospital Association to obtain the weights which were then applied to the unweighted data for weighted estimates of each year 16. NIS recently provided a new weight called “Trend Weight” for the 1993–2011 data, which were calculated in the same way as the weights for the redesigned NIS starting with 2012. Trend weights are designed to be used instead of the original NIS discharge weights for trend analysis.17 National estimates of demographics were obtained by summing up the weighted estimates of demographics in each year.
We calculated the absolute number of each extended procedure performed as well as the percentage of patients who underwent surgery who had each of the given extended procedures. To account for changes in the incidence of cancer over time, we used American Cancer Society statistics to derive the number of procedures performed per 1000 new cases of ovarian cancer. We calculated the weighted number of procedures divided by the number of new cases from ACS and expressed per 1000.18 Trends were assessed using linear regression approaches. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC). All hypothesis tests were two-sided.
Results
We identified a total of 57,390 women with ovarian cancer who underwent inpatient surgery. After weighting, our cohort included 274,639 patients. Demographic data are shown in Table 1. The median age of the cohort was 63 (IQR 53–73, unweighted). One-fourth were age 50–59 (24.9%), one-fourth 60–69 (24.2%) and one-fifth were 70–79 (18.9%). Most were white (63.0%), with commercial insurance (50.2%) or Medicare (36.5%), and in high (34.6%) or medium-high (26.2%) census-level income. More than two-thirds of the procedures were performed at large (71.8%) and urban teaching (68.0%) hospitals. One third of the procedures were performed in the South (33.8%), while less than one quarter were performed in the West (23.2%), Midwest (22.6%), and Northeast (20.4%) each.
Table 1.
Demographic and clinical characteristics of the cohort.
| N, weighted | %, weighted | |
|---|---|---|
| Age | ||
| <40 | 24,020 | 8.7% |
| 40–49 | 42,789 | 15.6% |
| 50–59 | 68,266 | 24.9% |
| 60–69 | 66,395 | 24.2% |
| 70–79 | 52,014 | 18.9% |
| ≥80 | 21,156 | 7.7% |
| Race | ||
| White | 173,087 | 63.0% |
| Black | 14,306 | 5.2% |
| Hispanic | 16,211 | 5.9% |
| Other/Unknown | 71,036 | 25.9% |
| Year | ||
| 1998 | 16,422 | 5.8% |
| 1999 | 17,595 | 6.2% |
| 2000 | 16,201 | 5.7% |
| 2001 | 17,985 | 6.4% |
| 2002 | 18,182 | 6.4% |
| 2003 | 16,017 | 5.7% |
| 2004 | 17,472 | 6.2% |
| 2005 | 18,714 | 6.6% |
| 2006 | 16,045 | 5.7% |
| 2007 | 17,833 | 6.3% |
| 2008 | 18,291 | 6.5% |
| 2009 | 17,436 | 6.2% |
| 2010 | 18,336 | 6.5% |
| 2011 | 15,720 | 5.6% |
| 2012 | 16,360 | 5.8% |
| 2013 | 16,030 | 5.7% |
| Income status | ||
| Low | 40,537 | 14.8% |
| Medium Low | 61,029 | 22.2% |
| Medium High | 71,944 | 26.2% |
| High | 95,056 | 34.6% |
| Unknown | 6,073 | 2.2% |
| Insurance status | ||
| Medicare | 100,259 | 36.5% |
| Medicaid | 17,823 | 6.5% |
| Commercial | 137,830 | 50.2% |
| Self Pay | 9,582 | 3.5% |
| Other/Unknown | 9,146 | 3.3% |
| Comorbidity (Elixhauser) | ||
| 0 | 97,720 | 35.6% |
| 1 | 80,560 | 29.3% |
| ≥2 | 96,360 | 35.1% |
| Hospital bed size | ||
| Small | 21,826 | 7.9% |
| Medium | 54,662 | 19.9% |
| Large | 197,199 | 71.8% |
| Unknown | 952 | 0.3% |
| Hospital location and teaching status | ||
| Rural | 14,294 | 5.2% |
| Urban non-teaching | 72,666 | 26.5% |
| Urban teaching | 186,727 | 68.0% |
| Unknown | 952 | 0.3% |
| Region | ||
| Northeast | 55,995 | 20.4% |
| Midwest | 62,130 | 22.6% |
| South | 92,851 | 33.8% |
| West | 63,665 | 23.2% |
The weighted N was rounded to the nearest integer.
Overall, the number of procedures performed annually ranged from 15,720 to 18,714. Concomitant extended procedures performed over this time period included 32,804 colon resections, 25,382 rectosigmoid resections, 10,104 small bowel resections, 8,112 colostomies, 6,646 diaphragm resections, 5,069 splenectomies, 2,813 ileostomies, 1,609 gastrectomies, 1,309 bladder resections, and 1,283 liver resections (Table 2).
Table 2.
National estimates of extended procedures
| Procedure | Total procedures | Weighted estimates | Annual Percent* | Annual Rate** | |||
|---|---|---|---|---|---|---|---|
| Weighted N | Mean | p-value | Mean | p-value | Mean | p-value | |
| Colon Resection | 32,805 | 2,050 | 0.40 | 11.9% | 0.40 | 90 | 0.03 |
| Rectosigmoid resection | 25,382 | 1,586 | 0.02 | 9.3% | 0.02 | 70 | <0.001 |
| Small Bowel Resection | 10,104 | 632 | 0.97 | 3.7% | 0.97 | 28 | 0.13 |
| Colostomy | 2,813 | 507 | 0.17 | 3.0% | 0.17 | 22 | 0.86 |
| Diaphragm resection | 6,646 | 415 | <0.001 | 2.4% | <0.001 | 19 | <0.001 |
| Splenectomy | 5,069 | 317 | <0.001 | 1.8% | <0.001 | 14 | <0.001 |
| Ileostomy | 2,813 | 176 | <0.001 | 1.0% | <0.001 | 8 | <0.001 |
| Gastrectomy | 1,609 | 101 | 0.11 | 0.6% | 0.11 | 4 | 0.03 |
| Bladder Resection | 1,309 | 82 | 0.28 | 0.5% | 0.28 | 4 | 0.19 |
| Liver resection | 1,283 | 80 | <0.001 | 0.5% | <0.001 | 4 | <0.001 |
Annual percent was calculated as the number of extended procedures per case of hysterectomy and bilateral salpingoophorectomy
Annual rate was calculated as the number of cases per 1000 new diagnoses
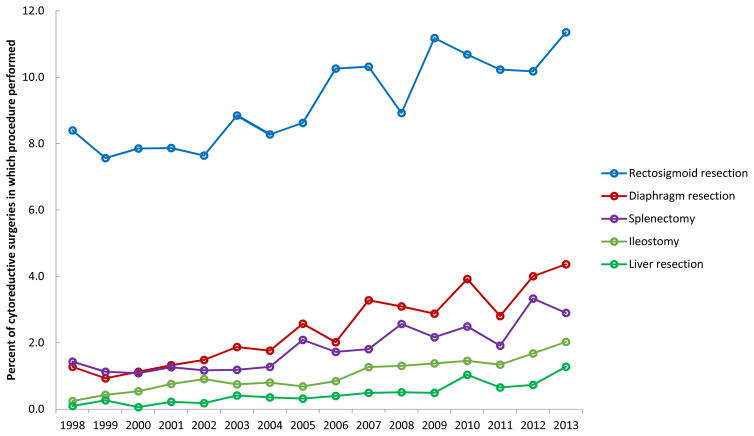
To account for variation in number of procedures performed each year, the annual percentages of extended procedures performed per case from 1998–2013 were calculated. On average, colon resection was performed in 11.9%, rectosigmoid resection in 9.3%, small bowel resection in 3.7%, colostomy in 3.0%, diaphragm resection in 2.4%, splenectomy in 1.8%, ileostomy in 1.0%, gastrectomy in 0.6%, bladder resection in 0.5%, and liver resection in 0.5% of cases (Table 2).
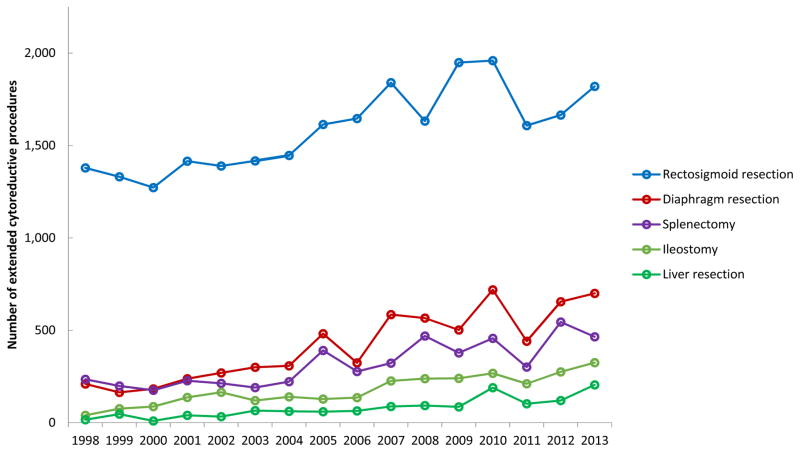
The annual number of procedures and percent of procedures per case over this time period was significantly different for rectosigmoid resections (P=0.02), diaphragm resections (P<0.001), splenectomies (P<0.001), ileostomies (P<0.001), and liver resections (P<0.001) (Figures 1 and 2). The overall trend appeared to be an increase in extended procedures from 1998 to 2010, followed by a brief decrease in 2011, then an increase through 2013.
Figure 1.
Figure 2.
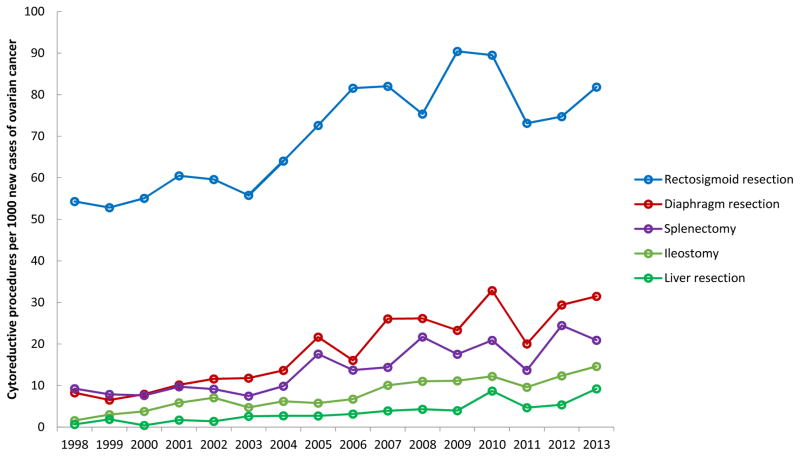
To account for changes in the incidence of ovarian cancer over time, the annual rate of extended procedures performed per 1000 new cases of ovarian cancer diagnosed from 1998–2013 were calculated. Per 1000 new cases, an average of 90 colon resections, 70 rectosigmoid resections, 28 small bowel resections, 22 colostomies, 19 diaphragm resections, 14 splenectomies, 8 ileostomies, 4 gastrectomies, 4 bladder resections, and 4 liver resections were performed annually. The annual rate of procedures performed over this time period was significantly different for colon resections (P=0.03), rectosigmoid resections (P<0.001), diaphragm resections (P<0.001), splenectomies (P<0.001), ileostomies (P<0.001), gastrectomies (P=0.03), and liver resections (P<0.001) (Figure 3). Similar to changes in absolute numbers of cases over time, the overall trend for the rate of extended procedures per 1000 new ovarian cancer cases showed a gradual increase from 1998 to 2010, followed by a brief decrease in 2011, then an increase through 2013.
Figure 3.
Conclusions
This study suggests that there was a significant increase in the use of extended procedures for women undergoing debulking surgery for the treatment of ovarian cancer from 1998–2013. These differences were significant for absolute numbers of procedures, the rate per 1000 new ovarian cancer cases, and the percent per hysterectomy/BSO for rectosigmoid resection, diaphragm resection, splenectomy, ileostomy, and liver resection. Specifically, the use of these procedures rose from 1998 to 2010, declined in 2011, and rose again in 2012 and 2013.
While our data suggests an overall increase in the use of extended procedures from 1998 to 2013, it is interesting to note the abrupt decrease in most of these procedures from 2010 to 2011, which coincides with the publication of the EORTC’s phase III trial that compared neoadjuvant chemotherapy with primary surgical cytoreduction.10 This study suggested no difference in overall survival for patients undergoing neoadjuvant chemotherapy compared to upfront primary debulking surgery, and may have had an impact on the decreased use of primary radical debulking procedures following its publication. Similar, albeit smaller decreases in the use of some extended procedures were also noted from 2008 to 2009, coinciding with the 2008 Biennial Meeting of the International Gynecologic Cancer Society, at which preliminary results of this trial were presented. These decreases appear to be transient, however, and the overall trends show increasing use of extended procedures, specifically after the 2010 decreases. In addition, over the period of the study the benefits of complete resection to no residual disease was recognized and numerous studies emerged suggesting the potential benefits of neoadjuvant chemotherapy as well as the morbidity of cytoreductive surgery. All of these factors may have contributed to temporal changes in practice patterns.
Following the release of preliminary EORTC data at national meetings in 2008 and 2009, Dewdney and colleagues sought to evaluate physicians’ opinions and patterns of care regarding NACT through a survey study in 2009. At the time of the survey, physicians reported that NACT was rarely employed, with 60% of respondents using it in less than 10% of cases and 30% using it in 10–25% of cases. While almost 40% believed that women with bulky upper abdominal disease on preoperative imaging would benefit from NACT versus primary debulking, greater than 80% did not believe that available evidence justified the use of NACT.19 Since then, new data from multiple clinical trials has been published suggesting that NACT decreases surgical morbidity, increases the likelihood of optimal cytoreduction, and decreases the need for extended procedures, without compromise of overall survival.10–12 Despite NACT trial results, our data suggest that extended procedures have not declined substantially. Based on the survey above, it appears that physicians were hesitant to change their practice patterns and were not convinced of the benefits of NACT prior to 2010.
As clinical trial data from the past decade continues to demonstrate the benefits of NACT, there has been increased adoption of its use. A recently published study by Melamed and colleagues examined the trends in NCAT use over time, and demonstrated an increase in use from 8.6% in 2004 to 22.6% in 2013 (P<0.001) 13. If these data are to be interpreted in the context of prior neoadjuvant studies, an increased use of NACT should result in a decreased need for extended procedures. With the exception of transient decreases, our data suggest that the use of extended procedures have not declined substantially. Why the use of extended radical procedures began to rise again in 2012 is not intuitively clear.
We recognize several limitations of this study. First, the Nationwide Inpatient Sample contains a random sample of hospital discharge diagnoses, which are used to provide weighted estimates, not absolute numbers. Second, we lack data to define which patients received neoadjuvant therapy versus primary cytoreduction. However, a priori the intent of our analysis was not to compare procedure use between these treatments, but rather to describe the overall use of extended cytoreductive procedures. Third, some important clinical data including cancer stage, intraoperative details, postoperative course, details surrounding administration and timing of chemotherapy (including which patients did or did not receive NACT) is not available in NIS. Lastly, as with any study using administrative data, there may be inaccurate coding or undercapture of a small number of procedures. However, all of the procedures analyzed represent major interventions likely to generate a billing code, thus under-capture of a significant number of procedures is unlikely.
These data demonstrate that while there was a transient decrease in the use of extended cytoreductive procedures from 2010 to 2011 after the publication of the EORTC data, use of these procedures again increased in 2012 and 2013. The reasons underlying the rise remain unknown. As neoadjuvant chemotherapy is used more frequently, further comparative effectiveness studies to examine the impact of this treatment on both short and long-term outcomes are greatly needed.
Supplementary Material
Acknowledgments
The authors have no conflicts of interest or disclosures.
Dr. Wright (NCI R01CA169121-01A1) and Dr. Hershman (NCI R01 CA166084) are recipients of grants from the National Cancer Institute. Dr. Hershman is the recipient of a grant from the Breast Cancer Research Foundation/Conquer Cancer Foundation.
Footnotes
The authors have no conflicts of interest.
References
- 1.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–59. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 2.Hoskins WJ, Bundy BN, Thigpen JT, Omura GA. The influence of cytoreductive surgery on recurrence-free interval and survival in small-volume stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecologic oncology. 1992;47:159–66. doi: 10.1016/0090-8258(92)90100-w. [DOI] [PubMed] [Google Scholar]
- 3.Hoskins WJ, McGuire WP, Brady MF, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. American journal of obstetrics and gynecology. 1994;170:974–9. doi: 10.1016/s0002-9378(94)70090-7. discussion 9–80. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. National Cancer Institute monograph. 1975;42:101–4. [PubMed] [Google Scholar]
- 5.Chi DS, Eisenhauer EL, Zivanovic O, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecologic oncology. 2009;114:26–31. doi: 10.1016/j.ygyno.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Aletti GD, Dowdy SC, Gostout BS, et al. Aggressive surgical effort and improved survival in advanced-stage ovarian cancer. Obstetrics and gynecology. 2006;107:77–85. doi: 10.1097/01.AOG.0000192407.04428.bb. [DOI] [PubMed] [Google Scholar]
- 7.Wright JD, Herzog TJ, Neugut AI, et al. Effect of radical cytoreductive surgery on omission and delay of chemotherapy for advanced-stage ovarian cancer. Obstetrics and gynecology. 2012;120:871–81. doi: 10.1097/AOG.0b013e31826981de. [DOI] [PubMed] [Google Scholar]
- 8.Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. Journal of Clinical Oncology. 2016 doi: 10.1016/j.ygyno.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vergote I, van Gorp T, Amant F, Leunen K, Neven P, Berteloot P. Timing of debulking surgery in advanced ovarian cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2008;18(Suppl 1):11–9. doi: 10.1111/j.1525-1438.2007.01098.x. [DOI] [PubMed] [Google Scholar]
- 10.Vergote I, Tropé CG, Amant F, et al. Neoadjuvant Chemotherapy or Primary Surgery in Stage IIIC or IV Ovarian Cancer. New England Journal of Medicine. 2010;363:943–53. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 11.Morrison J, Haldar K, Kehoe S, Lawrie TA. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. The Cochrane database of systematic reviews. 2012;8:Cd005343. doi: 10.1002/14651858.CD005343.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. The Lancet. 386:249–57. doi: 10.1016/S0140-6736(14)62223-6. [DOI] [PubMed] [Google Scholar]
- 13.Melamed A, Hinchcliff EM, Clemmer JT, et al. Trends in the use of neoadjuvant chemotherapy for advanced ovarian cancer in the United States. Gynecologic oncology. 2016 doi: 10.1016/j.ygyno.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP) Rockville, MD: Agency for Healthcare Research and Quality; 2012–2014. Available at: https://www.hcup-us.ahrq.gov/nisoverview.jsp. Retrieved September 22, 2016. [Google Scholar]
- 15.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity Measures for Use with Administrative Data. Medical Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Houchens R, Ross D, Elixhauser A. HCUP Methods Series Report # 2006-05 ONLINE. U.S. Agency for Healthcare Research and Quality; Jan 4, 2015. Using the HCUP National Inpatient Sample to Estimate Trends. 2015. 2016. Available: http://www.hcup-us.ahrq.gov/reports/methods/methods.jsp. [Google Scholar]
- 17.Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP) Rockville, MD: Agency for Healthcare Research and Quality; 1993–2011. Trend Weights for HCUP NIS Data. Available at: https://www.hcup-us.ahrq.gov/db/nation/nis/trendwghts.jsp. Retrieved January 23, 2017. [Google Scholar]
- 18.American Cancer Society. [Accessed January 23, 2017];Cancer Facts & Figures 1998 to 2013. Available: http://www.cancer.org/research/cancerfactsstatistics/allcancerfactsfigures/index.
- 19.Dewdney SB, Rimel BJ, Reinhart AJ, et al. The role of neoadjuvant chemotherapy in the management of patients with advanced stage ovarian cancer: survey results from members of the Society of Gynecologic Oncologists. Gynecologic oncology. 2010;119:18–21. doi: 10.1016/j.ygyno.2010.06.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.