Abstract
Current ISC culture systems face significant challenges such as animal-derived or undefined matrix compositions, batch-to-batch variability (e.g. Matrigel-based organoid culture), and complexity of assaying cell aggregates such as organoids which renders the research and clinical translation of ISCs challenging. Here, through screening for suitable ECM components, we report a defined, collagen based monolayer culture system that supports the growth of mouse and human intestinal epithelial cells (IECs) enriched for an Lgr5+ population comparable or higher to the levels found in a standard Matrigel-based organoid culture. The system, referred to as the Bolstering Lgr5 Transformational (BLT) Sandwich culture, comprises a collagen IV-coated porous substrate and a collagen I gel overlay which sandwich an IEC monolayer in between. The distinct collagen cues synergistically regulate IEC attachment, proliferation, and Lgr5 expression through maximizing the engagement of distinct cell surface adhesion receptors (i.e. integrin α2β1, integrin β4) and cell polarity. Further, we apply our BLT Sandwich system to identify that the addition of a bone morphogenetic protein (BMP) receptor inhibitor (LDN-193189) improves the expansion of Lgr5-GFP+ cells from mouse small intestinal crypts by nearly 2.5-fold. Notably, the BLT Sandwich culture is capable of expanding human-derived IECs with higher LGR5 mRNA levels than conventional Matrigel culture, providing superior expansion of human LGR5+ ISCs. Considering the key roles Lgr5+ ISCs play in intestinal epithelial homeostasis and regeneration, we envision that our BLT Sandwich culture system holds great potential for understanding and manipulating ISC biology in vitro (e.g. for modeling ISC-mediated gut diseases) or for expanding a large number of ISCs for clinical utility (e.g. for stem cell therapy).
Keywords: intestinal stem cells, Lgr5, BLT Sandwich culture, integrin α2β1
1. Introduction
Rapid continual intestinal epithelial turnover throughout life is driven by Lgr5+ (Leucine-rich repeat-containing G protein-coupled receptor 5-expressing) intestinal stem cells (ISCs) residing at the crypt base, which give rise to all mature epithelial lineages along the crypt-villus axis [1]. ISCs are responsible for the homeostasis and regeneration of gut epithelium during normal tissue function and following injury [2].
A robust in vitro ISC culture system is crucial for understanding ISC biology and exploiting it for therapeutic applications. Multiple ISC culture systems have been reported and can be generally categorized as three-dimensional (3D) organoid (or “mini-gut”) or two-dimensional (2D) monolayer culture [3–10]. Most existing 3D culture systems require Matrigel, a murine-derived gelatinous protein mixture with undefined composition, batch-to-batch variation, and inherent xenogeneic contamination [11, 12]. Matrigel also presents a number of practical challenges during processing and handling, including temperature sensitivity and unstable physical properties. Alternatively, a 2D ISC culture system with a combination of Matrigel coating and an irradiated fibroblast feeder layer has been developed. However, this system raises similar concerns due to the presence of Matrigel and irradiated fibroblasts [9]. Most recently, Gjorevski et al. have developed a polyethylene glycol (PEG)-based synthetic matrix that supports initial ISC self-renewal and subsequent differentiation to form branching organoids [13]. The synthetic system addresses multiple limitations linked with Matrigel-based systems and may broaden the utility of ISCs in basic and clinical research. While this approach is appealing, the technical complexity associated with matrix engineering including temporally controlled degradation kinetics may limit the broad application of such system. Furthermore, 3D organoids are often highly variable in size and shape. Due to their physically enclosed structure, extracellular access is restricted from the apical/luminal side of the organoids. Thus, 3D organoids are considered suboptimal for modeling the interactions between enteric pathogens and host epithelium at the apical side. As a result, it is often challenging to scale up the throughput of such models [14, 15]. Therefore, the development of a robust and chemically defined, yet simple ISC culture system is imperative. We envisioned that a chemically defined monolayer culture on transwells could represent a viable solution to expand mouse and human Lgr5+ cells and obviate the issues linked with 3D organoids by providing unobstructed access to both basal and apical sides.
Collagen I (Col I) has been widely explored as Matrigel-alternative substrate coating or scaffolds for IEC culture [4]. It offers several appealing advantages over Matrigel including defined composition, better availability (i.e. through multiple tissue or commercial sources) and FDA approval for a broad spectrum of biomedical applications [16]. For instance, adult Lgr5+ ISCs or minced neonatal intestinal fragments were able to expand and form cystic structures when embedded inside Col I gel. However, the percentage of resulting Lgr5+ cells was either not robustly characterized or was noticeably low, i.e. <10% [4, 5, 7, 12, 15, 17–20]. Moreover, some of the Col I gel based IEC culture systems rely on stromal feeder cells or their conditioned media, which represents another variable and raise similar concerns as Matrigel. Besides Col I, other defined ECM proteins (e.g. laminin) have been attempted as substrate coatings for growing IEC monolayers, which offer a simple yet scalable solution for IEC expansion. However, their capability of maintaining Lgr5+ population is generally poor or unclear [5, 21].
Aside from the surrounding matrix or ECM cues described above, fate and function of ISCs are also tightly regulated by paracrine signals from adjacent niche constituents [22]. These signals target a number of key pathways including Wnt, Bone Morphogenetic Proteins (BMP), epidermal growth factor (EGF) and Notch, and they crosstalk to precisely balance the proliferation and differentiation of Lgr5+ ISCs [23]. For instance, we previously identified two small molecules that serve as potent chemical surrogates of essential ISC niche factors. Through reinforcing the canonical Wnt and Notch signaling, these molecules (CHIR99021 and valproic acid, “CV”) synergistically boost the level of Lgr5+ ISCs in a Matrigel-based 3D organoid culture [24]. We envisioned these potent signaling molecules might be useful in a Matrigel-free chemically defined culture system to expand Lgr5+ cells.
To maximize the expansion of Lgr5+ ISCs, we examined a novel monolayer culture system comprising chemically defined ECM components and small molecule modulators. Specifically, via screening a panel of basement membrane related ECM components, we first identified an ECM coating (Col IV) that supports the optimal IEC attachment and growth; then, to leverage the biological benefits of Col I and overcome the limitations linked with 3D culturing in Col I gels, a thin layer of Col I gel was overlaid onto the pre-seeded IECs on Col IV coating. Here we show that the Col I gel overlay provides essential integrin-mediated apical IECs-ECM interactions that are lacking from using thin Col IV coating on transwells alone. We envisioned that the bulk gel layer (rather than a thin superficial ECM coating or media supplement) may also serve as a permeable physical shield/structural support to hold the underlying epithelial cells in place (i.e. to prevent epithelial extrusion) and hence minimize cell anoikis during expansion [25]. This BLT Sandwich configuration effectively promotes the attachment and proliferation of IECs with the percentage of Lgr5-GFP+ population higher than using Col IV coating or Col I gel alone. Our BLT Sandwich solution maximizes cell accessibility to distinct ECM ligands that are essential for engaging key integrin receptors and cell polarity elements (i.e. maximal ZO-1, F-actin and integrin β1, 4), which appeared to be associated with Lgr5 expression. The optimized ECM composition acts in concert with the potent small molecule modulators we identified in this study (LDN-193189, a specific BMP antagonist) and previously (“CV”), to maximize the expansion of Lgr5+ ISCs. The BLT Sandwich culture system yields mouse Lgr5-GFP+ cells with similar frequencies and stemness comparable to standard 3D Matrigel culture, and it is also capable of growing freshly isolated crypts from both mouse and human intestinal tissues with high levels of Lgr5 expression. This Matrigel-free, chemically defined monolayer culture system demonstrates the potential to engineer cell-ECM interactions to control ISC biology and validate key signaling pathways essential for ISC self-renewal. This approach may be broadly applicable to other epithelial stem cell types. Overall, our BLT Sanwich culture system could represent a superior alternative to 3D organoid culture for research and clinical applications.
2. Materials & Methods
All animal-related procedures were approved by the Committee on Animal Care (CAC) of the Massachusetts Institute of Technology (MIT). Studies involving human materials were approved by the Committee on the Use of Humans as Experimental Subjects (COUHES) at Massachusetts General Hospital and MIT.
Mouse crypt isolation and characterization
Heterozygous Lgr5-EGFP-IRES-CreERT2 and wild-type C57BL/6J mice were obtained from Jackson Labs and maintained at animal facilities at MIT. 8–12-week-old mice were used for intestinal crypt isolation. Small intestinal (SI) and colonic crypts were isolated as documented [24]. Briefly, entire small intestine and colon tissues were harvested and flushed with cold PBS to remove luminal content. The tissues were then opened longitudinally and gently scraped with the blunt end of a razor blade to remove mucosal content and villi. Tissues were cut into 2–4 mm pieces followed by washing in cold PBS until the supernatant was clear. Tissue fragments were then incubated with EDTA (2 mM for small intestine, 5 mM for colon) for 10–20 min with gentle shaking. After removal of EDTA, the fragments were vigorously shaken with 2% FBS in cold PBS for 3–4 times to ensure crypt release. Crypt-containing supernatant fractions were pooled together, passed through a 70-μm cell strainer and centrifuged at 50 g for 1 min. The crypts were then washed thrice in basal media supplemented with 10 μM Y-27632 (see details in Supplement Table 1) to remove villi and single cells before further experiments or flow cytometric characterization. For flow cytometry, crypts were incubated with StemPro Accutase (Thermo Fisher) for 10 min, mechanically dissociated into single cells using a 23-Gauge syringe needle (BD), and passed through a 20-μm cell strainer (Miltenyi Biotec). The single cells were negative stained with 7-aminoactinomycin D (7-AAD, 1 μg mL−1, Thermo Fisher) and analyzed with BD LSR II flow cytometer or sorted for the Lgr5-GFPhigh population with BD FACS Aria I as previously reported [3].
Human crypt isolation
De-identified human SI or colon biopsies from patients undergoing gastrointestinal surgery were obtained through Massachusetts General Hospital Tissue Repository. Muscle and submucosa layers of the biopsy were carefully dissected and the remaining tissue was treated with dithiotreitol (10 mM, Sigma) for 5 min before being cut into 2–4 mm small fragments. The fragments were then incubated with EDTA (4 mM) with gentle agitation for 20–30 min at 4 °C, and the released crypts were harvested, filtered through a 200-μm strainer (pluriSelect) and washed following similar procedure with mouse crypt isolation.
3D Matrigel culture of Lgr5-GFP+ ISCs-containing organoids
Mouse or human crypts prepared from above steps were plated in Matrigel to form Lgr5-enriched organoids as previously reported [23]. Briefly, colonic or SI crypts from mice or patient biopsies were uniformly embedded in growth factor-reduced Matrigel (BD Bioscience). Cold Matrigel solution containing 150–200 crypts was plated at the center of pre-warmed 24-well plate (Fisher Scientific) and allowed to solidify for 20 min at 37 °C. Afterwards, full ISC maintenance media (500 μL) pre-determined for mouse and human intestinal/colonic epithelial stem cell culture was added and the culture was maintained for 4–6 days with media change every other day. Specific media formulations are detailed in Supplement Table 1. Upon completion of the culture, the organoids were vigorously mixed with a 200-μL pipette tip for 1–2 min in ice-cold basal media. Resulting organoid fragements (further referred to as intestinal epithelial cell (IEC) clusters) were passed through a 70-μm cell strainer and spun at 50 g for 1 min to ensure uniform cluster size and remove single cells. A proportion of the purified IEC clusters was taken for flow cytometric analysis as described above to confirm the baseline level of Lgr5-GFP+ population (i.e. 30–40% for mouse SI organoids) [24]. Remaining IEC clusters were counted and then either re-plated in fresh Matrigel or seeded in other culture settings as detailed below.
Screening of ECM coatings for optimal IEC attachment and growth
Matrigel-alternative ECM screen was perfomed using HTS Transwell-96 well plates with polycarbonate membrane (0.4 μm pore, Corning Life Sci.). Candidate ECM components included: fibulin 1 (human, Abcam), laminin-111 (mouse, Corning), fibronectin (bovine, Sigma), vitronectin (human, Stemcell Technologies), fibrinogen (salmon, Reagent Proteins), collagen I (Col I, rat tail, Corning), Col II (bovine, Corning), Col III (human, Advanced BioMatrix), Col IV (mouse, Corning). Individual ECM was diluted with Advanced DMEM/F12 media to a range of concentrations: 2, 10, 50, 100, and 150 μg/mL. The transwell was coated with different ECM dilutions for 22 μL/well for 2 hours at 37 °C. Coatings with 0.1% BSA and 30-fold dilution of Matrigel (hereinafter referred to as “Matri-coating”) in F12 media were included as negative and positive controls, respectively. 5,000 single IECs dissociated from Matrigel-expanded mouse SI organoids were seeded on different ECM coatings in 50 μL ISC maintenance media (i.e. ENR+CV) for 2 hours before unattached cells were washed off. In parallel, 20–30 IEC clusters derived from mouse SI organoids were seeded on different coatings and cultured for 2 days. Numbers of attached (2 hours after seeding with single cells) and proliferated IECs (2 days after seeding with clusters) were quantified by CyQUANT Cell Proliferation Assay Kit (Thermo Fisher). Since the assay kit relies on fluorescence reading (480nm/520nm Ex/Em) that overlaps with GFP, only the organoids cultured from SI crypts from GFP negative mice were used for this initial ECM screen. Matrigel-alternative ECM coating that yields the most significant IEC attachment and growth was identified and focused in further experiments.
Integrin α2β1 blocking assay
24-well transwell inserts with polyester membrane (0.4 μm pore, 1.12 cm2 surface area) were coated with predetermined ECM coating, i.e. 100 μg/mL Col IV, or 0.1% BSA in F12 media for 2 hours before cell seeding. Single IECs dissociated from mouse SI organoids were first treated with blocking buffer: basal media supplemented with 2% FBS, anti-mouse CD16/32 Fc blocker (2 μg/mL, BioLegend) and Y-27632 (10 μM) for 20 min. Afterwards, blocking buffer containing 100,000 IECs was respectively incubated with the following substances for 30 min on ice: integrin α2 antibody (10 μg/mL, BD Biosciences), integrin β1 antibody (10 μg/mL, BD Biosciences), combination of integrin α2 and β1 antibodies (each at 10 μg/mL), IgG1 κ isotype control (10 μg/mL, BD Biosciences), integrin α2β1 binding peptide (DGEA, 100 μg/mL, GenScript) [26], scrambled peptide control (RGES, 100 μg/mL, GenScript), EDTA (2 mM, negative control), and no treatment (positive control). IECs treated with different conditions were then allowed to attach for 2–3 hours on BSA- or Col IV-coated transwell. Attached single IECs were fixed with 4% paraformaldehyde (PFA, Fisher Scientific) and counterstained with Hoechst 33258 (Thermo Fisher) to visualize nuclei on a Nikon Eclipse TE2000 fluorescence microscope. 5–6 fields of view (2.1mm × 2.8mm) were acquired per transwell (n = 3 each condition) and cell number was quantified with ImageJ.
In addition to assessing integrin-mediated single IEC attachment, similar blocking experiment was performed with minor modifications to interrogate the role of integrin α2β1 played in ECM-mediated growth of Lgr5+ ISCs. Briefly, mouse IEC clusters were first incubated with single cell blocking buffer containing the combination of integrin α2 and β1 antibodies or their isotype controls for 30 min, then washed with basal media and seeded with 250–300 clusters per well in different culture systems with or without Col I overlay and Col IV coating as detailed below. A final concentration of 10 μg/mL of integrin α2 and β1 antibodies (each) was also mixed within the Col I overlay and supplemented in ENR+CV media over the entire course of a 4-day culture. The effect of integrin blocking on cell number, morphology and Lgr5 level was examined as detailed below.
Establishment of 2D BLT Sandwich culture
the transwells (0.4 μm pore, 1.12 cm2 surface area) was first coated with 170 μL Col IV (100 μg/mL). 200–250 freshly isolated crypts or Matrigel-expanded IEC clusters were suspended in 240 μL basal media and allowed to settle on the insert for 30 min. The supernatant was then carefully aspirated from the insert followed by immediately overlaying 170 μL ice-cold neutralized Col I solution (1.6 mg/mL) pre-mixed with Jagged-1 peptide (1 μM, AnaSpec). Prior to adding media, total number of IECs initially seeded per well and their percentage of Lgr5-GFP+ ISCs, was confirmed using 2 extra inserts by live cell counting and flow cytometry. Meanwhile, other inserts were kept at 37 °C for 20–25 min to ensure Col I gelation before adding full ISC maintenance media (500 μL inside insert and 600 μL outside), resulting in a Sandwich configuration consisting of a bottom Col IV coating, IEC middle layer and Col I gel overlay (i.e. Sandwich culture, see schematic in Figure 1). A silicone O-ring (medical grade, 1.7 mm width, 11.2 mm outer diameter, McMaster-Carr) was then gently fit on top of the overlay inside insert to minimize gel dislodgement during culture. The culture was maintained for 4–5 days with media change every other day before reaching 80–85% confluence. Afterwards, the capability of expanding ISCs was compared between Sandwich culture and analogous culture settings as follows: 3D encapsulation in Col I gel, Sandwich culture with Matri-coating replacing Col IV coating, Sandwich culture in between two Col I gel layers (i.e. soft Col I gel bed replacing thin Col IV coating on transwell), and standard 3D Matrigel organoid culture (as positive control). The thin coating and bulk gels involved in these culture settings were prepared following similar procedure detailed above for Col IV/Col I-based Sandwich culture and 3D Matrigel culture. All culture settings were compared using Matrigel-expanded mouse SI IEC clusters (with 30–40% Lgr5-GFP+ ISCs) and maintained in ENR+CV media for 4 days. Resulting organoids or IEC monolayers were incubated with dispase (0.2 mg/mL) and collagenase (50 U/mL) in trypsin-EDTA (0.25%, all from Sigma) for 20–30 min at 37 °C, mechanically dissociated into single cells, followed by counting of total live IECs (trypan blue exclusion) and flow cytometric analysis of 7-AAD-negative, Lgr5-GFP+ ISCs. Fold expansion of ISCs was calculated as: (number of total IECs multiplied by % of 7-AAD−/Lgr5-GFP+ ISCs)/baseline number of ISCs. Cell morphology (nuclear staining with Hoescht 33342, Thermo Fisher) and distribution of GFP+ ISCs (green) was imaged using Olympus FV2000 confocal microscope.
Figure 1.
Schematic of the 2D BLT Sandwich culture system. Intestinal crypts isolated from Lgr5-GFP donor mice or human intestinal biopsies are directly seeded onto Col IV-coated transwells. Alternatively, the isolated crypts can be pre-expanded in Matrigel to form Lgr5+ ISCs-enriched organoids following established methods [1]. The organoids are then mechanically dissociated into ISC-containing IEC clusters and seeded onto Col IV-coated transwells. Next, a thin layer of type I collagen solution is gently overlaid onto the settled cells and gelled, resulting in a sandwich configuration comprising Col IV coating/IECs/Col I gel overlay. Chemically defined ISC maintenance media containing essential signaling modulators (i.e. CV + BMP inhibitor LDN) is then added into the transwell insert. An epithelial monolayer containing high levels of Lgr5+ ISCs is rapidly generated via the combination of potent signaling factors and unique presentation of defined ECM cues.
Flow cytometric analysis of Ki67, lysozyme and integrins
IECs from different culture settings were washed with flow wash buffer (2% FBS, 0.09% NaN3 in PBS) and stained with Zombie UV Fixable Viability Kit (Biolegend) for dead cell exclusion. Afterwards, the cells were incubated with flow blocking buffer: 5% goat serum (Vector Labs) and mouse Fc blocker (1:100) for 10 min followed by staining with Alexa Fluor 647-integrin β4 (CD104) and PE-integrin β1 (CD29) antibodies (Biolegend) for 25 min on ice. Surface stained cells were immediately analyzed with BD LSR II flow cytometer. IECs without surface staining were fixed with 4% PFA for 10–15 min, permeabilized with 0.1% saponin (Sigma) in PBS for 10 min, and then treated with flow blocking buffer supplemented with 0.08% saponin for 20 min. Thereafter the samples were incubated with primary antibodies against Ki67 (ab15580 from Abcam, 1:300) and lysozyme (A0099 from Dako, 1:400), as well as their respective isotype controls with equal dilution: polyclonal rabbit IgG (ab171870 from Abcam), polyclonal rabbit Ig fraction (X0936 from Dako) and monoclonal rabbit IgG (ab172730 from Abcam). After 30 min incubation with gentle shaking, cells were washed twice with flow wash buffer containing 0.08% saponin, subsequently stained with Alexa Fluor 647 goat anti-rabbit IgG secondary antibody (Thermo Fisher, 400X) and washed thoroughly before being analyzed with BD LSR II flow cytometer. All flow cytometry data was analyzed by FlowJo.
Immunocytochemistry staining
Samples from the BLT Sandwich culture or 3D Matrigel control were first rinsed with PBS and fixed with 4% PFA for 30 min. 3D organoids in Matrigel were then mechanically disrupted and carefully transferred to BSA-coated low-retention tubes (Fisher Scientific). The IEC monolayer on transwell, i.e. Sandwich culture, or organoids were then permeabilized with 0.4% Triton X-100 (Sigma) for 30 min, blocked with 5% goat serum and stained overnight at 4 °C with the same primary Ki67 and lysozyme antibodies detailed above for flow cytometry, as well as the following: anti-Muc2 (sc-15334 from Santa Cruz, 1:75), anti-ZO1 tight junction protein (ab59720 from Abcam, 1:50), anti-DLL1 (ab10554 from Abcam, 1:100), anti-Chromogranin A (ChgA, sc-13090 from Santa Cruz, 1:100). Alexa Fluor 594 or 647 conjugated antibodies (Thermo Fisher, 500X) were used as secondary antibodies and co-stained with TRITC-phalloidin (Thermo Fisher, 200X) for 1 hour to visualize F-actin if desired. Samples were then thoroughly rinsed with 0.05% Tween-20 in PBS and counterstained with Hoescht 33342 before confocal imaging. GFP/green stain represents the presence of Lgr5-GFP+ stem cells unless otherwise specified. All fluorescence images shown were acquired by Olympus FV2000 confocal microscope and merged from 15–20 sections/images of 3D Z-stacks (total 100–150 μm scan depth). Moreover, intestinal alkaline phosphatase (Alpi) was stained by Alkaline Phosphatase Staining Kit II (00-0055, Stemgent) in accordance with vendor’s instruction, and imaged using Nikon Eclipse TE2000 inverted microscope.
Quantitative real-time PCR (qPCR) analysis
RNAs from 2D Sandwich and 3D Matrigel culture were extracted using TRI Reagent (Sigma) according to the manufacture’s procedure. Total RNA (1 μg) were reverse transcribed into cDNA using QuantiTect Reverse Transcription Kit (Qiagen). 15 μL reaction volume per well containing 15 ng cDNA, 0.4 μM forward and reverse primers (each) and 7.5 μL Power SYBR Green PCR master mix (2X, Thermo Fisher) was loaded onto MicroAmp Optical 384-well reaction plate (Thermo Fisher). PCR reactions were performed using ABI 7900HT fast real-time sequence detection system following thermal cycling program as per manufacturer’s recommendation. qPCR primers were designed using Primer3 online tool and obtained from Integrated DNA Technologies. Specificity and efficiency of each primer pair were validated using cDNA derived from mouse IECs following previously established guideline and procedure [27, 28]. The information of specific primer sequences targeting mouse mRNA encoding ChgA, Muc2, Lyz1, Alpi, Lgr5, and house-keeping genes (i.e. GUSB, B2M, Vil1, and HPRT) was listed in Supplement Table 2. Comparative Ct method (ΔΔCt) using geometric mean Ct from the four house-keeping genes as internal control was employed for the calculation of relative mRNA expression [29]. qPCR analysis for human IECs was performed following the procedures as we previously described [24]. All groups were tested with at least 3 biological replicates and repeated three times with crypts or organoids derived from three different donor mice and patients.
Statistical analysis
Sample size (n) and method of data presentation are specified in each figure legends. Statistical difference between groups was determined using one-way analysis of variance (ANOVA) and Tukey–Kramer post hoc test for multiple comparisons. Significance levels are indicated as: ** p < 0.01, *** p < 0.001. Groups without significant difference, i.e. p > 0.05, are labeled as “NS”.
3. Results & Discussion
Here, we implemented a step-by-step approach to establish an efficient 2D ISC culture system via the rational combination of ECM cues and small molecule signaling modulators. First, we identified a Matrigel-alternative ECM coating which maximizes the attachment and proliferation of IECs. We then refined the configuration of ECM cues (i.e. BLT Sandwich culture, see schematic in Figure 1) to significantly enrich the Lgr5+ ISC population. To identify relevant ECM components in the BLT sandwich culture system, we used full ISC maintenance media (ENR+CV, details in Supplement Table 1), given its ability to enrich Lgr5+ ISCs in 3D Matrigel culture [24]. Finally, we identified a small molecule inhibitor of BMP receptors, which further improves the proliferation of Lgr5+ cells in our BLT Sandwich culture.
3.1. Identification of Type IV Collagen as a Matrigel Alternative Substrate for the Growth of Intestinal Epithelial Cells (IECs)
It has been difficult to establish primary cultures of monolayer IECs with high frequency of stem cells. This is due to the stem cells’ intrinsic dependence on multiple niche factors (e.g. balanced Wnt/BMP activity) and appropriate cell-cell/cell-matrix contact [30–32]. Lacking the presentation of these factors could lead to rapid cell death/anoikis or differentiation in vitro [33].
The proliferation, migration and differentiation of crypt cells in vivo are susceptible to changes in basement membrane (BM) ECM compositions and the corresponding integrin receptors (e.g. integrin α2β1) along the crypt-villus axis [31, 34–37]. Inspired by these findings, we first sought to identify an ECM coating that could maximize IEC attachment and growth in vitro, through screening the major ECM components found in intestinal BM. Consistent IEC attachment efficiency of around 60% (defined as number of cells attached normalized to number of total cells added per well) was observed on coatings of different Matrigel dilutions: 10X, 20X, 30X, 50X (standard dilution range used for Matrigel thin coating, data not shown) [38]. Therefore, as we sought to identify an equally effective, yet Matrigel-free coating, 30X Matrigel dilution was selected as positive coating control for this experiment and is further referred to as “Matri-coating”. Col IV emerged as the lead ECM coating supporting both the initial attachment of single IECs (Figure 2A) and the growth of IEC clusters (within 2 days, Supplement Figure 1). Specifically, we found that a coating concentration of Col IV of 100 μg/mL (i.e. surface-adsorbed density ~3.4 μg/cm2, Supplement Figure 2) led to the highest IEC attachment efficiency: 56 ± 7%, which is comparable to that of the Matri-coating. Similar attachment efficiency (55–65%) has also been reported for epidermal cells and corneal epithelial cells plated on Col IV-coated substrates with similar coating concentrations [39, 40].
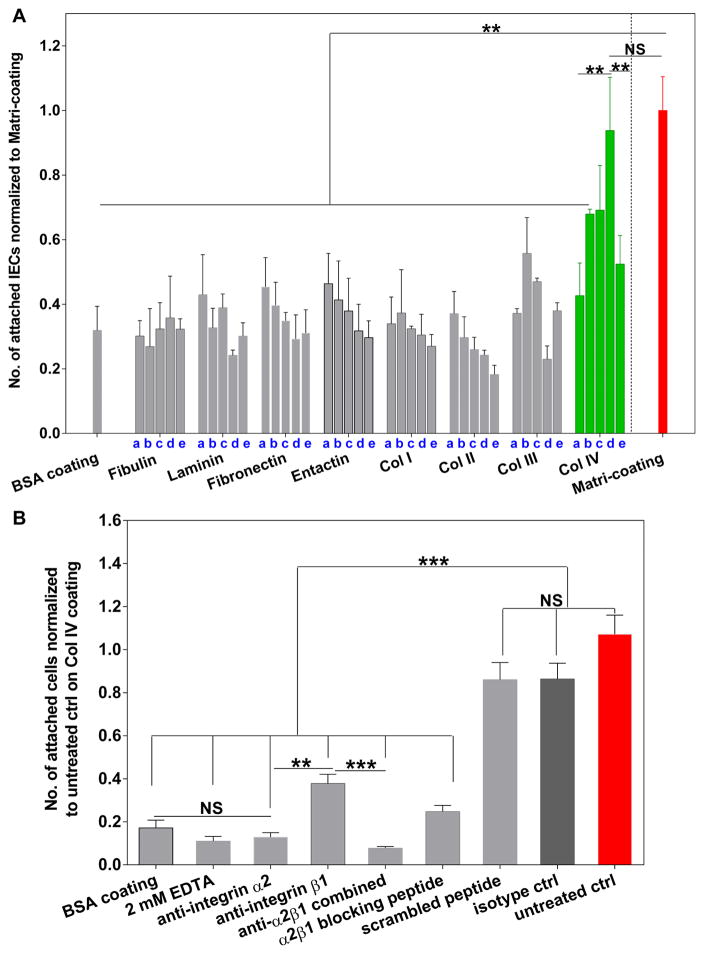
Figure 2.
Identification of Col IV as a Matrigel alternative coating for the maximal attachment of IECs. A: Define BM ECMs with different coating concentrations that are most supportive for the initial attachment of single IECs on transwells. a–e: 2, 10, 50, 100 and 150 μg/mL. Two hours after seeding, the transwells were gently washed with 500 μL cold PBS for 3 times to remove unattached cells. The number of attached IECs per well on each coating was measured by Cyquant DNA quantification assay and normalized to the coating with 30X dilution of Matrigel in F12 media, i.e. Matri-coating (red bar). Transwells coated with 0.1% BSA was included as negative control. Data represents mean ± SD, n = 2–3 wells, representative from three independent trials. B: Towards understanding the mechanism of Col IV mediated single IEC attachment: image quantification for the number of attached IECs after 2 hour incubation on Col IV (100 μg/mL)-coated transwells. Transwells in all groups were pre-coated with Col IV prior to cell seeding, except the BSA coating group (seeded with untreated IECs) highlighted in grey bar. Single IECs derived from Matrigel-expanded SI organoids were pretreated with integrin α2, β1 antibodies, their combination and isotype controls, as well as integrin α2β1 binding peptide (DGEA) and the scrambled peptide sequence (RGES). To further confirm that the Col IV-mediated cell adhesion is integrin- or divalent cation-dependent, IECs were also seeded in the presence of 2 mM EDTA for the 2 hour incubation. Mean ± SEM, n = 10–12 fields of view per group, representative from three independent trials with single IECs derived from different passages in Matrigel.
Our findings align with previous studies which showed that the attachment of epithelial cells was greatest on Col IV-coated surfaces as compared to other ECM coatings. Col IV efficiently engages integrin (e.g. integrin β1) bindings and leads to rapid epithelial cell attachment within minutes [41–43]. Of note, Col IV coating has long been utilized to separate human epidermal stem cells from transit amplifying cells via the rapid adhesion of stem cells to Col IV. This suggests the potential role of Col IV for not only enhancing overall IEC attachment, but also enriching the ISC population [44]. Compared to other ECM components tested in this study, Col IV-mediated IEC attachment exhibits a distinct coating concentration dependence which peaks at 100 μg/mL (3.4 μg/cm2). Col IV adsorption density peaks at 150 μg/mL (Supplement Figure 2), which is indicative of a saturated surface coating. Coating concentrations higher than 100 μg/mL did not further enhance IEC attachment and growth (Figure 2A, Supplement Figure 1). We suspect that this might be due to the excessively high density of Col IV binding sites that restricts the availability of free surface integrins and results in limited cell motility or contractile forces, which are critical regulators of cell adhesion [45, 46].
Furthermore, we confirmed that the attachment of IECs on Col IV is divalent cation- or integrin α2β1-mediated, as evidenced by the dramatic reduction (>65%) of cell attachment upon treatment with EDTA, integrin α2β1 antibodies or the integrin α2β1 specific binding peptide, DGEA (Figure 2B). Integrin receptors of Col IV primarily belong to the β1 subgroup, namely α1β1 and α2β1 [47, 48]. In adult intestinal epithelium, α2β1 is predominantly associated with the lower crypt region where Lgr5+ cells reside [1, 49]. Crypt cells sit tightly on BM containing Col IV but lacking laminin, suggesting that in the intestinal epithelium, α2β1 integrin may primarily act as the receptor for collagen IV rather than laminin [37]. This hypothesis is also partially reflected by our ECM screening results where we found that laminin coating exhibited minimal support for IEC attachment and growth. Collectively, our results are reminiscent of in vivo findings which highlight the significance of the Col IV-integrin α2β1 axis for the function of intestinal epithelium and further substantiate our ECM screening results.
3.2. Establishment and Characterization of the BLT Sandwich Culture System
After optimizing the ECM coating for IEC attachment, we next decided to refine the configuration of the culture system to further enhance the percentage of Lgr5-GFP+ ISCs in the resulting IEC monolayer. During the initial ECM screen, we noted that when ECM-coated transwells were seeded with a single cell suspension of IECs initially containing ~35% Lgr5-GFP+ cells in ENR+CV media, > 90% cells did not survive more than 48 hours and the remaining/attached live cells were nearly all Lgr5-GFP negative. A similar trend was observed when the Col IV coating was seeded with Matrigel pre-expanded IEC clusters or organoids. Under these conditions, less than 5% of the cells were Lgr5-positive after 2 days and the Lgr5-GFP+ population was nearly abolished after 4–5 days (Figure 3A). It was also noticed that IEC clusters or crypts quickly collapsed into loose aggregates or single cells within 3–5 hours of seeding, and only those directly in contact with Col IV coating were able to attach and survive. We suspect that the rapid loss of Lgr5-GFP+ cells is largely due to the combination of ISC differentiation and cell death/anoikis, which has been linked to the insufficient ECM anchorage and cell-cell contact [50, 51]. Of note, during our initial ECM screen, we have observed comparably significant Lgr5 loss among all the coating conditions (Lgr5-GFP all less than 0.5% after 3 days, unpublished observation/data). To address this issue, inspired by existing epithelial cell culture systems where two Col gel layers were employed in a Sandwich configuration to maximize monolayer cell-ECM interactions, we introduced another layer of Col I gel onto the pre-seeded crypts or IEC clusters (Figure 1) [19, 52]. While taking advantage of the biocompatibility, superior availability/lower cost (relative to other commercially available ECM proteins such as Col III and laminin at their gelling concentrations), and well known cell-interactive properties of Col I gel, the unique top-bottom presentation of ECM cues will ensure sufficient cell-matrix/cell-cell interactions and presumably maintain appropriate cell orientation, which we considered to be essential for IEC growth and Lgr5 expression.
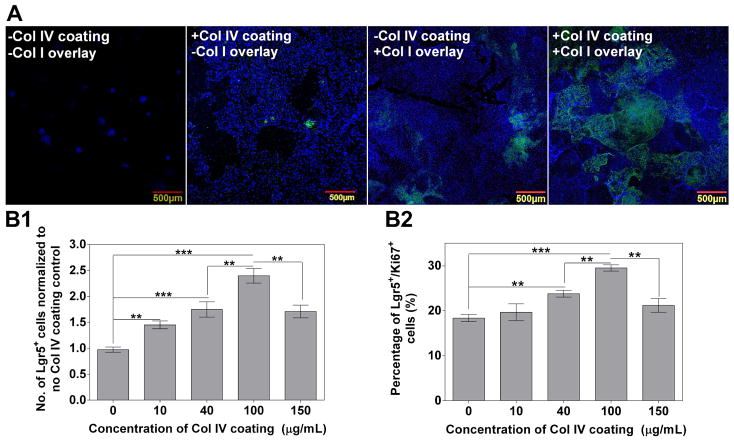
Figure 3.
Optimization of the BLT Sandwich culture system using mouse SI organoids under different culture settings for 4 days. A: Sandwich configuration composed of Col IV thin coating (100 μg/mL) and Col I gel overlay synergistically enhances the expansion of Lgr5+ cells. Representative confocal images (A) show the morphology and presence of Lgr5-GFP in IEC monolayer on transwells under different culture settings with or without Col IV coating and Col I overlay. B1–2: Col IV coating concentration dictates the number of Lgr5-GFP+ cells (B1, normalized to no Col IV coating control) and the percentage of Lgr5/Ki67 co-positive population (B2). n = 3–4 wells, data represents mean ± SD from one representative trial out of three independent trials using SI organoids derived from three donor mice.
As shown in Figure 3, Col I overlay and Col IV coating synergistically enhances the growth of total IECs and the percentage of Lgr5-GFP+ cells on transwells. The synergistic effect on ISC growth is more profound on porous transwells than on TCPS (Supplement Figure 3), likely due to ease of nutrient access to both sides of the cells on the transwells, which is also beneficial for epithelial cell polarization and proliferation [53, 54]. Therefore, we elected to use transwell inserts for the remainder of this study.
To simplify the discussion hereinafter, the BLT Sandwich culture with Col IV coating and Col I overlay is referred to as “+C4+C1”; the non-Sandwich condition that uses Col IV coating but does not have a Col I overlay is referred to as “+C4-C1”; the non-Sandwich condition that has neither a Col IV coating nor Col IV overlay is referred to as “−C4-C1” and the condition that uses a Col I overlay but no Col IV coating is referred to as “−C4+C1”. No difference in total IEC growth was observed between the “−C4+C1” and “+C4+C1” conditions on TCPS. However, the same conditions on transwells resulted in a greater than 1.5-fold increase from “−C4+C1” to “+C4+C1”, implying the synergy between Col IV coating and the substrate type. Furthermore, IEC clusters seeded in the “−C4+C1” condition yielded twice as many IECs than those in “+C4-C1” condition on transwells after a 4-day culture. Additionally, both conditions were significantly more conducive (> 5-fold) to IEC growth than the “−C4-C1” condition. Col I gel overlay plays a critical role for preserving Lgr5 levels regardless of the substrate type. The “−C4+C1” condition yielded a percentage of Lgr5-GFP+ population around 10%, which is markedly higher than “−C4-C1” and “+C4-C1” conditions on both transwells and TCPS (Supplement Figure 3). Among all four conditions, the BLT Sandwich culture, i.e. “+C4+C1”, most efficiently drove IEC proliferation and had the highest level of Lgr5-GFP+ cells: 35 ± 4 % on transwells and 20 ± 2 % on TCPS (Supplement Figure 3B).
Furthermore, by varying Col IV coating concentrations from 0–150 μg/mL in the “+C4+C1” condition, the number of resulting Lgr5-GFP+ cells showed a distinct concentration dependence that peaked at 100 μg/mL (Figure 3B1). A similar trend was observed for the percentage of Lgr5 and Ki67 co-positive cells (i.e. actively-dividing Lgr5-GFP+ cells) as shown in Figure 3B2, which is also consistent with our initial ECM screen results without Col I gel overlay (Figure 2A). These findings suggest that optimal presentation of matrix cues efficiently dictates IEC growth with a high percentage of Lgr5+ population and proliferative capability.
Next, we compared the performance of the BLT Sandwich culture system to existing similar culture systems which may be also capable of expanding IECs. In addition to standard 3D Matrigel culture, we compared 3D embedding in Col I gel, 2D Sandwich in between two layers of Col I gels and 2D Sandwich conditions with identical Col I gel overlay but distinct substrate coatings (100 μg/mL Col IV vs. Matri-coating). When seeded with identical number of IEC clusters and cultured in ENR+CV media for 4 days, all of the conditions yielded IECs with comparable levels of Lgr5-GFP+ population, between 30–40% (Supplement Figure 4). 2D Sandwich conditions with Col IV coating or Matri-coating formed island-like clusters with diameters greater than 500 μm. Similar cystic monolayer structures were observed in 3D Col I gel and 2D Sandwich between two Col I gel layers, but with much smaller cluster sizes than the other two Sandwich conditions. This observation is in agreement with the total IEC number quantification results (Supplement Figure 4B1). The slower growth of IECs found in 3D Col I gel and 2D Col I gel Sandwich culture conditions is associated with markedly lower number of Lgr5-GFP+ cells compared to the BLT Sandwich culture on Col IV-coated transwells (Supplement Figure 4B2). We speculate that different stiffness of the surrounding Col I gel matrices and the polyester-based transwell substrate may contribute to the distinct IEC proliferation rates, through a cellular contractile force-dependent mechanism [35, 55].
We then attempted to understand the mechanism governing the synergistic effect of Col IV coating and Col I overlay on the growth of Lgr5-GFP+ cells. The “+C4+C1” condition exhibits much stronger and more uniform F-actin and ZO-1 staining compared to the “-C4+C1” and “+C4-C1” conditions. The “+C4+C1” condition also results in decreased cell-to-cell distance (Supplement Figure 5A). F-actin denotes the apical side of epithelial cells, and it mediates actomyosin contractility which stabilizes integrin binding [56]. ZO-1 maintains the integrity of epithelial lining by concentrating at the apically localized tight junctions [57]. F-actin and ZO-1 expression in the “+C4+C1” conditions correlates with the augmented expression of integrin β1 and β4 relative to the other two conditions without Col I or Col IV (Supplement Figure 5B1–2). Of note, a similar pattern of ZO-1 and F-actin expression and localization was also observed in Matrigel-based intestinal organoid culture, where the maintenance of normal tissue organization and genotype has been linked to the presence of intact cell apical-basal polarity [58]. The integrin β1/β4 co-positive population was increased from 56.7% in the “+C4-C1” condition to 87.7% in the “+C4+C1” condition, suggesting the sandwiched presentation of distinct ECM cues effectively activates integrins which may be essential for cell adhesion, survival and maintaining the orientation of the cell polarity axis [58–60]. Such axis provide distinct domains (e.g. lateral and basal) through which multiple mutually reinforcing extracellular cues act on and regulate stem cell self-renewal, such as epithelial growth factor receptor and cadherin adhesion receptors [56, 61, 62]. Moreover, the heterogeneity of the levels of ISC proliferation and stemness in vitro appears to be mediated by their levels of surface β1 integrin expression. Higher levels of β1 integrin have been linked to more rapid epithelial stem cell adhesion to Col IV substrates, higher colony forming efficiency, and delayed onset of terminal differentiation [44].
The overall IEC growth and percentage of Lgr5-GFP+ population in our BLT Sandwich culture are primarily mediated by integrin α2β1 (Supplement Figure 5C), which is one of the major receptors for Col I and Col IV. Integrin α2β1 blocking significantly suppressed more than 70% of the total number of IECs and nearly completely abolished Lgr5 expression in the BLT Sandwich culture. With respect to the “+C4-C1” condition, >95% of attached IECs lost Lgr5 expression after 4 days but integrin α2β1 blocking did not significantly diminish their attachment or number. This implies that in the absence of Col I overlay, other integrins may also dictate IEC-Col IV interactions or the cells may attach to endogenously synthesized ECMs without relying on integrin α2β1. Noteworthy, mounting evidence suggests that Lgr5+ ISCs drive the polarization between intestinal regeneration and tumorigenesis via YAP-dependent stem cell reprogramming [63]. In in vitro 3D ISC culture settings, YAP-mediated oncogene activation is particularly relevant when matrix mechanical properties are taken into account [13]. Further mechanistic insight into how different ECM cues regulate ISC growth and fate decision in our 2D BLT system, i.e. potentially via Hippo/YAP pathway, remains a key aspect to be investigated in our future studies.
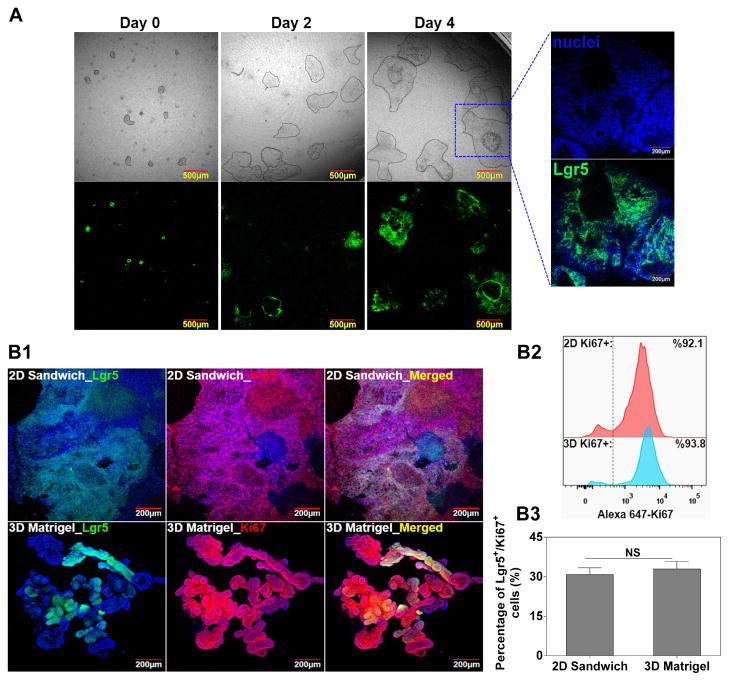
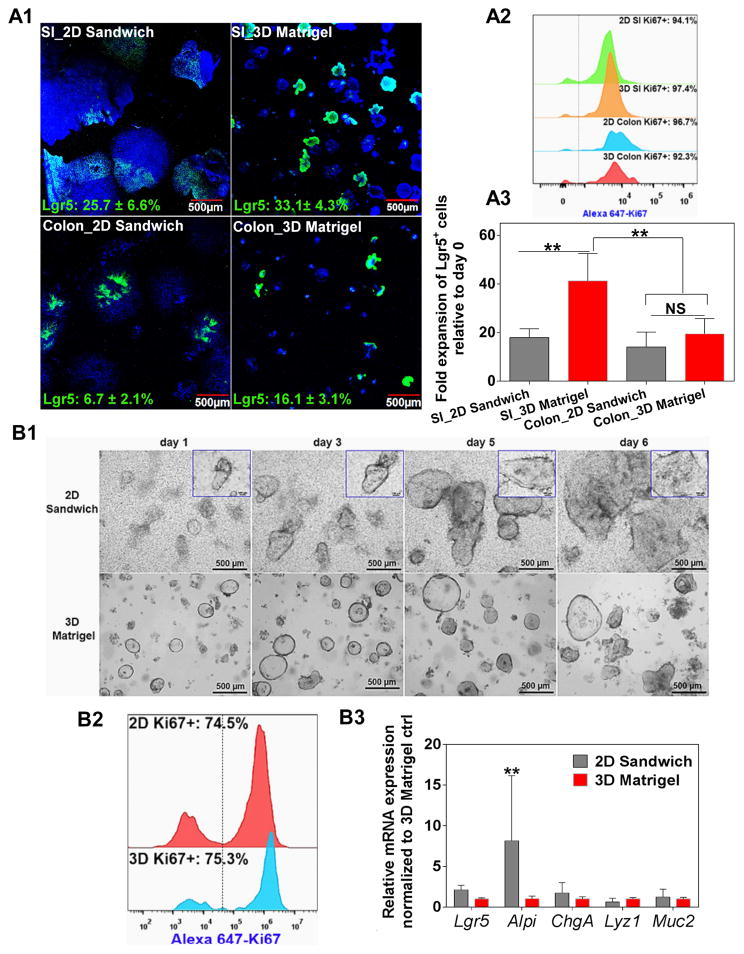
Morphologically, the BLT Sandwich culture-derived IEC monolayer exhibits an undulating pattern (Figure 4A) reminiscent of the topography of native intestinal epithelium. While this observation is intriguing, our confocal Z-stack imaging confirmed that it is indeed a monolayer structure without distinct local cell stratifications, as evidenced by Supplement Video 1 and the Z/side view of the Z-stack imaging of the BLT system (Supplement Figure 13). We argue that the observed undulating pattern may be associated with the heterogeneous cell-cell distance or cell junctions within the monolayer, which may indicate the heterogeneity of the ISC/IEC populations in our system and warrants further investigation. Moreover, when compared to 3D Matrigel culture, IECs from the BLT Sandwich culture were equally proliferative (> 90% Ki67+) and enriched with comparable percentage of Ki67/Lgr5 co-positive cells around 30% (Figure 4B). By varying the initial seeding density of IEC clusters, a relatively consistent fold expansion of Lgr5-GFP+ cells: 10.5 ± 2.8-fold, was revealed in the BLT Sandwich culture over 4 days. This is equivalent to approximately one cell division per 24–36 hours for the Lgr5+ cells, which is comparable to the cycling rate of native ISCs in vivo [1]. However, when an identical number of IEC clusters were plated 3D in Matrigel (35 μL/gel) for the same period (4 days), fold expansion of Lgr5-GFP+ cells varied significantly with the initial seeding density, and the numbers of Lgr5-GFP+ cells were generally higher than in BLT Sandwich culture (Supplement Figure 6). It is worth noting that we only compared the growth rate between 2D BLT Sandwich and 3D Matrigel systems within a relatively short culture period (4 days) before the IEC monolayer or organoids exhibited appreciable cell death due to space constraints. Significantly variable ISC fold expansion seen with the 3D Matrigel culture could be attributed to the complex mass transport and heterogeneous organoid size/shape, which may impair its robustness for large scale ISC production or serving as cellular models. Whereas this is clearly less of an issue for 2D BLT Sandwich culture owing to its monolayer nature, i.e. scalable by simply changing substrate surface area, and unobstructed mass transport mediated by the transwells.
Figure 4.
Characterization of the BLT Sandwich culture system. A: Representative phase contrast and fluorescence images showing the proliferation and presence of Lgr5-GFP of IEC monolayer at day 4. Contour of epithelial island/monolayer is delineated by dashed lines in phase contrast images for better visualization. Zoomed-in view depicts the undulating pattern of IEC monolayer/sheet that is closely associated with Lgr5-GFP+ cells. B1: Confocal images comparing the expression of Lgr5 and Ki67 between 2D Sandwich and 3D Matrigel culture systems at day 4 (a); B2–3: Flow cytometry results comparing the level of Ki67+ cells (B2) and Lgr5/Ki67 co-positive cells (B3) between the two culture systems. Mean ± SEM, averaged from n = 3–4 independent trials with different SI organoid donor mice.
With respect to the cellular composition, we speculate that the 60–70% of Lgr5−/Ki67+ cells expanded from our BLT Sandwich culture are actively dividing cells prone to differentiate into enterocytes through Lgr5−/Ki67+ and/or Lgr5−/Ki67− transient amplifying cells (TAs) or enterocyte precursors [64]. This is supported by our findings suggesting that IECs from the BLT Sandwich culture had an elevated expression of absorptive lineage/enterocyte marker (Alpi) and lacked both Dll1 (marker for early secretory progenitors) and mature secretory lineage markers (Lyz1, Muc2, ChgA) relative to the 3D Matrigel control in ENR+CV media (Supplement Figure 7) [65]. From an in vivo perspective, the highly proliferative Lgr5−/Ki67+ TAs or enterocyte lineage progenitors have been shown to transdifferentiate and replace lost Lgr5+ stem cells and promote crypt regeneration under tissue injury [66]. Therefore, in addition to the Lgr5+ cells, the remaining Lgr5−/Ki67+ cells found in the IEC populations we are expanding may be clinically beneficial as cell therapies for intestinal epithelial regeneration.
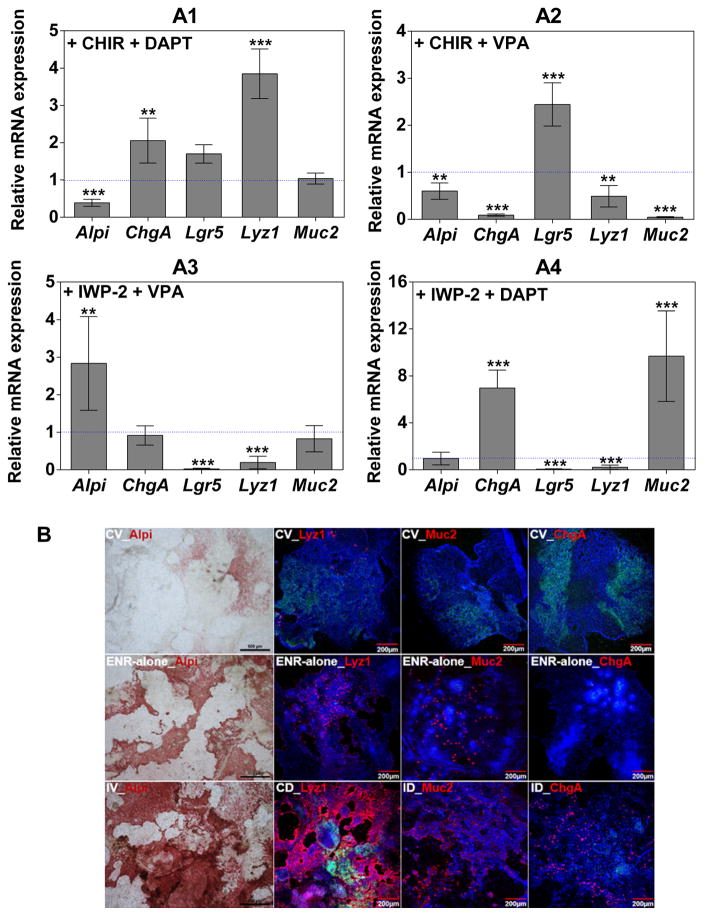
We sought to further characterize the system by validating the multipotency and stemness of Sandwich culture-derived Lgr5-GFP+ cells. We first assessed the multi-lineage differentiation potential of monolayer IECs (with ~35% Lgr5-GFP+ cells) under multiple chemical induction factors (Figure 5). After 4 days in ENR+CV media, the BLT Sandwich culture was switched to media containing different combinations of Wnt and Notch modulators for another 3 days to coax lineage-specific differentiation of ISCs [24]. The differentiation conditions are defined as ENR-alone media supplemented with the following combinations of small molecules: Wnt inhibitor + secretory lineage inhibitor (IWP-2 + VPA (IV), enterocytes); Wnt agonist + Notch inhibitor (CHIR + DAPT (CD), Paneth cells); Wnt inhibitor + Notch inhibitor (IWP-2 + DAPT (ID), goblet cells and enteroendocrine cells). The ENR-alone condition is known to drive the simultaneous differentiation of ISCs into multiple mature IEC lineages [24]. Compared to the ENR-alone condition, CD, IV and ID treatment significantly elevated the expression of different desired lineage specific markers as shown in Figure 5A. Additionally, ISC maintenance media (ENR+CV) notably upregulated Lgr5 mRNA expression by 2.45-fold relative to the ENR-alone control, whereas other lineage marker genes were all significantly suppressed by at least 40%. Such multi-lineage differentiation potential was also validated at the protein level by immunocytochemical staining (Figure 5B). The BLT Sandwich culture under different chemical induction cues resulted in significantly more apparent Alpi, lysozyme, mucin 2 and ChgA staining than their respective controls under ENR-alone and ENR+CV conditions. Appreciable levels of Lgr5-GFP+ cells remain in the CD condition where nearly 90% of IECs are lysozyme-positive (see image quantification results in Supplement Table 3). This finding may underscore the putative relationship or dependence between ISCs and their niche constituent—Paneth cells. However, it is practically challenging to directly correlate the percentage of differentiation in our in vitro system (e.g. under ENR-alone condition) to the in vivo settings, as more definitive IEC lineage markers will need to be further identified/evaluated, and it’s unclear how closely the existing in vitro differentiation protocols recapitulate the in vivo niche. We propose that these are important questions to be addressed together by the whole ISC field.
Figure 5.
Validation of the multi-lineage differentiation potential of BLT Sandwich culture-expanded ISCs. A1–4: qPCR analysis of relative mRNA expression of Lgr5 and mature IEC markers (Alpi, Lyz1, Muc2 and ChgA) from monolayer IECs (with ~35% baseline Lgr5-GFP level) treated with indicated differentiation cocktails for 3 days with ENR being present in all conditions. mRNA levels of target genes were normalized to ENR-alone conditions. Mean ± SEM, n = 3 independent trials each with 2–3 biological repeats. **, ***: significantly different versus ENR-alone control. B: Colorimetric staining (Alpi) and immunocytochemistry staining of mature IEC lineage markers after 3-day differentiation. Representative images from three independent trials with n = 2 wells (8–10 images) each condition. Scale bars for all Alpi images: 500 μm.
Finally, we validated that single Lgr5-GFP+ ISCs purified from 2D BLT Sandwich and 3D Matrigel cultures yielded comparable colony-forming efficiency (CFE) 60–70% and levels of Lgr5-GFP+ cells around 70% when subsequently cultured 3D in Matrigel for another 7 days in ENR+CV media, further confirming the stemness of BLT Sandwich culture-expanded Lgr5+ ISCs (Supplement Figure 8).
3.3. The BLT Sandwich Culture is Capable of Expanding Mouse Colonic Stem Cells and Human-Derived IECs
After establishing and characterizing the BLT Sandwich culture using Matrigel pre-expanded mouse SI organoids (or IEC clusters), we then explored the BLT Sandwich culture’s utility for growing Matrigel pre-expanded colonic organoids derived from both mouse and human colon crypts as well as freshly isolated mouse and human small intestinal crypts. From a translational perspective, the capability of culturing patient-derived intestinal stem cells in a Matrigel-free system offers significant impact towards personalized stem cell therapy or intestinal disease modeling.
Mouse colonic IEC clusters were able to attach to and expand into large (> 500 μm) island structures within 4 days in the BLT Sandwich culture, similar to SI IEC clusters (Supplement Figure 9A–C). The resulting monolayer consists of more than 92% Ki67+ cells and 11 ± 3.5% Lgr5-GFP+ colonic stem cells (CSCs), which were expanded around 4-fold in mouse CSC maintenance media in 4 days. When initially seeded with 150–180 clusters per well, fold expansion of CSCs was slightly lower in the BLT Sandwich culture than 3D Matrigel control, and the percentage of resulting Lgr5-GFP+ CSCs was about half of the Matrigel control (~20% overall). This suggests that the BLT Sandwich culture-expanded colonic IECs contain a large proportion of proliferating cells that are Lgr5-GFP negative, and they generally expand faster than those in Matrigel (hence diluting the % of resulting Lgr5-GFP+ CSCs more). Additionally, Matrigel pre-expanded human colonic organoids with initial diameter < 100 μm can be expanded in the BLT Sandwich culture and form large monolayers (> 1 mm2 in surface area coverage) in human ISC maintenance media (ENR+gWNASP, refer to Supplement Table 1) over 6 days (Supplement Figure 9D). This is of particular relevance when the resultant monolayers are used as autologous cell patches to regenerate the damaged intestinal epithelium. The large surface area of the cell patches may allow better in vivo engraftment efficiency than their smaller counterparts, which is crucial to the success of the cell therapy [67].
Furthermore, we have demonstrated that freshly isolated mouse SI and colonic crypts can be directly seeded in our BLT Sandwich culture, without Matrigel pre-expansion, and grow substantially in their respective maintenance media (Figure 6). The capability to expand freshly-isolated crypts which have never been exposed to Matrigel is highly desirable, especially when the cells are expanded for clinical transplantation, because even trace amounts of Matrigel contaminant may pose significant regulatory hurdles, for reasons stated previously. Additionally, primary culture in Matrigel-free conditions can avoid the potential crosstalk or interference derived from undefined Matrigel components. Reducing interference is particularly desirable when the culture is applied as disease modeling and drug screening platforms.
Figure 6.
(A) BLT Sandwich culture is capable of expanding freshly isolated mouse SI, colon crypts and (B) patient-derived SI crypts. Equal number of freshly isolated crypts were evenly seeded into 2D Sandwich and 3D Matrigel systems and cultured for 4 days (mouse) or 6 days (human). A1: Confocal images showing the morphology and Lgr5-GFP expression of SI and colonic IECs in 2D and 3D systems. Percentage of Lgr5-GFP+ cells was noted inside each picture in green. A2: Flow cytometry comparing Ki67 expression of IECs expanded from both systems. A3: Comparison of the fold expansion of mouse SI and colonic Lgr5-GFP+ cells between 2D and 3D systems. Mean ± SEM, n = 4 donor mice each with 3–5 wells/group. B1: Photographs showing the proliferation and morphological change of human SI IECs cultured under both systems for 6 days. Insets show the growth of a representative single crypt into a monolayer patch with diameter ~ 1 mm at day 6 (scale bar: 100 μm). B2: Flow cytometry comparing Ki67 expression between both systems at day 6 (representative from n = 3 patients). B3: qPCR analysis comparing the mRNA expression of key IEC lineage markers between both systems at day 6. Mean ± SEM, n = 3 trials using freshly isolated SI crypts from three patients. ** p<0.01: significantly different between 2D and 3D cultures.
After 4 days of Sandwich culture, freshly isolated mouse SI crypts (initially containing 9% Lgr5-GFP+ cells) underwent ~20-fold expansion of Lgr5-GFP+ cells, which constitute 25.7 ± 6.6% of the resulting total IECs. While exhibiting lower fold expansion of Lgr5-GFP+ cells compared to 3D Matrigel, the BLT Sandwich culture showed comparably high level of Ki67+ (> 92%) and only a slightly lower percentage of Lgr5-GFP+ cells. Overall, freshly isolated SI crypts yielded much faster expansion of Lgr5-GFP+ cells than Matrigel pre-expanded SI IEC clusters, when both were seeded with equal density in the Sandwich culture. This may be due to the differential baseline levels of lysozyme-positive cells, i.e. Wnt ligand source or Paneth cells, present in freshly isolated crypts and Matrigel-expanded organoids (Supplement Figure 10). With respect to the freshly isolated mouse Lgr5-GFP+ CSCs, no significant difference of fold expansion was observed between 2D BLT Sandwich culture and 3D Matrigel control after 5-day culture (Figure 6A). However, the percentage of resulting Lgr5-GFP+ population in 2D BLT Sandwich culture (6.7 ± 2.1%) was notably lower than 3D Matrigel control (16.1 ± 3.1%). This discrepancy between the percentage and fold expansion of Lgr5-GFP+ CSCs implies that colonic IECs overall grow significantly faster in the BLT Sandwich culture than in Matrigel, as also evidenced by the higher count of total live IECs from the BLT Sandwich culture (data not shown). Mouse Lgr5-GFP+ CSCs generally grow slower than their SI counterparts (Figure 6A3), which is likely due to the absence of Paneth cells or the presence of more apoptotic/dead cells in the freshly isolated colonic crypts (Supplement Figure 11), or due to the inherited distinct proliferative capacity of SI and colon IECs in vivo [1].
Importantly, human-derived freshly isolated human SI crypts were expanded into large monolayer patches in the BLT Sandwich culture, as opposed to the cystic or budding structures typically seen in 3D Matrigel (Figure 6B1). Human SI IECs grown in the BLT Sandwich culture were ~ 75% Ki67+ (similar with 3D Matrigel control) and maintained LGR5 mRNA level slightly higher than that of 3D Matrigel control (Figure 6B2–3), suggesting that the BLT Sandwich culture system may be a superior alternative to 3D Matrigel culture for expanding patient-specific intestinal stem cells. We envision that this novel culture system holds great promise for not only large scale production of ISCs/CSCs as cell therapy, but also serving as a robust platform for studying and manipulating ISC biology to identify new therapeutics targeting intestinal epithelial disorders in a patient-specific/personalized manner. For instance, the BLT Sandwich culture system could be used to establish a monolayer ISC biobank by expanding intestinal crypts derived from biopsies from multiple subjects with distinct genetic backgrounds. This biobank could be further utilized to screen novel therapies, or to validate clinically accessed drugs to establish the correlation between different drug response and patient genotype, which is highly relevant towards precision medicine for predicting clinical responders or non-responders [68].
3.4. Identification of LDN-193189 to Further Improve Lgr5-GFP+ ISC Expansion in the BLT Sandwich Culture
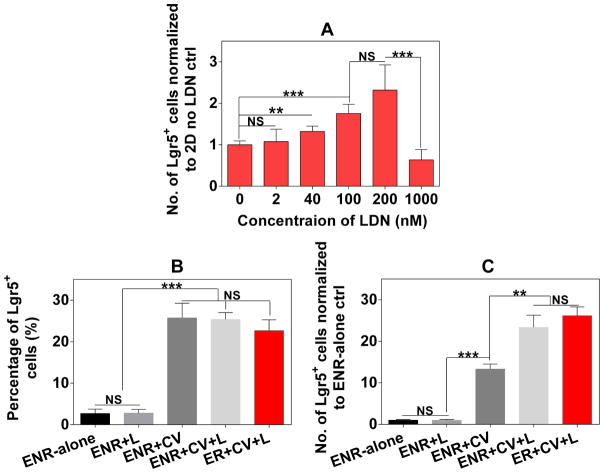
To validate the utility of the BLT Sandwich culture system, we then explored small molecule approaches to further boost the Lgr5+ ISC expansion based on our established ENR+CV media, and demonstrated the key differences between 2D BLT sandwich and 3D Matrigel systems. Using freshly isolated mouse SI crypts in the BLT Sandwich culture, we identified LDN-193189 (or LDN), a selective inhibitor of BMP type I receptor kinase that markedly improves the fold expansion of Lgr5-GFP+ cells (Figure 7A). 100–200 nM LDN profoundly enhanced the proliferation of Lgr5-GFP+ cells by over 1.5-fold after a 4-day culture in ENR+CV media. However, this mitogenic effect declined to baseline levels (i.e. no LDN control) at the higher concentration of 1000 nM. BMP signaling is known to mitigate ISC self-renewal through Wnt suppression and the exogenous BMP antagonist polypeptide, Noggin, is required for ISC-containing organoid growth in Matrigel [3, 30]. LDN has been reported to enhance lung epithelium repair by promoting the self-renewal of airway epithelial stem cells and increasing the number of differentiating progenitors in vivo [69]. However, its role in ISC growth was previously unknown. Our data suggests that in the BLT Sandwich culture, the combination of CV and LDN synergistically boosted the number of Lgr5-GFP+ cells by more than 20-fold compared to the ENR-alone condition (Figure 7B), whereas 200 nM LDN without CV did not significantly impact the percentage and total number of Lgr5-GFP+ cells relative to ENR-alone condition (Figure 7B–C).
Figure 7.
Positive effect of LDN-193189 (L) on the expansion of Lgr5-GFP+ cells cultured from freshly isolated mouse SI crypts for 4 days in 2D BLT Sandwich culture under different culture media conditions. A: Number of Lgr5-GFP+ cells normalized to the control without LDN in response to different concentrations of LDN in ISC maintenance media (i.e. ENR+CV). B: Percentage of resulting Lgr5-GFP+ cells under different media conditions; C: Normalized number of Lgr5-GFP+ cells (to ENR-alone control) under different conditions with or without CV, LDN (200 nM) and Noggin (N). Data represents mean ± SEM, n = 3–4 independent trials with different donor mice.
The impact of CV on the proliferation of total IECs and the Lgr5-GFP+ population is more potent compared to the small molecule BMP inhibitors that we have tested thus far including dorsomorphin, LDN and DMH1, in both 2D BLT Sandwich and 3D Matrigel cultures. There is no significant difference between the percentage of Lgr5-GFP+ cells in the ENR+CV, ENR+CV+LDN and ER+CV+LDN (i.e. replacing Noggin with LDN) conditions in the BLT Sandwich culture. The number of resulting Lgr5-GFP+ cells in the ER+CV+LDN condition was marginally higher than the number of Lgr5-GFP+ cells in the ENR+CV+LDN condition, and a similar trend was observed with other BMP inhibitors (dorsomorphin and DMH1, data not shown). This suggests that Noggin can be replaced with small molecule BMP inhibitors for expanding Lgr5+ ISCs in the BLT Sandwich culture. This is beneficial for reducing the cost and improving the scalability of the culture system. LDN boosts the number of Lgr5-GFP+ cells in Sandwich culture by increasing the proliferation of overall IECs (primarily TAs), rather than directly enriching the Lgr5-GFP+ population. In contrast, adding LDN in ENR+CV media markedly suppressed the number of Lgr5-GFP+ cells in 3D Matrigel culture (Supplement Figure 12A). Unlike the Sandwich culture, LDN cannot replace Noggin for expanding Lgr5-GFP+ ISCs in Matrigel (Supplement Figure 12B). The opposing effects of LDN on 2D BLT Sandwich and 3D Matrigel cultures suggest their varying dependence on BMP signaling for Lgr5+ ISC expansion, and further underscore the complex crosstalk between Wnt, Notch, BMP pathways and the ECM. Future studies are warranted to unveil the mechanisms underlying the differential responses of different culture settings (2D vs. 3D) to defined chemical cues. Such findings will be instrumental for better understanding of ISC biology and realizing the full potential of our BLT Sandwich culture system for research and therapeutic applications.
4. Conclusion
Here we report a compositionally defined, native ECM component-based culture system that supports the expansion of intestinal organoids or crypts with an enriched Lgr5-GFP+ ISC population. This simple, monolayer ISC culture system overcomes some of the major limitations (e.g. inter-lot variability, inaccessibility to apical side, scalability issues) associated with existing 3D organoid cultures. The unique Sandwich presentation of distinct collagen cues synergistically mediates IEC attachment, proliferation and Lgr5 expression in an integrin α2β1-dependent manner. In conjunction with ECM cues and known potent Wnt/Notch modulators, the BMP inhibitor LDN-193189, was found to significantly boost the expansion of Lgr5-GFP+ cells from mouse small intestinal crypts. Moreover, the system is capable of expanding mouse colonic ISCs and human-derived IECs with high levels of Lgr5 expression. The system may also have broad utility for the culture of other epithelial progenitor cell types, which we are currently exploring in the laboratory. We envision that our chemically defined BLT monolayer Sandwich culture system holds great promise for intestinal disease related research and therapeutic applications.
Supplementary Material
Acknowledgments
We thank Mrs. Mervelina Saturno-Condon and Dr. Glenn Paradis at MIT Koch Institute Flow Cytometry Core for generously assisting Lgr5 cell sorting. We also acknowledge Miss. Carolyn McDonagh at MGH Tissue Repository for helping with patient gut biopsy collection. This work was supported by National Institutes of Health (NIH) grant R01DE13023 to R.L. and NIH grant R01HL095722 to J.M.K.
Footnotes
Disclosure Statement
No competing financial interests exist. J.M.K. R.S.L. and X.Y. hold equity in Frequency Therapeutics, a company that has an option to license IP generated by J.M.K., R.S.L. and X.Y. and that may benefit financially if the IP is licensed and further validated.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 2.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15(1):19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 3.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–5. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 4.Jabaji Z, Sears CM, Brinkley GJ, Lei NY, Joshi VS, Wang J, Lewis M, Stelzner M, Martin MG, Dunn JC. Use of collagen gel as an alternative extracellular matrix for the in vitro and in vivo growth of murine small intestinal epithelium. Tissue engineering. Part C, Methods. 2013;19(12):961–9. doi: 10.1089/ten.tec.2012.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott A, Rouch JD, Jabaji Z, Khalil HA, Solorzano S, Lewis M, Martin MG, Stelzner MG, Dunn JC. Long-term renewable human intestinal epithelial stem cells as monolayers: A potential for clinical use. Journal of pediatric surgery. 2016 doi: 10.1016/j.jpedsurg.2016.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fordham RP, Yui S, Hannan NR, Soendergaard C, Madgwick A, Schweiger PJ, Nielsen OH, Vallier L, Pedersen RA, Nakamura T, Watanabe M, Jensen KB. Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell. 2013;13(6):734–44. doi: 10.1016/j.stem.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K, Clevers H, Watanabe M. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5(+) stem cell. Nat Med. 2012;18(4):618–23. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda M, Mizutani T, Mochizuki W, Matsumoto T, Nozaki K, Sakamaki Y, Ichinose S, Okada Y, Tanaka T, Watanabe M, Nakamura T. Small intestinal stem cell identity is maintained with functional Paneth cells in heterotopically grafted epithelium onto the colon. Genes Dev. 2014;28(16):1752–7. doi: 10.1101/gad.245233.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Yamamoto Y, Wilson LH, Zhang T, Howitt BE, Farrow MA, Kern F, Ning G, Hong Y, Khor CC, Chevalier B, Bertrand D, Wu L, Nagarajan N, Sylvester FA, Hyams JS, Devers T, Bronson R, Lacy DB, Ho KY, Crum CP, McKeon F, Xian W. Cloning and variation of ground state intestinal stem cells. Nature. 2015;522(7555):173–8. doi: 10.1038/nature14484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, van Houdt W, van Gorp J, Taylor-Weiner A, Kester L, McLaren-Douglas A, Blokker J, Jaksani S, Bartfeld S, Volckman R, van Sluis P, Li VS, Seepo S, Sekhar Pedamallu C, Cibulskis K, Carter SL, McKenna A, Lawrence MS, Lichtenstein L, Stewart C, Koster J, Versteeg R, van Oudenaarden A, Saez-Rodriguez J, Vries RG, Getz G, Wessels L, Stratton MR, McDermott U, Meyerson M, Garnett MJ, Clevers H. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161(4):933–45. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10(9):1886–90. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- 12.Jabaji Z, Brinkley GJ, Khalil HA, Sears CM, Lei NY, Lewis M, Stelzner M, Martin MG, Dunn JC. Type I collagen as an extracellular matrix for the in vitro growth of human small intestinal epithelium. PloS one. 2014;9(9):e107814. doi: 10.1371/journal.pone.0107814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordonez-Moran P, Clevers H, Lutolf MP. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539(7630):560–564. doi: 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- 14.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nature biotechnology. 2014;32(8):760–72. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, DiSalvo M, Gunasekara DB, Dutton J, Proctor A, Lebhar MS, Williamson IA, Speer J, Howard RL, Smiddy NM, Bultman SJ, Sims CE, Magness ST, Allbritton NL. Self-renewing Monolayer of Primary Colonic or Rectal Epithelial Cells. Cellular and Molecular Gastroenterology and Hepatology. doi: 10.1016/j.jcmgh.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matarasso SL. The use of injectable collagens for aesthetic rejuvenation. Seminars in cutaneous medicine and surgery. 2006;25(3):151–7. doi: 10.1016/j.sder.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Ootani A, Li X, Sangiorgi E, Ho QT, Ueno H, Toda S, Sugihara H, Fujimoto K, Weissman IL, Capecchi MR, Kuo CJ. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med. 2009;15(6):701–6. doi: 10.1038/nm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chevillard M, Hinnrasky J, Pierrot D, Zahm JM, Klossek JM, Puchelle E. Differentiation of human surface upper airway epithelial cells in primary culture on a floating collagen gel. Epithelial cell biology. 1993;2(1):17–25. [PubMed] [Google Scholar]
- 19.Du Y, Han R, Wen F, Ng San San S, Xia L, Wohland T, Leo HL, Yu H. Synthetic sandwich culture of 3D hepatocyte monolayer. Biomaterials. 2008;29(3):290–301. doi: 10.1016/j.biomaterials.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Swift B, Pfeifer ND, Brouwer KL. Sandwich-cultured hepatocytes: an in vitro model to evaluate hepatobiliary transporter-based drug interactions and hepatotoxicity. Drug Metab Rev. 2010;42(3):446–71. doi: 10.3109/03602530903491881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong Z, Solanki A, Hamilos A, Levy O, Wen K, Yin X, Karp JM. Application of biomaterials to advance induced pluripotent stem cell research and therapy. EMBO J. 2015;34(8):987–1008. doi: 10.15252/embj.201490756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469(7330):415–8. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li VS, Clevers H. In vitro expansion and transplantation of intestinal crypt stem cells. Gastroenterology. 2012;143(1):30–4. doi: 10.1053/j.gastro.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Yin X, Farin HF, van Es JH, Clevers H, Langer R, Karp JM. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat Methods. 2014;11(1):106–12. doi: 10.1038/nmeth.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenhoffer GT, Rosenblatt J. Bringing balance by force: live cell extrusion controls epithelial cell numbers. Trends in cell biology. 2013;23(4):185–192. doi: 10.1016/j.tcb.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staatz WD, Fok KF, Zutter MM, Adams SP, Rodriguez BA, Santoro SA. Identification of a tetrapeptide recognition sequence for the alpha 2 beta 1 integrin in collagen. J Biol Chem. 1991;266(12):7363–7. [PubMed] [Google Scholar]
- 27.Svec D, Tichopad A, Novosadova V, Pfaffl MW, Kubista M. How good is a PCR efficiency estimate: Recommendations for precise and robust qPCR efficiency assessments. Biomolecular Detection and Quantification. 2015;3:9–16. doi: 10.1016/j.bdq.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong Z, Duncan RL, Jia X. Modulating the behaviors of mesenchymal stem cells via the combination of high-frequency vibratory stimulations and fibrous scaffolds. Tissue Eng Part A. 2013;19(15–16):1862–78. doi: 10.1089/ten.tea.2012.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tong Z, Zerdoum AB, Duncan RL, Jia X. Dynamic vibration cooperates with connective tissue growth factor to modulate stem cell behaviors. Tissue Eng Part A. 2014;20(13–14):1922–34. doi: 10.1089/ten.tea.2013.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36(10):1117–21. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 31.Beaulieu JF, Vachon PH, Chartrand S. Immunolocalization of extracellular matrix components during organogenesis in the human small intestine. Anat Embryol (Berl) 1991;183(4):363–9. doi: 10.1007/BF00196837. [DOI] [PubMed] [Google Scholar]
- 32.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strater J, Wedding U, Barth TF, Koretz K, Elsing C, Moller P. Rapid onset of apoptosis in vitro follows disruption of beta 1-integrin/matrix interactions in human colonic crypt cells. Gastroenterology. 1996;110(6):1776–84. doi: 10.1053/gast.1996.v110.pm8964403. [DOI] [PubMed] [Google Scholar]
- 34.Wang C-C, Jamal L, Janes KA. Normal morphogenesis of epithelial tissues and progression of epithelial tumors. Wiley interdisciplinary reviews. Systems biology and medicine. 2012;4(1):51–78. doi: 10.1002/wsbm.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Bio. 2014;15(12):786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vllasaliu D, Falcone FH, Stolnik S, Garnett M. Basement membrane influences intestinal epithelial cell growth and presents a barrier to the movement of macromolecules. Exp Cell Res. 2014;323(1):218–31. doi: 10.1016/j.yexcr.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 37.Beaulieu JF. Integrins and human intestinal cell functions. Front Biosci. 1999;4:D310–21. doi: 10.2741/beaulieu. [DOI] [PubMed] [Google Scholar]
- 38.Watkin H, Streuli CH. Adenoviral-mediated gene transfer in two-dimensional and three-dimensional cultures of mammary epithelial cells. Methods in Cell Biology. 2002;69:403–423. doi: 10.1016/s0091-679x(02)69025-9. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Miao C, Guo W, Jia L, Zhou J, Ma B, Peng S, Liu S, Cao Y, Duan E. Enrichment of putative human epidermal stem cells based on cell size and collagen type IV adhesiveness. Cell Res. 2008;18(3):360–71. doi: 10.1038/cr.2007.103. [DOI] [PubMed] [Google Scholar]
- 40.Cameron JD, Skubitz AP, Furcht LT. Type IV collagen and corneal epithelial adhesion and migration. Effects of type IV collagen fragments and synthetic peptides on rabbit corneal epithelial cell adhesion and migration in vitro. Invest Ophthalmol Vis Sci. 1991;32(10):2766–73. [PubMed] [Google Scholar]
- 41.Moore R, Madara JL, MacLeod RJ. Enterocytes adhere preferentially to collagen IV in a differentially regulated divalent cation-dependent manner. Am J Physiol. 1994;266(6 Pt 1):G1099–107. doi: 10.1152/ajpgi.1994.266.6.G1099. [DOI] [PubMed] [Google Scholar]
- 42.Olivero DK, Furcht LT. Type IV collagen, laminin, and fibronectin promote the adhesion and migration of rabbit lens epithelial cells in vitro. Invest Ophthalmol Vis Sci. 1993;34(10):2825–34. [PubMed] [Google Scholar]
- 43.Groulx J-F, Gagné D, Benoit YD, Martel D, Basora N, Beaulieu J-F. Collagen VI is a basement membrane component that regulates epithelial cell–fibronectin interactions. Matrix Biology. 2011;30(3):195–206. doi: 10.1016/j.matbio.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73(4):713–24. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 45.Gaudet C, Marganski WA, Kim S, Brown CT, Gunderia V, Dembo M, Wong JY. Influence of Type I Collagen Surface Density on Fibroblast Spreading, Motility, and Contractility. Biophysical Journal. 2003;85(5):3329–3335. doi: 10.1016/S0006-3495(03)74752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DiMilla PA, Stone JA, Quinn JA, Albelda SM, Lauffenburger DA. Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J Cell Biol. 1993;122(3):729–37. doi: 10.1083/jcb.122.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Des Parkin J, San Antonio JD, Pedchenko V, Hudson B, Jensen ST, Savige J. Mapping structural landmarks, ligand binding sites and missense mutations to the collagen IV heterotrimers predicts major functional domains, novel interactions and variation in phenotypes in inherited diseases affecting basement membranes. Human mutation. 2011;32(2):127–143. doi: 10.1002/humu.21401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vandenberg P, Kern A, Ries A, Luckenbill-Edds L, Mann K, Kuhn K. Characterization of a type IV collagen major cell binding site with affinity to the alpha 1 beta 1 and the alpha 2 beta 1 integrins. J Cell Biol. 1991;113(6):1475–83. doi: 10.1083/jcb.113.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beaulieu JF. Differential expression of the VLA family of integrins along the crypt-villus axis in the human small intestine. J Cell Sci. 1992;102(Pt 3):427–36. doi: 10.1242/jcs.102.3.427. [DOI] [PubMed] [Google Scholar]
- 50.Heijmans J, van Lidth de Jeude Jooske F, Koo B-K, Rosekrans Sanne L, Wielenga Mattheus CB, van de Wetering M, Ferrante M, Lee Amy S, Onderwater Jos JM, Paton James C, Paton Adrienne W, Mommaas AM, Kodach Liudmila L, Hardwick James C, Hommes Daniël W, Clevers H, Muncan V, van den Brink Gijs R. ER Stress Causes Rapid Loss of Intestinal Epithelial Stemness through Activation of the Unfolded Protein Response. Cell Reports. 2013;3(4):1128–1139. doi: 10.1016/j.celrep.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 51.Avivar-Valderas A, Salas E, Bobrovnikova-Marjon E, Diehl JA, Nagi C, Debnath J, Aguirre-Ghiso JA. PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Molecular and cellular biology. 2011;31(17):3616–29. doi: 10.1128/MCB.05164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dunn JC, Tompkins RG, Yarmush ML. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991;7(3):237–45. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- 53.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- 54.Paz AC, Javaherian S, McGuigan AP. Tools for micropatterning epithelial cells into microcolonies on transwell filter substrates. Lab on a chip. 2011;11(20):3440–8. doi: 10.1039/c1lc20506d. [DOI] [PubMed] [Google Scholar]
- 55.Provenzano PP, Keely PJ. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. Journal of Cell Science. 2011;124(8):1195. doi: 10.1242/jcs.067009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elbediwy A, Vincent-Mistiaen ZI, Thompson BJ. YAP and TAZ in epithelial stem cells: A sensor for cell polarity, mechanical forces and tissue damage. Bioessays. 2016;38(7):644–53. doi: 10.1002/bies.201600037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cereijido M, Contreras RG, Shoshani L, Flores-Benitez D, Larre I. Tight junction and polarity interaction in the transporting epithelial phenotype. Biochim Biophys Acta. 2008;1778(3):770–93. doi: 10.1016/j.bbamem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 58.Fatehullah A, Appleton PL, Nathke IS. Cell and tissue polarity in the intestinal tract during tumourigenesis: cells still know the right way up, but tissue organization is lost. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2013;368(1629):20130014. doi: 10.1098/rstb.2013.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dowling J, Yu QC, Fuchs E. Beta4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. The Journal of Cell Biology. 1996;134(2):559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Myllymaki SM, Teravainen TP, Manninen A. Two distinct integrin-mediated mechanisms contribute to apical lumen formation in epithelial cells. PloS one. 2011;6(5):e19453. doi: 10.1371/journal.pone.0019453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Banon-Rodriguez I, Galvez-Santisteban M, Vergarajauregui S, Bosch M, Borreguero-Pascual A, Martin-Belmonte F. EGFR controls IQGAP basolateral membrane localization and mitotic spindle orientation during epithelial morphogenesis. EMBO J. 2014;33(2):129–45. doi: 10.1002/embj.201385946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.den Elzen N, Buttery CV, Maddugoda MP, Ren G, Yap AS. Cadherin adhesion receptors orient the mitotic spindle during symmetric cell division in mammalian epithelia. Mol Biol Cell. 2009;20(16):3740–50. doi: 10.1091/mbc.E09-01-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 2015;526(7575):715–8. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- 64.Basak O, van de Born M, Korving J, Beumer J, van der Elst S, van Es JH, Clevers H. Mapping early fate determination in Lgr5+ crypt stem cells using a novel Ki67-RFP allele. Embo j. 2014;33(18):2057–68. doi: 10.15252/embj.201488017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Es JH, Sato T, van de Wetering M, Lyubimova A, Gregorieff A, Zeinstra L, van den Born M, Korving J, Martens ACM, van den Oudenaarden A, Clevers H. Dll1 marks early secretory progenitors in gut crypts that can revert to stem cells upon tissue damage. Nature cell biology. 2012;14(10):1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tetteh PW, Basak O, Farin HF, Wiebrands K, Kretzschmar K, Begthel H, van den Born M, Korving J, de Sauvage F, van Es JH, van Oudenaarden A, Clevers H. Replacement of Lost Lgr5-Positive Stem Cells through Plasticity of Their Enterocyte-Lineage Daughters. Cell Stem Cell. 2016;18(2):203–13. doi: 10.1016/j.stem.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 67.Holmberg FEO, Seidelin JB, Yin X, Mead BE, Tong Z, Li Y, Karp JM, Nielsen OH. Culturing human intestinal stem cells for regenerative applications in the treatment of inflammatory bowel disease. EMBO Molecular Medicine. 2017;9(5):558–570. doi: 10.15252/emmm.201607260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dekkers JF, Berkers G, Kruisselbrink E, Vonk A, de Jonge HR, Janssens HM, Bronsveld I, van de Graaf EA, Nieuwenhuis EE, Houwen RH, Vleggaar FP, Escher JC, de Rijke YB, Majoor CJ, Heijerman HG, de Winter-de Groot KM, Clevers H, van der Ent CK, Beekman JM. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Science translational medicine. 2016;8(344):344ra84. doi: 10.1126/scitranslmed.aad8278. [DOI] [PubMed] [Google Scholar]
- 69.Tadokoro T, Gao X, Hong CC, Hotten D, Hogan BL. BMP signaling and cellular dynamics during regeneration of airway epithelium from basal progenitors. Development (Cambridge, England) 2016;143(5):764–73. doi: 10.1242/dev.126656. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reference
- 1.Yin X, Farin HF, van Es JH, Clevers H, Langer R, Karp JM. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat Methods. 2014;11(1):106–12. doi: 10.1038/nmeth.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.