Abstract
Background
Uterine carcinosarcomas (UCSs) are a rare but clinically aggressive form of cancer. They are biphasic tumors consisting of both epithelial and sarcomatous components. Most uterine carcinosarcomas are clonal, with the carcinomatous cells undergoing metaplasia to give rise to the sarcomatous component. The objective of this study was to identify novel somatically mutated genes in UCSs.
Methods
We whole exome sequenced paired tumor and non-tumor DNAs from 14 UCSs and orthogonally validated 464 somatic variants using Sanger sequencing. Fifteen genes that were somatically mutated in at least two tumor exomes were Sanger sequenced in another 39 primary UCSs.
Results
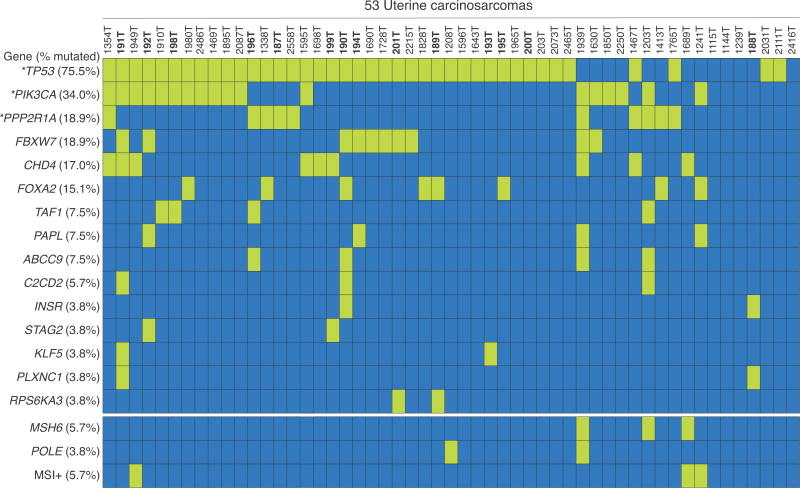
Overall, among 53 UCSs in this study, the most frequently mutated of these 15 genes were TP53 (75.5%), PIK3CA (34.0%), PPP2R1A (18.9%), FBXW7 (18.9%), CHD4 (17.0%), and FOXA2 (15.1%). FOXA2 has not previously been implicated in UCSs and was predominated by frameshift and nonsense mutations. One UCS with a FOXA2 frameshift mutation expressed truncated FOXA2 protein by immunoblotting. Sequencing of FOXA2 in 160 primary ECs revealed somatic mutations in 5.7% of serous, 22.7% of clear cell, 9% of endometrioid and 11.1% of mixed ECs, most of which were frameshift mutations.
Conclusions
Collectively, our findings provide compelling genetic evidence that FOXA2 is a pathogenic driver gene in the etiology of primary uterine cancers including UCSs.
Keywords: Endometrial cancer, endometrial carcinoma, mutation, exome, uterine cancer, carcinosarcoma
Introduction
Uterine carcinosarcomas (UCSs), also referred to as malignant mixed Müllerian tumors of the uterus, are rare biphasic tumors consisting of both carcinomatous and sarcomatous elements. UCSs have an aggressive clinical course; one study reported 5-year relative survival rates of 53.7% at all AJCC (American Joint Committee on Cancer) stages, 73.7% at stage I, 43.3% at stage II, 26.2% at stage III, and 13.6% at stage IV 1. Although early studies reported that the carcinomatous component determines tumor aggressiveness 2, 3, a recent meta-analysis of 906 UCS cases found that both the carcinoma and sarcoma components can influence prognosis 4.
Molecular studies that analyzed the carcinoma and sarcoma components of individual tumors separately, found evidence for shared alterations between each component in a majority of cases 5–10. Thus, most UCSs are believed to be monoclonal in origin, resulting from a metaplastic transition of carcinoma cells to sarcoma cells 11. The carcinoma component is often of serous, clear cell, undifferentiated, high grade endometrioid, or mixed histology; lower-grade endometrioid carcinomas are also observed although they are less common 4. The sarcoma component can be of uterine (“homologous”) or extra-uterine (“heterologous”) origin including rhabdomyosarcoma, chondrosarcoma, or osteosarcoma 12.
Targeted gene sequencing and whole exome sequencing of UCSs have uncovered frequent somatic mutations in genes involved in the DNA damage response (TP53, MLH1), the PI3-kinase pathway (PIK3CA, PTEN, PIK3R1), chromatin remodeling (ARID1A, MLL3/KMT2C, ARID1B, BAZ1A), ubiquitin-mediated protein degradation (FBXW7, SPOP), signal transduction (KRAS, NRAS, JAK2, ERBB2), WNT signaling (CTNNB1) and protein dephosphorylation (PPP2R1A) 5, 10, 13–21. Microsatellite instability (MSI) and loss of mismatch repair (MMR) protein expression, which are indicative of mismatch repair defects, have also been observed (reviewed in 22). The recent integrated genomic analysis of 57 UCSs by The Cancer Genome Atlas (TCGA) further implicated the mutational disruption of ARHGAP35, RB1, U2AF1, and ZBTB7B in the molecular pathogenesis of UCS 8.
The objective of this study was to identify novel somatically mutated genes in UCSs. Here, we whole exome sequenced 14 primary UCSs and paired normal DNAs. Follow-up Sanger sequencing of 15 genes that were mutated in at least two discovery screen tumors was performed in another 39 UCSs. Our finding that FOXA2 is somatically mutated in 15.1% of UCSs and is predominated by frameshift and nonsense mutations, strongly implicates FOXA2 as a novel pathogenic driver gene in UCSs. We also report frequent FOXA2 mutations in other histological subtypes of EC, further supporting FOXA2 as a driver of uterine carcinomas.
Materials and Methods
Clinical specimens
The NIH Office of Human Subjects Research determined that this research was not “human subjects research” per the Common Rule (45 CFR 46), and therefore that no IRB review was required for whole exome sequencing or Sanger sequencing of these samples. Anonymized, matched tumor and non-tumor (normal) samples sequenced in the discovery and prevalence screen were obtained as fresh frozen tissues from the Co-Operative Human Tissue Network (CHTN), which is funded by NCI (Supplementary Table 1). Genomic DNA for 38 UCSs from Washington University in St. Louis (Supplementary Table 2) was also included in the prevalence screen. Endometrial carcinoma (EC) DNAs included in this study were previously isolated from 53 serous ECs, 22 clear cell ECs, 67 endometrioid ECs, and 18 mixed histology ECs 23–25. Clinical follow-up information is not available for the 15 UCSs obtained from CHTN or for the 160 ECs in this study.
Estimation of neoplastic cellularity and genomic DNA extraction
An H&E section prepared for each frozen tumor specimen received from CHTN was evaluated by a pathologist (M.J.M) to assess histotype and demark regions of greater than 70% neoplastic cellularity for subsequent DNA isolation (Puregene DNA extraction kit, Qiagen) (Supplementary Table 1). Prior to whole exome sequencing, 14 paired tumor-normal DNAs were further purified by phenol-chloroform extraction. For Washington University cases, a gynecologic pathologist performed histologic review of the diagnostic slides from formalin-fixed paraffin embedded tumor tissue, as well as sections prepared from the fresh-frozen tumor tissue used for DNA isolation (Supplementary Table 2). Identity testing was performed on all tumor-normal DNA pairs using the Coriell Identity Mapping kit (Coriell) according to the manufacturer’s instructions.
Whole exome capture and sequencing
Sequencing libraries of matched tumor and normal DNAs were generated using the Illumina TruSeqV2:28 capture kit (Supplementary Table 3). Enriched libraries were sequenced with the Illumina HiSeq 2000 platform until at least 85% of targeted bases had adequate coverage to produce a Most Probable Genotype (MPG) score of 10 26. The average depth of coverage for individual exomes is provided (Supplementary Table 3).
Read mapping, variant calling, and variant filtering
Short sequence reads were aligned to the UCSC human reference “hg19” using novoalign version 2.08.02, and genotypes were called using the program bam2mpg with the –qual_filter 20 option 26. VarSifter 27 was used to identify coding and splice somatic variants with an MPG score ≥10 in the normal exome, an MPV score ≥10 in the tumor exome, and at least 5 reads covering the site in each sample. SNVs detected in any normal sample, probable false-positive variants consistent with sequencing artifacts or misaligned reads as determined by manual data curation, and variants annotated as non-clinical SNVs in dbSNP v135 were filtered out.
Primer design, PCR amplification and Sanger sequencing
Primer sequences and PCR conditions are available on request. Sanger sequencing and analysis were performed as previously described 25. In the mutation prevalence screen, variant calling was restricted to exonic sequence; splice donor and acceptor sequences were not assessed. Exonic variants were discriminated as somatic or germline by sequencing independent PCR products generated from relevant tumor and matched normal DNAs.
Microsatellite instability analysis
Microsatellite instability (MSI) analysis of tumor-normal DNA pairs was performed using the Promega MSI Analysis System v 1.2, according to the manufacturer’s instructions. MSI-high tumor genotypes (≥2 unstable mononucleotide markers) are reported here as MSI+. MSI-low (one unstable mononucleotide marker) and MSI-negative (no unstable mononucleotide markers) tumor genotypes are reported here as microsatellite stable (MSS).
Cell lines
The RL-95-2, HEC1A, HEC1B, KLE and AN3CA endometrial cancer (EC) cell lines were obtained from the American Type Culture Collection or the National Cancer Institute Developmental Therapeutics Program cell line repository. MFE-280 and MFE-296 were purchased from Sigma-Aldrich. HEC-59 was purchased from AddexBio. Dr. Alessandro A. Santin kindly provided ARK1, ARK2, ARK4, and ARK6 serous EC cell lines, Dr. Bo Rueda kindly provided the SK-UT-2 EC cell line, and Dr. John J. Risinger kindly provided the NCI-EC2 clear cell EC cell line.
Protein extraction, quantification and immunoblotting
Protein was extracted from seven primary UCSs (UCS1-7) using a protocol modified from Peña-Llopis and Brugarolas 28. Briefly, tissue was homogenized in 10 volumes of RIPA buffer (Thermo Fisher Scientific) containing complete tablet protease inhibitors (Roche), 0.1mM NaVO4, and 1mM NaF (New England BioLabs). Lysate was centrifuged twice through a QIAshredder column (Qiagen), then cleared using centrifugation. EC cells were lysed in RIPA buffer (Thermo Fisher Scientific) containing complete tablet protease inhibitors (Roche), 0.1mM NaVO4, and 1mM NaF (New England Biolabs) 30 min on ice, then cleared using centrifugation. Protein was quantified using the Quick Start Bradford reagent (Bio-Rad) and equal amounts of total protein were denatured in LDS sample buffer (Thermo Fisher Scientific) at 80°C prior to SDS-PAGE and wet transfer to PVDF membranes (Bio-Rad). Primary and HRP-conjugated secondary antibodies were: anti-FOXA2 (Cell Signaling #8186), β-actin (Sigma-Aldrich, A2228), goat anti-rabbit HRP (Cell Signaling) and goat anti-mouse HRP (Cell Signaling).
DNA and RNA extraction from frozen tissue sections
The following extractions were completed according to the manufacturers’ protocols. DNA was extracted from frozen sections that corresponded to regions targeted for protein extraction using The Pinpoint Slide DNA Isolation System (Zymo Research). For tumor UCS-5, RNA was extracted with TRIzol® Reagent (Thermo Fisher Scientific) pipetted directly onto the frozen section corresponding to the region used for DNA and protein extraction. RNA was converted to cDNA using the Superscript III First strand synthesis kit (Thermo Fisher Scientific).
Accession code
Whole exome sequencing data have been deposited into dbGAP, under accession code phs001153.v1.p1.
Results
Mutation discovery screen by whole exome sequencing
Whole exome sequencing of 14 primary UCS tumor-normal pairs achieved an average depth of coverage of 77× for aligned reads. On average, 89% of targeted bases had sufficient coverage and quality for variant calling (Supplementary Table 3). After data filtering, we identified 882 exonic (614 nonsynonymous and 268 synonymous) and 21 splice-junction somatic variant calls among the 14 tumors (Supplementary Table 4). We could orthogonally assess 519 of the nonsynonymous and splice junction variants by Sanger sequencing; 89.4% (464 of 519) of assessed variants orthogonally validated as bona fide somatic mutations (448 nonsynonymous, and 16 splice-junction mutations) (Supplementary Table 4). There was a mean of 33 validated nonsynonymous/splice junction somatic mutations per tumor (range 13–71 mutations/tumor); validated mutations were distributed among 426 protein-encoding genes. Seventeen protein-encoding genes, including six consensus cancer genes, were somatically mutated in two or more UCS exomes (Supplementary Table 4). Two of the 17 genes (TTN and DNAH2) had relatively large open reading frames and were not analyzed further; the other 15 genes were Sanger sequenced in a mutation prevalence screen.
Mutation prevalence screen
We Sanger sequenced the coding exons of TP53, FBXW7, PIK3CA, PPP2R1A, CHD4, RPS6KA3, FOXA2, INSR, KLF5, TAF1, PAPL, PLXNC1, C2CD2, ABCC9, and STAG2 in another 39 primary UCSs. TP53, PPP2R1A, and PIK3CA were previously sequenced by targeted next-generation sequencing in 36 of 39 prevalence screen tumors as reported by McConechy et al 13. Given the high frequency of frameshift FOXA2 mutations in the prevalence screen tumors (described below), and the known challenges in calling insertions and deletions by next generation sequencing 29, we subsequently also Sanger sequenced FOXA2 in the 14 discovery screen tumors.
Among the 53 tumors in the combined discovery and prevalence screens, the most frequently mutated of the 15 genes were TP53 (75.5% of UCSs somatically mutated), PIK3CA (34.0%), PPP2R1A (18.9%), FBXW7 (18.9%), CHD4 (17.0%), and FOXA2 (15.1%) (Figure 1, Table 1, Supplementary Table 5, and Supplementary Figure 1). Of the six most frequently mutated genes, FOXA2 is the only gene that has not previously been implicated in UCS. Most FOXA2 mutations were frameshift or nonsense mutations predicted to encode truncated proteins that would disrupt one or more functional domains, including the carboxy-terminal transactivation domain (Figure 2a, and Table 1). Two missense mutations in FOXA2 localized to the central DNA binding (Winged helix) domain (Figure 2a), which is highly conserved across proteins in the forkhead box family 30. Both missense mutations are predicted to impact protein function by three in silico algorithms (Mutation Assessor, PolyPhen, and SIFT). Only one of eight FOXA2–mutated tumors (1241T; FOXA2-Met142Profs*8) was MSI+ and none were MSH6- or POLE-mutated (Figure 1). In terms of histology, FOXA2 mutations were identified in UCSs for which the sequenced DNA was isolated from admixed carcinoma/sarcoma (three cases) or from predominantly carcinoma (five cases) (Supplementary Table 1 and Supplementary Table 2).
Figure 1. Oncoprint displaying the occurrence of somatic mutations in 15 prevalence screen genes and the MSI, MSH6 and POLE status, of 53 primary UCSs forming the discovery and prevalence screens.
The frequency (% of altered cases) of each aberration is shown (left). Coded tumor numbers are shown at top. Somatically mutated tumors are shown by the green bars; dark blue bars indicate that no somatic mutation was detected. Discovery screen tumors are indicated in bold. (*) Mutations in TP53, PPP2R1A and PIK3CA among 36 of 39 prevalence screen tumors were detected here by Sanger sequencing and previously detected in these tumors by targeted next generation sequencing (Supplementary Table 7) 13.
Table 1.
Somatic mutations of FOXA2 in uterine carcinosarcomas and endometrial carcinomas
| Tumor ID | Tumor histology | Nucleotide change§ | Somatic mutation type | Predicted protein change |
|---|---|---|---|---|
| Uterine carcinosarcoma (UCS) mutations | ||||
| 189T | UCS | c.621C>G | Missense | p.I207M |
| 190T | UCS | c.710C>T | Missense | p.P237L |
| 195T¶ | UCS | c.1034_1053delCCGCGGCCCACCTGCTGGGC | Frameshift | p.A347Pfs*8 |
| 1241T | UCS | c.401_402insCC | Frameshift | p.M136Pfs*8 |
| 1338T | UCS | c.587delA | Frameshift | p.Y196Sfs*23 |
| 1413T | UCS | c.462_463insT | Frameshift | p.Y155Lfs*84 |
| 1828T | UCS | c.716C>A | Nonsense | p.S239* |
| 1980T | UCS | c.912_913insACTCGAGCGCCTT | Frameshift | p.P310Lfs*56 |
| Endometrial carcinoma (EC) mutations | ||||
| 84T | EEC | c.1039_1055delGCCCACCTGCTGGGCCC | Frameshift | p.H348Pfs*8 |
| 94T | EEC | c.445_446insCCCGCGCGCCCGCGACCCCA | Frameshift | p.K149Tfs*39 |
| 97T | EEC | c.201-202insACGG | Frameshift | p.Y68Tfs*172 |
| 124T | EEC | c. 1035_1046delCGCGGCCCACCTinsTGCG | Frameshift | p.H348Gfs*11 |
| 134T | EEC | c.318_325delGAGTCCCA | Frameshift | p.S107Pfs*129 |
| 146T | EEC | c.1039_1046delGCCCACCT | Frameshift | p.H348Gfs*11 |
| 43T | SEC | c.816delC | Frameshift | p.S272Rfs*51 |
| 49T | SEC | c.1044_1045delCC | Frameshift | p.L349Afs*12 |
| 75T | SEC | c.1036_1049delGCGGCCCACCTGCT | Frameshift | p.A346Gfs*11 |
| 24T | CCEC | c.453_454insA | Frameshift | p.R152Kfs*87 |
| 28T | CCEC | c.807_820delCGCCGGCAGCGGCAinsTGC | Frameshift | p.G271Efs*87 |
| 34T | CCEC | c.315_316insT | Frameshift | p.L106Ffs*133 |
| 46T | CCEC | c.743_756delACCTGCGCCGCCAG | Frameshift | p.Y248* |
| 77T | CCEC | c.806C>T | Missense | p.A269V |
| 15T | Mixed EC | c.594_604delGAACCAGCAGCinsA | Frameshift | p.N199Afs*17 |
| 176T | Mixed EC | c.1028_1029insG | Frameshift | p.Q344Afs*18 |
FOXA2 Transcript and protein accession numbers for variant annotation: NM153675 and NP710141
FOXA2 mutation detected in 195T was discovered by Sanger sequencing the 14 discovery screen tumors.
Abbreviations: UCS, uterine carcinosarcoma; EEC, endometrioid endometrial carcinoma; SEC, serous endometrial carcinoma; CCEC, clear cell endometrial carcinoma; mixed EC, mixed histology endometrial carcinoma.
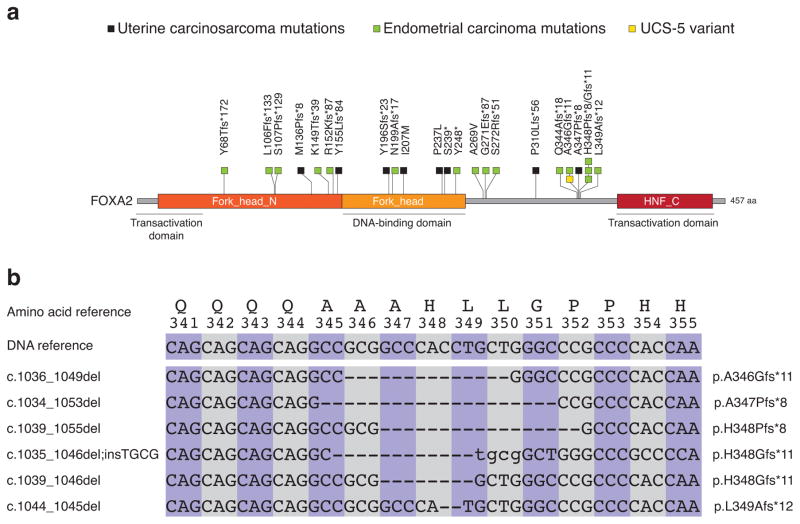
Figure 2. Localization of somatic FOXA2 mutations uncovered in uterine carcinosarcomas and in endometrial carcinomas relative to functional domains of the encoded protein.
(a) Location of somatic FOXA2 mutations in endometrial carcinomas (green squares) and in uterine carcinosarcomas (black squares), relative to mapped functional domains of the protein; the A346Gfs*11 variant found in UCS-5, which did not have paired normal DNA available for analysis, is indicated (yellow square) (b) Overlapping nucleotide deletions result in a cluster of somatic frameshift mutations in the C-terminal region of FOXA2. Deleted nucleotides are indicated by dashes; inserted nucleotides are shown in lower case.
FOXA2 is somatically mutated in endometrial carcinomas (ECs)
Because most UCSs are believed to originate from ECs 11, we extended our study to Sanger sequence the coding exons of FOXA2 from 160 primary ECs of diverse histotypes (53 serous, 22 clear cell, 67 endometrioid, 18 mixed). We uncovered somatic FOXA2 mutations in 5.7% of serous ECs, 22.7% of clear cell ECs, 9.0% of endometrioid ECs, and 11.1% of mixed histology ECs (Table 1). Differences in the mutation frequency of FOXA2 in serous, clear cell, and endometrioid ECs were not statistically significant (p=0.07, Chi-Square test). Five frameshift mutations in ECs and one frameshift mutation in a UCS formed a FOXA2 mutation cluster, resulting from overlapping genomic deletions in which the first positions of the frameshift were located within amino acids 346–349 (Figure 2b). All but one of 16 FOXA2 mutations in ECs are predicted to prematurely truncate FOXA2 (Figure 2a), and most FOXA2-mutated ECs (13 of 16 cases) were microsatellite stable (Supplementary Table 6) consistent with our observations in UCSs (Figure 1).
A recurrent mutation in FOXA2 encodes a truncated protein
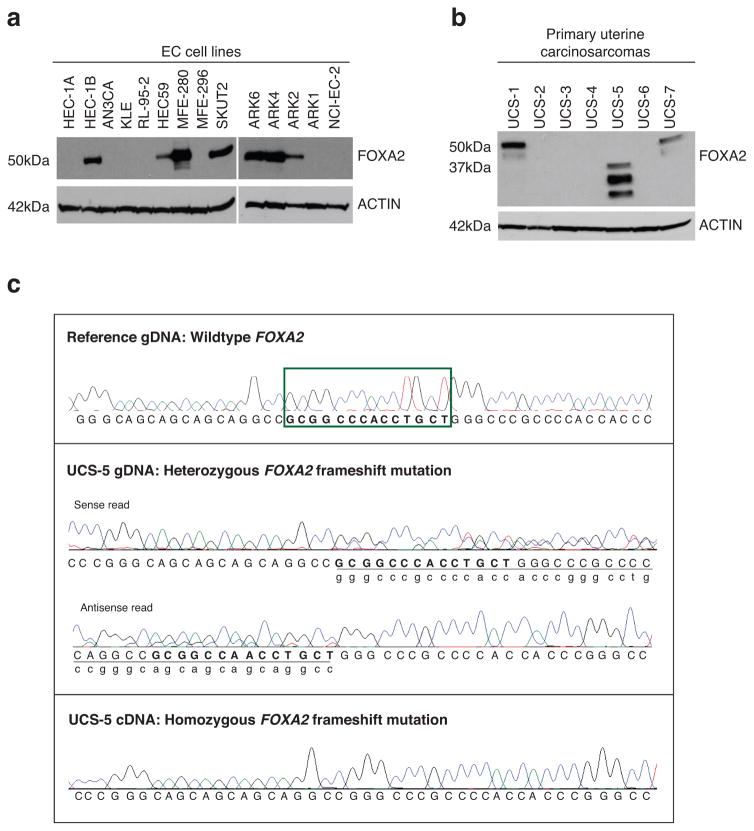
We next sought to evaluate FOXA2 expression in EC cell lines and in primary UCSs. We performed immunoblotting for FOXA2 on protein lysates from 14 EC cell lines and seven primary UCSs. These UCSs lacked matched normal tissue and therefore were not included in our discovery or prevalence mutation screens. Immunoblotting showed variable levels of FOXA2 expression in EC cell lines (Figure 3a and Supplementary Figure 2). Specifically, FOXA2 protein expression was readily detected in seven of 14 EC cell lines, including three of four serous EC cell lines (ARK2, ARK4, and ARK6) (Figure 3a); the single clear cell EC cell line evaluated (NCI-EC-2) expressed lower levels of FOXA2 (Supplementary Figure 2). Three of the seven primary UCSs assessed by immunoblotting had readily detectable FOXA2 protein expression (Figure 3b). Strikingly, one tumor (UCS-5) exhibited a shorter form of FOXA2 than expected (Figure 3b); sequencing all coding exons of FOXA2 from the genomic DNA of this tumor revealed that it harbored the A346Gfs*11 variant (Figure 3c). Matched normal DNA for UCS-5 was not available for sequencing but we predict that the A346Gfs*11 variant in this tumor is more likely to be somatic than germline because this alteration also occurred as a somatic mutation in a serous EC in our study (Table 1), and because it occurred within the recurrently mutated N-terminal region of FOXA2 (Figure 2). Although the A346Gfs*11 variant in UCS-5 appeared to be heterozygous at the genomic level, only the variant allele was detectable in cDNA generated from this tumor (Figure 3c). No FOXA2 exonic sequence variants were detected within the genomic DNA of UCS-1, -2, -4, -6, or -7; UCS-3 was not sequenced.
Figure 3. Variable expression of the FOXA2 protein in uterine cancer cell lines and expression of truncated FOXA2 in a UCS harboring a FOXA2 frameshift mutation.
Immunoblots showing variable FOXA2 protein expression levels among (a) endometrial carcinoma (EC) cell lines and (b) primary UCS tumors; a truncated FOXA2 protein (~30KDa) was detected in one tumor (UCS-5), (c) Partial FOXA2 nucleotide sequence traces for tumor UCS-5 showing heterozygosity for a frameshift mutation (c.1036_1049delGCGGCCCACCTGCT; p.A346Gfs*11) in the genomic DNA (gDNA) (middle panels) and homozygosity for this mutation in the cDNA (lower panel); an unmatched normal FOXA2 sequence is shown for reference, the nucleotides boxed in green (upper panel) are deleted in UCS-5.
Discussion
UCSs are rare gynecologic malignances that are associated with a poor clinical outcome 1–3. Here, using a combination of whole exome sequencing and targeted Sanger sequencing, we show that the FOXA2 (HNF3B) “pioneer” forkhead transcription factor, which has established roles in uterine glandular epithelium development and uterine biology 31–35, undergoes frequent somatic mutations in UCSs. The occurrence of FOXA2 mutations in 15.1% of UCSs in our study coupled with the fact that they are predominated by frameshift mutations predicted to prematurely truncate FOXA2 strongly suggests they are pathogenic driver mutations in some UCSs. FOXA2 has recently been proposed to be a tumor suppressor gene in endometrioid ECs36. Our observation of FOXA2 mutations in 9% of endometrioid ECs corroborates the recent report of FOXA2 mutations in 9.4% of such tumors 36. We extend these observations to further implicate FOXA2 as a candidate driver gene in other EC histological subtypes: within our cohort, 22.7% of clear cell, 11.1% of mixed histology and 5.7% of serous ECs harbored FOXA2 mutations.
The FOXA2 transcription factor has three major functional domains, a N-terminal transactivation domain (residues 1–52), a central winged-helix DNA binding domain (residues 157–257), and a C-terminal transactivation domain. All somatic frameshift/nonsense mutations we uncovered in UCSs and ECs are predicted to encode truncated proteins lacking the C-terminal transactivation domain. Twenty-five-percent (4 of 20) of somatic frameshift/nonsense mutations identified in our study are predicted to also disrupt the central winged-helix DNA-binding domain, and another 35% (7 of 20) of such mutations are initiated in the N-terminal forkhead region.
The genomic DNA sequences of most FOXA2-mutated tumors in our study also had evidence of a wildtype allele. One UCS that exhibited wildtype and mutated FOXA2 in genomic DNA had only the mutated allele in the cDNA and exhibited only a truncated protein by immunoblotting. These observations suggest that this tumor is either homozygous for the mutated allele with the wildtype allele in the genomic DNA being contributed by admixed normal cells that do not express the FOXA2 protein, or that the tumor is heterozygous at the genomic level but expresses FOXA2 exclusively from the mutant allele. Further studies are required to determine whether other FOXA2-mutated UCSs and ECs exclusively express the mutant allele.
TP53, PIK3CA, PPP2R1A were somatically mutated in 75.5%, 34.0%, and 18.9% of our cohort of 53 UCSs. These genes were previously sequenced by targeted next-generation sequencing in 36 UCSs included in our study but matched normal DNAs were not evaluated 13. By Sanger sequencing these three genes, we found 43 of 46 variants reported by McConechy et al 13 to be somatic, and we identified 9 additional somatic mutations not previously noted among the 36 UCSs (Supplementary Table 7).
Our combined whole exome sequencing and targeted gene sequencing of UCSs also revealed frequent somatic mutations in FBXW7 and CHD4. The incidence of somatic FBXW7 mutations among the UCSs in our study (18.9%) is in keeping with the frequency at which this tumor suppressor gene is mutated in other UCS cohorts (10.7% to 39%) 5, 8, 20, 21. All FBXW7 mutations in the UCSs within our study localized to the substrate-binding WD repeats (Supplementary Figure 1), consistent with the FBXW7 mutation spectrum in other cancers 37, 38, including endometrial cancers25, 39. We first nominated CHD4 as a candidate cancer gene based on mutational observations we made in endometrial carcinomas 25. Our finding that 17% of UCSs had somatically mutated CHD4, which encodes a catalytic subunit of the NuRD chromatin remodeling complex, is in line with recent reports of CHD4 mutations in 5.9%–18% of UCS cohorts 5, 8, 19. Most (6 of 10) CHD4 mutations in our study localized to the catalytic ATPase-helicase and helicase domains (Supplementary Figure 1), and two of these mutations involved arginine-975 and arginine-1162, which are two prominent pan-cancer mutation hotspots in CHD4 40, 41.
In conclusion, using a combination of whole exome sequencing and targeted gene sequencing we have identified a high frequency of FOXA2 frameshift mutations in UCSs. In addition, we also find frequent FOXA2 frameshift mutations in uterine carcinomas including serous ECs and clear cell ECs. Collectively, these findings lead us to nominate FOXA2 as a pathogenic driver gene in some of the most clinically aggressive forms of uterine cancer.
Supplementary Material
Acknowledgments
Funding: This work was supported by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health (HG200379) to D.W.B.
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
Competing Financial Interests: The authors declare no competing financial interests.
Author Contributions: D.W.B., designed and directed the study, and wrote the first draft of the manuscript. M.L.R., M.E.U., and P.J.G. provided critical comments and edits on the manuscript. D.G.M. and P.J.G., contributed clinical specimens and clinicopathological interpretation. M.J.M., performed histologic review and determined neoplastic cellularity of tumor specimens obtained from CHTN. M.L.R., isolated DNA and RNA samples provided by CHTN, performed identity testing, and microsatellite instability analysis. NISC performed library construction and exome sequencing. NISC and N.F.H. performed variant calling in exomes. M.L.G., M.L.R., and M.E.U., curated and orthogonally validated exome sequencing data. M.L.G., M.L.R., M.E.U., and D.W.B. designed, performed, analyzed, and/or interpreted the mutation discovery and prevalence screens. M.E.U., isolated protein and DNA for unmatched primary uterine carcinosarcomas, performed immunoblotting, and cell culture. P.J.G. performed statistical analysis.
References
- 1.Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J. Patient and Tumor Characteristics. Vol. 2007. National Cancer Institute; Bethesda, MD: 2007. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988–2001. SEER Program, NIH Pub. No. 07-6215. [Google Scholar]
- 2.Silverberg SG, Major FJ, Blessing JA, et al. Carcinosarcoma (malignant mixed mesodermal tumor) of the uterus. A Gynecologic Oncology Group pathologic study of 203 cases. Int J Gynecol Pathol. 1990;9:1–19. doi: 10.1097/00004347-199001000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Bitterman P, Chun B, Kurman RJ. The significance of epithelial differentiation in mixed mesodermal tumors of the uterus. A clinicopathologic and immunohistochemical study. Am J Surg Pathol. 1990;14:317–328. doi: 10.1097/00000478-199004000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo K, Takazawa Y, Ross MS, et al. Significance of histologic pattern of carcinoma and sarcoma components on survival outcomes of uterine carcinosarcoma. Ann Oncol. 2016;27:1257–1266. doi: 10.1093/annonc/mdw161. [DOI] [PubMed] [Google Scholar]
- 5.McConechy MK, Hoang LN, Chui MH, et al. In-depth molecular profiling of the biphasic components of uterine carcinosarcomas. J Pathol Clin Res. 2015;1:173–185. doi: 10.1002/cjp2.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abeln EC, Smit VT, Wessels JW, de Leeuw WJ, Cornelisse CJ, Fleuren GJ. Molecular genetic evidence for the conversion hypothesis of the origin of malignant mixed mullerian tumours. J Pathol. 1997;183:424–431. doi: 10.1002/(SICI)1096-9896(199712)183:4<424::AID-PATH949>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.Taylor NP, Zighelboim I, Huettner PC, et al. DNA mismatch repair and TP53 defects are early events in uterine carcinosarcoma tumorigenesis. Mod Pathol. 2006;19:1333–1338. doi: 10.1038/modpathol.3800654. [DOI] [PubMed] [Google Scholar]
- 8.Cherniack AD, Shen H, Walter V, et al. Integrated molecular characterization of uterine carcinosarcoma. Cancer Cell. 2017;31:411–423. doi: 10.1016/j.ccell.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin Z, Ogata S, Tamura G, et al. Carcinosarcomas (malignant mullerian mixed tumors) of the uterus and ovary: a genetic study with special reference to histogenesis. Int J Gynecol Pathol. 2003;22:368–373. doi: 10.1097/01.pgp.0000092134.88121.56. [DOI] [PubMed] [Google Scholar]
- 10.de Jong RA, Nijman HW, Wijbrandi TF, Reyners AK, Boezen HM, Hollema H. Molecular markers and clinical behavior of uterine carcinosarcomas: focus on the epithelial tumor component. Mod Pathol. 2011;24:1368–1379. doi: 10.1038/modpathol.2011.88. [DOI] [PubMed] [Google Scholar]
- 11.McCluggage WG. Uterine carcinosarcomas (malignant mixed Mullerian tumors) are metaplastic carcinomas. Int J Gynecol Cancer. 2002;12:687–690. doi: 10.1136/ijgc-00009577-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 12.El-Nashar SA, Mariani A. Uterine carcinosarcoma. Clin Obstet Gynecol. 2011;54:292–304. doi: 10.1097/GRF.0b013e31821ac635. [DOI] [PubMed] [Google Scholar]
- 13.McConechy MK, Ding J, Cheang MC, et al. Use of mutation profiles to refine the classification of endometrial carcinomas. J Pathol. 2012;228:20–30. doi: 10.1002/path.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Growdon WB, Roussel BN, Scialabba VL, et al. Tissue-specific signatures of activating PIK3CA and RAS mutations in carcinosarcomas of gynecologic origin. Gynecol Oncol. 2011;121:212–217. doi: 10.1016/j.ygyno.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 15.Biscuola M, Van de Vijver K, Castilla MA, et al. Oncogene alterations in endometrial carcinosarcomas. Human Pathol. 2013;44:852–859. doi: 10.1016/j.humpath.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 16.Murray S, Linardou H, Mountzios G, et al. Low frequency of somatic mutations in uterine sarcomas: a molecular analysis and review of the literature. Mutat Res. 2010;686:68–73. doi: 10.1016/j.mrfmmm.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Cheung LW, Hennessy BT, Li J, et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Discov. 2011;1:170–185. doi: 10.1158/2159-8290.CD-11-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bashir S, Jiang G, Joshi A, et al. Molecular alterations of PIK3CA in uterine carcinosarcoma, clear cell, and serous tumors. Int J Gynecol Cancer. 2014;24:1262–7. doi: 10.1097/IGC.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 19.Jones S, Stransky N, McCord CL, et al. Genomic analyses of gynaecologic carcinosarcomas reveal frequent mutations in chromatin remodelling genes. Nat Commun. 2014;5:5006. doi: 10.1038/ncomms6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hembree TN, Teer JK, Hakam A, Chiappori AA. Genetic investigation of uterine carcinosarcoma: Case report and cohort analysis. Cancer Control. 2016;23:61–66. doi: 10.1177/107327481602300111. [DOI] [PubMed] [Google Scholar]
- 21.Zhao S, Santin AD. Mutational landscape of uterine and ovarian carcinosarcomas implicates histone genes in epithelial–mesenchymal transition. Proc Natl Acad Sci U S A. 2016;113:12238–12243. doi: 10.1073/pnas.1614120113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoang LN, Ali RH, Lau S, Gilks CB, Lee CH. Immunohistochemical survey of mismatch repair protein expression in uterine sarcomas and carcinosarcomas. Int J Gynecol Pathol. 2014;33:483–491. doi: 10.1097/PGP.0b013e31829ff239. [DOI] [PubMed] [Google Scholar]
- 23.Rudd ML, Price JC, Fogoros S, et al. A unique spectrum of somatic PIK3CA (p110alpha) mutations within primary endometrial carcinomas. Clin Cancer Res. 2011;17:1331–1340. doi: 10.1158/1078-0432.CCR-10-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urick ME, Rudd ML, Godwin AK, Sgroi D, Merino M, Bell DW. PIK3R1 (p85alpha) is somatically mutated at high frequency in primary endometrial cancer. Cancer Res. 2011;71:4061–4067. doi: 10.1158/0008-5472.CAN-11-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Gallo M, O’Hara AJ, Rudd ML, et al. Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin-remodeling and ubiquitin ligase complex genes. Nat Genet. 2012;44:1310–1315. doi: 10.1038/ng.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teer JK, Bonnycastle LL, Chines PS, et al. Systematic comparison of three genomic enrichment methods for massively parallel DNA sequencing. Genome Res. 2010;20:1420–1431. doi: 10.1101/gr.106716.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teer JK, Green ED, Mullikin JC, Biesecker LG. VarSifter: visualizing and analyzing exome-scale sequence variation data on a desktop computer. Bioinformatics. 2012;28:599–600. doi: 10.1093/bioinformatics/btr711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pena-Llopis S, Brugarolas J. Simultaneous isolation of high-quality DNA, RNA, miRNA and proteins from tissues for genomic applications. Nat Protoc. 2013;8:2240–2255. doi: 10.1038/nprot.2013.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zighelboim I, Mutch DG, Knapp A, et al. High frequency strand slippage mutations in CTCF in MSI-positive endometrial cancers. Hum Mutat. 2014;35:63–65. doi: 10.1002/humu.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 31.Bazer FW. Uterine adenogenesis and pregnancy: multiple roles for Foxa2 in mice. Biol Reprod. 2010;83:319–321. doi: 10.1095/biolreprod.110.086694. [DOI] [PubMed] [Google Scholar]
- 32.Filant J, Lydon JP, Spencer TE. Integrated chromatin immunoprecipitation sequencing and microarray analysis identifies FOXA2 target genes in the glands of the mouse uterus. FASEB J. 2014;28:230–243. doi: 10.1096/fj.13-237446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeong JW, Kwak I, Lee KY, et al. Foxa2 is essential for mouse endometrial gland development and fertility. Biol Reprod. 2010;83:396–403. doi: 10.1095/biolreprod.109.083154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelleher AM, Peng W, Pru JK, Pru CA, DeMayo FJ, Spencer TE. Forkhead box a2 (FOXA2) is essential for uterine function and fertility. Proc Natl Acad Sci U S A. 2017;11:E1018–E1026. doi: 10.1073/pnas.1618433114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamagami K, Yamauchi N, Kubota K, et al. Expression and regulation of Foxa2 in the rat uterus during early pregnancy. J Reprod Dev. 2014;60:468–475. doi: 10.1262/jrd.2014-086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith B, Neff R, Cohn DE, et al. The mutational spectrum of FOXA2 in endometrioid endometrial cancer points to a tumor suppressor role. Gynecol Oncol. 2016;143:398–405. doi: 10.1016/j.ygyno.2016.08.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moberg KH, Bell DW, Wahrer DC, Haber DA, Hariharan IK. Archipelago regulates Cyclin E levels in Drosophila and is mutated in human cancer cell lines. Nature. 2001;413:311–316. doi: 10.1038/35095068. [DOI] [PubMed] [Google Scholar]
- 38.Akhoondi S, Sun D, von der Lehr N, et al. FBXW7/hCDC4 is a general tumor suppressor in human cancer. Cancer Res. 2007;67:9006–9012. doi: 10.1158/0008-5472.CAN-07-1320. [DOI] [PubMed] [Google Scholar]
- 39.Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.