Abstract
Marrow mesenchymal stem cells supply bone osteoblasts and adipocytes. Exercise effects to increase bone and decrease fat involve transfer of signals from the cytoplasm into the nucleus to regulate gene expression. We propose that exercise control of stem cell fate relies on structural connections that terminate in the nucleus and involve intranuclear actin structures that regulate epigenetic gene expression.
Keywords: stem-cell, actin, LINC-complex, telomere, Arp2/3, osteoblast, adipocyte
Introduction
Exercise is known to be salutary to a host of physiological systems, improving quality and quantity of life through effects on musculoskeletal, metabolic, and neural functions at the very least. As such, exercise is a cornerstone of treatments for osteoporosis, obesity, diabetes – and even aging – conditions which are accompaniments of life in the 21st century.
Obesity and osteoporosis appear as antipodal body types. In the first, improvements due to exercise are largely thought to arise due to energy expenditure causing a decrease in fat depots, thereby improving associated metabolic derangements. In the low bone density of osteoporosis, patients are, in contrast, characteristically thin. Here load bearing exercise is thought to improve bone formation and limit bone resorption through effects on the output from the mesenchymal and hematopoietic precursors of the osteoblast and osteoclast effector cells (1). Because bone marrow mesenchymal stem cells (MSC) in adults give rise to the osteoblasts and adipocytes found in bone, recent research has considered a strategy which states that limiting the output of adipocytes from MSC should improve the pool of osteoblasts, thereby improving osteoporosis (1).
Exercise induced signaling involves transfer of signals - both mechanical and biochemical - from the plasma membrane into the nucleus via the LINC (Linker of Nucleoskeleton and Cytoskeleton) complex (2). Penetrating the inner nuclear membrane, the SUN protein components of LINC connect to the main structural components of the nucleoskeleton, lamin and chromatin. Lamin regulates gene expression via epigenetic control through as of yet unknown mechanisms and its absence promotes adipocyte differentiation (3). Current thinking holds that these dynamic physical structures in the MSC translate physical signals - both dynamic (exercise) and static (e.g., stiffness) - from the local environment into regulatory signals that control cell fate. We hypothesize that actin structures, outside and within the nucleus, not only support cytoskeletal and nuclear architecture, but contribute substantively to control of gene expression and MSC fate selection (4, 5).
Reciprocal relationship of bone marrow osteoblasts and adipocytes
The primary output of bone marrow MSC include the bone osteoblast and the bone marrow adipocyte, the latter which serves an unclear function that might include energy storage (6). Bone marrow MSC output appears to reflect a reciprocal relationship between these osteoblast and adipocyte lineages. The reciprocity of fat and bone in the marrow is apparent in the case of the constitutive Lrp5 activation associated with high bone mass (7), where increased trabecular bone is accompanied by decreased fat in bone marrow (8). Further, possessing but a single allele for the fat master transcription factor PPARγ, results in an increased bone mass as adipocytes are diminished (9). Such reciprocity also pertains in the increased fat phenotype in the leptin null ob/ob mouse as well as in the fatless A-ZIP/F-1 mouse, with decreased and increased bone mass, respectively (10).
Likewise, in pathological states a proportionality ratio between osteoblasts and adipocytes appears to predict bone and fat mass in the skeleton (11). Such a zero sum game includes the osteoporosis that accompanies aging (12) and estrogen deficiency (13). PPARγ agonists lead to increases in marrow adipocytes while diminishing bone strength in aged mice and humans (14). A lack of exercise restrains osteogenesis, and favors accrual of marrow adipose (15). In this way, the output of the marrow MSC suggests a response to signals that promote one output while suppressing the other.
Exercise/mechanical factors limit adipocyte differentiation
Exercise is a salutary factor known to generate bone anabolism (16) and increase muscle mass (17) through alteration of stem cell output. With regard to bone progenitor cells in the bone marrow, load bearing exercise generates forces within medullary cavities via at least fluid flow and compression of surrounding and interspersed bone, and can be expected to be experienced directly and/or indirectly whereby sensing cells transmit directions to the bone marrow stem cells through humoral factors (18, 19).
Concentrating on how exercise generated mechanical signals modulates MSC differentiation, our lab has shown that mechanical input delivered via substrate stretch - resulting in cell strain - effectively counteracts an adipogenic stimulus, inhibiting adipogenesis of primary marrow derived MSCs and embryonic C3H10T1/2 MSCs (20) as evidenced by reduction in lipid and expression of PPARγ and adiponectin. Mechanical repression of adipogenesis depends on preservation of βcatenin activity which is effected via inhibition of GSK3β (21). Mechanical activation of βcatenin preserves MSC multipotentiality (22) in the face of adipogenic stimuli, and promotes accelerated osteogenesis in response to BMP2 (20). The anti-adipogenic effect of mechanical force is βcatenin dependent: βcatenin knockdown limits the ability of strain to prevent adipogenesis.
In vivo, running exercise also prevents not only generalized adipogenesis, certainly by promoting energy utilization, but also prevents expansion of marrow adipose tissue mass. Marrow adipose accumulation due to high fat diet or PPARγ agonist can be suppressed by daily running exercise in mice (6, 23), and exercise suppression of MAT expansion occurs simultaneously with new bone formation (6). Our findings that running exercise decreases marrow fat in young and old mice fed either a control or high fat diet (6) indicate that exercise might shrink marrow adipocyte size by energy use, and also suggests that force experienced in local skeletal bone during exercise might bias MSC away from environmental stimuli that promote adipogenesis. That exercise can also decrease marrow fat induced by the strong PPARγ agonist, rosiglitazone, which promotes fat cell differentiation in vitro (22, 24), supports that mechanical signaling inhibits fat cell development in the whole animal (23). The predominant effect of maintaining βcatenin in marrow derived MSC is preservation of multipotentiality, thus increasing osteogenic potential (22). In this way, exercise may represent a non-energy based mechanism to prevent fat development.
Cellular effects of physical force are transmitted through cytoskeletal elements
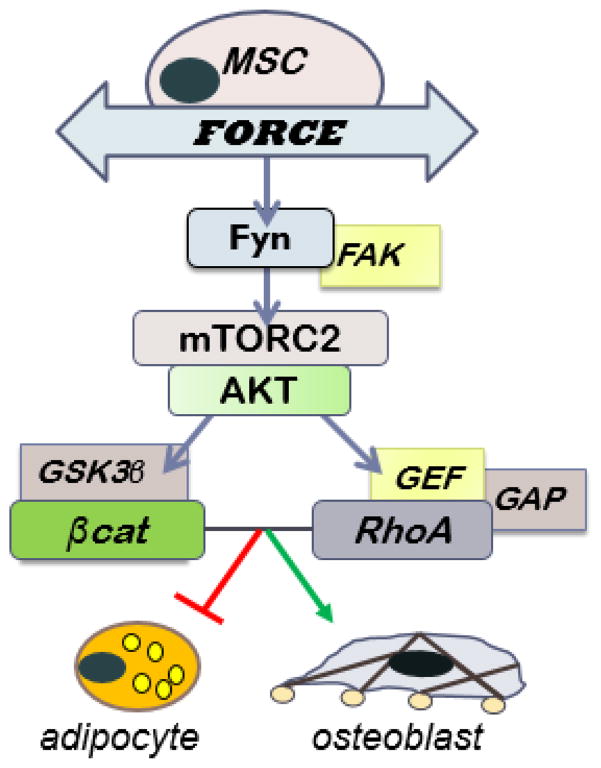
To understand the signaling responsible for preventing adipogenesis, our targets moved proximally from the GSK3β/βcatenin signaling node toward the plasma membrane, where the tug of substrate stretch is transmitted into the cell through integrins clustered into focal adhesions (Figure 1). Such focal adhesions, which connect the external substrate to bundled actin inside the cell (22), are critical to propagation of the strain-activated βcatenin signaling cascade: focal adhesion kinase operating in conjunction with Fyn to activate mTORC2, which then initiates the signal cascade of ↑Akt→ ↓GSK3β→ ↑βcatenin (21, 26). At the same time, in a parallel signaling pathway where Akt stimulates RhoA, we showed that strained cells develop significant bundled actin struts (F-actin) connecting new focal adhesions from the plasma borders (25, 27). That insulin also stimulates the mTORC2 signal pathway, yet does not stimulate RhoA, points to the importance of compartmentalization of signals in cells, and perhaps as well to the periodicity of the incoming signal (25). The enhanced cytoskeletal structure promotes MSC disposition into lineages which supply tissue competence (i.e., bone, cartilage), perhaps the cellular basis whereby dynamic physical exercise also reinforces the skeleton (28).
Figure 1. Signal pathway involved in strain regulated MSC fate decisions.
Two complementary pathways emerge after Akt is phosphorylated by mTORC2 after strain in MSC. One of these protects βcatenin from proteasomal degradation, and the other leads to activation of RhoA and actin structure. Both are anti-adipogenic, and may enhance osteogenesis.
Recent work also identifies the nucleus, and its membrane, as mechanosensory organelles, whereby anchoring to the cytoskeleton via Linker of Nucleoskeleton and Cytoskeleton (the LINC complex) complexes enables transmission of mechanical force from the outside inwards (29). We have further shown that cellular connectivity provided by these LINC complexes to be critical for activating FAK signaling at focal adhesions (2). This suggests that LINC-mediated connectivity may enable force propagation within the cell to activate mechanosignaling. Further, LINC connectivity appears to be dynamically regulated at both sides of the nuclear membrane. For example, a diaphanous formin FHOD1 (Formin Homology 2 domain containing 1) interacts with one form of many nesprins, nesprin-2, a force-bearing and actin binding element of LINC, and form secondary structures between actin and nesprin (30) to strengthen the nuclear connectivity. Similarly, formation thick actin fibers on the nuclear surface recruit LINC complexes to indentation sites (31) and cause Lamin A/C to accumulate at those sites inside the nuclear envelope. In this way, mechanical signals have the potential to further regulate connections between the nucleus and the cell cytoskeleton, generating a complementary level whereby mechanical input can control cell behavior.
Importantly, the LINC complex connects to internal nuclear chromatin, such that changes in nuclear shape are thought to be able to alter gene silencing and activation through regulating the internal nucleoskeleton, largely made up of lamin (32) through both force mediated geographies (33) and interaction with transcription factors (34) and signaling effectors (35). Recent findings suggest that the loss of LINC-mediated mechanical connectivity interferes with the changes in nuclear shape in response to substrate rigidity as well as the substrate rigidity-regulated epigenetic control in MSCs which are modulated by adaptations in nucleoskeletal Lamin A/C (36).
Interestingly, beyond intermediate filament family proteins which include Lamin A/C, the nucleus is rich with actin cytoskeleton related proteins which are capable of forming polymerized structures in a LINC-dependent manner (37). The possibility thus presents itself that intranuclear actin might also participate in structural rearrangements of chromatin and heterochromatin. To this end, we disrupted the MSC cytoskeleton using continuous cytochalasin D over the several days necessary to induce differentiation from the multipotential state. Expecting that this would induce adipogenesis, we found instead that MSC rapidly and robustly entered osteogenic lineage (5). Such osteogenesis occurred even in the absence of osteogenic medium generally used to promote the osteogenic gene program through ascorbate-directed formation of an extracellular matrix. Finally, 10 days after cytochalasin D actin disruption, cultured cells formed bone nodules that intensely stained with alizarin red (5). Sustained F-actin disruption similarly induced osteogenic differentiation of human marrow derived MSC (5).
While it now accepted that genetic elements within the nucleus respond to mechanical challenges indirectly via force transduction into intermediary biochemical cascades, it has only recently been considered that applied forces might also directly alter chromosomal conformations, thus influencing the accessibility of genetic information for binding of transcriptional enhancers or repressors (38). Indeed application of force to the cell surface has been shown to directly control gene expression (34). Inside the nucleus, structure is supported by a lamin network that makes up the internal nuclear scaffold (39). Chromatin is arranged on the Lamin scaffolding, in many cases leading to silencing of gene expression through heterochromatization. Lamin is further directly connected to the inter-leaflet LINC Sun proteins, and thereby is subject to cytoskeletal tension. That external force might control heterochromatization is consistent with force induced gene rearrangement (32). This suggests a new and unstudied area to explain how mechanical force might alter gene expression: through force directed control of nuclear structural elements.
MSC: epigenetic plasticity and programmatic silencing
Marrow derived MSC in culture, by virtue of the historical bone marrow environment from which they were derived – including the physical components of bone loading - have already begun progression toward the osteoblast lineage (40). To readjust, and progress toward adipogenic lineage instead, requires a chromatin conformation that allows the master fat transcription factor, PPARg, to access its effective cistrome. This suggests that the nuclear structure that permits access to previously silenced gene targets is crucial to differentiation.
How might intranuclear actin and lamin structures influence gene transcription? For osteogenesis, progression further in the osteogenic lineage requires the master osteogenic transcription factor, Runx2, to interact with its target cistrome (40). The PY motif of Runx2 has been previously shown to recruit YAP to Runx2 binding sites at heterochromatin, where its presence represses Runx2 activity (41). Our data suggest that Runx2 activation may be regulated through nuclear availability of YAP (5). Another possibility is that internal nuclear structure itself controls heterochromatization, a mechanism supported by the binding of lamin A/C to DNA causing specific silencing, perhaps through recruiting polycomb complexes (42). A structural level of gene expression is supported by our finding that actin polymerization occurs within the nucleus (5), and our finding that osteoblastogenesis depends on Arp2/3-induced secondary branching (4).
In terms of adipogenic differentiation, the loss of lamin leads to adipocyte differentiation in musculoskeletal tissue (43). Our data shows that an absence of branched (Arp2/3 dependent) actin structures contributes to adipogenesis (4). The gene loci that control adipogenic commitment move from a silenced state at the nuclear periphery to the center region of the nucleus to be activated and cause adipogenic commitment (44). As such, unbranched actin filaments may induce movement of adipogenic genes away from the nuclear periphery.
Mechanical control of gene arrangement within the nucleus
As a continuous polymerized network, nuclear lamina provides a framework to organize and regulate DNA (Figure 2). For example, diseases due to mutations in nuclear lamin and lamin associated proteins such as progeria (45) and muscular dystrophy cause redistribution of chromatin, altering expression patterns. Changes in nuclear envelope composition due to deletion of lamin A/C or the lamin B receptor (LBR), both which contribute to heterochromatin positioning, lead to changes in gene expression in myoblasts (46). How intra-nuclear structure is maintained by lamina, nuclear actin and the nuclear envelope, and how the whole contributes to the cellular physiology, is an area of increasing study. Indeed, interest in the interaction of telomeres with the nucleoskeleton and intranuclear actin has shown that such structural connections are critical for cell functions. With regard to intranuclear actin, inhibition of actin polymerization reduces subtelomeric dynamics, suggesting actin structure also protects telomere integrity (47). As well, during post mitotic genome reorganization Sun-1 and the sheltering subunit RAP-1 mediate physical tethering of telomeres to the nuclear envelope (48), suggesting that nucleoskeletal composition imposes a powerful influence on telomere function and maintenance. As telomeres protect chromosomal termini from being processing as “damaged” DNA fragments, (49), it is possible that exercised induced changes in nucleoskeletal composition and architecture could combat aging.
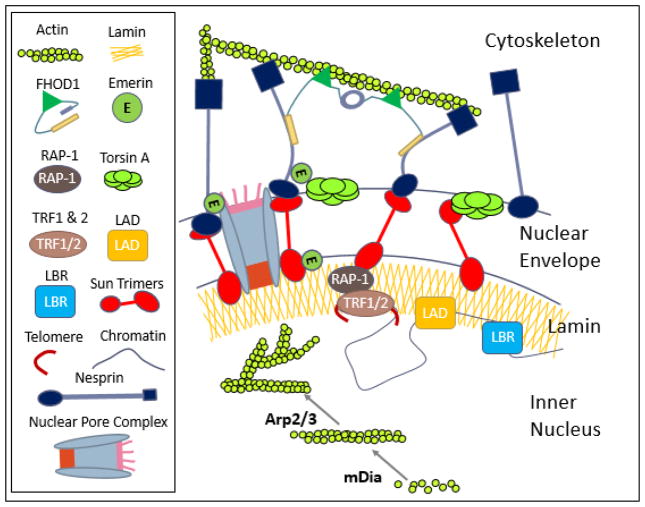
Figure 2. Hypothesis: Nucleoskeleton as a structural and regulatory organelle.
The schematic shows the nuclear envelope, nucleoskeleton and their binding partners that potentially play a role in MSC fate selection as well as facilitating the mechanical coupling between cytoplasmic and nuclear cytoskeletons. LINC complexes composed of Sun trimers and Giant Nesprin mechanically couple the actin cytoskeleton. For simplicity giant nesprin isoforms were indicated as nesprin and sun trimers were drawn as monomers. Mechanical coupling of actin and LINC involves a cytoplasmic formin FHOD1 that attaches nesprin and actin at multiple points for a more robust association. Torsin A may also facilitate the LINC assembly at the nuclear envelope. Inside the nucleus, G-actin is assembled into linear rods via mDia and into branched networks via the Arp2/3 complex, both of which can regulate availability of transcription factors and chromatin positioning. Sun1 directly binds to nuclear pore complexes as well as with RAP-1 to localize telomeres to the nuclear envelope. Chromatin interacts with nuclear structural elements to regulate gene expression including lamin B (through lamin B receptor, LBR), lamin A/C (via lamin associated domains, LADs), and actin filaments.
Interestingly, dynamic mechanical signals generated during moderate to vigorous load bearing exercise have been associated with salutary effects on telomere shortening at the cellular level in some tissues (50). While the possible mechanisms by which exercise may control telomeres remains to be explored, we recently showed that the application mechanical signals increase expression of LINC elements in mesenchymal stem cells (2). This suggests that mechanically-induced changes in nucleoskeletal architecture might underlie some of exercise effects to protect telomere length.
Conclusion: Force modifies the nuclear landscape
Growing evidence supports that gene expression in multipotential stem cells is dependent on nuclear structures that permit access of transcription factors to cistromic targets required for acquisition of cell phenotype. Nuclear structure appears to control heterochromatization as well as sequestration and transport of transcription factors. For example, the lamin nucleoskeleton silences genes through associated binding domains. The arrangement of lamin at the periphery of the nucleus, where genes tend to be silenced within massed heterochromatin, is modulated by tension transmitted from the periphery of the cell, where it is connected to its substrate via focal adhesions. In the cytoplasm, actin is critical to the cytoskeletal scaffolding that transmits force into the nucleus and regulates nuclear shape, thus participates in gene expression. As noted, we have found that actin structures within the nucleus may be able to provide a level of control for gene expression which can modify, or even circumvent, the need for extra-nuclear structure. As such, we propose that actin polymerization within the nucleus also affects gene expression through controlling availability of genes to their transcription factors, as well as providing anchors for telomere repair (Figure 2). As actin polymerization is a dynamic process, and responds to mechanical force, the transport of actin monomers into the nucleus, and their active reassembly within, are likely to play a role in the mechanical control of cell lineage.
In sum, our work has shown that mechanical force generated through dynamic loading of mesenchymal stem cells affects actin polymerization in the cell cytoplasm through affecting specific signal transduction pathways. Cytoplasmic actin structure in turn affects nuclear shape and gene expression. Our studies of mechanical control of actin structure have identified a new role for actin polymerization in controlling gene expression within the nucleus.
Summary.
This article presents a hypothesis regarding exercise control of mesenchymal stem cell fate.
Key Points.
Growing evidence supports that gene expression in multipotential stem cells is dependent on nuclear structure, which allow access of transcription factors to cistromic targets that define cell phenotype.
Nuclear structure is affected by force connections transmitted from focal adhesions at the cell membrane through actin bundles to LINC elements on the nuclear membrane. Such connectivity underlies force induced rearrangements of the intranuclear landscape.
As actin polymers are critical to transfer of force from cytoskeletal scaffolding into the nucleus - and actin polymerization is dynamic and responds to mechanical force – we hypothesize that the transport of actin monomers into the nucleus, and their reassembly within the nucleoskeleton, may be critical to mechanical control of cell lineage.
Acknowledgments
This work was supported by NIH Grants AR066616 to JR, AR062097 to MS and P20GM109095 to GU
References
- 1.Ozcivici E, Luu YK, Adler B, Qin YX, Rubin J, Judex S, et al. Mechanical signals as anabolic agents in bone. Nature reviews Rheumatology. 2010;6(1):50–9. doi: 10.1038/nrrheum.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uzer G, Thompson WR, Sen B, Xie Z, Yen SS, Miller S, et al. Cell Mechanosensitivity to Extremely Low-Magnitude Signals Is Enabled by a LINCed Nucleus. Stem Cells. 2015;33(6):2063–76. doi: 10.1002/stem.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akter R, Rivas D, Geneau G, Drissi H, Duque G. Effect of lamin A/C knockdown on osteoblast differentiation and function. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24(2):283–93. doi: 10.1359/jbmr.081010. [DOI] [PubMed] [Google Scholar]
- 4.Sen B, Uzer G, Samsonraj R, Xie Z, McGrath C, Styner M, et al. Intranuclear actin structure modulates mesenchymal stem cell differentiation. Stem Cells. 2017 doi: 10.1002/stem.2617. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sen B, Xie Z, Uzer G, Thompson WR, Styner M, Wu X, et al. Intranuclear Actin Regulates Osteogenesis. Stem Cells. 2015;33(10):3065–76. doi: 10.1002/stem.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Styner M, Thompson WR, Galior K, Uzer G, Wu X, Kadari S, et al. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone. 2014;64:39–46. doi: 10.1016/j.bone.2014.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, et al. High bone density due to a mutation in LDL-receptor-related protein 5. The New England journal of medicine. 2002;346(20):1513–21. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 8.Qiu W, Andersen TE, Bollerslev J, Mandrup S, Abdallah BM, Kassem M. Patients with high bone mass phenotype exhibit enhanced osteoblast differentiation and inhibition of adipogenesis of human mesenchymal stem cells. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007;22(11):1720–31. doi: 10.1359/jbmr.070721. [DOI] [PubMed] [Google Scholar]
- 9.Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. The Journal of clinical investigation. 2004;113(6):846–55. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460(7252):259–63. doi: 10.1038/nature08099. Epub 2009/06/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fazeli PK, Horowitz MC, MacDougald OA, Scheller EL, Rodeheffer MS, Rosen CJ, et al. Marrow fat and bone--new perspectives. The Journal of clinical endocrinology and metabolism. 2013;98(3):935–45. doi: 10.1210/jc.2012-3634. Epub 2013/02/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wren TA, Chung SA, Dorey FJ, Bluml S, Adams GB, Gilsanz V. Bone marrow fat is inversely related to cortical bone in young and old subjects. The Journal of clinical endocrinology and metabolism. 2011;96(3):782–6. doi: 10.1210/jc.2010-1922. Epub 2010/12/24. [DOI] [PubMed] [Google Scholar]
- 13.Cohen A, Dempster DW, Stein EM, Nickolas TL, Zhou H, McMahon DJ, et al. Increased marrow adiposity in premenopausal women with idiopathic osteoporosis. The Journal of clinical endocrinology and metabolism. 2012;97(8):2782–91. doi: 10.1210/jc.2012-1477. Epub 2012/06/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harslof T, Wamberg L, Moller L, Stodkilde-Jorgensen H, Ringgaard S, Pedersen SB, et al. Rosiglitazone decreases bone mass and bone marrow fat. The Journal of clinical endocrinology and metabolism. 2011;96(5):1541–8. doi: 10.1210/jc.2010-2077. Epub 2011/03/04. [DOI] [PubMed] [Google Scholar]
- 15.Casazza K, Hanks LJ, Hidalgo B, Hu HH, Affuso O. Short-term physical activity intervention decreases femoral bone marrow adipose tissue in young children: a pilot study. Bone. 2012;50(1):23–7. doi: 10.1016/j.bone.2011.08.032. Epub 2011/09/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Styner M, Pagnotti GM, McGrath C, Wu X, Sen B, Uzer G, et al. Exercise Decreases Marrow Adipose Tissue Through ss-Oxidation in Obese Running Mice. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2017 doi: 10.1002/jbmr.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vega RB, Konhilas JP, Kelly DP, Leinwand LA. Molecular Mechanisms Underlying Cardiac Adaptation to Exercise. Cell Metab. 2017;25(5):1012–26. doi: 10.1016/j.cmet.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. The Journal of biological chemistry. 2008;283(9):5866–75. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 19.Wallace IJ, Pagnotti GM, Rubin-Sigler J, Naeher M, Copes LE, Judex S, et al. Focal enhancement of the skeleton to exercise correlates with responsivity of bone marrow mesenchymal stem cells rather than peak external forces. J Exp Biol. 2015;218(Pt 19):3002–9. doi: 10.1242/jeb.118729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sen B, Xie Z, Case N, Ma M, Rubin C, Rubin J. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology. 2008;149(12):6065–75. doi: 10.1210/en.2008-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sen B, Styner M, Xie Z, Case N, Rubin CT, Rubin J. Mechanical loading regulates NFATc1 and beta-catenin signaling through a GSK3beta control node. The Journal of biological chemistry. 2009;284(50):34607–17. doi: 10.1074/jbc.M109.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Case N, Thomas J, Xie Z, Sen B, Styner M, Rowe D, et al. Mechanical input restrains PPARgamma2 expression and action to preserve mesenchymal stem cell multipotentiality. Bone. 2013;52(1):454–64. doi: 10.1016/j.bone.2012.08.122. Epub 2012/09/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Styner M, Pagnotti GM, Galior K, Wu X, Thompson WR, Uzer G, et al. Exercise Regulation of Marrow Fat in the Setting of PPARgamma Agonist Treatment in Female C57BL/6 Mice. Endocrinology. 2015;156(8):2753–61. doi: 10.1210/en.2015-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Styner M, Meyer MB, Galior K, Case N, Xie Z, Sen B, et al. Mechanical strain downregulates C/EBPbeta in MSC and decreases endoplasmic reticulum stress. PloS one. 2012;7(12):e51613. doi: 10.1371/journal.pone.0051613. Epub 2012/12/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sen B, Xie Z, Case N, Thompson WR, Uzer G, Styner M, et al. mTORC2 regulates mechanically induced cytoskeletal reorganization and lineage selection in marrow-derived mesenchymal stem cells. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014;29(1):78–89. doi: 10.1002/jbmr.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Case N, Thomas J, Sen B, Styner M, Xie Z, Galior K, et al. Mechanical regulation of glycogen synthase kinase 3beta (GSK3beta) in mesenchymal stem cells is dependent on Akt protein serine 473 phosphorylation via mTORC2 protein. The Journal of biological chemistry. 2011;286(45):39450–6. doi: 10.1074/jbc.M111.265330. Epub 2011/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sen B, Guilluy C, Xie Z, Case N, Styner M, Thomas J, et al. Mechanically induced focal adhesion assembly amplifies anti-adipogenic pathways in mesenchymal stem cells. Stem Cells. 2011;29(11):1829–36. doi: 10.1002/stem.732. Epub 2011/09/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gross TS, Srinivasan S, Liu CC, Clemens TL, Bain SD. Noninvasive loading of the murine tibia: an in vivo model for the study of mechanotransduction. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2002;17(3):493–501. doi: 10.1359/jbmr.2002.17.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lombardi ML, Jaalouk DE, Shanahan CM, Burke B, Roux KJ, Lammerding J. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. The Journal of biological chemistry. 2011;286(30):26743–53. doi: 10.1074/jbc.M111.233700. Epub 2011/06/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arsenovic PT, Ramachandran I, Bathula K, Zhu R, Narang JD, Noll NA, et al. Nesprin-2G, a Component of the Nuclear LINC Complex, Is Subject to Myosin-Dependent Tension. Biophysical journal. 2016;110(1):34–43. doi: 10.1016/j.bpj.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Versaevel M, Braquenier JB, Riaz M, Grevesse T, Lantoine J, Gabriele S. Super-resolution microscopy reveals LINC complex recruitment at nuclear indentation sites. Sci Rep. 2014;4:7362. doi: 10.1038/srep07362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martins RP, Finan JD, Guilak F, Lee DA. Mechanical regulation of nuclear structure and function. Annual review of biomedical engineering. 2012;14:431–55. doi: 10.1146/annurev-bioeng-071910-124638. Epub 2012/06/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang W, Worman HJ, Gundersen GG. Accessorizing and anchoring the LINC complex for multifunctionality. The Journal of cell biology. 2015;208(1):11–22. doi: 10.1083/jcb.201409047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tajik A, Zhang Y, Wei F, Sun J, Jia Q, Zhou W, et al. Transcription upregulation via force-induced direct stretching of chromatin. Nat Mater. 2016;15(12):1287–96. doi: 10.1038/nmat4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thakar K, May CK, Rogers A, Carroll CW. Opposing roles for distinct LINC complexes in regulation of the small GTPase RhoA. Molecular biology of the cell. 2017;28(1):182–91. doi: 10.1091/mbc.E16-06-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341(6149):1240104. doi: 10.1126/science.1240104. Epub 2013/08/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plessner M, Melak M, Chinchilla P, Baarlink C, Grosse R. Nuclear F-actin formation and reorganization upon cell spreading. The Journal of biological chemistry. 2015;290(18):11209–16. doi: 10.1074/jbc.M114.627166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le HQ, Ghatak S, Yeung CC, Tellkamp F, Gunschmann C, Dieterich C, et al. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nature cell biology. 2016 doi: 10.1038/ncb3387. [DOI] [PubMed] [Google Scholar]
- 39.Schreiber KH, Kennedy BK. When lamins go bad: nuclear structure and disease. Cell. 2013;152(6):1365–75. doi: 10.1016/j.cell.2013.02.015. Epub 2013/03/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer MB, Benkusky NA, Sen B, Rubin J, Pike JW. Epigenetic Plasticity Drives Adipogenic and Osteogenic Differentiation of Marrow-derived Mesenchymal Stem Cells. The Journal of biological chemistry. 2016;291(34):17829–47. doi: 10.1074/jbc.M116.736538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaidi SK, Sullivan AJ, Medina R, Ito Y, van Wijnen AJ, Stein JL, et al. Tyrosine phosphorylation controls Runx2-mediated subnuclear targeting of YAP to repress transcription. The EMBO journal. 2004;23(4):790–9. doi: 10.1038/sj.emboj.7600073. Epub 2004/02/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramdas NM, Shivashankar GV. Cytoskeletal control of nuclear morphology and chromatin organization. Journal of molecular biology. 2015;427(3):695–706. doi: 10.1016/j.jmb.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Tong J, Li W, Vidal C, Yeo LS, Fatkin D, Duque G. Lamin A/C deficiency is associated with fat infiltration of muscle and bone. Mechanisms of ageing and development. 2011;132(11–12):552–9. doi: 10.1016/j.mad.2011.09.004. Epub 2011/10/11. [DOI] [PubMed] [Google Scholar]
- 44.Szczerbal I, Foster HA, Bridger JM. The spatial repositioning of adipogenesis genes is correlated with their expression status in a porcine mesenchymal stem cell adipogenesis model system. Chromosoma. 2009;118(5):647–63. doi: 10.1007/s00412-009-0225-5. [DOI] [PubMed] [Google Scholar]
- 45.Goldman RD, Shumaker DK, Erdos MR, Eriksson M, Goldman AE, Gordon LB, et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(24):8963–8. doi: 10.1073/pnas.0402943101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solovei I, Wang AS, Thanisch K, Schmidt CS, Krebs S, Zwerger M, et al. LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell. 2013;152(3):584–98. doi: 10.1016/j.cell.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Spichal M, Brion A, Herbert S, Cournac A, Marbouty M, Zimmer C, et al. Evidence for a dual role of actin in regulating chromosome organization and dynamics in yeast. Journal of cell science. 2016;129(4):681–92. doi: 10.1242/jcs.175745. [DOI] [PubMed] [Google Scholar]
- 48.Crabbe L, Cesare AJ, Kasuboski JM, Fitzpatrick JA, Karlseder J. Human telomeres are tethered to the nuclear envelope during postmitotic nuclear assembly. Cell Rep. 2012;2(6):1521–9. doi: 10.1016/j.celrep.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Czapiewski R, Robson MI, Schirmer EC. Anchoring a Leviathan: How the Nuclear Membrane Tethers the Genome. Front Genet. 2016;7:82. doi: 10.3389/fgene.2016.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loprinzi PD, Loenneke JP, Blackburn EH. Movement-Based Behaviors and Leukocyte Telomere Length among US Adults. Medicine and science in sports and exercise. 2015;47(11):2347–52. doi: 10.1249/MSS.0000000000000695. [DOI] [PMC free article] [PubMed] [Google Scholar]