Abstract
Objectives
Cardiovascular fat (CF) is associated with greater coronary heart disease (CHD) risk. Postmenopausal women have greater CF volumes than premenopausal women and the association between specific CF depot volumes and CHD risk is more pronounced after menopause. Race, central adiposity, and visceral adiposity are important factors that could impact CF volumes. Whether racial differences in CF volumes and in their associations with central (visceral fat (VAT)) and general adiposity (body mass index (BMI)) exist in midlife women have not been addressed before.
Methods
A total of 524 participants from the Study of Women’s Health Across the Nation (mean age: 50.9 ± 2.9 years; 62% White and 38% Black) who had data on CF volumes (epicardial fat (EAT), paracardial fat (PAT), total heart fat (TAT), and aortic perivascular fat), VAT, and BMI were studied.
Results
Black women had less volumes of all CF depots than White women, independent of study covariates, and BMI or VAT (P<0.05). Race significantly modified associations between adiposity measures and CF volumes. Every 1-SD higher BMI was associated with 66.7% greater PAT volume in White compared with 42.4% greater PAT volume in Black women (P=0.004); while, every 1-SD higher VAT was associated with 32.3% greater EAT volume in Black compared with 25.3% greater EAT volume in White women (P=0.039).
Conclusions
Racial differences were found in CF volumes and in their associations with adiposity measures among midlife women. Future research should determine how race-specific changes in CF volumes impact CHD risk in women.
Keywords: Paracardial fat, perivascular fat, epicardial fat, race, adiposity, women
Introduction
Cardiovascular fat (CF), defined as the fat surrounding the heart and arteries,1 has been shown to be a metabolically active organ that secretes numerous pro- and anti-inflammatory substances.2 In addition, literature suggests that individual CF depots (epicardial fat (EAT), paracardial fat (PAT), total heart fat (TAT), and thoracic aortic perivascular fat) may be embryonically and metabolically different;3,4 therefore, it may be important to look at these depots separately.3 The current theory asserts that CF depots become dysfunctional in states of excess adiposity.1,5 The close proximity and paracrine effects of CF depots make them potentially important fat depots.2,3 Indeed, many studies have found CF to be positively associated with the presence6,7 and severity8,9 of subclinical atherosclerosis, independent of other adiposity measures.
Postmenopausal women have greater volumes of EAT, PAT and TAT compared to premenopausal women independent of obesity status.10 Interestingly, volumes of PAT are greater in women with higher declines in estradiol levels10 supporting the notion that menopause is associated with fat redistribution. Most recently, postmenopausal women with greater volumes of PAT showed greater risk of coronary heart disease (CHD) compare with premenopausal women.11 Hence, understanding potential factors that may link to greater volumes of CF depots is especially important in midlife women.
Limited information is available regarding determinants of CF, but a few studies have shown positive correlations between CF and adiposity measures (body mass index (BMI) and abdominal visceral fat).12–14 Racial differences in the CF volumes have been identified in middle-aged men, with Black men having less CF compared with White men, independent of overall adiposity.14 Studies have shown that, in general, Blacks compared with Whites have more subcutaneous fat,15,16 and less hepatic fat17 and visceral fat,15 but higher incidence rates of heart failure and stroke,18,19 higher rates of diabetes,20 and a greater risk of cardiovascular disease mortality,19 indicating a racial-obesity paradox. The recent finding indicating that visceral fat has a stronger influence on CF in Black compared with White men introduces a potential mechanism involved in this racial-obesity paradox.14 Interestingly, race has been found to modify the associations between adiposity measures and individual CF depots, with White men having more EAT and TAT than Black men for increasing BMI levels.14 Although research regarding racial differences in CF among women is limited, some evidence suggests that Black women have a more favorable fat distribution with less EAT21 and visceral fat15 when compared with White women. However, none of these studies has focused on understanding the contribution of race and other adiposity measures to CF depots in women at midlife.
Since postmenopausal women have a higher risk of CHD22,23 and less favorable fat distribution with more CF and visceral fat10, 24 compared with premenopausal women, the relationships between race and adiposity in regards to CF in midlife women are intriguing. Therefore, our objectives were to determine whether race, overall adiposity, and central adiposity were associated with the quantity of individual CF depots (EAT, PAT, TAT, and perivascular fat) and to evaluate whether cross-sectional associations between individual adiposity measures (BMI, subcutaneous fat, and visceral fat) and individual volumes of CF depots vary by race in midlife women. We hypothesized that similar to men, CF volumes would differ by race with Black women having lesser volumes of CF compared to White women at midlife; that CF volumes would be positively associated with BMI, subcutaneous fat, and visceral fat; and that associations between adiposity measures and CF volumes would differ by race, with stronger associations between BMI and CF found in White women.
Methods
Study Population
The Study of Women’s Health Across the Nation (SWAN) is a community-based longitudinal multisite study of women transitioning through menopause. The study design and objectives have been reported previously.25 Briefly, from seven sites (Boston, MA; Detroit, MI; Oakland, CA; Los Angeles, CA; Pittsburgh, PA; Chicago, IL; and Newark, NJ) 3302 participants aged 42–52 years were recruited between 1996 and 1997.25 The eligibility criteria for the SWAN study included having an intact uterus and at least one ovary and at least one menstrual period and no hormone therapy use within the past 3 months. SWAN Heart was conducted at the Pittsburgh and Chicago SWAN sites which enrolled White and Black women at 2 time points, an average of 2.4 years (range 1–5 years) apart. The baseline SWAN Heart visit took place across visits 04-07 of the SWAN parent study, while the SWAN Heart follow-up visit took place across visits 06-09 of the SWAN parent study. The SWAN Cardiovascular Fat ancillary study was designed to quantify CF among SWAN Heart study participants at the SWAN Heart baseline visit.10 A total of 562 out of the 608 SWAN Heart participants who had a readable CF measure (EAT, PAT, TAT, or perivascular fat) were included in these analyses. Participants were excluded if they were missing adiposity measures or had undergone surgical menopause (n=38). A total of 524 women were included in the perivascular fat analyses. Due to either poor image quality or scans that did not encompass the designated anatomical boundaries for the EAT, PAT, or TAT depots, 39 additional participants were excluded from EAT, PAT, and TAT analyses, leaving a total of 485 women. All participants signed informed consent and the institutional review board at each site approved the study protocol.
CF Depots Measurements and Quantification
EAT, PAT, and TAT (EAT+PAT) volumes were quantified at the Los Angeles Biomedical Research Institute, Harbor-UCLA Medical Center, CA, USA, using images previously acquired during the electron-beam CT scanning to measure coronary artery calcification (3-mm-thick transverse images obtained with a GE-Imatron C150 Electron Beam Tomography scanner (GE Medical Systems, South San Francisco, CA, USA)).10 EAT, PAT, and TAT volumes were determined from 15 mm above to 30 mm below the superior extent of the left main coronary artery to include the fat around the proximal coronary arteries. The chest wall served as the anterior border and the aorta and the bronchus served as the posterior border. Using volume analysis software (GE Healthcare, Waukesha, WI, USA), adipose tissue was distinguished from the remainder of the heart tissue by a threshold of −190 to −30 Hounsfield units. CF was measured by manually tracing the borders of the area of interest every 2–3 CT slices beginning at the starting point and then using the software to automatically trace the segments in between these selected slices. As previously described, EAT was defined as the fat inside the pericardium, PAT was defined as the fat outside of the pericardium, and TAT was defined as the total fat within the above described anatomical borders.10 PAT volume was measured by subtracting the EAT volume from the TAT volume. These fat measures have excellent reproducibility with between- and within-reader spearman correlation coefficients of 0.97.10
Perivascular fat was measured using images previously acquired from the electron-beam CT scanning performed to quantify aortic calcification (6 mm thick cross-sectional images obtained with a GE-Imatron C150 Electron Beam Tomography scanner (GE Medical Systems, South San Francisco, CA, USA)).10 Perivascular fat was quantified using Slice-o-Matic v4.3 (Tomovision, Magog, Quebec, Canada) at the University of Pittsburgh Ultrasound Research Lab. Perivascular fat was defined as the adipose tissue surrounding the descending aorta and was distinguished from other tissues by a threshold of −190 to −30 Hounsfield units. The pulmonary bifurcation served as the proximal border, while the first lumbar vertebrae marked the distal border. The vertebral foramen served as the posterior border, while the anterior borders included a horizontal line through the left bronchus which progressed distally until eventually the interior border of the crus of the diaphragm. The borders were manually traced for every slice. This fat measure has excellent intra-reader and inter-reader reproducibility (intra-class coefficient 0.999 and 0.998, respectively).7
Readers of all cardiovascular fat depots at the Los Angeles Biomedical Research Institute and the Ultrasound Research Lab were blinded to participants’ characteristics. The lead author performed all statistical analyses and did not read any scan.
Adiposity Measures
Abdominal fat was measured with a single 6 mm thick cross-sectional image obtained between the L4 and L5 vertebral space with a GE-Imatron C150 Electron Beam Tomography scanner (GE Medical Systems, South San Francisco, CA, USA) as described elsewhere.26 Briefly, scans were read by a single reader at the University of Pittsburgh. Adipose tissue was distinguished from other tissues by a threshold of −190 to −30 Hounsfield units using image analysis software (AcuImage software, South San Francisco, CA). A region of interest line along a fascial plane was drawn at the interior of the abdominal musculature and adipose tissue within this area was considered visceral fat. subcutaneous fat area was calculated as the difference between the total abdominal fat area and visceral fat. Excellent inter-observer reliability was reported with intra-class coefficients of 0.94 and 0.97 for visceral fat and total abdominal fat, respectively.26 Weight and height were measured in light clothing and without shoes. Weight was measured using a standardized, calibrated scale and height was measured using a stadiometer. BMI was calculated as weight in kilograms divided by height in square meters.
Study Covariates
Blood pressure was measured in the right arm with the participant seated using a mercury sphygmomanometer after 5 minutes of rest. Blood pressure readings were taken twice and averaged. Hypertension was defined as present if the following criteria were met: SBP ≥ 140, or DBP ≥ 90, or taking blood pressure medication. Serum glucose was measured using a hexokinase-coupled reaction (Boehringer Mannheim Diagnostics) and diabetes was defined as present if the fasting serum glucose was greater than or equal to 126 or if taking diabetes medication.
Race, age, financial strain, alcohol consumption, cholesterol medication, current smoking status, and physical activity were self-reported. Financial strain was derived from the interview question, “How hard is it for you to pay for the very basics like food, housing, medical care, and heating?” For analyses, the answers were dichotomized as “somewhat hard to very hard” and “not hard at all”. Alcohol consumption was categorized into the following: less than or equal to one drink per month; more than one drink per month to one drink per week; and two or more drinks per week. Physical activity was measured via a modified Baecke score of exercise frequency with higher scores indicating more routine physical activity.27
Menopausal status was categorized into the following groups using self-reported bleeding patterns: premenopausal (menses in the last 3 months with no change in regularity in the last 12 months); early peri-menopausal (menses in the last 3 months with some change in regularity during the prior 12 months); late peri-menopausal (no menses within the last 3 months, but some menstrual bleeding over the prior 12 months); and postmenopausal (no menses for the last 12 months). Due to small numbers in some of these categories, premenopausal and early peri-menopausal women were combined in one group and late peri-menopausal and postmenopausal women were combined in another group.10 Only 51(9.7%) out of 524 women included in our analysis reported using hormone therapy (HT). This group of HT users included both postmenopausal women who reported HT use after menopause (n=29) and women for whom HT use precluded correct menopausal status classification (n=22), “unknown menopausal status”. Given that our analysis is focusing on racial modifying effect, assessing HT use as a separate variable from menopausal status variable would result in very small numbers for “unknown menopausal status” category by race (n=5 for black). Therefore, HT use and unknown menopausal status due to HT use were combined as a separate category for menopausal status variable used in the current analyses. The approach of combing postmenopausal HT users and unknown menopausal status due to HT use has the advantage of resulting in a single, relatively larger group. The final menopausal status variable included 3 categories as follow: 1) Pre-/early peri-menopausal status, 2) late peri-/postmenopausal status, and 3) HT use.
Statistical Analyses
The characteristics of the study population were summarized and presented as mean ± standard deviation for normally distributed variables; median (Q1, Q3) for skewed variables; and frequency (percentage) for categorical variables. Normality was assessed for all continuous variables and EAT, PAT, TAT, perivascular fat, and visceral fat were log-transformed. Chi-square and t-tests were used to determine whether participant characteristics, CF measures, and adiposity measures differed by race.
Separate univariate linear regression models were created to assess the relationships between the characteristics of the study population and CF volumes (Supplemental Table 1). Multivariable linear regression was used to determine whether race as the primary independent variable was associated with CF volumes (EAT, PAT, TAT, and perivascular fat; separate models). Age, study site, and menopausal status were a priori selected covariates to be included in all analyses. To determine which additional covariates to include in the multivariable analyses, we assessed all variables that were significantly associated with CF volumes (P<0.05) using backward elimination. To determine the most parsimonious model, variables were removed in a stepwise manner based on significance and whether or not they improved the fit of the model. Sensitivity analyses were conducted to determine if triglycerides and low-density lipoprotein cholesterol improved the fit of the model and they did not; therefore, they were not included in the final model. The following covariates were included in the final model: age, study site, menopausal status, diabetes, alcohol consumption, and physical activity. All continuous variables were centered at the mean.
Racial differences in CF volumes were calculated. To provide results that are easily interpreted, % differences and % changes in CF volumes with 95% confidence intervals were calculated.31 Beta coefficients and related 95% confidence intervals from linear regression were presented as the % differences in CF between Blacks and Whites using the formula (eβ-1)*100; and % change in CF per standard deviation in BMI and subcutaneous fat for Whites and Blacks using the formula (eβ*SD-1)*100.28 One standard deviation above the geometric mean in visceral fat was approximately a 55% increase; therefore the following formula was used to calculate the % change in CF per standard deviation in visceral fat for Whites and Blacks (eβ*(log(1.55)-1)*100.28 Additional adjustments for individual adiposity measures (BMI, visceral fat, and subcutaneous fat; separate models) were performed to determine if adiposity measures explained the relationships between race and CF volumes. Scatter plots of the associations between adiposity measures and CF measures by race were created to examine the data. To determine whether race significantly modified the associations between adiposity measures and CF, interactions were assessed between race and adiposity measures as related to CF volumes (separate models) adjusting for the above listed covariates. The race-specific effect sizes of adiposity measures on CF measures (separate models) were calculated to facilitate comparisons between the effect sizes of adiposity measures on CF in the individual races. Interactions between menopausal status and race in regards to CF volumes were assessed in each model and no statistically significant interactions were found (all p>0.05). All analyses were conducted using SAS v9.3 (SAS Institute, Cary, North Carolina).
Results
The characteristics of the study population overall and by race are presented in Table 1. The women in our study were 50.9 ± 2.9 years old, 38% Black, and 55% pre/early peri-menopausal. Black women were more likely to be hypertensive, consume less alcohol, have lower physical activity, have higher BMI levels, have greater subcutaneous fat, and have lower volumes of EAT and TAT compared with White women.
Table 1.
Characteristics of the study population overall and by race
| Variables | Total (n=524) |
White (n=324) (62%) |
Black (n=200) (38%) |
P-value |
|---|---|---|---|---|
| Age, years | 50.9 ± 2.9 | 50.9 ± 2.9 | 51.0 ± 2.8 | 0.605 |
| Menopausal Status, n (%) Pre-/early peri-menopausal Late peri-/postmenopausal Hormone Users |
290 (55.3) 183 (34.9) 51 (9.7) |
183 (56.5) 103 (31.8) 38 (11.7) |
107 (53.5) 80 (40.0) 13 (6.5) |
0.048 |
| Financial Strain, n (%) | 158 (31.6) | 72 (23.2) | 86 (45.5) | <0.001 |
| Current Smoker, n (%) | 94 (17.9) | 56 (17.3) | 38 (19.0) | 0.619 |
| Alcohol Use, n (%) ≤ 1/month > 1/month to 1/week ≥ 2/week |
196 (37.8) 191 (36.4) 135 (25.8) |
87 (26.9) 134 (41.4) 103 (31.8) |
111 (55.5) 57 (28.5) 32 (16.0) |
<0.001 |
| Hypertension, n (%) | 133 (25.4) | 53 (16.4) | 80 (40.0) | <0.001 |
| Diabetes, n (%) | 26 (5.0) | 12 (6.0) | 14 (4.3) | 0.390 |
| Cholesterol Medication, n (%) | 28 (5.3) | 16 (4.9) | 12 (6.0) | 0.600 |
| Physical Activity | 7.8 ± 1.7 | 8.2 ± 1.6 | 7.2 ± 1.6 | <0.001 |
| BMI, kg/m2 | 29.2 ± 6.2 | 28.1 ± 5.7 | 31.0 ± 6.5 | <0.001 |
| Abdominal visceral fat, cm2 | 110.6 (72.0, 161.5) | 107.6 (69.0, 165.6) | 114.0 (79.2, 158.0) | 0.956 |
| Abdominal subcutaneous fat, cm2 | 335.1 ± 151.2 | 315.6 ± 146.7 | 362.6 ± 154.3 | <0.001 |
| EAT, cm3 | 36.5 (27.9, 50.5) | 38.2 (28.1, 51.6) | 35.4 (25.7, 49.7) | 0.014 |
| PAT, cm3 | 9.0 (5.4, 14.8) | 8.9 (5.4, 14.8) | 9.5 (5.2, 14.8) | 0.615 |
| TAT, cm3 | 46.7 (34.8, 64.9) | 48.0 (35.0, 65.2) | 45.2 (33.9, 64.9) | 0.044 |
| Aortic perivascular fat, cm3 | 29.6 (24.1, 39.0) | 30.6 (24.5, 39.2) | 28.6 (23.0, 37.6) | 0.088 |
Data presented as mean ± standard deviation, median (interquartile range), or frequency (percentage); BMI, body mass index; EAT, epicardial fat; PAT, paracardial fat; TAT, total heart fat
After adjusting for age, study site, menopausal status, hypertension, diabetes, alcohol consumption, and physical activity, Black women had 19.8% less EAT, 24.5% less PAT, 20.4% less TAT, and 13.2% less perivascular fat than White women (all p<0.001) (Table 2). These racial differences remained significant after further adjustment for BMI and subcutaneous fat (separate models). Although these significant racial differences persisted after adjusting for visceral fat, the magnitude of reported effect sizes were somewhat attenuated with Black women having 10.6% less EAT, 11.0% less PAT, 10.2% less TAT, and 5.0% less perivascular fat than White women (all p<0.05).
Table 2.
Adjusted percent differences in volumes of CF depots by race
| EAT (n=485) | PAT (n=485) | TAT (n=485) | Aortic perivascular fat (n=524) | |
|---|---|---|---|---|
| % Difference (95% CI) |
% Difference (95% CI) |
% Difference (95% CI) |
% Difference (95% CI) |
|
| Model 1: adjusted for age, study site, menopausal status, hypertension, diabetes, alcohol consumption, and physical activity | ||||
| Black vs White | −19.8c (−26.7, −12.4) |
−24.5c (−34.6, −12.9) |
−20.4c (−27.3, −12.7) |
−13.2c (−19.2, −7.0) |
| Model 2: model 1 + BMI | ||||
| Black vs White | −22.4c (−27.9, −16.6) |
−28.2c (−36.3, −19.2) |
−23.2c (−28.5, −17.6) |
−16.0c (−20.5, −11.4) |
| Model 3: model 1 + Visceral fat | ||||
| Black vs White | −10.6b (−16.6, −4.2) |
−11.0a (−20.8, −0.1) |
−10.2b (−16.0, −4.0) |
−5.0a (−9.7, −0.03) |
| Model 4: model 1 + Subcutaneous fat | ||||
| Black vs White | −21.0c (−27.1, −14.5) |
−26.3c (−35.2, −16.1) |
−21.6c (−27.8, −15.1) |
−14.0c (−19.2, −8.6) |
p-value < 0.05;
p-value <0.01;
p-value <0.001; BMI, body mass index; EAT, epicardial fat; PAT, paracardial fat; TAT, total heart fat; Visceral fat, aortic perivascular fat, EAT, PAT, and TAT were log transformed; Beta coefficients and related 95% CI from linear regression were presented as % differences between Blacks and Whites using the following formula: (eβ-1)*100.28
In general, higher levels of adiposity were significantly associated with higher volumes of CF for all depots (data not shown). Race modified the associations between BMI and PAT, as well as between visceral fat and EAT after adjusting for age, study site, menopausal status, hypertension, diabetes, alcohol consumption, and physical activity. White women had significantly more PAT for higher BMI levels when compared with Black women (interaction p-value=0.004) (Figure 1-A). In contrast, Black women had significantly more EAT for higher visceral fat levels when compared with White women (Interaction p-value=0.039) (Figure 1-B). The race-specific changes in CF volumes per 1-standard deviation increments in adiposity measures within each race are shown in Table 3. Every 1-standard deviation higher BMI corresponded to 66.7% greater PAT in White women compared with only 42.4% greater PAT in Black women (interaction p-value=0.004). In contrast, every 1-standard deviation higher visceral fat corresponded to 32.3% greater EAT in Black women compared with only 25.3% greater EAT in White women (interaction p-value = 0.039). These differences were independent of age, study site, menopausal status, hypertension, diabetes, alcohol consumption, and physical activity.
Figure 1. Scatterplots of race-specific slopes of A) BMI on PAT and B) visceral fat on EAT.
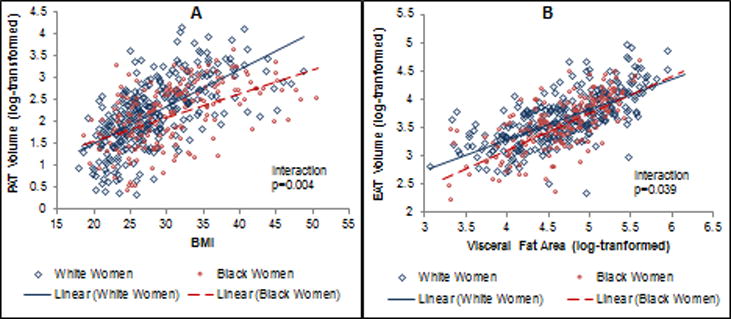
A) BMI on PAT volume by race: Every 1-SD higher BMI was associated with 66.7% greater PAT volume in White compared with 42.4% greater PAT volume in Black women (P=0.004); B) Visceral fat on EAT volume by race: Every 1-SD higher visceral fat was associated with 32.3% greater EAT volume in Black compared with 25.3% greater EAT volume in White women (P=0.039). Models were adjusted for age, study site, menopausal status, hypertension, diabetes, alcohol consumption, and physical activity.
Table 3.
Race-specific changes in volumes of CF depots per 1-SD increment changes in adiposity measures
| EAT | PAT | TAT | Aortic perivascular fat | |
|---|---|---|---|---|
| % Change (95% CI) |
% Change (95% CI) |
% Change (95% CI) |
% Change (95% CI) |
|
| Per 1-SD increment of BMI | ||||
| Whites | 34.9 (28.7, 41.5) | 66.7 (54.2, 79.9) | 40.4 (34.0, 47.1) | 31.3 (26.8, 36.0) |
| Blacks | 30.9 (24.5, 37.6) | 42.4 (31.3, 54.5)b | 32.5 (26.2, 39.2) | 26.0 (21.4, 30.8) |
| Per 1-SD increment of visceral fat | ||||
| Whites | 25.3 (21.4, 29.2) | 45.7 (38.3, 53.4) | 29.2 (25.3, 33.1) | 22.3 (19.6, 25.0) |
| Blacks | 32.3 (26.6, 38.1)a | 40.3 (30.5, 50.9) | 33.6 (28.1, 39.3) | 25.6 (21.7, 29.6) |
| Per 1-SD increment of subcutaneous fat | ||||
| Whites | 21.5 (15.7, 28.0) | 43.8 (32.5, 55.8) | 25.3 (19.0, 31.7) | 17.2 (13.0, 21.5) |
| Blacks | 25.7 (18.8, 32.7) | 32.3 (21.0, 44.6) | 26.2 (19.4, 33.5) | 22.3 (17.0, 27.6) |
Whites differ from Blacks, p-value <0.05;
Whites differ from Blacks, p-value <0.01; BMI, body mass index; EAT, epicardial fat; PAT, paracardial fat; TAT, total heart fat; EAT, PAT,TAT, aortic perivascular fat, and visceral fat were log transformed; all models were adjusted for age, menopausal status, hypertension, diabetes, alcohol consumption, and physical activity; Beta coefficients and related 95% CI from linear regression were presented as %changes in Blacks and Whites using the formula (eβ*SD-1)*100 for BMI and subcutaneous fat; and the formula (eβ*(log(1.55)-1)*100 for visceral fat.28
Discussion
In a population of White and Black midlife women, we found racial differences in volumes of CF depots with Black women having significantly less CF volumes in all four depots compared with White women adjusted for age, study site, menopausal status, hypertension, diabetes, alcohol consumption, and physical activity. These racial differences remained significant even after additional adjustment for BMI and subcutaneous fat (separate models). Although Black women still had significantly less CF volumes after adjusting for visceral fat, the results were somewhat attenuated, suggesting a potential role of visceral fat in understanding racial differences in CF. In addition, we found that race modified the associations between some of the adiposity measures and CF volumes. The magnitude of the association between BMI and PAT was greater among White women compared with Black women; while the magnitude of association between visceral fat and EAT was greater among Black women compared with White women.
Our findings of Black women having significantly less CF volumes compared to White women independent of adiposity measures are consistent with results among men14 and a population of combined men and women.21 To the best of our knowledge, the ERA-JUMP study, conducted among midlife men, is the only other study that evaluated whether racial differences exist independent of several measures of adiposity, and whether race modifies the associations between adiposity measures and CF volumes. Interestingly, the partial attenuation in the racial differences in CF that we found after adjusting for visceral fat was similar to the diminution found in the ERA-JUMP population of men.14 In addition, the interactions reported between race and adiposity measures in our study were comparable to the effect modifications previously reported among men, with the magnitude of associations between BMI and CF volumes greater in Whites compared to Blacks.14
Although our results on racial differences in CF volumes were generally consistent with findings previously reported among men, the effect modifications of race on the associations between adiposity measures and CF depots differed in regards to the specific location of the evaluated CF depot.14 In midlife White men, stronger associations were found between BMI and EAT and BMI and TAT compared to Blacks; however, in midlife White women, higher BMI was significantly associated with greater PAT, but not EAT or TAT, compared with Black women. There are several possible reasons for these discrepancies. Interestingly, we previously showed that greater declines in estradiol over a four-year period were significantly associated with greater PAT volume, but not EAT or TAT volumes.10 This suggests that hormones may play an important role in PAT deposition. Limited number of women reported HT use among the current study participants which limited our ability to assess potential association between CF and HT use. Future studies should assess this interesting association.
Considerable variability exists in the methods, definitions, and assessments of CF depots. Some studies combine the fat inside (EAT) and outside (PAT) the pericardial sac into one measure and rarely do studies evaluate PAT (using our definition) as a separate fat depot. Literature suggests that the fat inside and the fat outside the pericardial sac may differ in embryonic origin, adipocyte characteristics, and metabolic activity and, therefore, some researchers suggest assessing each CF depot separately.3, 4 Our recent findings showing significant effect modification of menopausal status and estradiol levels on associations of coronary artery calcification risk and PAT but not EAT, strongly support the importance of evaluating these two fat depots separately in women.11 Due to the close proximity, lack of muscle fascia separating it from the myocardium,29 and the possible shared microcirculation with the coronary arteries,30 it has been hypothesized that excess EAT may be especially important in regards to cardiovascular risk.29 Very little information is available assessing the associations between all three heart depots volumes (EAT, PAT, and TAT; using our definitions) and cardiovascular disease; however, findings support the importance of evaluating these fat depots separately.10, 11, 31 Our current results are in line with this notion.
More research is needed to understand the racial-obesity paradox among Black men and women in regards to cardiovascular disease. Blacks tend to have a more favorable adipose tissue distribution profile with less visceral fat15 and CF;14 however, they tend to have a higher risk of cardiovascular disease18,19 and diabetes.20 In our study population, Black women had 20% less visceral fat compared to White women after adjusting for BMI (p<0.001; data not shown). Despite having less visceral fat, there was a stronger association between visceral fat and EAT among Black compared to White women suggesting that higher CVD risk in Black could possibly be due to the greater impact of visceral fat on EAT volumes than in White. Interestingly, the association between TAT and CVD risk factors weakened after additional adjustment for visceral fat among Black participants from the Jackson heart study supporting this thought.32 The pathways by which visceral fat could impact EAT in black women are not clear. One possible hypothesis is that Black women are better able to accommodate excess energy in subcutaneous fat depots;33 however, once fat becomes dysfunctional and begins to accumulate in ectopic areas,34 the rate of fat accumulation is accelerated compared with White women. However, this is a speculative hypothesis that still should be tested.
Strengths and Limitations
This study has some limitations, including the cross-sectional design which prevents us from assessing temporality. Our findings are limited in generalizability to Black and White midlife women who share similar sociodemographic and behavioral characteristics. In addition, because we did not have percent body fat for this population we used BMI as a surrogate marker of overall adiposity. Given the small number of women on HT, our analyses could not adequately assess the potential impact of HT use on the tested associations. On the other hand, our study has several strengths that included the accessibility to data from the well-established parent SWAN study. We had high-quality measurements of CF depots, subcutaneous fat, and visceral fat. This is the first study evaluating racial differences in several CF depots independent of separate adiposity measures among women at midlife.
Conclusions
In conclusion, Black women had significantly lesser volumes of CF compared with White women, independent of individual measures of adiposity. Race-modified the associations between adiposity and CF with stronger associations between BMI and PAT in White women compared with Black women, and stronger associations between visceral fat and EAT in Black women compared with White women. Future studies should determine racial differences in the associations between longitudinal changes in adiposity measures and changes in CF volumes. In addition, research should look at how reductions in individual CF depots influence future cardiovascular disease risk in different races/ethnicities. Assessing these research questions may help to better understand the racial-obesity paradox and to identify critical areas for cardiovascular risk reduction in women.
Supplementary Material
Acknowledgments
Source of financial support: This work was supported by financial support: The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495).
SWAN Heart was supported by the National Heart, Lung, and Blood Institute (NHLBI) (Grants HL065581, HL065591). The SWAN Cardiovascular Fat Ancillary Study was supported by an award from the American Heart Association (AHA) Great River Affiliation Clinical Research Program: 12CRP11900031.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
Conflict of interest/Financial disclosure: Dr. Hanley has nothing to disclose; Dr. El Khoudary reports grants from AHA, during the conduct of the study; Dr. Matthews has nothing to disclose; Dr. Brooks reports grants from Gilead Science Inc, outside the submitted work; Dr. Janssen reports grants from NIH, during the conduct of the study; Dr. Budoff reports grants from NIH, during the conduct of the study; grants from GE, outside the submitted work; Dr. Sekikawa has nothing to disclose; Dr. Mulukutla has nothing to disclose.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Chhanda Dutta 2016- present; Winifred Rossi 2012–2016; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
References
- 1.Fitzgibbons TP, Czech MP. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. J Am Heart Assoc. 2014;3:e000582. doi: 10.1161/JAHA.113.000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iacobellis G, Barbaro G. The double role of epicardial adipose tissue as pro- and anti-inflammatory organ. Horm Metab Res. 2008;40:442–445. doi: 10.1055/s-2008-1062724. [DOI] [PubMed] [Google Scholar]
- 3.Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab. 2011;22:450–457. doi: 10.1016/j.tem.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Greenstein AS, Khavandi K, Withers SB, et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–1670. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 6.Wassel CL, Laughlin GA, Araneta MR, et al. Associations of pericardial and intrathoracic fat with coronary calcium presence and progression in a multiethnic study. Obesity. 2013;21:1704–1712. doi: 10.1002/oby.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shields KJ, Barinas-Mitchell E, Gingo MR, et al. Perivascular adipose tissue of the descending thoracic aorta is associated with systemic lupus erythematosus and vascular calcification in women. Atherosclerosis. 2013;231:129–135. doi: 10.1016/j.atherosclerosis.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahabadi AA, Lehmann N, Kalsch H, et al. Association of Epicardial Adipose Tissue With Progression of Coronary Artery Calcification Is More Pronounced in the Early Phase of Atherosclerosis Results From the Heinz Nixdorf Recall Study. JACC Cardiovasc Imaging. 2014;7:909–916. doi: 10.1016/j.jcmg.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Bettencourt N, Toschke AM, Leite D, et al. Epicardial adipose tissue is an independent predictor of coronary atherosclerotic burden. Int J Cardiol. 2012;158:26–32. doi: 10.1016/j.ijcard.2010.12.085. [DOI] [PubMed] [Google Scholar]
- 10.El Khoudary SR, Shields KJ, Janssen I, et al. Cardiovascular Fat, Menopause, and Sex Hormones in Women: The SWAN Cardiovascular Fat Ancillary Study. J Clin Endrocinol Metab. 2015;100:3304–3312. doi: 10.1210/JC.2015-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Khoudary SR, Shields KJ, Janssen I, et al. Postmenopausal Women With Greater Paracardial Fat Have More Coronary Artery Calcification Than Premenopausal Women: The Study of Women’s Health Across the Nation (SWAN) Cardiovascular Fat Ancillary Study. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iacobellis G, Ribaudo MC, Assael F, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: A new indicator of cardiovascular risk. J Clin Endrocrinol Metab. 2003;88:5163–5168. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 13.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 14.El Khoudary SR, Shin C, Masaki K, et al. Ectopic cardiovascular fat in middle-aged men: effects of race/ethnicity, overall and central adiposity. The ERA JUMP study. In J Obesity. 2015;39:488–494. doi: 10.1038/ijo.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanaley JA, Giannopoulou I, Tillapaugh-Fay G, Nappi JS, Ploutz-Snyder LL. Racial differences in subcutaneous and visceral fat distribution in postmenopausal black and white women. Metabolism. 2003;52:186–191. doi: 10.1053/meta.2003.50024. [DOI] [PubMed] [Google Scholar]
- 16.Hill JO, Sidney S, Lewis CE, Tolan K, Scherzinger AL, Stamm ER. Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Clin Nutr. 1999;69:381–387. doi: 10.1093/ajcn/69.3.381. [DOI] [PubMed] [Google Scholar]
- 17.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 18.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke Incidence and Survival Among Middle-Aged Adults 9-Year Follow-Up of the Atherosclerosis Risk in Communities (ARIC) Cohort. Stroke. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 19.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 20.Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA. 2000;283:2253–2259. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 21.Salami SS, Tucciarone M, Bess R, et al. Race and epicardial fat: the impact of anthropometric measurements, percent body fat and sex. Ethn Dis. 2013;23:281–285. [PubMed] [Google Scholar]
- 22.Gorodeski GI. Impact of the menopause on the epidemiology and risk factors of coronary artery heart disease in women. Exp Gerontol. 1994;29:357–375. doi: 10.1016/0531-5565(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 23.Pitha JAO, Kovar J, Leskova J, et al. Changes in cardiovascular risk profile in women after menopause (Prague Pre and Post Menopausal Female study) Cor Vasa. 2014;56:e113–e7. [Google Scholar]
- 24.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obesity. 2008;32:949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sower MCS, Sternfeld B, Morganstein D, et al. SWAN: a multicenter, multiethnic, community-based cohort study of owmen and the menopausal transition. In: Lobo RAKJ, Marcus R, editors. Menopause: Biology and Pathobiology. New York, NY: Academic Press; 2000. pp. 175–188. [Google Scholar]
- 26.Thurston RC, Sowers MR, Sutton-Tyrrell K, et al. Abdominal adiposity and hot flashes among midlife women. Menopause. 2008;15:429–434. doi: 10.1097/gme.0b013e31815879cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med. 1999;28:313–323. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 28.Benoit K. Linear regression models with logarithmic transformations: Methodology Institute. London School of Economics; Mar 17, 2011. Available from: http://www.kenbenoit.net/courses/ME104/logmodels2.pdf. Accessed April 6, 2017. [Google Scholar]
- 29.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–543. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 30.Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 2015;11:363–371. doi: 10.1038/nrendo.2015.58. [DOI] [PubMed] [Google Scholar]
- 31.Ahmadi N, Nabavi V, Yang E, et al. Increased epicardial, pericardial, and subcutaneous adipose tissue is associated with the presence and severity of coronary artery calcium. Acad Radiol. 2010;17:1518–1524. doi: 10.1016/j.acra.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Fox CS, Hickson D, Sarpong D, et al. Pericardial adipose tissue, atherosclerosis, and cardiovascular disease risk factors: the Jackson heart study. Diabetes Care. 2010 Jul;33:1635–1639. doi: 10.2337/dc10-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 34.Britton KA, Fox CS. Ectopic fat depots and cardiovascular disease. Circulation. 2011;124:e837–e841. doi: 10.1161/CIRCULATIONAHA.111.077602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


