Abstract
Background
In a phase 1 study of pulse-continuous dose erlotinib, no patient had disease progression in the central nervous system. This expansion cohort of the phase 1 study tests this same regimen in a cohort of individuals with EGFR-mutant lung cancers with untreated brain metastases.
Methods
Patients had not received epidermal growth factor receptor (EGFR) TKIs or radiation for brain metastases. All received of erlotinib 1200 mg on days 1&2 and 50 mg on days 3–7 weekly. The primary endpoints were overall and central nervous system (CNS) response rates by RECIST 1.1 and progression free survival.
Results
Between May 2015 to August 2016, we enrolled 19 patients. 42% of patients had target brain lesions and the median size of target brain lesions was 13 mm. Overall, 14 patients (74%, 95% confidence interval (CI) 51 to 89%) had partial responses. The response rate in brain metastases was 75%. The overall median progression free survival was 10 months (95% CI 7 to NR). Only 3 patients (16%) had CNS progression. To date, 4 patients required CNS radiation at any time in their course. Adverse events (any grade) seen in ≥ 10% of patients were rash, diarrhea, nausea, ALT increase, and fatigue.
Conclusions
Pulse-continuous dose erlotinib produced a 74% overall and 75% response rate in brain metastases in patients with EGFR-mutant lung cancers with untreated brain metastases. CNS control persisted even after progression elsewhere. Although this regimen did not improve progression-free survival or delay the emergence of EGFR T790M, it prevented progression in the brain and could be useful in situations where CNS control is critical.
Keywords: erlotinib, EGFR, non-small cell lung cancer, brain metastases, EGFR T790M
INTRODUCTION
Lung cancers are the leading cause of brain metastases and these brain metastases are a common cause for cancer related morbidity and mortality. In patients with epidermal growth factor receptor (EGFR)- mutant lung cancers, nearly 25% of patients have brain metastases at the time of diagnosis of metastatic disease.1 The majority of patients with EGFR mutant lung cancers will respond initially to EGFR TKI, but resistance to these agents typically develops.2,3
Coupled with the fact that central nervous system (CNS) is also a frequent site of disease progression, the cumulative incidence of CNS metastases approaches 60% in patients with EGFR-mutant lung cancers. Up to 33% of patients with EGFR-mutant lung cancers have CNS progression while on initial EGFR TKI therapy. Isolated CNS progression often occurs in the setting of continued systemic control.4,5 CSF concentrations of erlotinib are 3–5% of that in concurrent plasma samples6 suggesting that CNS-only progression may be due to inadequate drug delivery as well as tumoral drug resistance. Pulse dose erlotinib results in higher CSF concentrations and may be more effective in the treatment of CNS metastases.7,8 In addition, when resistance mechanisms are assessed by molecular testing on tumor samples from both the CNS and systemic disease sites, the CNS metastases often do not harbor resistance mutations such as EGFR T790M.9
Preclinical work incorporating evolutionary mathematical modeling suggested intermittent pulse doses of erlotinib in conjunction with continuous low-dose administration would delay the establishment of resistant cell populations.10 Based on these concepts and preclinical data,10 we previously tested the schedule of twice weekly pulse (high dose) erlotinib and continuous daily erlotinib (low dose) as initial treatment in patients with EGFR-mutant lung cancers. We defined the maximum tolerated dose (MTD) of erlotinib 1200mg on days 1 and 2 and 50mg on days 3–7.11 While this regimen did not delay the time to acquired resistance or prevent the emergence of T790M, no patient developed progression in the CNS. To follow up on this unexpected observation, we treated an additional cohort of patients with untreated brain metastases or leptomeningeal disease with this regimen.
METHODS
This trial was an expansion cohort of a prospective, open-label, single-center phase I dose-escalation study in patients with EGFR-mutant lung cancers.11 The primary endpoints of the study were overall and CNS response by RECIST 1.1. Secondary endpoints included progression-free survival and overall survival.
Patients had stage IV or recurrent EGFR-mutant lung adenocarcinomas with brain metastases or leptomeningeal disease. Patients were eligible if they had received no prior treatment with an EGFR TKI, and no prior radiation to the CNS. Prior cytotoxic chemotherapy was allowed. Patients were required to have measurable disease per RECIST (version 1.1). Patients must have had adequate organ function and a Karnofsky Performance Status ≥ 70%.
Study Design
All patients received initial daily doses of erlotinib 1200mg day 1 and 2, and 50mg days 3–7 weekly, with no planned treatment breaks, the same dose, schedule, and dose reduction scheme used in the earlier phase I study.11 Erlotinib was continued until disease progression or intolerable toxicity.
Study Assessments
Patients were assessed weekly during the initial 28 days and then every 21 days. Patient history, physical examination, complete blood count and serum chemistries were performed at each visit. Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 4. Response to therapy was assessed every 6 weeks with a contrast CT scan and head MRI scan with response evaluated per RECIST 1.1. After 18 weeks on treatment, we performed radiographic assessments every 12 weeks.
Statistical Analysis
Progression-free survival was estimated using the Kaplan-Meier method, and defined as the time from start of erlotinib until progression or death. Patients were censored at the date they came off study or date of last assessment if still receiving study therapy. Response rates were calculated using binomial proportions and exact 95% CIs. All statistical analyses were performed using R 3.2.2 (R Development Core Team).
RESULTS
Patients
We enrolled 19 patients with EGFR-mutant lung cancers and untreated CNS metastases from May 2015 to August 2016. The median age was 61 years (range 45–80). 74% of the patients were women; 32% of patients had received platinum doublet chemotherapy. 42% of patients had target brain lesions (per RECIST 1.1) with the remainder having non-target lesions. The median size of the target brain lesions was 13 mm (range 10 to 19 mm) and the median number of brain metastases per patient was 4 (range 1–78). One patient had leptomeningeal disease with positive CSF cytology at enrollment. 32% of patients were on dexamethasone for cerebral edema at study entry. The clinical characteristics of all patients are listed in Table 1.
Table 1.
Baseline Patient and Disease Characteristics
| Characteristic | n=19 (%) |
|---|---|
| Age, Median (range), years | 61 (45–80) |
|
| |
| Sex | |
| Female | 14 (74) |
| Male | 5 (26) |
|
| |
| KPS (%) | |
| ≥90 | 9 (47) |
| 80 | 9 (47) |
| 70 | 1 (5) |
|
| |
| Smoking status | |
| Former (pack-year range) | 12 (<1–35) |
| Never | 22 |
|
| |
| EGFR sensitizing mutation | |
| L858R | 13 (68) |
| Exon 19 deletion | 6 (32) |
|
| |
| Prior chemotherapy | |
| Yes | 6 (32) |
| No | 13 (68) |
|
| |
| CNS involvement at diagnosis | |
| No | 0 (0) |
| Yes | 19 (100) |
Response and Progression-Free Survival
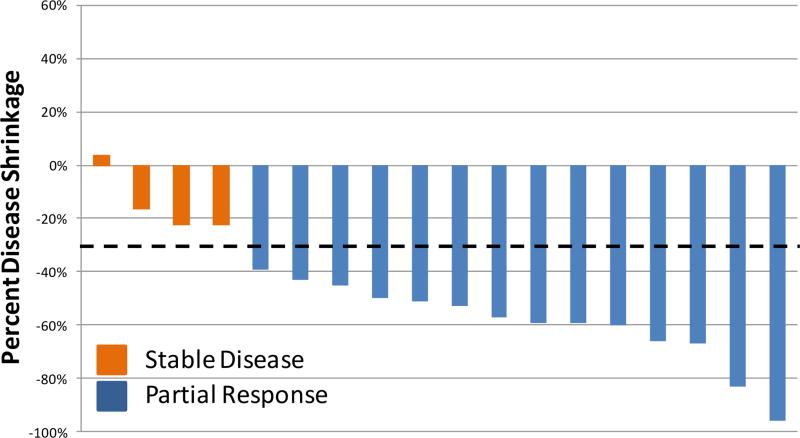
Fourteen patients had partial responses (overall response rate of 74%, 95% CI 51–89%, Figure 1). One patient came off study due to toxicity prior to the first follow up radiographic assessment and was counted as a non-responder. The median progression free survival was 9.7 months (95% CI 7.0-NR). Seventeen of the 19 patients are still alive, with a median follow-up of 15 months. Thirteen discontinued pulse erlotinib due to progression of disease. All 13 patients had EGFR T790M testing performed at progression and 5 (38%, 95% CI 18–65%) were found to have acquired EGFR T790M. All biopsies were of systemic sites of disease.
Figure 1.
Best overall response of target lesions (RECIST 1.1) in 18 patients with a radiographic assessment of response. One patient without follow up imaging was excluded.
CNS Activity
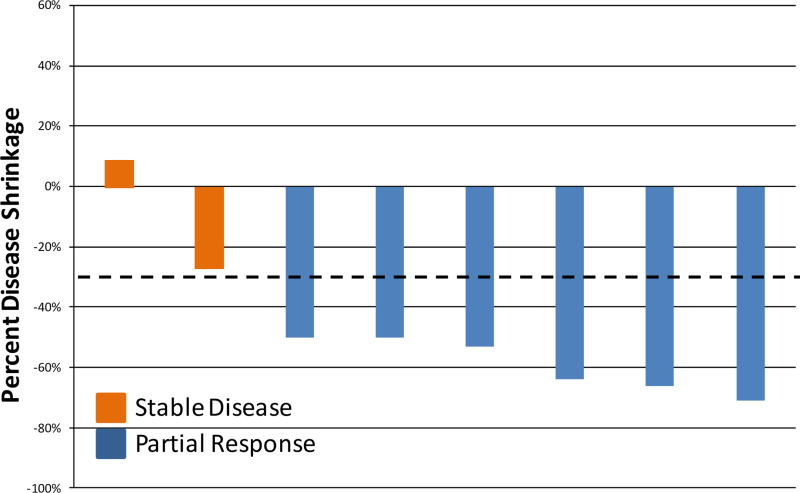
The objective response rate in the 8 patients with measurable brain metastases was 75% (95% CI 40–94%, Figure 2). In addition, in 11 patients with non-target brain lesions, we observed 6 patients with lesions initially present that were then noted to be absent on study treatment. The patient with leptomeningeal disease had clinical improvement with decreased facial pain and numbness as well as a radiologic improvement with decreased enhancement of the meninges. Four of the 6 patients on dexamethasone at study entry were able to discontinue it.
Figure 2.
Best CNS response in 8 patients with target brain lesions (RECIST 1.1).
Only 3/19 patients, all with brain metastases at presentation, developed disease progression in the CNS. One had progression only in existing brain metastases, one developed a new brain metastasis, and one patient had both CNS and systemic progression.
Toxicity
All 19 patients were evaluable for toxicity (Table 2). No grade 4 toxicities or deaths from any cause on study. Three patients were removed from the study for toxicity (1 each for dizziness, nausea and vomiting, and transaminitis). All 3 then continued on daily erlotinib. Fourteen of the 19 patients required reduction of pulse dose. The median pulse dose delivered after 3 months on study was 1050 mg on days 1–2 of each week.
Table 2.
Study drug-related adverse events seen in ≥10% of patients
| CTCAE Term | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total (any grade) |
|---|---|---|---|---|---|
| Rash | 10 (52%) | 7 (37%) | 2 (11%) | 0 | 19 (100%) |
| Diarrhea | 14 (74%) | 4 (21%) | 1 (5%) | 0 | 19 (100%) |
| Nausea | 12 (63%) | 1 (5%) | 0 | 0 | 13 (68%) |
| ALT increase | 11 (58%) | 1 (5%) | 1 (5%) | 0 | 12 (63%) |
| Fatigue | 6 (32%) | 6 (32%) | 0 | 0 | 12 (63%) |
| Dry skin | 9 (47%) | 2 (11%) | 0 | 0 | 11 (58%) |
| Vomiting | 6 32%) | 2 (11%) | 0 | 0 | 8 (42%) |
| Mucositis | 4 (21%) | 3 (16%) | 0 | 0 | 7 (37%) |
| Pruritus | 5 (26%) | 2 (11%) | 0 | 0 | 7 (37%) |
| AST increase | 7 (37%) | 0 (0%) | 0 | 0 | 7(37%) |
| Bilirubin increase | 2 (11%) | 2(11%) | 2(11%) | 0 | 6 (32%) |
| Alopecia | 5 (26%) | 1 (5%) | 0 | 0 | 6 (32%) |
| Dizziness | 2 (11%) | 2 (11%) | 1 (5%) | 0 | 5(26%) |
| Paronychia | 4 (21%) | 0 | 0 | 0 | 4(21%) |
| Anorexia | 3 (16%) | 1 (5%) | 0 | 0 | 4 (21%) |
| Anemia | 1 (5%) | 3 (16%) | 0 | 0 | 4 (21%) |
| Abdominal Pain | 2(11%) | 1(5%) | 0 | 0 | 3(16%) |
| Platelet count decrease | 3(16%) | 0 | 0 | 0 | 3(16%) |
| Dry Eye | 1 (5%) | 1 (5%) | 0 | 0 | 2(11%) |
| Dysgeusia | 2 (11%) | 0 | 0 | 0 | 2(11%) |
| GERD | 2 (11%) | 0 | 0 | 0 | 2(11%) |
| Hypertrichosis | 2 (11%) | 0 | 0 | 0 | 2(11%) |
| Trichomegaly | 2 (11%) | 0 | 0 | 0 | 2(11%) |
CTCAE (common terminology criteria for adverse events), GERD (gastroesophageal reflux disease)
Patient Disposition
As of April 2017, 3 patients remain on study. 13 patients discontinued study therapy due to progressive disease, and 3 others for adverse events. Four individuals required radiation to brain metastases it at any time during their illness. No patient died on study.
DISCUSSION
This is the first prospective trial to evaluate the use of pulse-continuous dose erlotinib in patients with untreated brain metastases. The 74% objective response rate overall and 10 month median progression free survival were similar to reports with standard doses of erlotinib.3 While this dose and schedule of erlotinib did not improve overall outcomes, CNS control was impressive with only 3 patients progressing in the CNS. This low rate of progression is especially notable for this patient population who all had CNS metastases untreated with radiation or surgery at the study start. Furthermore, in this population, all of whom could have received CNS radiation at diagnosis, only 4 of 19 patients have received it subsequently, potentially sparing them from the potential adverse effects of CNS radiation. Pulse-continuous dose erlotinib may prevent pharmacologic erlotinib resistance in the CNS but did not delay the emergence of T790M.
The duration of control of CNS disease is challenging to compare to standard dosing schedules of erlotinib as none of the reports of large studies of EGFR TKIs include this information.12 Prospective trials in patients with brain metastases have included a substantial portion of patients who had received prior whole brain or stereotactic radiation13 or evaluated the combination of EGFR TKI with radiation.14 While there have been reports of the use of high pulse doses of EGFR TKIs to treat CNS metastases, these are all retrospective with partial responses uncommon and rarely durable.7 These previous reports utilized pulse dose EGFR TKI in the setting of progressive disease while on EGFR TKI therapy, not as first-line EGFR TKI treatment. The efficacy of pulse dose EGFR TKI would presumably be markedly different in the salvage setting compared to first-line treatment. There is emerging evidence that newer EGFR TKIs such as osimertinib and AZD3759 have superior CNS penetration (16–17), and prospective clinical trials testing these agents as first-line treatment in patients with EGFR-mutant lung cancers are currently ongoing (NCT02228369)..
In our earlier phase I trial, pulse-continuous dose erlotinib demonstrated side effects comparable to those seen in studies of erlotinib 150 mg daily.15 This report again demonstrates that pulse-continuous dose erlotinib is tolerated by most people with CNS metastases. The regimen was discontinued for toxicity in three patients, all of whom remained on erlotinib.
This study of pulse continuous dose erlotinib demonstrated that systemic therapies for persons with lung cancers work equally well in extracranial sites and the CNS for patients with untreated CNS metastases. This regimen controlled CNS disease and could be useful in situations where CNS control is critical. Pulse-continuous dose erlotinib is an effective regimen for patients with EGFR mutant lung cancers with brain metastases, even in patients with symptomatic CNS disease. With CNS metastases affecting a large proportion of patients with EGFR-mutant lung cancers, a treatment regimen that effectively treats and prevents CNS metastases and may postpone or obviate the need to CNS radiotherapy is valuable option for selected patients.
Acknowledgments
H.A.Y. reports research funding given to her institution from Astellas, AstraZeneca, Clovis Oncology, Incyte and has done consulting for AstraZeneca and Boehringer Ingelheim. G.J.R. reports research funding given to his institution from Novartis, Millenium, GSK, Pfizer, Infinity Pharmaceuticals and Ariad and has done consulting for Novartis and Roche. H.A.Y. and G.J.R. are named on a patent pending for pulse and continuous erlotinib for CNS metastases pending to Astellas. M.G.K. has done consulting for Ariad, AstraZeneca and Genentech/Roche. W.P. is currently employed by Roche and he also has rights to EGFR T790M testing that were licensed on his behalf and others by MSKCC to Molecular MD.
Funding: U.S. Department of Health and Human Services National Institutes of Health National Cancer Institute P30 CA008748
Footnotes
Conflict of Interest: All other authors have nothing to disclose.
Author Contributions: Conceptualization: KCA, MGK, GJR, HAY; Methodology: KCA, MGK, GJR, AI, HAY; Validation: KCA, AI, CRR, HAY; Formal analysis: KCA, AI; Investigation: SAH, RJY, CRR, LA HAY; Resources: MGK, GJR, AI, HAY; Data curation: KCA, CRR, AI; Writing – original draft: KCA, MGK, GJR, HAY; Writing – review and editing: KCA, KB, MD, SAH, RJY, WP, MGK, GJR; Visualization: KCA, MGK, GJR, HAY; Supervision: MGK, HAY; Project administration: HAY; Funding acquisition: MGK, GJR, HAY
References
- 1.Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015;88(1):108–111. doi: 10.1016/j.lungcan.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sequist LV, Yang JC-H, Yamamoto N, et al. Phase III Study of Afatinib or Cisplatin Plus Pemetrexed in Patients With Metastatic Lung Adenocarcinoma With EGFR Mutations. J Clin Oncol. 2013;31(27):3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 3.Jänne PA, Wang X, Socinski MA, et al. Randomized Phase II Trial of Erlotinib Alone or With Carboplatin and Paclitaxel in Patients Who Were Never or Light Former Smokers With Advanced Lung Adenocarcinoma: CALGB 30406 Trial. J Clin Oncol. 2012;30(17):2063–2069. doi: 10.1200/JCO.2011.40.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heon S, Yeap BY, Lindeman NI, et al. The Impact of Initial Gefitinib or Erlotinib versus Chemotherapy on Central Nervous System Progression in Advanced Non–Small Cell Lung Cancer with EGFR Mutations. Clin Cancer Res. 2012;18(16):4406–4414. doi: 10.1158/1078-0432.CCR-12-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omuro AMP, Kris MG, Miller VA, et al. High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer. 2005;103(11):2344–2348. doi: 10.1002/cncr.21033. [DOI] [PubMed] [Google Scholar]
- 6.Togashi Y, Masago K, Fukudo M, et al. Cerebrospinal Fluid Concentration of Erlotinib and its Active Metabolite OSI-420 in Patients with Central Nervous System Metastases of Non-small Cell Lung Cancer. J Thorac Oncol. 2010;5(7):950–955. doi: 10.1097/JTO.0b013e3181e2138b. [DOI] [PubMed] [Google Scholar]
- 7.Grommes C, Oxnard GR, Kris MG, et al. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro-Oncol. 2011;13(12):1364–1369. doi: 10.1093/neuonc/nor121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke JL, Pao W, Wu N, Miller VA, Lassman AB. High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol. 2010;99(2):283–286. doi: 10.1007/s11060-010-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hata A, Katakami N, Yoshioka H, et al. Spatiotemporal T790M Heterogeneity in Individual Patients with EGFR-Mutant Non-Small-Cell Lung Cancer after Acquired Resistance to EGFR-TKI. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2015;10(11):1553–1559. doi: 10.1097/JTO.0000000000000647. [DOI] [PubMed] [Google Scholar]
- 10.Chmielecki J, Foo J, Oxnard GR, et al. Optimization of Dosing for EGFR-Mutant Non–Small Cell Lung Cancer with Evolutionary Cancer Modeling. Sci Transl Med. 2011;3(90):90ra59–90ra59. doi: 10.1126/scitranslmed.3002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu HA, Sima C, Feldman D, et al. Phase 1 study of twice weekly pulse dose and daily low-dose erlotinib as initial treatment for patients with EGFR-mutant lung cancers. Ann Oncol. 2017;28(2):278–284. doi: 10.1093/annonc/mdw556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuler M, Wu Y-L, Hirsh V, et al. First-Line Afatinib versus Chemotherapy in Patients with Non-Small Cell Lung Cancer and Common Epidermal Growth Factor Receptor Gene Mutations and Brain Metastases. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2016;11(3):380–390. doi: 10.1016/j.jtho.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Ceresoli GL, Cappuzzo F, Gregorc V, Bartolini S, Crinò L, Villa E. Gefitinib in patients with brain metastases from non-small-cell lung cancer: a prospective trial. Ann Oncol. 2004;15(7):1042–1047. doi: 10.1093/annonc/mdh276. [DOI] [PubMed] [Google Scholar]
- 14.Welsh JW, Komaki R, Amini A, et al. Phase II Trial of Erlotinib Plus Concurrent Whole-Brain Radiation Therapy for Patients With Brain Metastases From Non–Small-Cell Lung Cancer. J Clin Oncol. 2013;31(7):895–902. doi: 10.1200/JCO.2011.40.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 16.Ahn MJ, Kim DW, Kim TM, Lin CC, Rathayake J, et al., editors. Phase I study of AZD3759, a CNS penetrable EGFR inhibitor, for the treatment of non-small-cell lung cancer (NSCLC) with brain metastasis (BM) and leptomeningeal metastasis. J Clin Oncol. 2017;34 (suppl; 9003) [Google Scholar]
- 17.Yang CH, Kim DW, Kim SW, Cho BC, Lee JS, et al. Osimertinib activity in patients (pts) with leptomeningeal (LM) disease from non-small cell lung cancer (NSCLC): Updated results from BLOOM, a phase 1 study. J Clin ONcol. 2017;34 (suppl; 9002) [Google Scholar]