Abstract
Background and Aims
Primary sclerosing cholangitis (PSC) patients with ulcerative colitis (UC) have a high risk of colonic neoplasia. As histologic inflammation is an independent risk factor for the development of neoplasia, we hypothesized that patients with UC and PSC have more subclinical disease activity than patients with UC alone.
Methods
We performed a retrospective evaluation of patients with ulcerative pancolitis who were in clinical remission and compared endoscopic and histologic activity between those with and without PSC. Disease activity was scored per colonic segment using a modified Mayo endoscopic subscore and histologic assessment. In each colonic segment, differences in disease activity and the degree of discordance between endoscopic and histologic inflammation among UC patients with and without PSC were compared.
Results
143 patients (23 UC-PSC, 120 UC) with 205 exams (36 UC-PSC, 169 UC) were included in the analysis. UC-PSC patients had significantly more endoscopic (OR=4.21, 95% CI 1.67–10.63) and histologic activity (OR=5.13, 95% CI 2.25–11.68) in the right colon as well as greater degree of histologic than endoscopic inflammation in the proximal colon (OR = 3.14, 95% CI 1.24–7.97) than UC without PSC. UC-PSC patients had significantly less histologic activity in the rectum on multivariate analysis (OR=0.24, 95% CI 0.08–0.72).
Conclusion
UC patients with PSC who are in clinical remission are significantly more likely to have endoscopic and histologic inflammation in the right colon compared to UC patients without PSC. As neoplasia frequently develops in the right colon in patients with PSC, these findings provide novel insight into the cause of colorectal cancer in UC patients with PSC.
Keywords: ulcerative colitis, primary sclerosing cholangitis, mucosal inflammation, histologic inflammation
Introduction
Patients with ulcerative colitis (UC) are at an increased risk for developing dysplasia and colorectal cancer (CRC).1 Numerous risk factors for neoplasia in UC patients have been described, including chronic mucosal inflammation, early age disease onset, increased disease duration, and extensive mucosal involvement.1–3 One of the most significant risk factors is having concomitant primary sclerosing cholangitis (PSC), a progressive disease characterized by biliary ductal inflammation and fibrosis.4, 5 A meta-analysis performed by Soetikno and colleagues described an odds ratio of CRC of 4.09 (95% CI, 2.89–5.76) when compared to UC patients without PSC.6 Despite the significant increased risk of CRC among UC patients with concurrent PSC (UC-PSC), the etiology and pathogenesis of CRC remain unknown in this population.
Observational studies of UC-PSC patients describe a unique UC disease phenotype as well as CRC presentation. UC patients with PSC are more likely to have pancolitis, backwash ileitis, and rectal sparing than UC patients without PSC.7–10 Importantly, colitis in patients with PSC is often asymptomatic or milder than patients with UC alone.9, 10 This has led to a theory that the increased risk of CRC stems from subclinical disease and delayed UC diagnosis. Subclinical disease activity and discordance between clinical and endoscopic disease activity has been well described in patients with UC alone,11 although it has not been assessed in patients with UC-PSC compared to UC patients without PSC. We therefore hypothesized that patients with UC and PSC have a greater degree of subclinical disease activity compared to patients with UC alone.
Materials and Methods
The study protocol was approved by the Institutional Review Board at the University of Chicago (IRB-1030). Patients with UC-PSC and UC without PSC were identified retrospectively from an IRB-approved prospective registry of patients with confirmed IBD at the University of Chicago that is linked to patients’ electronic medical records.
Using standard database management software, we identified patients with the diagnosis of ulcerative pancolitis who had a clinic visit within 3 months of a surveillance colonoscopy; clinic notes on patients seen with UC at the University of Chicago IBD Center since 2011 contain a Simple Colitis Activity Index (SCCAI). We then manually reviewed the electronic medical records of each patient. Only those who were confirmed to be in clinical remission as defined as a SCCAI score ≤2 within 3 months of a surveillance colonoscopy and had a histologically and clinically confirmed diagnosis of ulcerative colitis with documented pancolitis by endoscopy and pathology were included in the final analysis. The identified patients were extracted into REDcap (Research Electronic Data Capture, Memphis, TN) and additional data were collected by chart review including date of UC and PSC diagnosis, age, sex, race, ethnicity, medication usage [corticosteroids, 5-aminosalicylic acids (5-ASA), anti-tumor necrosis factor therapies (anti-TNF), immunomodulators (IMM) (6-mercaptopurine, azathioprine, and methotrexate), and anti-integrins].
We reviewed all colonoscopy, pathology, and clinical encounters that met criteria from May, 2011 until May, 2016. Disease activity was scored based on the description of endoscopic severity (normal/quiescent, mild, moderate or severe), photographic images, and when specified, the Mayo endoscopic subscore from the endoscopic report. Using this information, we assigned a modified Mayo subscore12 of 0,1,2,3 based on the highest degree of inflammation present during the endoscopic exam to each of three distinct colorectal segments: the right colon (including the cecum, ascending and transverse colon), the left colon (including the sigmoid, descending colon and splenic flexure), and the rectum. Additionally, each patient was assigned an overall endoscopic Mayo score based on the highest degree of disease activity described in any segment.12
Gastrointestinal pathologists at the University of Chicago routinely grade histologic severity of disease activity during standard of care interpretation of specimens as either normal, quiescent, mild, moderate, or severe using the following criteria: normal is defined as no features of acute or chronic injury; quiescent (inactive) is defined as evidence of chronic injury (i.e. basal lymphoplasmocytic infiltrate, architectural distortion, or metaplastic changes), increased lamina propria lymphocytes, but no neutrophilic involvement of the epithelium; mild is defined as neutrophils within the epithelium, but not spilling over into the crypts; moderate is defined by neutrophils within crypts; and severe disease is defined by mucosal erosions.13 For this study, histologic reporting of normal/quiescent, mild, moderate or severe inflammation were converted to ordinal numbers of 0, 1, 2 or 3. If the degree of activity varies regionally or within the biopsies from one region of the gut, it is standard for the reviewing pathologist to state the range of activity. For the purposes of this analysis, we used the worst activity reported in the right colon, left colon, and rectum. We excluded exams without adequate histologic labeling of the segmental location of origin. We also recorded an overall score per patient based on the highest level of histologic disease activity seen in an individual examination.
Statistical Analysis
Subjects in the two groups were evaluated for statistically significant differences in demographic characteristics including sex, race, medication usage and age at the time of their first colonoscopy evaluated in the study analysis using a chi-square test or two-sample t-test. We compared disease activity between the UC-PSC and UC groups by dichotomizing our results in two ways: (1) normal/quiescent disease versus active disease (including all degrees of disease activity, score of 1–3) and (2) normal/quiescent or mild disease (score of 0–1) versus moderate or severe disease activity (score of 2–3). Using similar dichotomization of disease activity, we also evaluated the impact of ursodiol on endoscopic and histologic inflammation in UC-PSC patients. Variables were evaluated utilizing univariate and multivariate generalized estimating equation (GEE) logistic regression models, which account for the correlation among multiple observations from the same patient. All analyses were performed for both endoscopic and histologic activity for each of the three segments of the colon, and separately for the “worst” segment (the highest degree of inflammation) per exam. Additionally, using the ordinal scale of 0–3, we compared the degree of discordance between endoscopic and histologic inflammation in UC-PSC compared to UC patients. A multivariate analysis was performed to adjust for age at colonoscopy, sex, race, and use of corticosteroids.
RESULTS
Characteristics of the study population
After an electronic query of the IBD database, we initially identified 38 UC-PSC patients with 89 exams and 140 UC patients with 196 exams who had pancolitis and a clinic visit within 3 months of a surveillance colonoscopy. Following confirmation of inclusion criteria, 143 patients [23 (16%) UC-PSC and 120 (84%]) UC] who had a total of 205 exams [36 (18%) UC-PSC and 169 (82%) UC] were included in the final analysis. The two groups were similar in their demographic characteristics and medication usage (Table 1) except UC-PSC patients were younger with median age of 35.6 years old (range 20.1–66.7) compared to 44.8 years old (range 18.4–87.7) in the UC group (p=0.01). A total of 10 of 23 (43.5%) UC-PSC patients were on ursodiol therapy at the time of clinical remission and colonoscopic evaluation. The median dose (mg/kg/day) was 12.2 (range 5.9–17.6).
Table 1.
Demographics and medication usage in the study population. Age represents the age of the patient at the time of their first colonoscopy used in the study analysis.
| UC-PSC n=23 |
UC n=120 |
p value | |
|---|---|---|---|
| Age [Mean (SD)] | 38.1 (13.7) | 46.4 (16.8) | 0.01 |
| Female gender (%) | 6 (26.1%) | 52 (43.3%) | 0.12 |
| White (%) | 19 (82.6%) | 93 (79.5°%) | 0.73 |
| Ever used anti-TNF (%) | 8 (34.8%) | 36 (30.0%) | 0.65 |
| Ever used IMM (%) | 13 (56.5%) | 59 (49.2%) | 0.52 |
| Ever used steroids (%) | 5 (21.7%) | 26 (21.7%) | 0.99 |
| Ever used 5-ASA (%) | 13 (56.5%) | 70 (58.3%) | 0.87 |
| Ever used vedolizumab (%) | 3 (13.0%) | 7 (5.8%) | 0.21 |
IMM=immunomodulator. 5-ASA= 5-aminosalicylic acid
Global endoscopic and histologic disease activity are not different between UC patients with and without PSC
On evaluation of patients using the most inflamed segment per exam (the most severe degree of inflammation identified per exam), 21 (58%) exams among UC-PSC patients and 110 (65%) among UC patients had active disease on endoscopic evaluation in at least one colonic segment (unadjusted OR=1.19, 95% CI 0.47–3.02, p=0.72). Likewise, there was no statistically significant difference between groups when patients were dichotomized into those with quiescent or mild disease versus those who had moderate or severe disease (unadjusted OR=0.73, 95% CI 0.28–1.90, p=0.52). There was also no difference in endoscopic disease activity based on the use of ursodiol in the UC-PSC group (p=0.71).
On histologic evaluation, 25 (69%) exams among UC-PSC patients and 97 (58%) among UC patients had active disease on at least one biopsy (unadjusted OR=1.98, 95% CI 0.84–4.70, p=0.12). There was not a statistically significant difference between the groups when patients were dichotomized to quiescent/normal or mild disease versus moderate or severe disease (unadjusted OR=1.18, 95% CI 0.50–2.78, p=0.71. Furthermore, use of ursodiol was not associated with difference in histologic inflammation in patients with UC and PSC (p=0.32).
UC patients with PSC have increased subclinical endoscopic disease activity in the right colon compared to UC patients without PSC
Endoscopic disease activity was compared between the two groups in each segment of the colon. In the right colon, 20 (56%) exams of UC-PSC patients had active disease on endoscopic evaluation compared to 52 (32%) exams of the UC patients without PSC (unadjusted OR=4.12, 95% CI 1.67–10.20, p=0.002). This remained significant after adjusting for gender, age at colonoscopy, corticosteroid use, and race (OR=4.21, 95% CI 1.67–10.63, p=0.002). In order to explore the impact of ursodiol use on these findings, endoscopic inflammation of UC-PSC patients was compared between those taking or not taking ursodiol at the time of colonoscopy. In UC-PSC patients taking ursodiol, 53% had proximal colonic inflammation compared to 58% of patients not taking the medication (p=0.92).
No differences in endoscopic disease activity in the left colon were observed between groups where 19 (53%) UC-PSC exams had endoscopic disease activity in the left colon compared to 86 (51%) UC exams (unadjusted OR=1.69, 95% CI 0.69–4.16, p=0.25). Similarly, the proportion of patients who had active endoscopic disease in the rectum was comparable in each group with 14 (39%) UC-PSC exams and 77 (47%) UC exams (unadjusted OR=1.00, 95% CI 0.42–2.37, p=1.00) (Table 2). A comparison of the distribution of endoscopic scores between the two groups is shown in Figure 1A.
Table 2.
Segmental endoscopic and histologic activity in UC patients with PSC compared to UC patients without PSC. Odds ratios and 95% confidence interval calculated using a multivariate analysis adjusting for gender, corticosteroid exposure, race, and age.
| OR (95% CI) | p value | |
|---|---|---|
| Right colon | ||
| Endoscopic activity | 4.21 (1.67–10.63) | 0.002 |
| Histologic activity | 4.87 (2.04–11.61) | <0.001 |
| Left colon | ||
| Endoscopic activity | 1.54 (0.61–3.90) | 0.36 |
| Histologic activity | 1.51 (0.62–3.68) | 0.37 |
| Rectum | ||
| Endoscopic activity | 0.80 (0.33–1.96) | 0.63 |
| Histologic activity | 0.24 (0.08–0.72) | 0.01 |
Figure 1.
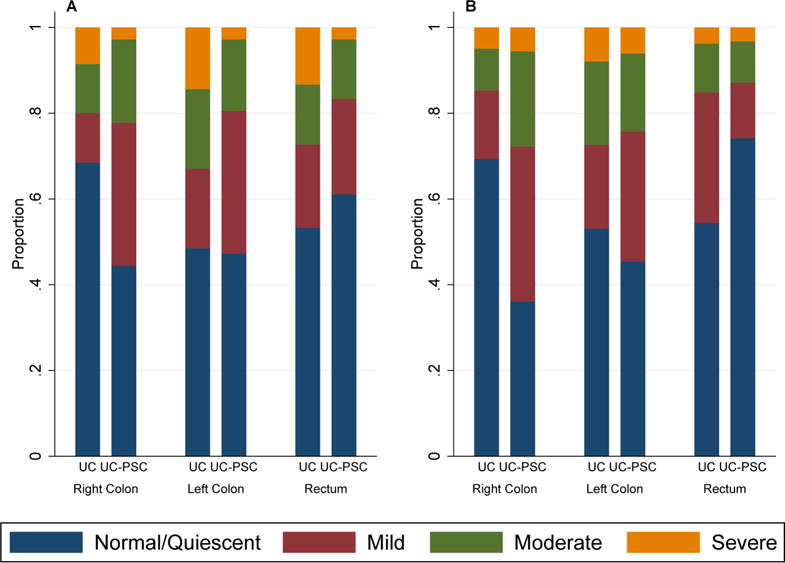
Comparison of subclinical colonic inflammation between UC patients with and without PSC. A) Endoscopic activity using the Mayo endoscopic subscore where ordinal subscores of 0, 1, 2,and 3 represent normal, mild, moderate, and severe mucosal inflammation, respectively. B) Histologic activity using clinical pathology reports of quiescent, mild, moderate or severe inflammation.
UC patients with PSC who are in clinical remission have increased histologic activity in the right colon and decreased histologic activity in the rectum compared to UC patients without PSC
When histologic disease of the right colon was evaluated, patients with PSC had significantly greater histologic disease activity than those without PSC; 23 (64%) exams in the UC-PSC group and 50 (31%) exams in the UC group had active disease on histologic examination (OR= 5.13, 95% CI 2.25–11.68, p<0.001), this remained statistically significant after adjusting for gender, age at colonoscopy, corticosteroid use, and race (OR=4.87, 95% CI 2.04–11.61, p<0.001). In the left colon, however, 18 (55%) exams of UC-PSC patients and 77 (47%) exams of UC patients had active histologic disease (unadjusted OR 1.52, 95% CI 0.67–3.47, p=0.32). In contrast to the subclinical histologic inflammation observed in the proximal colon in patients with PSC, 8 (26%) UC-PSC exams and 72 (46%) UC exams had active disease in the rectum. This was not statistically significant in univariate analysis (OR 0.47, 95% CI 0.17–1.29, P=0.14); however, when adjusted for gender, age at colonoscopy, corticosteroid use, and race, PSC patients had significantly less histologic disease activity in the rectum (OR=0.24, 95% CI 0.08–0.72, p=0.01) A breakdown of the histologic scores within each group is demonstrated in figure 1B.
In a separate analysis examining the impact of ursodiol on these findings, there was no association with histologic disease activity in any colonic segment in the UC-PSC group (proximal colon: p=0.49, left colon: p=0.5, rectum: p=0.81).
Patients with PSC have a more severe grade of histologic than endoscopic inflammation in the proximal colon
When comparing the degree of histologic inflammation to endoscopic inflammation between the two groups in the right colon, patients with PSC had significantly greater odds of having a higher grade of histologic inflammation compared to their endoscopic disease activity (unadjusted OR = 3.14, 95% CI 1.24–7.97, p=0.02). In contrast, there was no significant discordance among grades of endoscopic and histologic activity in either the left colon or rectum between patients with and without PSC (Left colon: unadjusted OR = 1.57, 95% CI 0.62–3.97, p=0.35; Rectum: unadjusted OR = 0.54, 95% CI 0.15–1.94, p=0.34).
DISCUSSION
In this study of patients with UC in clinical remission, we found a greater degree of active endoscopic and histologic inflammation in the proximal colon of patients with concomitant PSC compared to patients without PSC. Furthermore, there was a discordance observed between histologic and endoscopic activity in the proximal colon of PSC patients, where histologic findings were frequently more severe than the endoscopic description of disease activity. In addition, UC-PSC patients had significantly less histologic disease activity in the rectum compared to UC patients without PSC. This is aligned with the previously described phenotype of colitis with decreased rectal inflammation in UC-PSC and provides a potential explanation for the reduced symptomatology in such patients despite proximal disease activity. To our knowledge, this is the first study to assess endoscopic and histologic inflammation in patients in clinical remission with PSC compared to IBD patients without PSC, and we believe that these novel observations provide a potential mechanistic explanation for the predominance of right-sided neoplasia that has been well described in UC-PSC patients.
PSC is a chronic progressive syndrome characterized by intrahepatic and extrahepatic biliary ductal inflammation and fibrosis. Its progressive nature leads to cholestasis and eventually may result in cirrhosis and liver failure.14 PSC is a known co-existing condition with IBD and has a prevalence of 8% with UC and approximately 1%–3% with Crohn’s disease.15, 16 IBD patients with PSC are at increased risk for numerous gastrointestinal malignancies,17–19 including CRC. The increased risk of CRC among UC-PSC patients has been of great interest, with studies describing a cumulative risk as high as 20–30% by 20 years from the time of diagnosis,17, 20, 21 making CRC one of the leading causes of death in this patient group.17 Because of this well-described and strong risk, regular colonoscopic surveillance has been recommended. Current guidelines recommend annual surveillance colonoscopy with random biopsies starting from the time concurrent PSC and IBD are diagnosed.22–24
The etiology and pathogenesis of CRC in UC-PSC is unknown. However, the fact that patients with IBD and PSC have a much higher risk of predominately proximal colonic neoplastic lesions has led to a number of proposed theories. One hypothesis suggests that bile acids may be the major culprit. Cholestasis observed in patients with PSC leads to a build-up of secondary bile acids,5 which in animal models have been found to have a carcinogenic effect.25 An observed increased prevalence of right-sided tumors in IBD-PSC patients,26 where bile acids concentration is the highest, has led to the hypothesis that bile acids may have a key role in CRC development.5,20,26,27 This position gained support when studies examining ursodeoxycholic acid (UDCA) suggested a potential reduction in the carcinogenic effect by reducing the levels of the secondary bile acid, deoxycholic acid.28 In a randomized controlled trial by Pardi and colleagues evaluating the effect of UDCA in UC-PSC patients, 10% of the patients in the interventional arm developed colorectal neoplasia compared to 35% in the control group (RR = 0.26, 95%CI: 0.06–0.92).29 Furthermore, a number of meta-analyses also demonstrated a chemoprotective effect for the development of high-grade dysplasia and CRC using low dose UDCA.30, 31 However, these findings have been challenged by additional studies demonstrating no reduction in the incidence of CRC with UDCA32 and an even higher rate of CRC development when a high dose of UDCA was used (HR=4.44, 95% CI: 1.30–20.1).33 Further supporting the argument against toxic bile acids contributing to neoplasia is the fact that the risk of colonic neoplasia does not decrease following a liver transplant.34 In our cohort, 43.5% of UC-PSC patients were on ursodiol therapy. Although the impact of ursodiol on the risk of neoplasia was not evaluated in this study, there was no association between use of ursodiol with subclinical endoscopic or histologic activity in this cohort of patients.
A second mechanistic hypothesis proposed for the development of CRC in this patient group is related to a prolonged course of subclinical inflammatory disease activity.4 As a milder clinical disease course has been reported in patients with IBD and PSC than IBD without PSC,9 a lack of clinical symptoms may lead to untreated active histologic inflammation or delayed diagnosis of PSC. Therefore, the effective disease duration (and exposure to inflammation) may be much longer than appreciated at the time of diagnosis. This hypothesis is supported by previous work that identified a correlation between mucosal inflammation and colorectal neoplasia in patients with IBD.1, 2 Although not focused on patients with PSC, previous studies demonstrate a 4 to 5-fold increased risk for colorectal neoplasia for every 1-unit increase in histological inflammation in patients with IBD, including patients with histologic inflammation without associated endoscopic disease activity.35
There are several limitations to this study. As the study was retrospective, there are limitations to the data collection, including exclusion of patients related to lack of available data, and the potential for ascertainment errors. Second, we used non-standard measures of inflammation, by extrapolating data from endoscopy and pathology reports. Although the grading system utilized by the pathologists is standardized at our center, it is not validated. We have previously described inter-observer agreement with this histologic grading approach2, however, and these endoscopic evaluations were performed by experienced IBD clinicians who routinely provide detailed evaluation of disease activity by segment. Finally, we were not able to measure severity of the liver disease in PSC patients, which may have influenced these findings.
In conclusion, UC patients with PSC who are in clinical remission have a greater degree of endoscopic and histologic inflammation in the proximal colon compared to UC patients without PSC. As long-standing mucosal inflammation is a known independent risk factor for colonic neoplasia, we believe that these findings may provide an explanation to prior observations of a greater prevalence of right-sided CRC and more advanced CRC at time of cancer diagnosis in patients with PSC. Based on this study, we recommend that clinicians routinely assess histologic disease activity in the proximal colon of UC-PSC patients who are in clinical remission, even in patients without endoscopic inflammation. As the field of IBD continues to move towards so-called “tight disease control” and treating to achieve the objective target of mucosal healing, understanding the distinctive pattern of inflammation in patients with PSC and UC will be key to managing these patients more effectively. It seems logical that the goal of management in these patients should be mucosal healing, but it is acknowledged that it is not known whether this can actually be achieved in such patients, or if doing so will reduce the risk of cancer. Therefore, longer-term prospective studies are needed to assess the impact of achieving mucosal healing on the prevention of neoplasia in this population.
Acknowledgments
Funding for this study came from the Scholtz Family Foundation, P30DK42086, K08DK40826 (JP), The Helmsley Charitable Trust
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no relevant conflicts of interest.
Writing Assistance: None
Author Contributions:
Study Concept: NKC, DTR, BJ, KW, JP
Data Collection: NKC, KM, ALT, ASA, IP, AG, JNG, KWi
Data Analysis: NKC, DTR, KW, JP
Drafting of manuscript: NKC
Critical review of manuscript: DTR, KM, KWi, KW, BJ, JP, JH, CW
Reviewed final version of manuscript: All authors
References
- 1.Rutter M, Saunders B, Wilkinson K, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–459. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Rubin DT, Huo D, Kinnucan JA, et al. Inflammation is an independent risk factor for colonic neoplasia in patients with ulcerative colitis: a case-control study. Clin Gastroenterol Hepatol. 2013;11:1601–8. e1–4. doi: 10.1016/j.cgh.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong NA, Harrison DJ. Colorectal neoplasia in ulcerative colitis-recent advances. Histopathology. 2001;39:221–34. doi: 10.1046/j.1365-2559.2001.01292.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang R, Leong RW. Primary sclerosing cholangitis as an independent risk factor for colorectal cancer in the context of inflammatory bowel disease: a review of the literature. World J Gastroenterol. 2014;20:8783–9. doi: 10.3748/wjg.v20.i27.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torres J, de Chambrun GP, Itzkowitz S, et al. Review article: colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34:497–508. doi: 10.1111/j.1365-2036.2011.04753.x. [DOI] [PubMed] [Google Scholar]
- 6.Soetikno RM, Lin OS, Heidenreich PA, et al. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002;56:48–54. doi: 10.1067/mge.2002.125367. [DOI] [PubMed] [Google Scholar]
- 7.Boonstra K, van Erpecum KJ, van Nieuwkerk KM, et al. Primary sclerosing cholangitis is associated with a distinct phenotype of inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2270–6. doi: 10.1002/ibd.22938. [DOI] [PubMed] [Google Scholar]
- 8.de Vries AB, Janse M, Blokzijl H, et al. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J Gastroenterol. 2015;21:1956–71. doi: 10.3748/wjg.v21.i6.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loftus EV, Jr, Harewood GC, Loftus CG, et al. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91–6. doi: 10.1136/gut.2004.046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaeffer DF, Win LL, Hafezi-Bakhtiari S, et al. The phenotypic expression of inflammatory bowel disease in patients with primary sclerosing cholangitis differs in the distribution of colitis. Dig Dis Sci. 2013;58:2608–14. doi: 10.1007/s10620-013-2697-7. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg L, Lawlor GO, Zenlea T, et al. Predictors of endoscopic inflammation in patients with ulcerative colitis in clinical remission. Inflamm Bowel Dis. 2013;19:779–84. doi: 10.1097/MIB.0b013e3182802b0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–9. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 13.Weber CR, Nalle SC, Tretiakova M, et al. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest. 2008;88:1110–20. doi: 10.1038/labinvest.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendes F, Lindor KD. Primary sclerosing cholangitis: overview and update. Nat Rev Gastroenterol Hepatol. 2010;7:611–9. doi: 10.1038/nrgastro.2010.155. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen HH, Fallingborg JF, Mortensen PB, et al. Hepatobiliary dysfunction and primary sclerosing cholangitis in patients with Crohn’s disease. Scand J Gastroenterol. 1997;32:604–10. doi: 10.3109/00365529709025107. [DOI] [PubMed] [Google Scholar]
- 16.Olsson R, Danielsson A, Jarnerot G, et al. Prevalence of primary sclerosing cholangitis in patients with ulcerative colitis. Gastroenterology. 1991;100:1319–23. [PubMed] [Google Scholar]
- 17.Fevery J, Henckaerts L, Van Oirbeek R, et al. Malignancies and mortality in 200 patients with primary sclerosering cholangitis: a long-term single-centre study. Liver Int. 2012;32:214–22. doi: 10.1111/j.1478-3231.2011.02575.x. [DOI] [PubMed] [Google Scholar]
- 18.Bergquist A, Ekbom A, Olsson R, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321–7. doi: 10.1016/s0168-8278(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 19.Harnois DM, Gores GJ, Ludwig J, et al. Are patients with cirrhotic stage primary sclerosing cholangitis at risk for the development of hepatocellular cancer? J Hepatol. 1997;27:512–6. doi: 10.1016/s0168-8278(97)80356-x. [DOI] [PubMed] [Google Scholar]
- 20.Claessen M, Lütgens M, van Buuren H, et al. More right - sided IBD - associated colorectal cancer in patients with primary sclerosing cholangitis. Inflamm Bowel Dis. 2009;15:1331–1336. doi: 10.1002/ibd.20886. [DOI] [PubMed] [Google Scholar]
- 21.Terg R, Sambuelli A, Coronel E, et al. Prevalence of primary sclerosing cholangitis in patients with ulcerative colitis and the risk of developing malignancies. A large prospective study. Acta Gastroenterol Latinoam. 2008;38:26–33. [PubMed] [Google Scholar]
- 22.Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–78. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 23.Itzkowitz SH, Present DH. Consensus conference: colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:314–321. doi: 10.1097/01.mib.0000160811.76729.d5. [DOI] [PubMed] [Google Scholar]
- 24.Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–89. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 25.Bernstein H, Bernstein C, Payne CM, et al. Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res. 2005;589:47–65. doi: 10.1016/j.mrrev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Marchesa P, Lashner BA, Lavery IC, et al. The risk of cancer and dysplasia among ulcerative colitis patients with primary sclerosing cholangitis. Am J Gastroenterol. 1997;92:1285–8. [PubMed] [Google Scholar]
- 27.Shetty K, Rybicki L, Brzezinski A, et al. The risk for cancer or dysplasia in ulcerative colitis patients with primary sclerosing cholangitis. Am J Gastroenterol. 1999;94:1643–9. doi: 10.1111/j.1572-0241.1999.01156.x. [DOI] [PubMed] [Google Scholar]
- 28.Batta AK, Salen G, Holubec H, et al. Enrichment of the more hydrophilic bile acid ursodeoxycholic acid in the fecal water-soluble fraction after feeding to rats with colon polyps. Cancer Res. 1998;58:1684–7. [PubMed] [Google Scholar]
- 29.Pardi DS, Loftus EV, Jr, Kremers WK, et al. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003;124:889–93. doi: 10.1053/gast.2003.50156. [DOI] [PubMed] [Google Scholar]
- 30.Singh S, Khanna S, Pardi DS, et al. Effect of ursodeoxycholic acid use on the risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2013;19:1631–8. doi: 10.1097/MIB.0b013e318286fa61. [DOI] [PubMed] [Google Scholar]
- 31.Hansen JD, Kumar S, Lo WK, et al. Ursodiol and colorectal cancer or dysplasia risk in primary sclerosing cholangitis and inflammatory bowel disease: a meta-analysis. Dig Dis Sci. 2013;58:3079–87. doi: 10.1007/s10620-013-2772-0. [DOI] [PubMed] [Google Scholar]
- 32.Lindström L, Boberg KM, Wikman O, et al. High dose ursodeoxycholic acid in primary sclerosing cholangitis does not prevent colorectal neoplasia. Aliment Pharmacol Ther. 2012;35:451–7. doi: 10.1111/j.1365-2036.2011.04966.x. [DOI] [PubMed] [Google Scholar]
- 33.Eaton JE, Silveira MG, Pardi DS, et al. High-dose ursodeoxycholic acid is associated with the development of colorectal neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Am J Gastroenterol. 2011;106:1638–45. doi: 10.1038/ajg.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanouneh IA, Macaron C, Lopez R, et al. Risk of colonic neoplasia after liver transplantation for primary sclerosing cholangitis. Inflamm Bowel Dis. 2012;18:269–74. doi: 10.1002/ibd.21692. [DOI] [PubMed] [Google Scholar]
- 35.Colman RJ, Rubin DT. Histological inflammation increases the risk of colorectal neoplasia in ulcerative colitis: a systematic review. Intest Res. 2016;14:202–10. doi: 10.5217/ir.2016.14.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]


