Abstract
Endothelium-dependent vasodilation is reduced following acute exercise, or following high intraluminal pressure in isolated arterioles from sedentary adults, but not in arterioles from regular exercisers. The preserved vasodilation in arterioles from exercisers is hydrogen peroxide (H2O2)-dependent, whereas resting dilation is nitric oxide (NO)-dependent. We hypothesize chronic exercise elicits adaptations allowing for maintained vasodilation when NO bioavailability is reduced.
Keywords: high blood pressure, oxidative stress, anti-oxidants, endothelium, endothelial dysfunction
Introduction
The endothelium controls vascular function by regulating platelet activation and aggregation, leukocyte adhesion, and proliferation of vascular smooth muscle (1). In response to various chemical (e.g. acetylcholine) and physical (i.e. shear stress) stimuli, the endothelium mediates vasomotor tone of the microcirculation by synthesizing and releasing a number of compounds that act to dilate or constrict adjacent vascular smooth muscle cells. These compounds include nitric oxide (NO), which is largely agreed to be the primary vasodilator (2), prostacyclin (PGI2) (3), hydrogen peroxide (H2O2) (4, 5), and other undefined endothelium-derived hyperpolarizing factors (EDHF). Endothelium–derived vascular smooth muscle-contracting agents, include Endothelin–1 (ET-1), widely considered the most potent constricting molecule (6).
Reduction in either flow induced dilation, or acetylcholine induced vasodilation (both of which are normally mediated by NO), is a hallmark of the development of cardiovascular (CV) disease, and endothelial dysfunction. Endothelial dysfunction is thought be an initiating event in the development of atherosclerosis (1). However, in humans, in vivo and in vitro studies have demonstrated that relaxing factors, other than NO, compensate to maintain endothelium-dependent dilation to flow, when NO availability is reduced. For example, over the last two decades, several studies have demonstrated that H2O2 serves as a compensatory vasodilator in disease (4, 5, 7), and following certain environmental insults including high arterial pressure and acute aerobic or resistance exercise (4, 5, 7). We hypothesize that chronic exercise elicits adaptations allowing for maintained vasodilation when NO bioavailability is reduced. This review will outline the normal mechanisms of vasodilation, and data supporting the novel observation that H2O2 serves as a compensatory vasodilator to maintain vasodilation following acute resistance exercise and exposure to high intraluminal pressure in arterioles from regular exercisers.
Normal Mechanisms of Vasodilation
As noted above, NO is regarded as the primary vasodilator in the vasculature. NO production in the endothelium is catalyzed by the activation of endothelial nitric oxide synthase (eNOS), a constitutively Ca2+-dependent member of the NOS family of enzymes. NOS isozymes catalyze the oxidation of the amino acid L-arginine to L-citrulline and gaseous NO in the presence of NADPH and the cofactors flavin adenine dinucleotide, flavin mononucleotide, and tetrahydrobiopterin (BH4) (2). Vascular NO production is contingent upon eNOS phosphorylation of serine (S) residues, predominately S117 (2, 6). When NO is formed in the vascular endothelium, it rapidly diffuses into adjacent vascular smooth muscle cells binding to a heme ion within Guanylyl Cyclase stimulating an increase in the hydrolysis of guanosine trisphosphate to cyclic guanosine monophosphate (cGMP), thus increasing intracellular cGMP. Cyclic GMP is a second messenger that promotes vasodilation via activation of protein kinase G, which causes the concentration of Ca2+ to decrease through inhibiting Ca2+ mobilization from the sarcoplasmic reticulum, and increasing the conductance of Ca2+-activated K+ (KCa) channels with subsequent hyperpolarization and reduction in Ca2+ influx through voltage-gated channels leading to relaxation of the vascular smooth muscle cells (2).
Inhibition of NOS via NG-Nitro-L-arginine Methyl Ester (L-NAME) or NG- Monomethyl-L-arginine, monoacetate (L-NMMA) blocks the majority of endothelium-dependent dilation under resting conditions (4, 5). A litany of clinical studies have used brachial artery flow mediated dilation (FMD), a noninvasive assessment of endothelial function first described by Anderson and Mark (8), and popularized by Celermajer et al. (9). This assessment includes measuring endothelial function via ultrasonographic imaging of arteries at rest and following a cuff-induced ischemia and release used to generate an increase in shear stress and a subsequent endothelium-dependent vasodilation (10).
Initially, brachial artery FMD was thought to serve as a bioassay for NO. However a recent study demonstrated that inhibition of NO production via L-NMMA did not abolish brachial artery FMD, which is in agreement with previous studies showing NO inhibition studies in the radial artery (10). Cuff placement of the occlusion cuff during the FMD procedure has been shown to alter the NO dependency of FMD with a distal placement leading to less NO-dependent dilation compared to a proximal cuff placement (11). Taken together, these findings suggest that other factors contribute to endothelium-dependent vasodilation. Nonetheless, NO does play an essential role in normal endothelial health. Disease states associated with impaired vascular function have been shown to be associated with impaired NO bioavailability.
Effect of cardiovascular disease on vasodilator mechanisms
Patients with CV risk factors such as hypertension, hypercholesterolemia, insulin resistance, and smoking exhibit endothelial dysfunction, which is mainly characterized by insufficient production of NO, and impaired flow induced dilation (9, 11). Several mechanisms have been proposed to explain the impaired NO production associated with endothelial dysfunction, including increased production of reactive oxygen species (ROS), suppression of the upstream eNOS activator-phosphatidylinositol 3-kinase (particularly in the case of insulin resistance) (6), and increased levels of asymmetric dimethyl-L-arginine (ADMA) a competitive inhibitor of L-arginine that results in reduced NO production (12). Of the mechanisms thought to contribute to endothelial dysfunction and the etiology of CV disease, excessive ROS has gained the most attention likely due to the complex nature of ROS in regulating vascular tone in disease.
There are several sources of ROS production in the endothelium; these include the NADPH oxidase (NOX) family of enzymes, xanthine oxidase, enzymes of the mitochondrial respiratory chain, lipoxygenase, and uncoupled eNOS (2, 4, 13–15). ROS production is associated with increased risk of CV disease (11). Furthermore, a commonality of most conditions that precede CV disease such as insulin resistance, hyperglycemia, hypercholesterolemia, smoking, etc. is increased ROS production. Biomarkers of increased oxidative stress such as oxidised-LDL, myeloperoxidase, and plasma F2-Isoprostanes are detected at higher levels in patients with coronary artery disease (CAD), atherosclerosis, ischemic heart disease, and heart failure (3). Hypertension which is a major risk factor for stroke and myocardial infarction is also accompanied by elevated levels of ROS (13, 16).
There are several mechanisms through which ROS interfere with eNOS activity and NO bioavailability. Paradoxically, ROS can convert eNOS from an NO producing enzyme to a superoxide (O2−)-producing enzyme via eNOS uncoupling. This process is mediated via oxidation of eNOS cofactor BH4 to BH2, accumulation of endogenous ADMA, or S-glutathionylation of eNOS (17). ROS also interfere with NO availability through reacting with the free NO resulting in the formation of the highly reactive oxidant, peroxynitrite (2). Our group has demonstrated that even an acute transient elevation of intraluminal pressure is sufficient to increase ROS generation, reduce NO bioavailability and impair flow induced dilation in isolated adipose arterioles from healthy humans (5) and mice (7). High pressure-induced reductions in endothelium-dependent dilation is prevented by sepiapterin, a precursor of BH4, thus preventing uncoupling of eNOS (17). Collectively, our findings along with other clinical and experimental studies demonstrate the detrimental effects of oxidative stress on vascular function.
Apart from ROS, individuals at increased CV risk also exhibit a higher basal level of ET-1 (6). Previous studies have shown that blocking the ET-1 vasoconstrictor pathway might improve the response to aerobic exercise in at-risk populations. For example, Schreuder et al. (18) demonstrated that ET-1 receptor blockade increased aerobic exercise-induced brachial artery blood flow in type 2 diabetics, and Barrett-O’Keefe et al. (19) demonstrated that ET-1 receptor blockade increased leg kicking exercise-induced leg blood flow in the elderly. Interestingly, both of these studies found that ET-1 vasoconstrictor pathway did not influence exercise blood flow in healthy individuals. The increased reliance to ET-1 in the elderly may have to do with impaired α-adrenergic and myogenic vasoconstriction that occurs with aging (20). However, regular exercise has been shown to partially restore normal α-adrenergic and myogenic vasoconstriction in a rodent model of aging (20). Several of the studies highlighted in this review focus on young and middle-aged humans and animal models. How these findings apply to the elderly and other at-risk populations such as diabetics and pre-hypertensives remains to be investigated.
Effect of Acute Exercise on Vasodilation in Human Microcirculation
The pluripotent, beneficial health effects of regular exercise are indisputable, and have been thoroughly reviewed (21). Vascular-specific adaptations to chronic exercise will also be discussed in a later section of this review. Here, the effects of acute bouts of resistance exercise on the vasculature will be reviewed. Observational studies suggest acute strenuous physical exertion increases the risk of CV events in sedentary adults (22). The experimental evidence on this matter is equivocal (for a thorough review see (23)). Some studies demonstrate that acute aerobic or resistance exercise impairs vascular function, some demonstrate no effect, and some demonstrate an increase in vascular function following acute aerobic or resistance exercise. The divergent findings are due to multiple factors including differences in participant sex, age and health/fitness status, varied intensities of the acute exercise stimulus, and assessments being taken at varied time points (23). It should be noted that most of these studies investigated the effects of acute exercise on conduit artery function. In regard to the microcirculation, our group has found that acute resistance exercise impairs endothelium-dependent dilation in arterioles in sedentary individuals, but not exercisers (4, 5), which mirrors our findings using brachial artery FMD (4, 5, 7, 24).
Dating back to 2006, Jurva et al. (24) hypothesized that impaired brachial artery FMD following acute resistance exercise was mediated via resistance exercise-induced elevations in arterial pressure. These findings were later corroborated by Phillips et al. (25) who demonstrated an immediate reduction in brachial artery FMD after a bout of acute resistance exercise in untrained subjects, but not in regular exercisers. Brachial blood pressures of above 200 mmHg were reported in both studies. These early findings led our group to develop the high intraluminal pressure model we currently use in isolated ex vivo arterioles. Lending further support to the notion of high pressure mediating endothelial dysfunction, MacDougall et al. reported arterial pressures reach up to 400 mmHg during lower body resistance exercise using beat-to-beat measures of blood pressure (24). Finally, a recent eloquent study by Buchanan et al. (26) demonstrated that restricting elevation in brachial blood pressure with the use of a proximal pressure cuff inflated to 100 mmHg prevented reduced endothelium-dependent dilation following acute lower body resistance exercise. Several other studies have since found that regular exercisers are protected from acute resistance exercise induced endothelial dysfunction as well (4, 5).
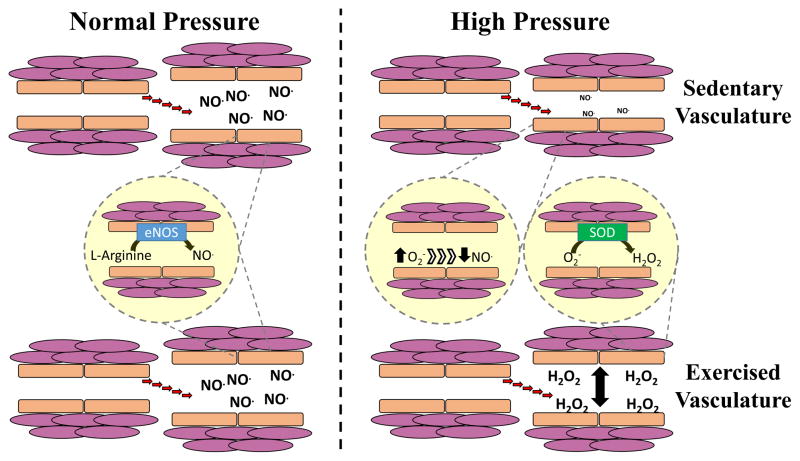
Interestingly, in the microcirculation we have demonstrated that a shift from NO to H2O2 mediated dilation mediated preserves endothelium-dependent dilation following acute resistance exercise using ex vivo arterioles from exercise-trained individuals (4, 5) (see switching pattern outlined in Figure 1). This paradoxical phenomenon indicates an increased production of ROS during acute bouts of exercise which, in turn, elicits alternative vasodilation mechanisms similar to those previously observed in patients with CAD (27, 28). Durand et al. (5) were the first to demonstrate that H2O2 contributed to the maintained vasodilation to acetylcholine in isolated adipose arterioles obtained from exercise trained subjects after acute resistance exercise, in contrast to NO mediated dilation at rest. Robinson et al. (4) demonstrated this same pattern after eight weeks of aerobic exercise training in overweight and obese participants (i.e. switch from NO to H2O2 mediated dilation). Important mechanistic insights were also gleaned from these studies including that treating arterioles with the angiotensin type I receptor blocker Losartan prevented an increase in ROS, and restored endothelium-dependent dilation in arterioles from sedentary individuals (5). This suggests that acute resistance exercise elicited activation of the local renin angiotensin system (RAS) resulting in increased ROS production, and resultant endothelial dysfunction. Our studies also indicate differential detoxification capacity of ROS via the superoxide dismutase (SOD) antioxidant system between regular exercisers and sedentary individuals likely determines the microvascular response to acute exercise (covered in detail below). To date, no studies have investigated if this phenotypic switch to H2O2 mediated dilation plays a role in conduit artery function following acute aerobic or resistance exercise.
Figure 1. Divergent dilatory signaling between sedentary individuals and exercisers in response to high pressure.
At rest, arterioles from both sedentary individuals and regular aerobic or resistance exercisers exhibit nitric oxide (NO)-dependent vasodilation (left panel). This is supported by several studies demonstrating that this dilation is nearly abolished by the NO synthase (NOS) inhibitor L-NG-Nitroarginine methyl ester (L-NAME) (4, 5, 7). Following exposure to high intraluminal pressure or acute resistance exercise, arterioles from sedentary individuals demonstrate reduced vasodilation and reduced sensitivity to L-NAME and the H2O2 scavenger catalase. In contrast, arterioles from regular aerobic and resistance exercisers demonstrate preserved vasodilation, and an enhanced response to catalase, suggesting a greater reliance on H2O2-dependent dilation (4, 5, 7).
The effect of high vascular pressure on vasodilation
In 1990 Panza et al. (29) demonstrated, for the first time, that hypertensive individuals display impaired endothelium-dependent vasodilation. Larger cross-sectional studies have since confirmed these findings (30). Although, the temporal relation between hypertension and endothelial dysfunctions remains to be fully understood. In the large cohort (Multi-Ethnic Study of Atherosclerosis; MESA) study, impaired FMD was not an independent predictor of future hypertension (30). This finding suggests impaired endothelial function is not predictor of hypertension. However, the effect of high pressure on vasodilation has been studied using several models, and it appears high arterial pressure does induce endothelial dysfunction. Using a whole body approach, Millgard et al. (31) assessed endothelium-dependent forearm blood flow with methacholine before, and immediately after a one hour norepinephrine infusion, used to elevate blood pressure (DBP ≥95 mmHg). High pressure did elicit a decrease in endothelium-dependent blood flow though it must be noted that this model could have been confounded by norepinephrine infusion increasing ROS independent of blood pressure. As noted above, acute bouts of resistance exercise increase blood pressure and result in transient impairment of endothelium-dependent dilation (4, 5, 24, 25). However, there is evidence to suggest other mediators play a role in endothelial dysfunction following acute cycling exercise, particularly increased sympathetic nerve activity (32). Hence, our laboratory and others have used isolated arteries to investigate the role of high pressure alone, in eliciting endothelial dysfunction.
To isolate the effects of high pressure on vascular function, several studies have employed in vitro models of high pressure to induce endothelial dysfunction in vessels from both animal models and humans. Mouse carotid arteries subjected to 30 minutes of high pressure (180 mmHg) demonstrate impaired vasodilation to acetylcholine and increased O2− production, both of which are rescued via inhibition of NOX II signaling (33). In an in situ model using open-chest anesthetized dogs, subjecting the left anterior descending coronary artery to 30 minutes of hypertension (200 mmHg) augmented endothelium-dependent constrictor responses to serotonin (34). In time course studies, as little as one to five minutes of high pressure evoked increased serotonin constrictor sensitivity for up to two and half hours.
There are several studies in human vasculature that have studied the impact of high pressure on vascular function. For example, isolated human saphenous vein segments and internal thoracic artery segments subjected to170 mmHg of intraluminal pressure demonstrated reduced stimulated NO· release and increased immunocyte adhesion (35). To determine the effects of high pressure alone, on the microcirculation, our group has undertaken several studies using experimentally-induced high intraluminal pressure in ex vivo arterioles from humans and mice. Similar to acute resistance exercise, Durand et al. (5) demonstrated that high intraluminal pressure elicits endothelial dysfunction in arterioles from sedentary individuals, but not arterioles from exercise trained individuals, which undergo a phenotypic switch to H2O2 -mediated dilation. Robinson et al. (7) recently demonstrated this same pattern in mice that underwent two weeks of wheel running. Furthermore, exercised mice demonstrated increased arterial SOD expression (SOD generates H2O2 from O2−, discussed in detail below). These findings corroborate our previous finding that eight weeks of aerobic exercise prevented endothelial dysfunction in overweight and obese following acute resistance exercise via H2O2 mediated dilation, and increased plasma SOD levels (4).
Chronic exercise effects on the mechanisms of vasodilation following acute exercise
Shear stress is a primary factor in eliciting vascular adaptations to regular exercise in humans. Several studies have used local heating and compression cuffs to manipulate vascular shear stress in human participants, and found that shear alone can induce beneficial adaptations (36, 37). Furthermore, recent studies suggest that a reduction in popliteal artery FMD that occurs with prolonged sitting is prevented by lower limb heating (37) and “fidgeting” (36), both of which increase shear stress through the popliteal artery.
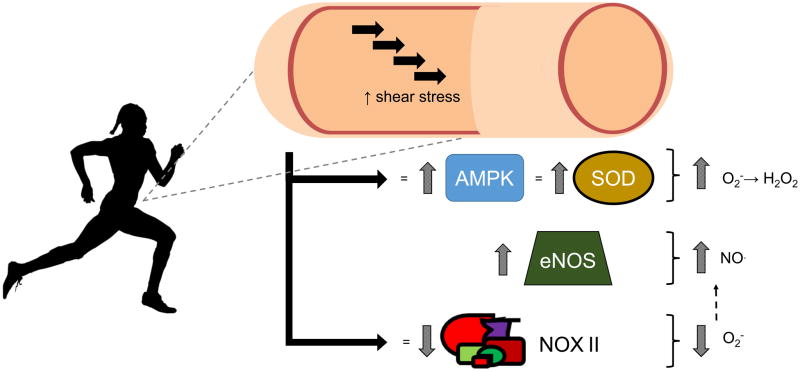
At the cellular level, signaling kinases including protein kinase B, protein kinase A, and adenosine monophosphate-activated protein kinase (AMPK) are all phosphorylated in response to shear stress in isolated microvessels (38). Further, AMPK promotes the increased expression of SOD enzymes (16) (see Figure 2). All SOD enzymes catalyze the dismutation of O2− into H2O2 thus increasing NO· bioavailability and providing H2O2 to act as an alternative vasodilator. SOD I localizes in the cytoplasm, SOD II is found in the mitochondria, and SOD III is extracellular (see Figure 3). SOD I and SOD III complex with copper and zinc, whereas SOD II complexes with manganese. Elevated shear stress has been found to increase SOD I gene expression, and SOD I, SOD II, and total SOD protein expression, in addition to increased NOS levels in endothelial cells (14) (see Figure 2). Aerobic exercise training increases SOD I protein expression in primary aortic endothelial cells (39) and coronary arterioles (15), in addition to reducing NOX II subunit expression (39).
Figure 2. Exercise and shear induced vascular adaptations.
Aerobic exercise and shear stress result in increased vascular AMPK (16) and SOD, resulting in greater conversion of (O2−) to H2O2 (15). By reducing O2−, there is likely a resultant increase NO bioavailability. In addition, some studies have demonstrated an increase in NOS expression (14) which would also result in increased NO. Aerobic exercise has also been shown to reduce NOX II subunit expression which results in less O2−, which theoretically should also yield increased NO bioavailability, as superoxide quenches NO to form peroxynitrite (ONOO−) (7, 14)
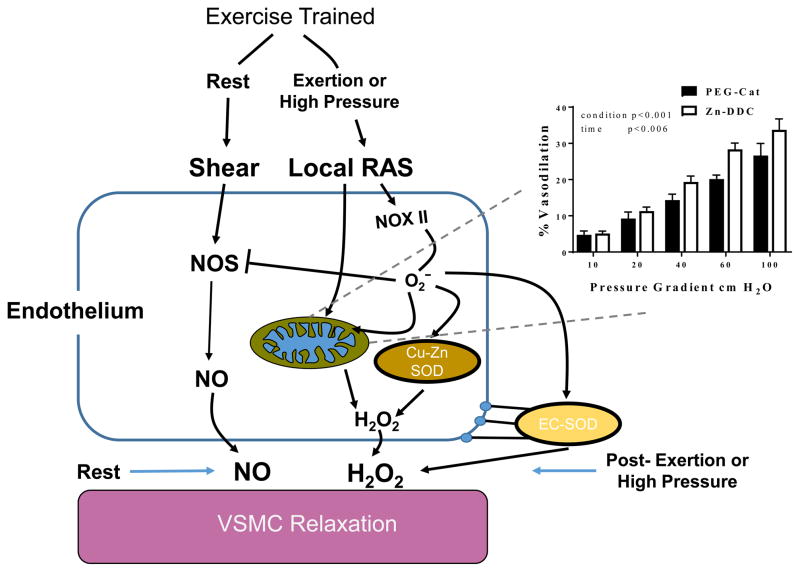
Figure 3. Cellular microenvironment at rest and during high pressure in the exercise trained vasculature.
Activation of local RAS results in increased NOX II activity and subsequent production of O2−. Increased SOD expression allows the exercised vasculature to convert this superoxide to H2O2 which can be used for vasodilation when NO bioavailability is reduced. Regular exercise results in several beneficial adaptions to the mitochondria (40, 41). Mitochondrial H2O2 appears to play a significant role in this maintained dilation as blockade of SOD I and SOD III does not reduce dilation to same extent as catalase which scavenges H2O2 from all three SOD isozymes (7).
We recently showed that regular aerobic exercise reduces NOX II expression, while increasing SOD expression (7). Interestingly, we found Zinc diethyldithiocarbamate, an inhibitor of SOD I and SOD III did not block dilatory responses to the same extent as catalase, which targets H2O2 from all SOD isozymes (see inset, Figure 3). This finding indicates that there may be a particularly important role for mitochondrial SOD in preserving dilation, which is line with previous studies suggesting that shear stress modulates mitochondrial physiology (40, 41). Shear stress increases expression of genes related to mitochondrial biogenesis including NRF-2 and the mitochondrial complexes involved in oxidative phosphorylation in endothelial cells, and shear reduces mitochondria membrane hyperpolarization (41). This is of importance as RAS induces mitochondrial membrane hyperpolarization leading to excessive oxidative stress and impaired endothelial function (13). In support of the hypothesis that regular exercise counteracts RAS and vascular mitochondrial dysfunction, Kim et al. (40) demonstrated shear stress mimics the effects of regular exercise in pre-hypertensives. Specifically shear increased mitochondrial biogenesis markers, and these effects were abolished using siRNA to abolish Sirtuin 1 signaling. This may explain the potential insulin sensitizing effect of shear stress. Collectively, these findings indicate regular aerobic exercise induces adaptations improve the microcirculatory redox environment (see Figure 2) which may manifest in preserved vasodilation in the face of RAS induced oxidative stress (activated by acute resistance exercise and high intraluminal pressure).
Several of the studies highlighted here demonstrate that regular aerobic exercise either directly increases NO· bioavailability by modifications to eNOS (38), or acts to indirectly increase NO· bioavailability by reducing O2− via a reduction pro-oxidative enzymes such as NOX II, or an increase in O2− scavenging enzymes such as the SODs (16, 39). However, in the context of the post-acute exercise period, we have shown inhibition of eNOS does not impact vasodilation differentially in regular exercisers versus sedentary individuals (i.e. in both populations there is less sensitivity to eNOS. Inhibition via L-NAME compared to resting condition). Therefore, it appears that the adaptations facilitating H2O2 mediated dilation supersede those promoting increased NO· bioavailability in this specific context. Non-NO mediated dilation is discussed at length below.
Non-NO mediated responses to shear
Shear stress-induced production/release of NO has been extensively studied and the effects of NO on vascular smooth muscle are well established. Non-NO mediated responses to shear is an area of increasing investigation. In this section, we discuss non-NO shear stress mediated responses and the mechanisms by which they mediate vascular smooth muscle relaxation. This includes shear responses mediated by PGI2, H2O2, and EDHFs. By name, EDHFs are expected to directly affect smooth muscle membrane potential thereby inducing vascular dilation, yet the specific molecule or molecules that comprise EDHF(s) are a topic of debate.
PGI2 contributes to endothelial cell-mediated vasodilator production which effect on vascular tone is tightly tied to thromboxane (TX)A2 (3). TXA2 acts as a vasoconstrictor on the vascular smooth muscle, whereas PGI2 functions as a vasodilator. PGI2:TXA2 is increased in certain physiological conditions including during acute exercise (42, 43) (these studies specifically looked at maximal aerobic exercise). Viinikka et al. (44) studied the effect of acute aerobic exercise on PGI2:TxA2 in a group of well-trained runners, and failed to detect significant changes in plasma prostacyclin. This would suggest that the changes of PGI2:TxA2-ratio induced by acute aerobic exercise are not enough to explain the protective effect of physical fitness against CVD. However Feng et al.(43) demonstrated that maximal aerobic exercise elicits an increase in plasma PGI2, while patients with endothelial dysfunction demonstrated a reduced PGI2 response to acute aerobic exercise. These findings are in agreement to that of Wennmalm et al who reported that both PGI2 and TXA2 were elevated following acute aerobic exercise in symptom free patients undergoing a stress test (overall the PGI2:TxA2 increased), while neither PGI2 or TxA2 were increased following acute aerobic exercise in those developing chest pain or ST depression during acute aerobic exercise. Taken together, these data suggest a continuum may exist whereby at-risk populations do not exhibit increased PGI2:TXA2 during acute aerobic exercise, healthy untrained individuals do, and well trained regular exercisers do not. These findings may relate to NO bioavailability and vascular plasticity, although this topic requires further investigation, including responses to acute resistance exercise.
In both large and resistance arteries shear stress-induced EDHF production is present, although EDHF contributes to vasodilation more so in smaller arteries (45). The contributions of EDHF in vasodilation to shear stress likely also vary between species and vascular beds (45). Mechanosensors including the glycocalyx, integrins, cell surface receptors, caveolae, cell-cell junctions, and ion channels transduce shear forces into signaling cascades involving EDHF and NO (45–47). Many of these mechanosensors are linked to EDHF production although most mechanisms remain unclear. For instance, members of the KCa channel family expressed in endothelium have been linked to EDHF in that pharmacological blockade of these channels, in combination with inhibiting eNOS, abolish dilation to flow (47). How these channels are activated by shear as well as the subsequent role played in EDHF production remains unknown. The location of these channels, particularly small KCa (SK) and intermediate KCa (IK) channels, at or near the myoendothelial junction suggests that K+ ions may serve as EDHF and activate inwardly rectifying K+ (Kir) channels on vascular smooth muscle. Interestingly, it has been found that the rapid exercise hyperemic response to acute, rhythmic handgrip exercise is mediated by Kir channels in vivo (48). However, not all vascular beds express Kir channels in smooth muscle (e.g. mesentery vascular smooth muscle) and SK and IK are still seemingly involved thus supporting a role for alternative mechanisms such as direct electrical connection of the endothelium to smooth muscle via gap junctions and/or activation of Na+/K+ ATPase (47, 49).
Cytochrome P450 2C9 (CYP2C9) metabolites of arachidonic acid known as epoxyeicosatrienoic acids (EETs) are produced by the vascular endothelium in response to chronic shear stress (50). Evidence for EETs leading to the activation of endothelial KCa channels supports the hypothesis that these molecules contribute to the shear-induced EDHF component of vasodilation (51). This is supported by a reduced EDHF component of flow induced dilation following blockade of TRPV4 or cytochrome CYP450 in the carotid arteries of mice (51). The proposed mechanism involves activation of transient receptor potential (TRP) channel V4 by EETs, subsequent Ca2+ influx, and finally KCa channel activation (51–53). However, the effects of acute increases in shear stress on CYP2C9 activity have yet to be determined.
While several reports provide evidence for a role of EETs as the EDHF in shear-induced vasodilation, Larsen et al. demonstrated that H2O2 inhibits CYP2C9 upon bradykinin stimulation and acts as an EDHF in lieu of EETs production (54). We have shown that flow stimulates H2O2 in an endothelium-dependent, yet NO-independent manner in aerobic or resistance exercise trained individuals and patients with CAD, thus H2O2 may serve as a soluble EDHF in these populations (4, 5, 28). Furthermore, smooth muscle K+ channels such as the large conductance KCa channel, are redox sensitive and have been shown to be activated by H2O2 (53). Activation of smooth muscle K+ channels hyperpolarizes membrane potential thereby reducing Ca2+ influx and promoting vasodilation. In regular aerobic or resistance exercisers performing acute resistance exercise, endothelium-dependent dilation switches from NO to H2O2 mediated dilation to maintain blood flow as NO becomes unavailable (4, 5, 7). However, at rest trained individuals rely on NO as the main dilator. Patients with CAD almost exclusively rely on H2O2 even at rest to sustain vasodilaton, presenting an interesting comparison between exercise induced mechanisms and disease (28). We recently linked NOX induced ROS to mitochondrial production of O2− and H2O2 in arterioles from CAD patients (28). These findings indicate compensatory mechanisms in disease states that may be present in regular exercisers during acute bouts of exercise, but exist as the driving vascular mediators in CAD. It is important to note that switching the meditator from NO to ROS in CAD may provide adequate short term vascular function, however, this likely introduces long term negative consequences and a direct route to severe CVD.
Implications and Conclusion
Physical activity and exercise prescription is an important component of CV disease and risk management. In terms of moderate and intense exercise training, evidence suggests that exercise is associated with reduced prevalence, and onset of CV disease (21). Compelling data show that higher aerobic exercise capacity (i.e. higher VO2peak) is closely associated with a reduction in mortality and morbidity (22). However, it is well recognized that sudden death and CV events occur at high frequency during, or soon after vigorous bouts of exercise (22). The assessment of endothelium-dependent vasodilation following perturbations such as intense resistance exercise and high fat meals is an emerging strategy used 1) to uncover vascular dysfunction in individuals at higher risk for CV disease; and 2) disentangle the unaccounted-for protection conferred by regular exercise (vs reduction in risk factors alone) (11). Endothelial dysfunction occurs early in the development of CV disease, and occurs in the microcirculation where changes in function may foster the development of cardiometabolic disease, such as hypertension and insulin resistance. Our findings suggest regular aerobic and resistance exercisers are protected from endothelial dysfunction induced by acute exertion (4, 5, 7). Regular exercise-induced upregulation of antioxidant defenses, and protection of NO bioavailability may be an important mechanistic link between exercise and CV protection from endothelial dysfunction to other stressors such as alcohol consumption, poor diet, and sudden physical exertion. Further, the evidence reviewed above suggests that H2O2 may play a more prominent role in protecting against endothelial dysfunction from high pressure induced by acute bouts of resistance exercise. While the mechanisms of preserved dilation following high pressure and exercise in trained individuals have not been fully elucidated, the available evidence suggests an increase in vascular SOD play a large role. In addition, shear stress itself may play a critical role in promoting H2O2 generation in the microcirculation during acute resistance exercise. This “exercise paradox” of chronic exercise that maintains endothelium-dependent vasodilation in the microcirculation through an H2O2 dependent mechanism and CV health on the one hand, and where acute, sudden exertion increases CV risk on the other hand, may be an important marker that identifies risk for vascular dysfunction in populations without overt CV disease.
Summary.
Regular exercise confers preservation of microvascular vasodilation in situations where nitric oxide bioavailability is reduced.
Key Points.
Endothelium-dependent vasodilation is reduced following acute exercise, or exposure to high intraluminal pressure in isolated arterioles from sedentary adults.
Endothelium-dependent vasodilation is preserved following acute exercise, or high intraluminal pressure in isolated arterioles from regular exercisers.
Preserved vasodilation is hydrogen peroxide (H2O2)-dependent, whereas resting dilation is nitric oxide (NO)-dependent, suggesting chronic exercise elicits adaptations allowing for maintained vasodilator function when NO bioavailability is reduced
Future studies are needed to determine if vasodilation in arterioles from regular exercisers are protected from other “real world” noxious stimuli, such as high-fat and/or high-sugar meals, second-hand smoke, mental stress, and excess alcohol consumption.
Abbreviations
- ADMA
asymmetric dimethyl-L-arginine
- AMPK
adenosine monophosphate-activated protein kinase
- BH4
tetrahydrobiopterin
- KCa channel
Ca2+-activated K+ channel
- CAD
coronary artery disease
- CV
cardiovascular
- CYP2C9
Cytochrome P450 2C9
- EDHF
endothelium-derived hyperpolarizing factors
- EETs
epoxyeicosatrienoic acids
- ET-1
Endothelin–1
- FMD
flow mediated dilation
- H2O2
hydrogen peroxide
- Kir channel
K+ inwardly rectifying channel
- L-NAME
NG-Nitro-L-arginine Methyl Ester
- L-NMMA
NG-monomethyl-L-arginine
- NOX
NADPH oxidases
- NO
nitric oxide
- PGI2
prostacyclin
- RAS
renin angiotensin system
- ROS
reactive oxygen species
- O2−
superoxide
- SOD
superoxide dismutase
- TX
thromboxane
- TRP channel
transient receptor potential channel
Footnotes
Disclosures: K23HL085614 (SAP), R01HL095701 (SAP), R01HL095701-03S1 (Assistantship for ATR), AHA 15POST24480172 (AMM), AHA 16POST27000011 (ISF)
Conflict of Interest: None
References
- 1.Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circulation research. 2001;88(8):756–62. doi: 10.1161/hh0801.089861. Epub 2001/04/28. [DOI] [PubMed] [Google Scholar]
- 2.Sessa WC. eNOS at a glance. Journal of cell science. 2004;117(Pt 12):2427–9. doi: 10.1242/jcs.01165. Epub 2004/05/26. [DOI] [PubMed] [Google Scholar]
- 3.Salvemini D, Kim SF, Mollace V. Reciprocal regulation of the nitric oxide and cyclooxygenase pathway in pathophysiology: relevance and clinical implications. Am J Physiol Regul Integr Comp Physiol. 2013;304:R473–87. doi: 10.1152/ajpregu.00355.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson AT, Franklin NC, Norkeviciute E, Bian JT, Babana JC, Szczurek MR, et al. Improved arterial flow-mediated dilation after exertion involves hydrogen peroxide in overweight and obese adults following aerobic exercise training. Journal of hypertension. 2016;34(7):1309–16. doi: 10.1097/hjh.0000000000000946. Epub 2016/05/04. [DOI] [PubMed] [Google Scholar]
- 5.Durand MJ, Dharmashankar K, Bian JT, Das E, Vidovich M, Gutterman DD, et al. Acute exertion elicits a H2O2-dependent vasodilator mechanism in the microvasculature of exercise-trained but not sedentary adults. Hypertension. 2015;65(1):140–5. doi: 10.1161/HYPERTENSIONAHA.114.04540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahmoud AM, Szczurek MR, Blackburn BK, Mey JT, Chen Z, Robinson AT, et al. Hyperinsulinemia augments endothelin-1 protein expression and impairs vasodilation of human skeletal muscle arterioles. Physiol Rep. 2016;4(16) doi: 10.14814/phy2.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson AT, Fancher IS, Sudhahar V, Bian JT, Cook M, Mahmoud AM, et al. Short-term Regular Aerobic Exercise Reduces Oxidative Stress Produced by Acute High Intraluminal Pressure in the Adipose Microvasculature. American Journal of Physiology - Heart and Circulatory Physiology. 2017 doi: 10.1152/ajpheart.00684.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson EA, Mark AL. Flow-mediated and reflex changes in large peripheral artery tone in humans. Circulation. 1989;79(1):93–100. doi: 10.1161/01.cir.79.1.93. Epub 1989/01/01. [DOI] [PubMed] [Google Scholar]
- 9.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340(8828):1111–5. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 10.Wray DW, Witman MA, Ives SJ, McDaniel J, Trinity JD, Conklin JD, et al. Does brachial artery flow-mediated vasodilation provide a bioassay for NO? Hypertension. 2013;62(2):345–51. doi: 10.1161/HYPERTENSIONAHA.113.01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension. 2011;57(3):363–9. doi: 10.1161/HYPERTENSIONAHA.110.167015. [DOI] [PubMed] [Google Scholar]
- 12.Xuan C, Tian QW, Li H, Zhang BB, He GW, Lun LM. Levels of asymmetric dimethylarginine (ADMA), an endogenous nitric oxide synthase inhibitor, and risk of coronary artery disease: A meta-analysis based on 4713 participants. Eur J Prev Cardiol. 2016;23(5):502–10. doi: 10.1177/2047487315586094. [DOI] [PubMed] [Google Scholar]
- 13.Dikalov SI, Nazarewicz RR. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxidants & redox signaling. 2013;19(10):1085–94. doi: 10.1089/ars.2012.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feairheller DL, Park JY, Rizzo V, Kim B, Brown MD. Racial differences in the responses to shear stress in human umbilical vein endothelial cells. Vasc Health Risk Manag. 2011;7:425–31. doi: 10.2147/vhrm.s22435. Epub 2011/07/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rush JW, Laughlin MH, Woodman CR, Price EM. SOD-1 expression in pig coronary arterioles is increased by exercise training. American journal of physiology Heart and circulatory physiology. 2000;279(5):H2068–76. doi: 10.1152/ajpheart.2000.279.5.H2068. Epub 2000/10/25. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Wang Q, Sun Z. Normal IgG Downregulates the Intracellular Superoxide Level and Attenuates Migration and Permeability in Human Aortic Endothelial Cells form a Hypertensive Patient. Hypertension. 2012;60(3):818–26. doi: 10.1161/hypertensionaha.112.199281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagi Z, Koller A. Lack of nitric oxide mediation of flow-dependent arteriolar dilation in type I diabetes is restored by sepiapterin. Journal of vascular research. 2003;40(1):47–57. doi: 10.1159/000068938. Epub 2003/03/20 68938. [DOI] [PubMed] [Google Scholar]
- 18.Schreuder TH, van Lotringen JH, Hopman MT, Thijssen DH. Impact of endothelin blockade on acute exercise-induced changes in blood flow and endothelial function in type 2 diabetes mellitus. Exp Physiol. 2014;99(9):1253–64. doi: 10.1113/expphysiol.2013.077297. [DOI] [PubMed] [Google Scholar]
- 19.Barrett-O’Keefe Z, Ives SJ, Trinity JD, Morgan G, Rossman MJ, Donato AJ, et al. Endothelin-A-mediated vasoconstriction during exercise with advancing age. The journals of gerontology Series A, Biological sciences and medical sciences. 2015;70(5):554–65. doi: 10.1093/gerona/glu065. Epub 2014/05/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis RT, 3rd, Stabley JN, Dominguez JM, 2nd, Ramsey MW, McCullough DJ, Lesniewski LA, et al. Differential effects of aging and exercise on intra-abdominal adipose arteriolar function and blood flow regulation. Journal of applied physiology. 2013;114(6):808–15. doi: 10.1152/japplphysiol.01358.2012. Epub 2013/01/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiuza-Luces C, Garatachea N, Berger NA, Lucia A. Exercise is the real polypill. Physiology. 2013;28(5):330–58. doi: 10.1152/physiol.00019.2013. [DOI] [PubMed] [Google Scholar]
- 22.Thompson PD, Franklin BA, Balady GJ, Blair SN, Corrado D, Estes NA, 3rd, et al. Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation. 2007;115(17):2358–68. doi: 10.1161/CIRCULATIONAHA.107.181485. [DOI] [PubMed] [Google Scholar]
- 23.Dawson EA, Green DJ, Cable NT, Thijssen DH. Effects of acute exercise on flow-mediated dilatation in healthy humans. Journal of applied physiology. 2013;115(11):1589–98. doi: 10.1152/japplphysiol.00450.2013. [DOI] [PubMed] [Google Scholar]
- 24.Jurva JW, Phillips SA, Syed AQ, Syed AY, Pitt S, Weaver A, et al. The effect of exertional hypertension evoked by weight lifting on vascular endothelial function. Journal of the American College of Cardiology. 2006;48(3):588–9. doi: 10.1016/j.jacc.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Phillips SA, Das E, Wang J, Pritchard K, Gutterman DD. Resistance and aerobic exercise protects against acute endothelial impairment induced by a single exposure to hypertension during exertion. J Appl Physiol (1985) 2011;110(4):1013–20. doi: 10.1152/japplphysiol.00438.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buchanan CE, Kadlec AO, Hoch AZ, Gutterman DD, Durand MJ. Hypertension during Weight Lifting Reduces Flow-Mediated Dilation in Nonathletes. Medicine and science in sports and exercise. 2017;49(4):669–75. doi: 10.1249/mss.0000000000001150. Epub 2016/11/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips SA, Hatoum OA, Gutterman DD. The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during CAD. Am J Physiol Heart Circ Physiol. 2007;292(1):H93–100. doi: 10.1152/ajpheart.00819.2006. [DOI] [PubMed] [Google Scholar]
- 28.Zinkevich NS, Fancher IS, Gutterman DD, Phillips SA. Roles of NADPH oxidase and mitochondria in flow-induced vasodilation of human adipose arterioles: ROS induced ROS release in coronary artery disease. Microcirculation. 2017 doi: 10.1111/micc.12380. Epub 2017/05/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panza JA, Quyyumi AA, Brush JE, Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. The New England journal of medicine. 1990;323(1):22–7. doi: 10.1056/nejm199007053230105. Epub 1990/07/05. [DOI] [PubMed] [Google Scholar]
- 30.Shimbo D, Muntner P, Mann D, Viera AJ, Homma S, Polak JF, et al. Endothelial dysfunction and the risk of hypertension: the multi-ethnic study of atherosclerosis. Hypertension. 2010;55(5):1210–6. doi: 10.1161/hypertensionaha.109.143123. Epub 2010/03/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millgard J, Lind L. Acute hypertension impairs endothelium-dependent vasodilation. Clin Sci (Lond) 1998;94(6):601–7. doi: 10.1042/cs0940601. Epub 1998/12/17. [DOI] [PubMed] [Google Scholar]
- 32.Atkinson CL, Lewis NC, Carter HH, Thijssen DH, Ainslie PN, Green DJ. Impact of sympathetic nervous system activity on post-exercise flow-mediated dilatation in humans. The Journal of physiology. 2015;593(23):5145–56. doi: 10.1113/jp270946. Epub 2015/10/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vecchione C, Carnevale D, Di Pardo A, Gentile MT, Damato A, Cocozza G, et al. Pressure-induced vascular oxidative stress is mediated through activation of integrin-linked kinase 1/betaPIX/Rac-1 pathway. Hypertension. 2009;54(5):1028–34. doi: 10.1161/hypertensionaha.109.136572. Epub 2009/09/23. [DOI] [PubMed] [Google Scholar]
- 34.Lamping KG, Dole WP. Acute hypertension selectively potentiates constrictor responses of large coronary arteries to serotonin by altering endothelial function in vivo. Circulation research. 1987;61(6):904–13. doi: 10.1161/01.res.61.6.904. Epub 1987/12/01. [DOI] [PubMed] [Google Scholar]
- 35.Bilfinger TV, Stefano GB. Human aortocoronary grafts and nitric oxide release: relationship to pulsatile pressure. The Annals of thoracic surgery. 2000;69(2):480–5. doi: 10.1016/s0003-4975(99)01083-8. Epub 2000/03/29. [DOI] [PubMed] [Google Scholar]
- 36.Morishima T, Restaino RM, Walsh LK, Kanaley JA, Fadel PJ, Padilla J. Prolonged sitting-induced leg endothelial dysfunction is prevented by fidgeting. American journal of physiology Heart and circulatory physiology. 2016 doi: 10.1152/ajpheart.00297.2016. ajpheart.00297.2016. Epub 2016/05/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Restaino RM, Walsh LK, Morishima T, Vranish JR, Martinez-Lemus LA, Fadel PJ, et al. Endothelial dysfunction following prolonged sitting is mediated by a reduction in shear stress. American journal of physiology Heart and circulatory physiology. 2016;310(5):H648–53. doi: 10.1152/ajpheart.00943.2015. Epub 2016/01/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang QJ, McMillin SL, Tanner JM, Palionyte M, Abel ED, Symons JD. Endothelial nitric oxide synthase phosphorylation in treadmill-running mice: role of vascular signalling kinases. J Physiol. 2009;587(Pt 15):3911–20. doi: 10.1113/jphysiol.2009.172916. Epub 2009/06/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rush JW, Turk JR, Laughlin MH. Exercise training regulates SOD-1 and oxidative stress in porcine aortic endothelium. Am J Physiol Heart Circ Physiol. 2003;284(4):H1378–87. doi: 10.1152/ajpheart.00190.2002. Epub 2003/02/22. [DOI] [PubMed] [Google Scholar]
- 40.Kim J-S, Kim B, Lee H, Thakkar S, Babbitt DM, Eguchi S, et al. Shear stress-induced mitochondrial biogenesis decreases the release of microparticles from endothelial cells. 2015 doi: 10.1152/ajpheart.00438.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim B, Lee H, Kawata K, Park JY. Exercise-mediated wall shear stress increases mitochondrial biogenesis in vascular endothelium. PloS one. 2014;9(11):e111409. doi: 10.1371/journal.pone.0111409. Epub 2014/11/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wennmalm A, Nowak J, Bjuro T. Excretion of thromboxane A2 and prostacyclin metabolites before and after exercise testing in patients with and without signs of ischemic heart disease. Circulation. 1990;82(5):1737–43. doi: 10.1161/01.cir.82.5.1737. Epub 1990/11/01. [DOI] [PubMed] [Google Scholar]
- 43.Feng DL, Murillo J, Jadhav P, McKenna C, Gebara OC, Lipinska I, et al. Upright posture and maximal exercise increase platelet aggregability and prostacyclin production in healthy male subjects. British journal of sports medicine. 1999;33(6):401–4. doi: 10.1136/bjsm.33.6.401. Epub 1999/12/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Viinikka L, Vuori J, Ylikorkala O. Lipid peroxides, prostacyclin, and thromboxane A2 in runners during acute exercise. Med Sci Sports Exerc. 1984;16(3):275–7. [PubMed] [Google Scholar]
- 45.Triggle CR, Dong H, Waldron GJ, Cole WC. Endothelium-derived hyperpolarizing factor(s): species and tissue heterogeneity. Clin Exp Pharmacol Physiol. 1999;26(2):176–9. doi: 10.1046/j.1440-1681.1999.03007.x. [DOI] [PubMed] [Google Scholar]
- 46.Deng Q, Huo Y, Luo J. Endothelial mechanosensors: the gatekeepers of vascular homeostasis and adaptation under mechanical stress. Sci China Life Sci. 2014;57(8):755–62. doi: 10.1007/s11427-014-4705-3. [DOI] [PubMed] [Google Scholar]
- 47.Ahn SJ, Fancher IS, Bian JT, Zhang CX, Schwab S, Gaffin R, et al. Inwardly rectifying K+ channels are major contributors to flow-induced vasodilatation in resistance arteries. J Physiol. 2017;595(7):2339–64. doi: 10.1113/JP273255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crecelius AR, Luckasen GJ, Larson DG, Dinenno FA. KIR channel activation contributes to onset and steady-state exercise hyperemia in humans. American journal of physiology Heart and circulatory physiology. 2014;307:H782–91. doi: 10.1152/ajpheart.00212.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crecelius AR, Richards JC, Luckasen GJ, Larson DG, Dinenno FA. Reactive hyperemia occurs via activation of inwardly rectifying potassium channels and Na+/K+-ATPase in humans. Circ Res. 2013;113(8):1023–32. doi: 10.1161/CIRCRESAHA.113.301675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang A, Sun D, Jacobson A, Carroll MA, Falck JR, Kaley G. Epoxyeicosatrienoic acids are released to mediate shear stress-dependent hyperpolarization of arteriolar smooth muscle. Circ Res. 2005;96(3):376–83. doi: 10.1161/01.RES.0000155332.17783.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loot AE, Popp R, Fisslthaler B, Vriens J, Nilius B, Fleming I. Role of cytochrome P450-dependent transient receptor potential V4 activation in flow-induced vasodilatation. Cardiovasc Res. 2008;80(3):445–52. doi: 10.1093/cvr/cvn207. [DOI] [PubMed] [Google Scholar]
- 52.Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, et al. Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005;97(9):908–15. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- 53.Thengchaisri N, Kuo L. Hydrogen peroxide induces endothelium-dependent and -independent coronary arteriolar dilation: role of cyclooxygenase and potassium channels. Am J Physiol Heart Circ Physiol. 2003;285(6):H2255–63. doi: 10.1152/ajpheart.00487.2003. [DOI] [PubMed] [Google Scholar]
- 54.Larsen BT, Gutterman DD, Sato A, Toyama K, Campbell WB, Zeldin DC, et al. Hydrogen peroxide inhibits cytochrome p450 epoxygenases: interaction between two endothelium-derived hyperpolarizing factors. Circ Res. 2008;102(1):59–67. doi: 10.1161/CIRCRESAHA.107.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]