Abstract
The aim of this study was to microencapsulate the reuterin produced by Lactobacillus reuteri BPL-36 strain for its long-term efficacy against food-borne pathogen Listeria monocytogenes. Lactobacillus reuteri BPL-36 strain previously isolated from a human infant fecal sample in lab was selected for the present study based on its ability to produce reuterin. The organism displayed a broad-spectrum antimicrobial activity. Reuterin concentration of 89.63 mM was obtained in the MRS–glycerol medium after 16 h incubation at 37 °C. The reuterin concentration required to inhibit the growth of Pseudomonas aeruginosa, Escherichia coli O157: H7, Salmonella typhi, Staphylococcus aureus, and Listeria monocytogenes was found to be 1.0, 2.0, 2.0, 4.0, and 10.0 AU/mL, respectively. Microencapsulation of reuterin to enhance long-term efficacy against food-borne pathogens was done. Results in this study indicated that the release characteristics of reuterin from the encapsulated particles were pH dependent. The release characteristics were unaffected by the storage of encapsulated reuterin at 4 °C for 2 weeks. The anti-listerial efficacy of the encapsulated reuterin was tested against L. monocytogenes in the BHI medium adjusted to pH 5.0 with a reuterin content equivalent to 16 mM, similar to un-encapsulated (free) reuterin. Encapsulated reuterin demonstrated enhanced efficacy against L. monocytogenes for longer duration of time when compared with un-encapsulated (free) reuterin. The present work demonstrated a novel antimicrobial delivery system that ensured much better capability of inhibiting the growth of L. monocytogenes throughout 24 h incubation at 37 °C.
Keywords: Lactobacillus reuteri, Reuterin, Microencapsulation, Pathogens, Safety
Introduction
Globally, contaminated food and drinking water cause deaths of one million people annually (Maurice 1994). Although tremendous efforts have been made by governments and food industry to reduce food-borne illnesses, there are sporadic outbreaks due to post-production contamination by food-borne pathogens (Wirjantoro et al. 2001; Cumming et al. 2008). Use of antimicrobial food preservatives in many food products has been followed to prevent post-processing contamination, extend shelf life, and maintain food quality while saving energy costs during production and transportation (Sofos et al. 1998).
Reuterin is a small antimicrobial compound that is produced as an intermediate metabolite during anaerobic fermentation of glycerol (Talarico et al. 1988). It consists of an equilibrium mixture of monomeric, hydrated monomeric, and cyclic dimeric forms of β-hydroxypropionaldehyde (Talarico and Dobrogosz 1989). Reuterin, produced by some strains of Lactobacillus reuteri, has shown antimicrobial activity against a range of food-borne pathogens and spoilage organisms, including Gram-positive and Gram-negative bacteria, yeasts, moulds, and protozoa (Axelsson et al. 1989; Mishra et al. 2012; Ortiz-Rivera et al. 2017). The inhibitory effect of reuterin is related to its action on DNA synthesis (Talarico and Dobrogosz 1989). Bioprotective effect of reuterin alone and in combination with nisin against various food-borne pathogens has been investigated in milk (Mishra et al. 2011).
Antimicrobials often have reduced efficacy in foods as compared to liquid solutions or microbiological growth media. Reactions between antimicrobial molecules and food components such as proteins, lipids, enzymes, ions, and surfactants may reduce antimicrobial effectiveness (Thomas 2005). A carrier material is used to encapsulate drugs in a designed structure, so that the drug is gradually released from the carrier–drug composite matrix by mass transfer, or released abruptly due to collapse of a carrier matrix triggered by environmental changes (Juliano 1978). Therefore, many researchers have developed novel delivery systems for antimicrobials to obtain sustained release so as to minimize interference of food components. These delivery systems include particulates (Salmaso et al. 2004; Jin et al. 2009; Narsaiah et al. 2014) and films (Lungu and Johnson 2005; Cao-Hoang et al. 2010; Nostro et al. 2010). Particulates can be used in food matrices, while films can be used to wrap solid foods to inhibit the growth of microorganisms on surfaces. Several studies have found that delivery systems can improve the efficiency of food antimicrobials by protecting antimicrobials from adverse environmental or processing effects and interactions with food components. Nisin encapsulated in liposomes demonstrated a 2-log greater inhibition of Listeria monocytogenes than free nisin (Were et al. 2004).
The first objective of this study was to microencapsulate the reuterin produced by L. reuteri BPL-36 strain for determination of its release pattern at different pH levels. The second objective was to compare its long-term efficacy with un-capsulated (free) reuterin against food-borne pathogen L. monocytogenes.
Materials and methods
Bacterial strains and culture conditions
Lactobacillus reuteri BPL-36 strain was previously isolated by our team from human infant fecal sample (Mishra et al. 2012), and was maintained and propagated in MRS broth (Hi-Media labs, Mumbai, India). Escherichia coli K12 (NCDC 135) was used as the indicator organism for determination of minimum inhibitory concentration (MIC). Listeria monocytogenes (ATCC 15303) and Staphylococcus aureus (NCDC 237), Pseudomonas aeruginosa (NCDC 105), and Salmonella typhi (NCDC 113), procured from National Collection of Dairy Cultures (NCDC), ICAR-NDRI, Karnal, India, were used as test organisms. Escherichia coli O157: H7 (ATCC 43888) was procured from American-Type Culture Collection (ATCC), USA. All the pathogenic strains were handled in class II type A2 biological safety cabinet and grown in Brain Heart Infusion broth (BHI; Hi-Media labs, Mumbai, India) at 37 °C for 18 h. Bacterial strains were maintained as stock cultures at − 80 °C in BHI broth supplemented with 15% glycerol. The organisms were propagated twice before use in the experiments. The purity of all the bacterial cultures was always ascertained by Gram staining prior to use for any experiment.
Production of reuterin
Over-night grown cultures of L. reuteri BPL-36 (1010 CFU/mL) were inoculated in MRS broth tubes supplemented with glycerol (360 mM) at 37 °C for 24 h in anaerobic conditions. After each 4 h interval, the cells from single tube were removed by centrifugation, cell-free supernatant was filter sterilized, and the presence of reuterin in it was detected by the colorimetric method of Circle et al. (1945). This method was based on the colorimetric determination of the acrolein formed from the 3-HPA produced from glycerol by the glycerol dehydratase. The concentration of reuterin in the BPL-36 cell-free supernatant was determined by comparing the spectral extinction values from the assay to those of a standard curve of acrolein (2-propenal).
Reuterin activity units
Reuterin activity was quantified by the MIC method using microtiter plates as described by Chung et al. (1989). From the over-night grown culture of E. coli K-12 (indicator organism), cells were harvested, washed twice with phosphate buffer (pH 7.2, 50 mM), suspended in the same buffer, and diluted to A420 of 0.2 measured using spectrophotometer. This suspension was diluted 1/10, which corresponds to about 1 × 106 CFU/mL. The diluted suspension (0.1 mL) was used to inoculate 0.2 mL of serial dilutions of reuterin diluted in Muller-Hinton medium (Himedia, India Laboratories). The microplate was incubated at 37 °C and growth was examined after 48 h. Reuterin concentration was defined as the reciprocal of the highest dilution that did not permit visible growth of the indicator strain and expressed as Arbitrary Units (AU) with 1 AU of reuterin being defined as the reciprocal of the highest dilution that did not permit visible growth of the indicator strain (El-Ziney et al. 1998).
Determination of minimum inhibitory concentration of reuterin to inhibit other food-borne test organisms
Listeria monocytogenes and E. coli O157: H7 were grown in Brain Heart Infusion broth (BHI) at 37 °C for 20 h. Salmonella typhi, Staphylococcus aureus, and Pseudomonas aeruginosa were grown at 37 °C for 20 h in Nutrient broth (Merck, Germany). Inocula of the cultures were then prepared (1 × 106 CFU/mL) as mentioned above and used (0.1 mL) to inoculate aliquots of the diluted reuterin (0.2 mL) in 96 wells microplates. Quantified reuterin was serially diluted in Muller-Hinton medium to obtain dilutions with 0.0, 2.0, 4.0, 6.0, 8.0, 10.0, and 12.0 AU/mL. The microplates were incubated at 37 °C and visible growth was checked after 48 h.
Encapsulation of reuterin
Reuterin was encapsulated (20:80 v/v) by mixing with 2% (w/v) sodium alginate and 0.4% (w/v) guar gum. Concentric two fluid glass nozzle with air atomization method (developed at CIPHET, Ludhiana, Punjab) was used for preparation of calcium alginate microcapsules incorporated with guar gum. Sodium alginate and guar-gum solution is prepared by dissolving polymers in distilled water using magnetic stirrer (Labco Instruments, New Delhi, India). The aqueous solution of sodium alginate–guar gum was infused from a peristaltic pump (Ravel Hiteks Pvt. Ltd., Chennai, India) at controlled flow rate into an air atomizing nozzle and sprayed under pressurized air into a reaction vessel containing 3 L of 0.2 M calcium chloride solution with continuous magnetic stirring at 1000 rpm for 30 min. The distance between the nozzle tip and the liquid level in reaction vessel was fixed at 12 in. The size of the orifice of inner nozzle (carrying guar gum and sodium alginate solution/matrix fluid) was 1 mm. It has 1.3 mm annular space (carrying pressurized air) between inner and outer nozzle. The pressurized air was used to break up the jet of matrix fluid. The kinetic energy of high-pressure air or inert air is used for break-up of matrix fluid jet. The small droplets produced fall in reaction vessel. The reaction vessel contains that the divalent calcium ions cross-link with sodium alginate on contact to form calcium alginate–guar-gum microcapsules by ionotropic gelification yielding microcapsules. The prepared microcapsules was sieved through buchner funnel and washed twice with sterile distill water and selected for further studies.
Evaluation of encapsulation properties
Various encapsulation properties given below were further studied:
Encapsulation efficiency
Reuterin content of supernatant was estimated. Reuterin content of polymer solution of alginate + guar gum before spraying was also estimated. Encapsulation efficiency was calculated using following formula:
Mass yield
Mass yield was calculated using following formula:
pH-dependent release characteristics of encapsulated reuterin
Release characteristics of encapsulated reuterin were checked by the method described by Oh et al. (2006) with slight modification. One gram of encapsulated reuterin was suspended in 100 mL of 50 mM sodium citrate buffer at pH levels ranging from 2 to 7. Changes in reuterin concentration after every hour of incubation at 37 °C were evaluated by the method prescribed by Circle et al. (1945). Similarly, changes in reuterin concentration up to 15 days of storage at 4 °C were evaluated.
Anti-listerial assay
Selected food-borne pathogen L. monocytogenes was incubated at 37 °C with two consecutive transfer to 9.31 logs CFU/mL. BPL-36 cell-free supernatant and encapsulated reuterin were added to 9 mL BHI broth (supplemented with 0.6% yeast extract) medium pre-adjusted to pH 6.0. Medium was then mixed with 1 mL different test organisms (106 CFU/mL) by vortexing. Overall reuterin concentration was used at 16 mM in all treatments. After incubation at 37 °C for 0, 1, 2, 4, 6, 8, 10, 12, 16, 20, and 24 h, the surviving bacterial population was determined by the plate count on BHI agar. Media without reuterin were used as positive control for all treatments.
Determination of mean and standard error of the mean (SEM)
The experimental data, as and when necessary, were presented as the mean and standard error of the mean (SEM) of different parameters studied in the present investigation. The mean and SEM were determined running Microsoft Excel 2000 Software Package, Microsoft Corporation, USA.
Results and discussion
Production of reuterin from L. reuteri BPL-36 strain
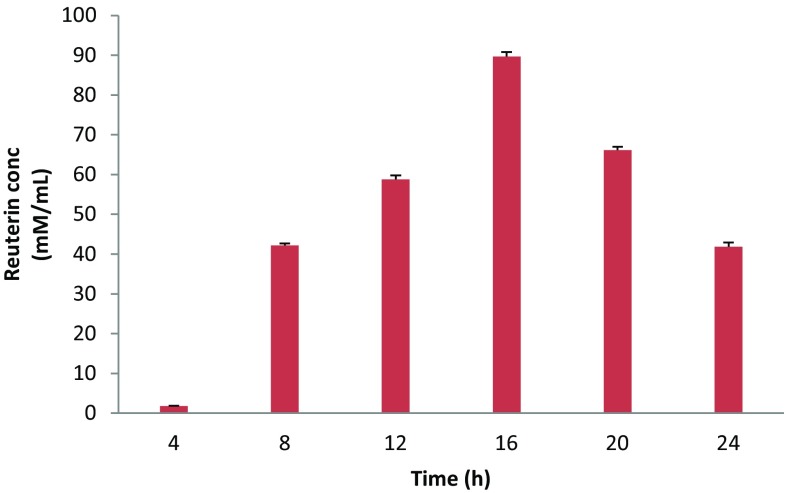
The reuterin production kinetics by BPL-36 resting cells in MRS–glycerol medium was studied. A sigmoidal increase in reuterin production was observed. The reuterin concentration increased markedly between 4 and 16 h and decreased after 16 h. Maximum reuterin concentration of 89.63 mM was obtained in the medium after 16 h of incubation at 37 °C (Fig. 1). Reuterin concentration was, however, declined on further incubation. This is probably because of the conversion of reuterin into 1,3-propanediol, the end-product of glycerol fermentation, by enzymation reaction of an NAD+-dependent oxidoreductase (Bieble et al. 1999; Daniel et al. 1999; Zeng and Bieble 2002), whose expression could possibly be triggered by the accumulation of reuterin.
Fig. 1.
Colorimetric quantification of reuterin produced by L. reuteri BPL-36 at different time intervals after incubation at 37 °C. Data points are means of triplicate readings with SEMs represented by vertical bars
Determination of minimum inhibitory concentration of reuterin to inhibit other food-borne test organisms
The reuterin concentrations required to inhibit the growth of E. coli K12, P. aeruginosa, E. coli O157: H7, S. typhi, S. aureus, and L. monocytogenes were 1.0, 1.0, 2.0, 2.0, 4.0, and 10.0 AU/mL, respectively (Table 1). It is evident from the above results that the strain L. monocytogenes was relatively resistant to reuterin as the higher concentration of reuterin is required to inhibit its growth in contrast to the other pathogens used in this study. Chung et al. (1989) reported the MIC of reuterin for Pseudomonas aeruginosa, Escherichia coli, Salmonella typhimurium, Staphylococcus aureus, and Listeria monocytogenes, respectively, as 2, 4, 4, 2, and 4–8 AU/mL which agrees to our results in some aspects.
Table 1.
Determination of minimum inhibitory concentration of reuterin to inhibit other food-borne test organisms
| Organisms | MIC (AU/mL) |
|---|---|
| Escherichia coli K12 | 1.0 |
| Pseudomonas aeruginosa | 1.0 |
| Escherichia coli O157: H7 | 2.0 |
| Salmonella typhi | 2.0 |
| Staphylococcus aureus | 4.0 |
| Listeria monocytogenes | 10.0 |
Evaluation of encapsulation properties
Summary of encapsulation performance of encapsulated reuterin is given in Table 2. Maximum encapsulation efficiency of 37.97% was observed with mass yield 71% at optimized controlled condition. Morphologically capsules were spherical in shape, were shiny and whitish in colour (Fig. 2). Surface of the capsules was not very smooth with small pits on its walls.
Table 2.
Summary of encapsulation performance of encapsulated reuterin
| Sample | Mass yield (%) | Encapsulation efficiency (%) |
|---|---|---|
| A | 74.36a | 36.35c |
| B | 74.55a | 37b |
| C | 71b | 37.55a |
| D | 71b | 37.97a |
Values containing the same superscript are not significant (P > 0.5)
Fig. 2.
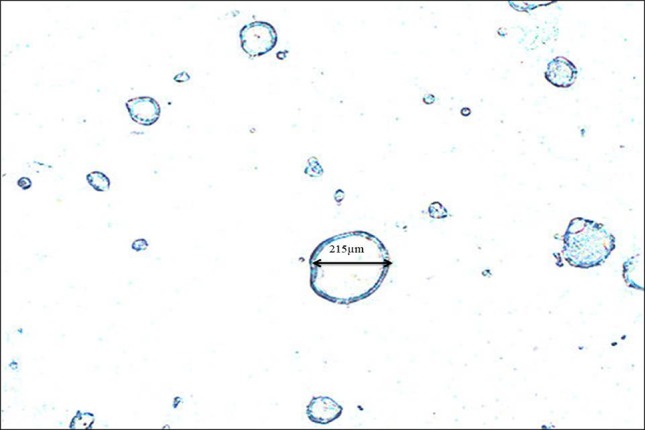
Morphology of microcapsules at 10× magnification
Effect of pH on release kinetics of encapsulated reuterin from alginate capsules
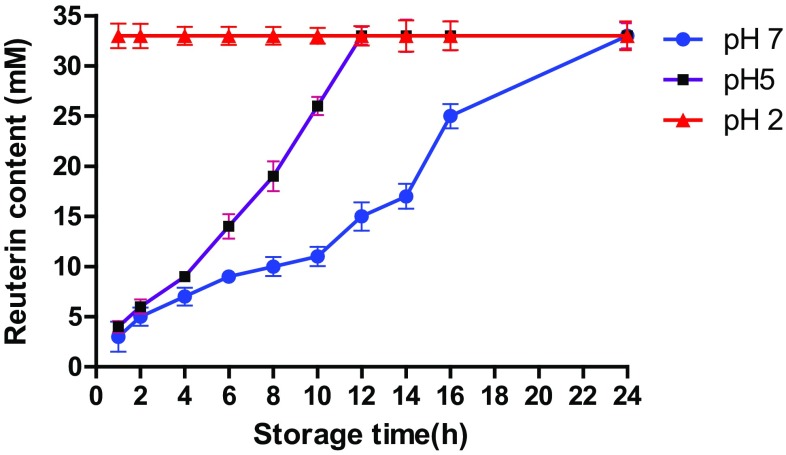
Release kinetics of encapsulated reuterin from alginate capsules are shown in Figs. 3 and 4. When encapsulated reuterin was suspended in sodium citrate buffer at pH level ranging from 2 to 7, 100% release of reuterin was observed during storage at 37 °C after 24 h. Reuterin activity was fully manifested at pH 2 and 5. All samples had complete release of reuterin in 30 min at pH 2.0. At pH 5, complete release of reuterin was observed only after 10 h, while at pH 7, complete release of reuterin was observed only after 24 h incubation at 37 °C (Fig. 3).
Fig. 3.
pH-dependent release pattern of encapsulated reuterin during storage at 37 °C. Data points are means of triplicate OD620 readings with SEMs represented by vertical bars
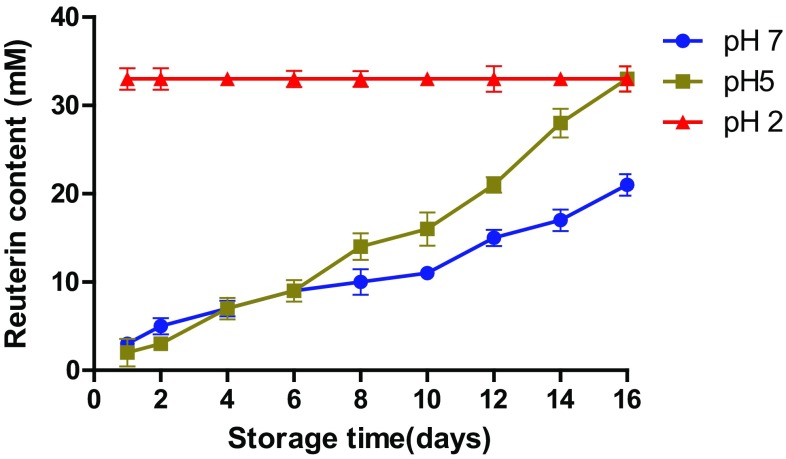
Fig. 4.
pH-dependent release pattern of encapsulated reuterin during storage at 4 °C. Data points are means of triplicate OD620 readings with SEMs represented by vertical bars
These release characteristics were unaffected by the storage of encapsulated reuterin at 4 °C for 2 weeks (Fig. 4), except at pH 7, where only about 64.24% of total encapsulated reuterin was released during storage at 4 °C. At pH 5, it took almost 15 days for the complete release of encapsulated reuterin in the buffer medium. These results indicate that the release characteristics of reuterin from the encapsulated particles are pH dependent. Our results are also in agreement with the published report of Oh et al. (2006), who had also encapsulated bacteriocin from Lactobacillus plantarum KU 107 in acid soluble coating polymer, Eudragit EPO in a hybridization system. He found no bacteriocin activity above pH 6 and at pH 6 only 25% of the total activity was detected. Stability of reuterin in aqueous solutions at pH 2.0–7.0 also is to be considered because Rollema et al. (1995) showed significant losses of nisin activity during incubation at different temperatures. As given in Figs. 3 and 4, the release of reuterin after 8 h incubation at 37 °C at pH 5 was found to be ~ 55%. Similar results were given by Degnan and Luchansky (1992) using un-encapsulated pediocin AcH in slurries of heated beef muscle and tallow.
Anti-listerial properties of reuterin delivery system
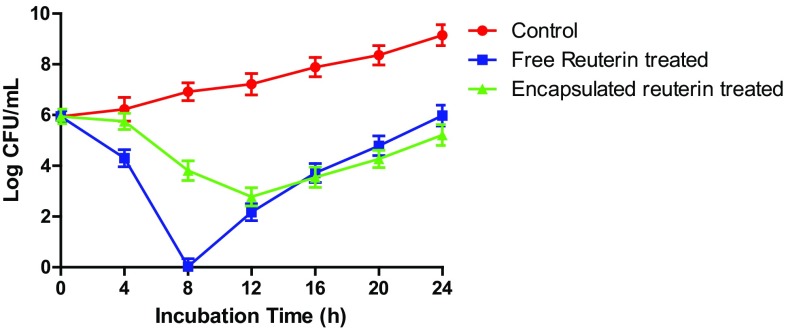
The anti-listerial efficacy of the encapsulated reuterin was tested against L. monocytogenes in the BHI medium adjusted to pH 5.0 with a reuterin amount equivalent to 16 mM, similar to un-encapsulated (free) reuterin. Free reuterin initially deactivated L. monocytogenes to undetectable level in < 7 h and the effectiveness against the growth of L. monocytogenes was observed for 8 h (Fig. 5). After 12 h, however, L. monocytogenes appeared to have recovered from the antimicrobial action and grew to a population no different from the initial log count of 0 h control sample (without antimicrobial treatment) after 24 h.
Fig. 5.
Log counts (CFU/mL, mean ± SD) of pathogens Listeria monocytogenes in BHI medium without or with free reuterin, encapsulated reuterin during incubation at 37 °C (reuterin 16 mM)
For encapsulated reuterin, the initial bactericidal and later inhibitory functions were lesser relatively as compared to the than free reuterin. When comparing to the release profile of encapsulated reuterin as given in Figs. 3 and 4, the release of reuterin after 8 h incubation at 37 °C at pH 5 was ~ 55% that may have accounted for less effectiveness than the free reuterin. Furthermore, sustained release of reuterin only lasted 11–12 h, corresponding to gradual recovery of L. monocytogenes population before reaching a level well below to the initial log count of 0 h control sample (without antimicrobial treatment) after 24 h. As observed from Fig. 5, it may be inferred that sustained release of reuterin to a sufficient concentration is needed to receive long-term efficacy to inhibit the growth of pathogenic bacteria in food systems. The similar conclusion was made by Chi-Zhang et al. (2004) who observed that effectiveness of nisin strongly depends on its mode of delivery. He also observed that L. monocytogenes cells developed resistance to higher concentration of nisin, compared to cells treated with slowly added nisin at the same total amount of antimicrobial which was similar to our finding with reuterin. Narsaiah et al. (2014) have also used the same encapsulator for encapsulation of nisin using sodium alginate and guar gum. Nisin encapsulated in liposomes produced a 2-log greater inhibition of L. monocytogenes than free nisin (Were et al. 2004). In our study sustained release of reuterin, only lasted 11–12 h, corresponding to gradual recovery of L. monocytogenes population before reaching a level well below to the initial log count of 0 h control sample (without antimicrobial treatment) after 24 h, better than the overall inhibition shown by free reuterin.
In conclusion, our work demonstrated a novel antimicrobial delivery system that had much better capability of inhibiting the growth of L. monocytogenes during long-term storage at 37 °C. The effectiveness might be longer if tests were performed at lower temperatures. Characterization of release profiles of reuterin unveiled that sustained release of reuterin was required to improve long-term efficacy of reuterin against the potent pathogen. The release profiles established in this work can be used as guidelines to identify applicability of a particular reuterin delivery system in appropriate food products. Because food products have long shelf lives and our delivery system is based on GRAS ingredients produced using commercially feasible processes, our work may be developed into practical intervention systems to enhance microbial safety of various food products.
Acknowledgements
Mr. Santosh Kumar Mishra is the recipient of scholarship from National Dairy Research Institute, Karnal, Haryana, India and duly acknowledges the financial assistance received. Funds received from NICRA project for carrying out this work are also acknowledged. He also acknowledges the help received from Dr. K. Narsaiah, Senior Scientist, CIPHET, Ludhiana for his generous help while using micro-encapsulator for encapsulation of reuterin.
Compliance with ethical standards
Ethical approval
This article does not contain any studies with human or animal subjects.
Informed consent
Additional informed consents were obtained from all the parents of infants from whom fecal samples were collected for isolation of lactic acid bacteria.
Conflict of interest
Santosh Kumar Mishra, R. K. Malik, Harsh Panwar, and Amit Kumar Barui declare that they have no conflict of interest.
References
- Axelsson LT, Chung TC, Dobrogosz WJ, Lindgren SE. Production of a broad spectrum antimicrobial substance by Lactobacillus reuteri. Microb Ecol Health Dis. 1989;2:131–136. doi: 10.3109/08910608909140210. [DOI] [Google Scholar]
- Bieble H, Mengel K, Zeng AP, Deckwer WD. Microbial production of I, 3-propanediol. Appl Microbiol Biotechnol. 1999;52:289–297. doi: 10.1007/s002530051523. [DOI] [PubMed] [Google Scholar]
- Cao-Hoang L, Chaine A, Gregoire L, Wache Y. Potential of nisin-incorporated sodium caseinate films to control Listeria in artificially contaminated cheese. Food Microbiol. 2010;27(7):940–944. doi: 10.1016/j.fm.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Chi-Zhang Y, Yam KL, Chikindas ML. Effective control of Listeria monocytogenes by combination of nisin formulated and slowly released into a broth system. Int J Food Microbiol. 2004;90:15–22. doi: 10.1016/S0168-1605(03)00168-5. [DOI] [PubMed] [Google Scholar]
- Chung TC, Axelsson L, Lindgren SE, Dobrogosz WJ. In vitro studies on reuterin synthesis by Lactobacillus reuteri. Microb Eco Health Dis. 1989;2(2):137–144. [Google Scholar]
- Circle SJ, Stone L, Boruff CS. Acrolein determination by means of tryptophane. Ind Eng Chem Anal Ed. 1945;17:259–262. doi: 10.1021/i560140a021. [DOI] [Google Scholar]
- Cumming M, Kludt P, Matyas B, DeMaria A, Stiles T, Han L, Gilchrist M, Neves P, Fitzgibbons E, Condon S. Outbreak of Listeria monocytogenes infections associated with pasteurized milk from a local dairy—Massachusetts, 2007. Morb Mortal Wkly Rep. 2008;57(40):1097–1100. [PubMed] [Google Scholar]
- Daniel R, Bobik TA, Gottschalk G. Biochemistry of coenzyme B12 dependent glycerol and diol dehydratase and organization of the encoding genes. FEMS Microbiol Rev. 1999;22:553–566. doi: 10.1111/j.1574-6976.1998.tb00387.x. [DOI] [PubMed] [Google Scholar]
- Degnan AJ, Luchansky JB. Influence of beef tallow and muscle on the antilisterial activity of pediocin AcH and liposome encapsulated pediocin AcH. J Food Prot. 1992;55(7):552–554. doi: 10.4315/0362-028X-55.7.552. [DOI] [PubMed] [Google Scholar]
- El-Ziney MG, Arneborg N, Uyttendaele M, Debevere J, Jakobsen M. Characterization of growth and metabolite production of Lactobacillus reuteri during glucose/glycerol co-fermentation in batch and continuous cultures. Biotechnol Lett. 1998;20(10):913–916. doi: 10.1023/A:1005434316757. [DOI] [Google Scholar]
- Jin T, Liu LS, Zhang H, Hicks K. Antimicrobial activity of nisin incorporated in pectin and polylactic acid composite films against Listeria monocytogenes. Int J Food Sci Technol. 2009;44(2):322–329. doi: 10.1111/j.1365-2621.2008.01719.x. [DOI] [Google Scholar]
- Juliano RL. Drug delivery systems: a brief review. Can J Physiol Pharmacol. 1978;56(5):683–690. doi: 10.1139/y78-112. [DOI] [PubMed] [Google Scholar]
- Lungu B, Johnson MG. Fate of Listeria monocytogenes inoculated onto the surface of model turkey frankfurter pieces treated with zein coatings containing nisin, sodium diacetate, and sodium lactate at 4 degrees C. J Food Prot. 2005;68(4):855–859. doi: 10.4315/0362-028X-68.4.855. [DOI] [PubMed] [Google Scholar]
- Maurice J. The rise and rise of food poisoning. New Sci. 1994;144:28–33. [Google Scholar]
- Mishra SK, Malik RK, Kaur G, Manju G, Pandey N, Singroha G. Potential bioprotective effect of reuterin produced by L. reuteri BPL-36 alone and in combination with nisin against food borne pathogens. Indian J Dairy Sci. 2011;64(5):406–411. [Google Scholar]
- Mishra SK, Malik RK, Manju G, Pandey N, Singroha G, Bahare P, Kaushik JK. Characterization of a reuterin-producing Lactobacillus reuteri BPL-36 strain isolated from human infant fecal sample. Probiot Antimicrob Proteins. 2012;4(3):154–161. doi: 10.1007/s12602-012-9103-1. [DOI] [PubMed] [Google Scholar]
- Narsaiah K, Jha SN, Wilson RA, Mandge HM, Manikantan MR. Optimizing microencapsulation of nisin with sodium alginate and guar gum. J Food Sci Technol. 2014;51(12):4054–4059. doi: 10.1007/s13197-012-0886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nostro A, Scaffaro R, Ginestra G, D’Arrigo M, Botta L, Marino A, Bisignano G. Control of biofilm formation by poly-ethylene-co-vinyl acetate films incorporating nisin. Appl Microbiol Biotechnol. 2010;87(2):729–737. doi: 10.1007/s00253-010-2598-z. [DOI] [PubMed] [Google Scholar]
- Oh SJ, Heo HJ, Park DJ, Kim SH, Lee SJ, Imm JY. Effect of encapsulated bacteriocin on acid production and growth of starter cultures in yoghurt. Food Sci Biotechnol. 2006;15(6):902–907. [Google Scholar]
- Ortiz-Rivera F, Sánchez-Vega R, Gutiérrez-Méndez N, León-Félix J, Acosta-Muñiz C, Sepulveda DR. Production of reuterin in a fermented milk product by Lactobacillus reuteri: inhibition of pathogens, spoilage microorganisms, and lactic acid bacteria. J Dairy Sci. 2017;100(6):4258–4268. doi: 10.3168/jds.2016-11534. [DOI] [PubMed] [Google Scholar]
- Rollema HS, Kuipers OP, Both P, De Vos WM, Siezen RJ. Improvement of solubility and stability of the antimicrobial peptide nisin by protein engineering. Appl Environ Microbiol. 1995;61(8):2873–2878. doi: 10.1128/aem.61.8.2873-2878.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmaso S, Elvassore N, Bertucco A, Lante A, Caliceti P. Nisin-loaded poly-l-lactide nano-particles produced by CO2 anti-solvent precipitation for sustained antimicrobial activity. Int J Pharm. 2004;287(2):163–173. doi: 10.1016/j.ijpharm.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Sofos JN, Beuchat LR, Davidson PM, Johnson EA. Naturally occurring antimicrobials in food. Regul Toxicol Pharmacol. 1998;28(2):71–72. doi: 10.1006/rtph.1998.1246. [DOI] [PubMed] [Google Scholar]
- Talarico TL, Dobrogosz WJ. Chemical characterization of an antimicrobial substance produced by Lactobacillus reuteri. Antimicrob Agents Chemother. 1989;33:674–679. doi: 10.1128/AAC.33.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarico TL, Casas IA, Chung TC, Dobrogosz WJ. Production and isolation of reuterin, a growth inhibitor produced by Lactobacillus reuteri. Antimicrob Agents Chemother. 1988;32:1854–1858. doi: 10.1128/AAC.32.12.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LVD. Nisin. In: Davidson PM, Sofos JN, Branen AL, editors. Antimicrobials in food. 3. Boca Raton: CRC Press Taylor & Francis Group, LLC; 2005. pp. 237–273. [Google Scholar]
- Were LM, Bruce B, Davidson PM, Weiss J. Encapsulation of nisin and lysozyme in liposomes enhances efficacy against Listeria monocytogenes. J Food Prot. 2004;67(5):922–927. doi: 10.4315/0362-028X-67.5.922. [DOI] [PubMed] [Google Scholar]
- Wirjantoro TI, Lewis MJ, Grandison AS, Williams GC, Delves-Broughton J. The effect of nisin on the keeping quality of reduced heat-treated milks. J Food Prot. 2001;64(2):213–219. doi: 10.4315/0362-028X-64.2.213. [DOI] [PubMed] [Google Scholar]
- Zeng AP, Bieble H. Bulk chemicals from biotechnology: the case of 1,3-propanediol production and the new trends. Adv Biochem Eng Biotechnol. 2002;74:239–259. doi: 10.1007/3-540-45736-4_11. [DOI] [PubMed] [Google Scholar]