Abstract
Several groups of birds have convergently evolved the ability to swim using their feet despite facing trade‐offs with walking. However, swimming relative to terrestrial performance varies across these groups. Highly specialized divers, such as loons and grebes, excel at swimming underwater but struggle to stand on land, whereas species that primarily swim on the water surface, such as Mallards, retain the ability to move terrestrially. The identification of skeletal features associated with a swimming style and conserved across independent groups suggests that the hindlimb of foot‐propelled swimming birds has adapted to suit the physical challenges of producing propulsive forces underwater. But in addition to skeletal features, how do hindlimb muscles reflect swimming ability and mode? This paper presents the first comparative myology analysis associated with foot‐based swimming. Our detailed dissections of 35 specimens representing eight species reveal trends in hindlimb muscle size and attachment location across four independent lineages of extant swimming birds. We expand upon our dissections by compiling data from historical texts and provide a key to any outdated muscle nomenclature used in these sources. Our results show that highly diving birds tuck the femur and proximal tibiotarsus next to the ribcage and under the skin covering the abdomen, streamlining the body. Several hindlimb muscles exhibit dramatic anatomical variation in diving birds, including the flexor cruris lateralis (FCL) and iliofibularis (IF), which reduce in size and shift distally along the tibiotarsus. The femorotibialis medius (FTM) extends along an expanded cnemial crest. The resulting increased moment arms of these muscles likely help stabilize the hip and knee while paddling. Additionally, distal ankle plantarflexors, including the gastrocnemius and digital flexors, are exceptionally large in diving birds in order to power foot propulsion. These patterns exist within distantly related lineages of diving birds and, to a lesser extent, in surface swimmers. Together, our findings verify conserved muscular adaptations to a foot‐propelled swimming lifestyle. The association of muscle anatomy with skeletal features and biomechanical movement demands can inform functional interpretation of fossil birds and reveal selective pressures underlying avian diversification.
Keywords: birds, diving, hindlimb, leg, muscle, myology, swimming
Introduction
Although birds are often known for their flying abilities, several species are excellent swimmers. Swimming birds that paddle with their feet face trade‐offs between moving on land and in the water. Despite this challenge, foot‐propelled swimming has evolved convergently in birds, arising independently within distantly related lineages (Prum et al. 2015). As a result of transitioning to a life in the water, these birds possess a suite of physiological and anatomical characteristics suitable for an aquatic environment. Previous research has focused on characterizing skeletal (Zeffer et al. 2003; Hinić‐Frlog & Motani, 2010) and organ‐level (Butler, 2004; Strod et al. 2004) adaptations for swimming; however, corresponding differences in muscle anatomy have never been studied. Comparing hindlimb myology across convergent lineages of foot‐propelled swimming birds provides a unique opportunity to investigate how hindlimb muscles have adapted over evolutionary time to suit the physical demands of foot‐propelled swimming.
The ability of birds to swim using their feet has evolved convergently in at least six avian lineages, although to varying degrees (Fig. 1A). Below we list and describe the extent of swimming ability in each of the six independent lineages. (1) The family Anatidae is one of the earliest diverging groups of extant birds and includes ducks, geese, and swans. All Anatidae species can swim. Most species are restricted to swimming on the surface, but some, particularly the diving ducks and seaducks, dive to considerable depths (up to 60 m for long‐tailed ducks, Clangula hyemalis) (Schorger, 1947). (2) The rails (Rallidae) and finfoots (Heliornithidae) include several wetland species, including coots (Fulica) and moorhens (Gallinule), capable of swimming on the surface for short distances. (3–5) Three independent lineages of birds are excellent divers that survive primarily by hunting fish underwater: grebes (Podicipedidae), loons (Gaviidae) and cormorants (Phalacrocoracidae). (6) An extinct lineage of birds from the Cretaceous period, Hesperornithes, closely resembled grebes and loons, suggesting a similar lifestyle and swimming ability (Hinić‐Frlog & Motani, 2010; Zinoviev, 2011). Other seabirds – including gulls, terns, auks, penguins, gannets, and some petrels – also occasionally swim using their feet. However, these groups are closely related to cormorants and loons, and are unlikely to represent an independent evolution of foot‐propelled swimming (Prum et al. 2015). Pelicans, however, are most closely related to non‐swimming shoebills and herons, indicating a potential seventh independent lineage.
Figure 1.

Evolution of foot‐based swimming in birds and general avian skeletal anatomy. (A) Foot‐based swimming has evolved at least six times within birds as denoted by black radiations within major gray bird lineages. The phylogeny is based on Prum et al. (2015). Branch lengths are not to scale. Dashed lines represent completely extinct groups. Common names of representative swimming birds from each lineage are listed to the right of the phylogeny. (B) General skeletal anatomy of birds annotated using the helmeted Guineafowl (MCZ 341648). tr., trochanter; i.c., iliac crest; i.f., iliac fossa; o.f., obturator foramen; il.f., ilioischiatic fenestra; is.f., ischiopubic fenestra; c.c., cnemial crest; MTP, metatarsophalangeal joint.
The hundreds of species represented in these lineages of foot‐propelled swimming birds can generally be separated into three functional groups. Surface swimming birds – such as dabbling ducks and coots – primarily stay on the water surface, escape threatening situations through flight, and are relatively capable of walking on land (Abourachid, 2001). Highly diving birds – such as grebes and loons – excel at swimming underwater, dive to escape threats, and struggle to stand or walk on land (Johnsgard, 1987). This reduced mobility on land, plus the loss of flight in some species, demonstrates a high‐level of specialization for swimming in these lineages. Lesser diving birds – such as seaducks and some cormorants – resemble highly diving birds in their ability to swim underwater and their tendency to escape by diving, but retain some ability to walk on land (Abourachid, 2001; White et al. 2008).
All foot‐propelled swimming birds must produce fluid forces, using their feet originally adapted for walking on land. Walking fundamentally differs from swimming. On land, the legs must resist gravity to support the body. In water, the legs no longer resist gravity, but when diving underwater the legs must produce forces to overcome an upward buoyancy force and resistive drag force acting on the body. Furthermore, generating fluid forces underwater differs from ground reaction forces on land. For a bird walking on land, the force its foot can exert against the ground to propel its body forward is limited only by the capacity of the hindlimb muscles to produce force and the structural integrity of the hindlimb musculoskeletal system. However, for a swimming bird, the force exerted against the water also depends on the size and shape of the foot, the orientation of the foot relative to its motion, and how quickly the foot is paddled (Vogel, 2008). To maximize fluid force production, and therefore swimming speed and maneuverability, an ideal swimmer would maximize the size of its feet and how quickly it pushes them through the water (Fish, 1993). However, large feet can make walking cumbersome. In addition, muscles face a trade‐off between contraction velocity and the capacity to produce force based on muscle architecture and intrinsic muscle properties (Lieber & Fridén, 2001; Biewener, 2003). Therefore, foot‐propelled swimming birds face different demands on the posture, structure, and muscular design of the hindlimb.
To contend with the challenges of producing forces underwater, all foot‐propelled swimming birds have evolved several specialized hindlimb features. Each species has an enlarged foot surface area due to webbing (three digits in Anatidae, loons, and seabirds, and four digits in cormorants) or keratinous lobes around the toes (in grebes, rails, and finfoots) (Johnsgard, 1987). Foot‐propelled swimming birds also have a thinner pelvis, shorter tarsometatarsus, a relatively short femur, and an enlarged cnemial crest (Zeffer et al. 2003; Hinić‐Frlog & Motani, 2010; Doube et al. 2012). All of these features are exaggerated in diving birds, with especially large feet (Raikow, 1973), thin pelvises (Johnsgard, 1987), and enlarged knees through an expanded tibiotarsal cnemial crest in loons (Shufeldt, 1904), patella in cormorants (Shufeldt, 1913; Owre, 1967), or both in grebes (Beddard, 1896). These anatomical patterns exist across all independent lineages of foot‐propelled swimming birds, suggesting that these features may provide a selective benefit for swimming efficacy.
Most known avian hindlimb adaptations identified for swimming have been based on functional interpretations of the skeleton, due to the relative ease of preserving and analyzing bone design. While the hindlimb skeleton helps support forces transmitted to the limbs, muscles more directly reflect the capacity to produce forces that control and determine limb motion. Muscle is an extremely plastic tissue (Wisdom et al. 2015). This plasticity allows muscle to accommodate to use throughout an animal's lifetime (Flück, 2006) as well as adapt over evolutionary time (Pennycuick, 1991). Muscle size, fiber type composition, and architectural structure determine a muscle's capacity to produce force and change length (Marsh, 1999; Lieber & Fridén, 2001). Where a muscle attaches to a bone defines the perpendicular distance between its line of action and a joint, its moment arm. Longer moment arms produce stronger torques around the joint for a given muscle force but result in small rotations relative to muscle length change (McMahon, 1984; Biewener, 2003). Across generations, selection acts on muscle anatomy to accommodate movement demands, with muscles changing in number (from fusion, loss or novel muscles arising), size, architecture, and attachment location (Kardong, 2015). Over long time scales, these gross anatomical changes result in macroevolutionary patterns where muscle anatomy represents an adaptation to an animal's lifestyle. As such, comparative muscle anatomy has the potential to reveal functional trends associated with modes of locomotion.
Although avian myology has been long studied, prior work has not provided comparative analysis of trends associated with foot‐propelled swimming. Most accounts survey anatomy across many species using one or two specimens per species and remark generally on foot‐propelled swimming birds (Garrod, 1873, 1874, 1875; Forbes, 1885; Gadow & Selenka, 1891; Hudson, 1937; Hudson et al. 1959; George & Berger, 1966; Raikow, 1987; Baumel & Witmer, 1993; Vanden Berge & Zweers, 1993; Bennett, 1996). A few studies comprehensively address the myology of a single species of paddling bird using a small sample size (Haughton, 1865; Garrod & Darwin, 1872; Shufeldt, 1890, 1904; Beddard, 1896; Wilcox, 1952; Owre, 1967; Rosser et al. 1982). But stylistic and naming variations among these texts have made comparisons challenging. Here we compile data from these sources while accounting for multiple source languages, discrepancies in muscle naming, and the use of outdated species names. We also provide comparative data for species never before studied and for previously studied species to evaluate past findings based on anatomical analysis of more specimens.
In this study we seek to identify functional trends in bird hindlimb anatomy relating to swimming performance. To address this aim, we directly compare hindlimb myology in species with varying levels of foot‐propelled swimming specialization that represent convergently evolved lineages. We present data from detailed dissections of all major hindlimb muscles (excluding only the intrinsic foot muscles) for 35 specimens representing seven species of foot‐propelled swimming birds and one, non‐swimming outgroup. We provide a convenient resource of accepted muscle names with common alternative names used in other works. Our scaled drawings, descriptions and discussion of the origins and insertions of the hindlimb muscles allow us, for the first time, to compare limb anatomy in the light of the functional demands of foot‐based swimming. Our findings provide insight into convergent evolution within a subset of swimming birds. Additionally, our work presents a framework for future research to compare anatomical trends associated with swimming in other birds and aquatic animals.
Materials and methods
Hindlimb dissections were performed for species representing four of five independent lineages of extant foot‐propelled swimming birds and one non‐swimming outgroup. We obtained bird carcasses from rehabilitation centers. The birds had either been brought to the center as carcasses or deceased while in care. Once at the rehabilitation center, all carcasses were frozen (−18 °C) before transport to the Concord Field Station (CFS, Bedford, MA, USA). The following six species were collected from local rehabilitation centers, including the Tufts Wildlife Clinic (North Grafton, MA, USA) and the New England Wildlife Center (Weymouth, MA, USA): Mallard (Anas platyrhynchos), Canada Goose (Branta canadensis), Mute Swan (Cygnus olor), Double‐crested Cormorant (Phalacrocorax auritus), Red‐throated Loon (Gavia stellate), and Common Loon (Gavia immer). Western Grebe (Aechmophorus occidentalis) carcasses were collected at the Wildlife Center of the North Coast (Astoria, Oregon), and then shipped on dry ice to the CFS. All collections were covered by federal (MB005348‐1) and state (Oregon 130‐12, Massachusetts 050.13SAL) scientific collecting permits. American Coots, representing the fifth independent lineage of extant foot‐based swimming bird have been comprehensively dissected in a prior study (Rosser et al. 1982). Of the species dissected here, the Canada Goose, Mute Swan, and Red‐throated Loon have not been described in previous studies. Non‐swimming Helmeted Guineafowl (Numida meleagris) were obtained from a local breeder and raised at the CFS before being humanely euthanized using an overdose of Euthasol administered intravenously following approved Harvard University IACUC procedures (AUP 98‐04). In total, 43 carcasses were collected between 2013 and 2015.
Before dissection, we thawed each carcass at 2 °C. Thawed carcasses were weighed (Ranger RC6RS, Omaha Corp., NJ, USA or PTHK, Mettler Toledo International, Inc., OH, USA) and emaciated birds were identified by comparison with published normal weight ranges (Rodewald, 2015). We removed all emaciated carcasses from the study and either donated the material to the Museum of Comparative Zoology (Cambridge, MA, USA) or disposed of the carcass as dictated by the collection permits. Data presented here represent 35 specimens, four per species except for five Guineafowl carcasses and six Western Grebe carcasses. The right hindlimb and pelvis were plucked of feathers and each hindlimb muscle was dissected (see Tables 1 and 2 for a list of all dissected muscles). Before removal, each muscle was photographed with the limb in an approximate mid‐stance or mid‐swimming stroke position. Midstance and midswing were estimated from previous literature (Gatesy, 1999; Biewener & Corning, 2001; Johansson & Norberg, 2001) when possible and estimated from high speed video of live animals swimming or walking. After removal, each muscle was weighed and analyzed for muscle length, fiber length, pennation angle, tendon mass, and tendon length, which will form the basis for a companion paper.
Table 1.
Proximal hindlimb muscles dissected and alternate names in other sources. The left column shows abbreviations used in this paper. The right column identifies alternate names used in major avian myology works. If an alternate name is not identified for a given source it is either not mentioned in that work or uses the name listed in the ‘Full Muscle Name’ column
| Full muscle name | Alternate names | |
|---|---|---|
| IC | Iliotibialis cranialis |
Extensor iliotibialis anteriorOw
Iliotibialis internus s. sartoriusGS SartoriusBe, Ga73, GB, Ha, Hu, Sh, Wi |
| ILPR | Iliotibialis lateralis pars preacetabularis |
Extensor iliotibialis lateralisOw
Gluteus maximusBe Gluteus primusFo, Sh IliotibialisGB, Hu Iliotibialis anterior and Iliotibialis medius/tensor fasciaeGS Tensor fasciae pars preacetaularGa73 Triple muscleHa |
| ILPO | Iliotibialis lateralis pars postacetabularis |
Extensor iliotibialis lateralisOw
Gluteus maximusBe Gluteus primusFo, Sh IliotibialisGB, Hu Iliotibialis posterior/gluteus posteriorGS Tensor fasciae pars postacetaularGa73 Triple muscleHa |
| ITCR | Iliotrochantericus cranialis |
Gluteus iiiGa73
Gluteus minimusBe, Sh IliacusFi Iliotrochantericus anteriorGS, GB, Hu Iliotrochantericus anticusWi Opponens quadratus femorisHa |
| ITM | Iliotrochantericus medius |
Gluteus minimusBe
IliacusHa |
| ITCA | Iliotrochantericus caudalis |
Gluteus iiGa73
Gluteus mediusBe, Ha, Sh Gluteus profundisOw Iliotrochantericus posteriorGS, GB, Hu Iliotrochantericus posticusWi |
| IF | Iliofibularis |
Biceps crurisGa73
Biceps femorisBe, GB, Ha, Hu, Wi Biceps flexor crurisSh Extensor iliofibularisGS, Ow |
| IFE | Iliofemoralis externus |
Gluteus medius et minimusGB, Hu
Gluteus minimusHa Gluteus ivGa73 PiriformisFi |
| IFI | Iliofemoralis internus |
IliacusGB, Hu, Wi
PectinaeusHa PsoasOw |
| FCLP | Flexor cruris lateralis pars pelvica |
CaudiliofexoriusGS
Flexor cruris lateralisOw SemintendinosusBe, Fo, Ga73, GB, Ha, Hu, Sh, Wi |
| FCLA | Flexor cruris lateralis pars accessoria |
Accesorius semitendinosi or Accessory semitendinosusBe, Fo, Ga73, GB, Ha, Hu, Sh
Caudiliofexorius accessoriusGS |
| CFC | Caudofemoralis pars caudalis |
Caudilofemoralis pars caudi‐femoralisGS
CaudofemoralisOw, Ra FemorocaudalBe, Fo, Ga73, Sh Piriformis pars caudofemoralisGB, Hu SemimembranosusHa |
| CFP | Caudofemoralis pars pelvica |
Accessory femorocaudalHe, Fo, Ga73, Sh
Caudilofemoralis pars ilio‐femoralisGS Piriformis pars iliofemoralisGB, Hu SemimembranosusHaO: |
| IS | Ischiofemoralis |
Obturator externusSh
Quadratus femorisHa |
| FCM | Flexor cruris medialis |
GracilisHa
IschioflexoriusGS SemimembranosusBe, Fo, Ga73, GB, Hu, Sh, Wi |
| PIFL | Pubo‐ischio‐femoralis lateralis |
Adductor longusSh
Adductor longus et brevisWi Adductor longus et brevis pars externaGB, Hu Adductor magnusGa73, Ha Adductor superficialisOw Pubischiofemoralis pars externaGS Puboischiofemoralis pars cranialisRa |
| PIFM | Pubo‐ischio‐femoralis medialis |
Adductor longus et brevisWi
Adductor longus et brevis pars internaGB, Hu Adductor magnusGa73, Ha, Sh Adductor profundusOw Pubischiofemoralis pars internaGS Puboischiofemoarlis pars caudalisRa |
| OB | Obturator externus and internus |
Gemellus and Obturator internusSh
Obturator and Accessorrii obturatorisGS Obturatorius lateralis and medialisRa |
| AM | Ambiens |
Sources: Be, Beddard, 1896; Bn, Bennett, 1996; Fo, Forbes, 1885; GS, Gadow & Selenka, 1891; Ga72, Garrod & Darwin, 1872; Ga73, Garrod, 1873, 1874; Ga75, Garrod, 1875; GB, George & Berger, 1966; Ha, Haughton, 1865; Hu, Hudson, 1937; Hudson et al. 1959; Ow, Owre, 1967; Ra, Raikow, 1987; Sh, Shufeldt, 1890; Wi, Wilcox, 1952.
Table 2.
Distal hindlimb muscles dissected and alternate names in other sources. The left column shows abbreviations used in this paper. The right column identifies alternate names used in major avian myology works. If an alternate name is not identified for a given source it is either not mentioned in that work or uses the name listed in the ‘Full Muscle Name’ column
| Full muscle name | Alternate names | |
|---|---|---|
| FTI | Femorotibialis internus |
Femoritibialis internusGS, Hu, Wi
Vastus internusHa, Sh |
| FTM | Femorotibialis medius |
CruraeusHa, Sh
Femoritibialis mediusGS, Hu, Wi Vastus medialisOw |
| FTE | Femorotibialis externus |
Femoritibialis externusGS, Hu, Wi
Vastus externusGa73, Ha, Sh Vastus lateralisOw |
| FL | Fibularis longus |
Peroneus longusBe, GB, Hu, Ow, Sh, Wi
Peroneus superficialisGS |
| FB | Fibularis brevis |
Peroneus brevisGB, Hu, Wi
Peroneus profundusGS Tibialis posticusSh |
| EDL | Extensor digitorum longus |
Extensor digitorum communisBe, GS, Ga72, Ha
Extensor longus digitorumSh |
| TC | Tibialis cranialis |
Tibialis anteriorGB, Hu, Ow
Tibialis anticusBe, GS, Ha, Sh, Wi |
| GM | Gastrocnemius medialis |
Gastrocnemido‐solaeusHa
Gastrocnemius anticusBe, GS Gastrocnemius internal headSh, Wi Gastrocnemius par medialis and pars supermedialisRa Gastrocnemius pars internaBe, GS, GB, Hu, Ow |
| GI | Gastrocnemius intermedia |
Gastrocnemido‐solaeusHa
Gastrocnemius anticusFo, Ga72 Gastrocnemius medial headWi Gastrocnemius pars intermediaRa Gastrocnemius pars mediaBe, GS, GB, Hu, Ow Gastrocnemius tibial headSh |
| GL | Gastrocnemius lateralis |
Gastrocnemido‐solaeusHa
Gastrocnemius external headSh, Wi Gastrocnemius pars externaBe, GS, GB, Hu, Ow Gastrocnemius posticusFo, Ga72 Gastronemius pars lateralisRa |
| SFPPDII | (Superficialis) Flexor perforans et perforatus digiti II |
Flexor perforatus et perforans digiti IIGS
Flexor perforatus indicis secundus pedisSh |
| SFPPDIII | (Superficialis) Flexor perforans et perforatus digiti III |
Flexor digiti interniGa72
Flexor perforatus et perforans digiti IIIBe, GS Flexor perforatus medius secundus pedisSh |
| SFPPDIV | (Superficialis) Flexor perforans et perforatus digiti IV |
Flexor externi digitiGa72
Flexor perforatus annularis primus pedisSh Flexor perforatus digiti IVBe, Bn, FS, GB, Hu,Ow,Ra,Wi |
| FPDII | Flexor perforatus Digiti II | Flexor perforatus indicis primus pedisSh |
| FPDIII | Flexor perforatus Digiti III |
Flexor magnusGa72
Flexor perforatus medius primus pedisSh |
| FHL | Flexor hallucis longus |
Flexor longus hallucisFo, Ga75, Sh
Flexor profundusGa72 |
| FDL | Flexor digitorum longus |
Flexor perforans digitorumFo, Ga75
Flexor profundusGa72 Flexor profundus s. perforansGS Flexor perforans digitorum profundusSh |
| PL | Plantaris | SoleusSh |
| PO | Popliteus |
Sources: Be, Beddard 1896; Bn, Bennett, 1996; Fo, Forbes, 1885; GS, Gadow & Selenka, 1891; Ga72, Garrod & Darwin, 1872; Ga73, Garrod, 1873, 1874; Ga75, Garrod, 1875; GB, George & Berger, 1966; Ha, Haughton, 1865; Hu, Hudson, 1937; Hudson et al. 1959; Ow, Owre, 1967; Ra, Raikow, 1987; Sh, Shufeldt, 1890; Wi, Wilcox, 1952.
Drawings of the pelvis and hindlimb skeleton were based from specimens from the Museum of Comparative Zoology Ornithology Collections. We photographed re‐articulated skeletal elements and scaled each picture to actual size to trace or use as reference for our drawings. The specimens used were: MCZ‐341648 (Guineafowl), MCZ‐347156 (Mallard), MCZ‐347645 (Canada Goose), MCZ‐347051 (Mute Swan), MCZ‐337606 (Double‐crested Cormorant), MCZ‐342951 (Western Grebe), MCZ‐337590 (Red‐throated Loon), and MCZ‐347914 (Common Loon). We digitally added muscles by overlaying dissection photos on scans of the skeletal drawings and tracing labeled muscle outlines. Our drawn muscles were compared for all specimens and carefully reviewed for accuracy during subsequent dissections of each species.
Results and Discussion
Diving birds incorporate the proximal hindlimb within the skin covering the abdomen. In non‐swimming and surface‐swimming birds (including Guineafowl and the Anatidae species analyzed in this study), the hindlimb extends away from the abdomen with a large range of motion in flexion and extension at the hip and knee (Figs 2A, 3, 4, 5, 6, 7D). In contrast, highly diving birds (grebes and loons) tuck the proximal portion of the leg against the ribs, keeping the femur and most of the tibiotarsus within the skin overlying the abdomen (Figs 2B, 8, 9, 10D). As a result, the body of loons and grebes looks like an ‘American football’ with only the feet protruding from the ‘ball’. The proximal hindlimb is tucked within the skin overlying the abdominal region of the bird's trunk. Lesser‐diving Double‐crested Cormorants represent an intermediate stage, incorporating only the femur and proximal tibiotarsus adjacent to the abdomen with half of the tibiotarsus extending away from the skin overlying the body (Fig. 7D). In comparison, only the foot of loons and grebes extends beyond the skin overlying the body. The intermediate pattern observed in Double‐crested Cormorants corresponds to an ability to both dive underwater and walk on land.
Figure 2.
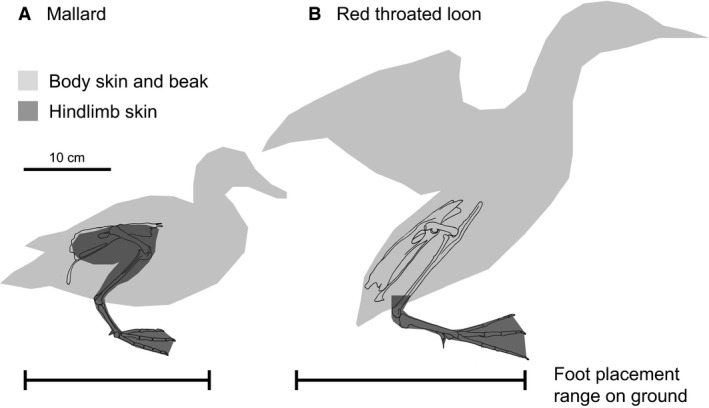
Hindlimb skin variation and standing ability in surface swimming and diving birds. (A) Surface swimming birds can easily stand on land, shown here using a Mallard. The entire lateral and medial surfaces of the limb are covered in skin separate from that overlying the body wall, allowing for a large range of motion at the hip and knee. (B) In contrast, highly diving birds, shown here using a Red‐throated Loon, struggle to stand on land. The medial surface of the proximal leg is not covered by skin and is instead tucked next to the ribs, restricting flexion‐extension mobility at the hip and knee. Only the feet extend away from the body with full skin coverage. Consequently, the body must be upright for the foot to reach under the body's center of mass, often requiring wing flapping to lift the body up to standing or while attempting to walk.
Figure 3.
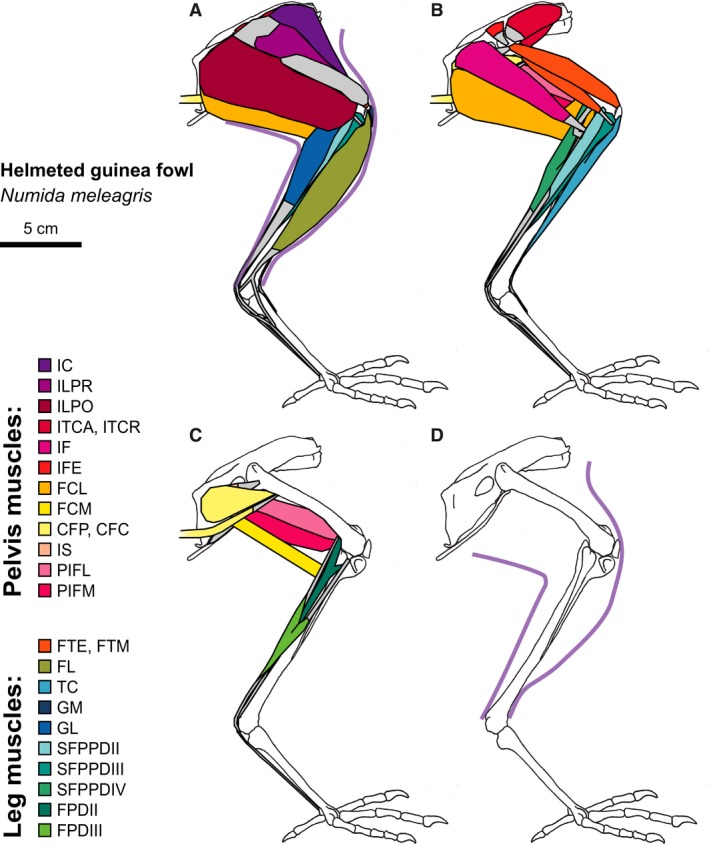
Helmeted guinea fowl hindlimb musculature. Major hindlimb muscles drawn based on dissections of several specimens. Panels show the hindlimb with (A) all muscles and outline of limb skin (in purple), (B) superficial muscles removed, (C) superficial and intermediate muscles removed, and (D) skeleton only and outline of limb skin (in purple). Skeleton drawing based on MCZ 341648. Muscle abbreviations listed in Table 1.
Figure 4.
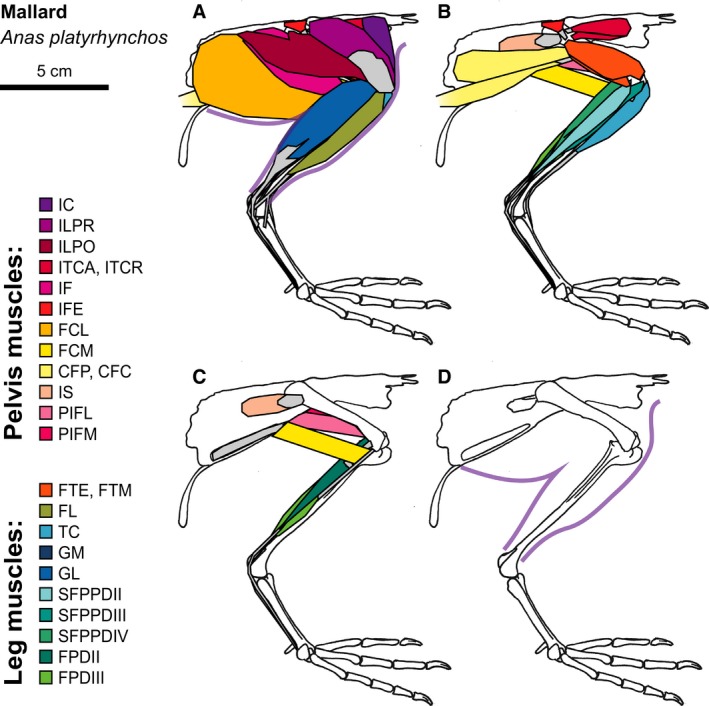
Mallard hindlimb musculature. Major hindlimb muscles drawn based on dissections of several specimens. Panels show the hindlimb with (A) all muscles and outline of limb skin (in purple), (B) superficial muscles removed, (C) superficial and intermediate muscles removed, and (D) skeleton only and outline of limb skin (in purple). Skeleton drawing based on MCZ 347156. Muscle abbreviations listed in Table 1.
Figure 5.
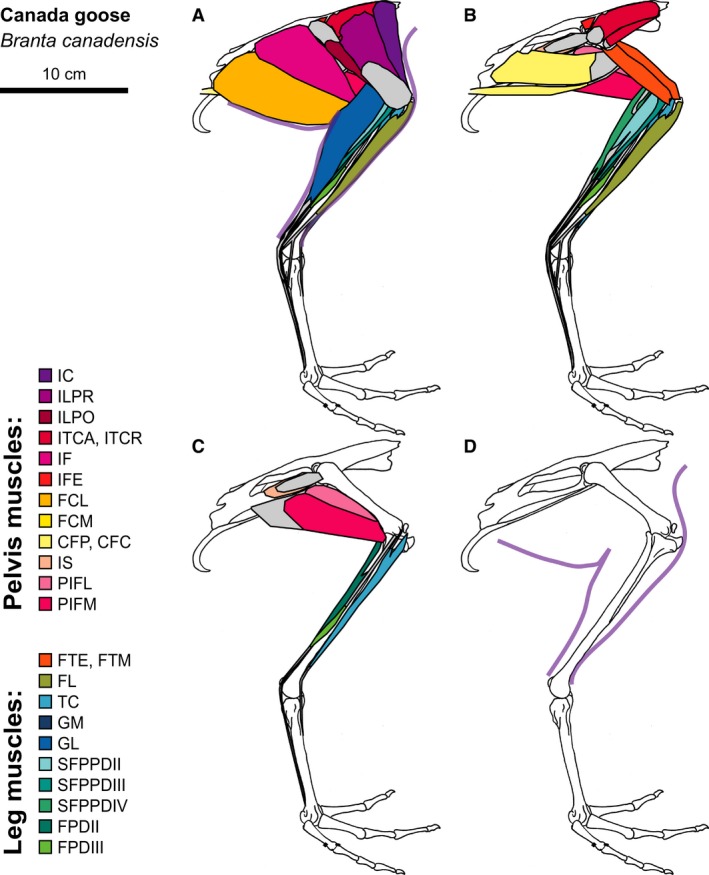
Canada Goose hindlimb musculature. Major hindlimb muscles drawn based on dissections of several specimens. Panels show the hindlimb with (A) all muscles and outline of limb skin (in purple), (B) superficial muscles removed, (C) superficial and intermediate muscles removed, and (D) skeleton only and outline of limb skin (in purple). Skeleton drawing based on MCZ 347645. Muscle abbreviations listed in Table 1.
Figure 6.
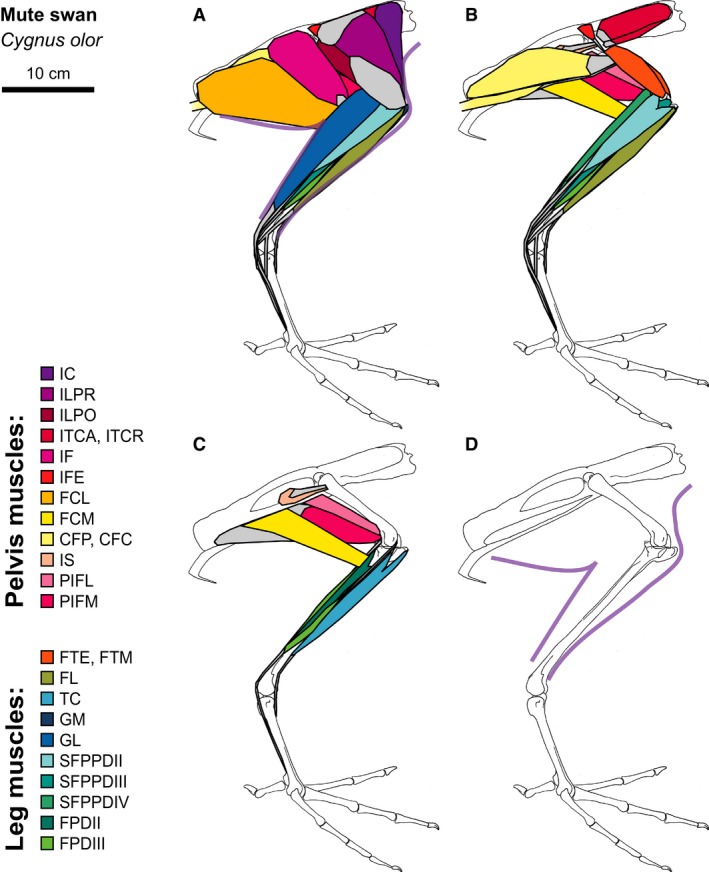
Mute Swan hindlimb musculature. Major hindlimb muscles drawn based on dissections of several specimens. Panels show the hindlimb with (A) all muscles and outline of limb skin (in purple), (B) superficial muscles removed, (C) superficial and intermediate muscles removed, and (D) skeleton only and outline of limb skin (in purple). Skeleton drawing based on MCZ 347051. Muscle abbreviations listed in Table 1.
Figure 7.
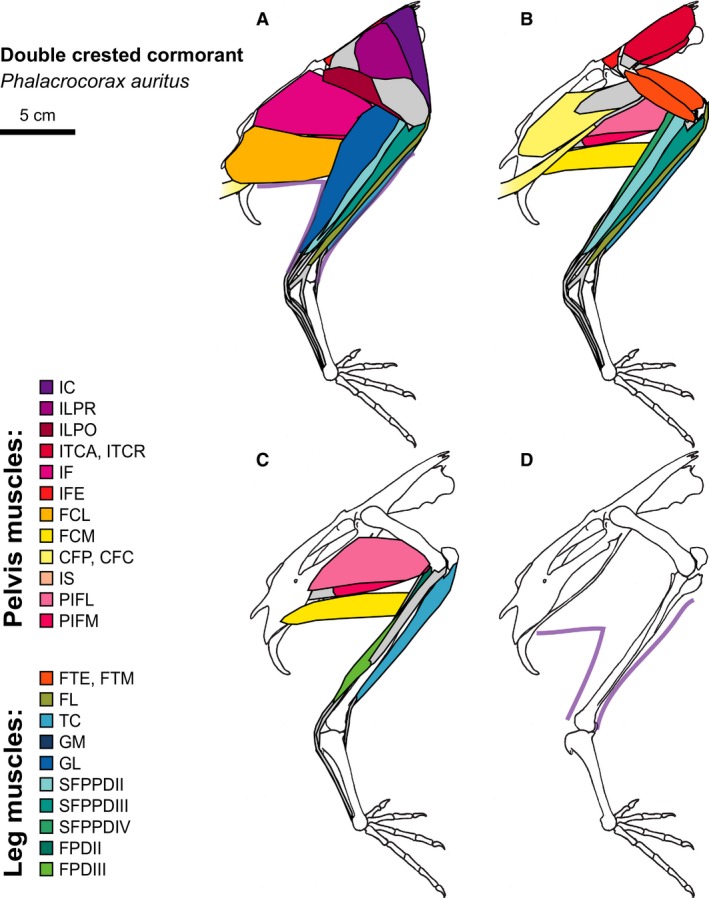
Double‐crested Cormorant hindlimb musculature. Major hindlimb muscles drawn based on dissections of several specimens. Panels show the hindlimb with (A) all muscles and outline of limb skin (in purple), (B) superficial muscles removed, (C) superficial and intermediate muscles removed, and (D) skeleton only and outline of limb skin (in purple). Skeleton drawing based on MCZ 337606. Muscle abbreviations listed in Table 1.
Figure 8.
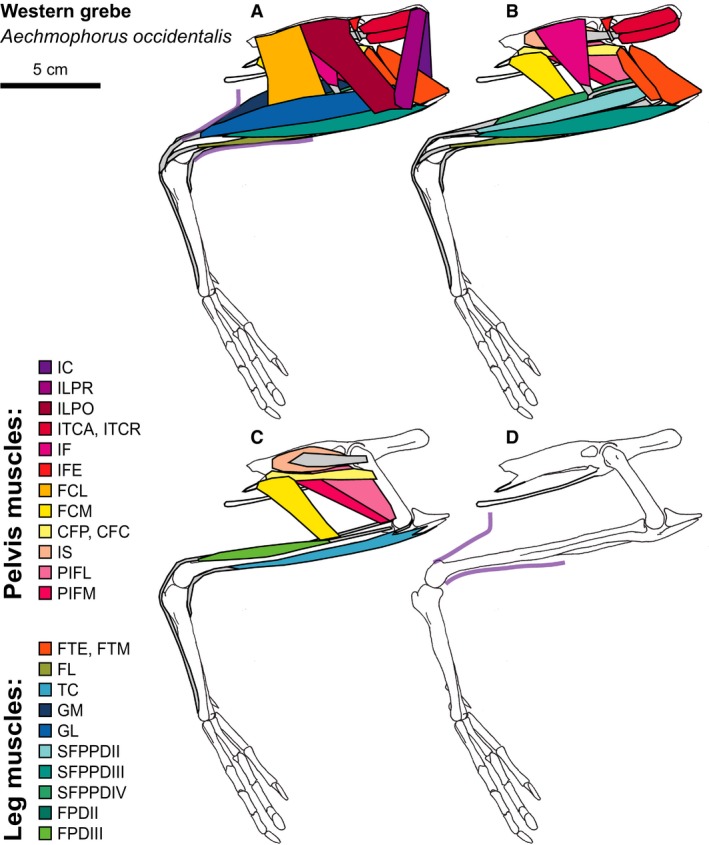
Western Grebe hindlimb musculature. Major hindlimb muscles drawn based on dissections of several specimens. Panels show the hindlimb with (A) all muscles and outline of limb skin (in purple), (B) superficial muscles removed, (C) superficial and intermediate muscles removed, and (D) skeleton only and outline of limb skin (in purple). Skeleton drawing based on MCZ 342951. Muscle abbreviations listed in Table 1.
Figure 9.
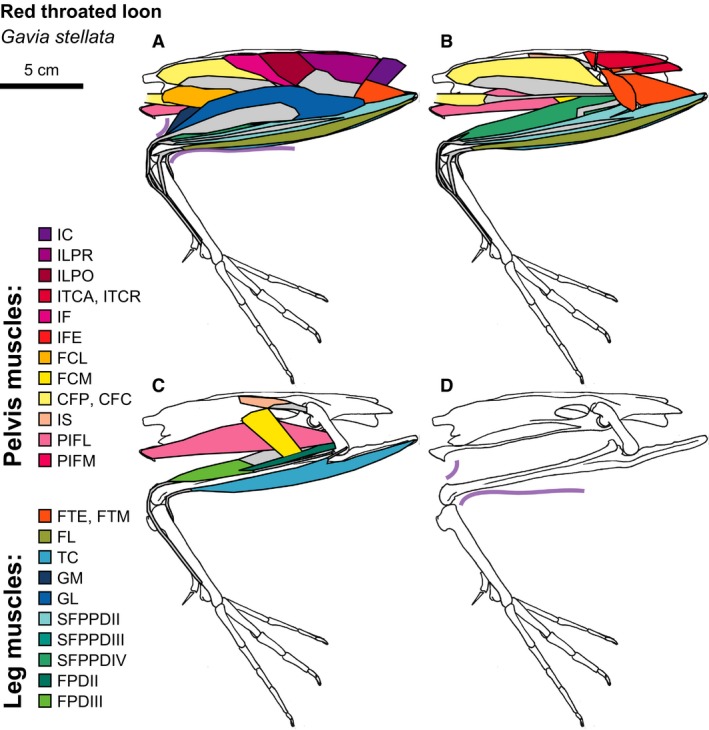
Red‐throated Loon hindlimb musculature. Major hindlimb muscles drawn based on dissections of several specimens. Panels show the hindlimb with (A) all muscles and outline of limb skin (in purple), (B) superficial muscles removed, (C) superficial and intermediate muscles removed, and (D) skeleton only and outline of limb skin (in purple). Skeleton drawing based on MCZ 337590. Muscle abbreviations listed in Table 1.
Figure 10.
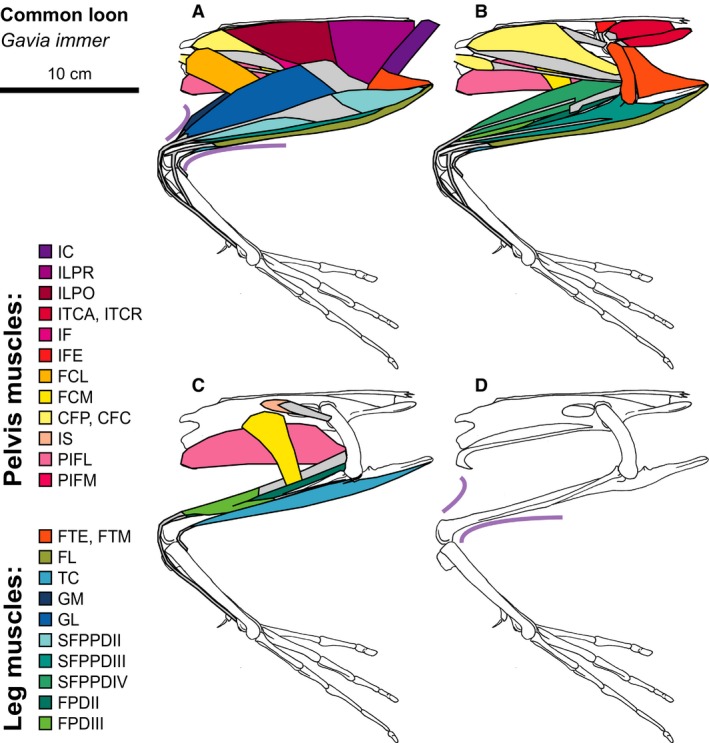
Common Loon hindlimb musculature. Major hindlimb muscles drawn based on dissections of several specimens. Panels show the hindlimb with (A) all muscles and outline of limb skin (in purple), (B) superficial muscles removed, (C) superficial and intermediate muscles removed, and (D) skeleton only and outline of limb skin (in purple). Skeleton drawing based on MCZ 347914. Muscle abbreviations listed in Table 1.
The tucked position of the proximal hindlimb in diving birds beneath the skin over the body relies on a convergent pelvis structure and increases hydrodynamic streamlining. Diving birds possess an enlarged antitrochanter, a flange of bone that extends above the acetabulum in all birds, which aids the positioning of the hindlimb. The antitrochanter increases femoral extension and laterally pivots the femur away from the vertebral column in highly diving birds (Hertel & Campbell, 2007). The abduction of the femur reduces hip flexion but permits integration of the proximal leg next to the wide and flat ribs. Integrating the proximal portion of the leg adjacent to the abdomen streamlines the body for swimming by creating a smooth surface that is less likely to create turbulence or incur detrimental drag force (Fish, 1993). An abducted femur also permits some diving birds to paddle their feet laterally instead of beneath (ventral to) the body as in surface swimmers (Johansson & Norberg, 2001; Ribak & Swallow, 2010; Provini et al. 2012). However, a laterally directed femur and the placement of the proximal hindlimb within the skin overlying the body prevent highly diving birds from positioning the feet forward underneath the body while on land (Fig. 2). Instead, diving birds must assume a more vertical body position while on land to prevent toppling forward, making walking precarious and less efficient (Abourachid, 2000). Highly diving birds that incorporate the proximal hindlimb into the abdominal skin streamline the body for swimming but face a trade‐off with stability on land.
Incorporating the proximal hindlimb inside of the body skin also facilitates large shifts in muscle attachments on the femur and tibiotarsus in highly diving birds. Most hindlimb muscles demonstrate some anatomical variation across the species studied (Supporting Information Tables S1 and S2). These results represent findings observed during our dissections, some of which confirm historical accounts, while others represent new data. Here we present a subset of the muscles dissected to highlight the most dramatic variation and discuss functional implications for swimming performance.
Pelvic musculature
Iliotibialis lateralis pars preacetabularis (ILPR)
The large superficial pelvic muscles covering the thigh in most birds are the iliotibialis lateralis pars preacetabularis (ILPR) and pars postacetabularis (ILPO). The preacetabular portion (ILPR) typically originates via an aponeurosis from the preacetabular iliac crest and inserts on a large aponeurosis that connects to the postacetabular portion of the muscle (ILPO) and the deep femorotibialis externus (FTE) muscle. This insertion aponeurosis then inserts on the patellar tendon, patella, and cnemial crest of the tibiotarsus. The ILPR acts to flex the hip and extend the knee.
Conserved across most species analyzed in this study, the ILPR originates on the caudal half of the preacetabular iliac crest. However, the ILPR of the Western grebe is completely fleshy and originates at the most cranial portion of the preacetabular iliac crest (Beddard, 1896). In grebes, the strap‐like muscle partially covers the iliotibialis cranialis (IC) muscle, with little connection to the ILPO. While other divers, cormorants and loons, do not mirror this trend, their ILPR and ILPO are comparatively well developed.
Iliotrochantericus cranialis (ITCR)
Three iliotrochantericus muscles lie deep to the ILPR along the preacetabular iliac fossa and rim, inserting onto the femoral trochanter and proximal shaft. The most superficial muscle of this group, and typically the largest, is the iliotrochantericus caudalis (ITCA) originating from the iliac fossa. The iliotrochantericus cranialis (ITCR) originates from the ventral rim of the preacetabular ilium, showing variation in the length and location of its attachment across species. The iliotrochantericus medicus (ITM) is the smallest of the muscles, originating from the caudal portion of the preacetabular ilium ventral rim. All three iliotrochantericus muscles act to abduct the hip and medially rotate the femur, causing abduction of the distal limb (Gatesy, 1994). In bipedal running birds, these muscles are active during stance phase to stabilize the hip and prevent pelvic roll (Gatesy, 1999).
While in most bird species the ITCR is much smaller than the ITCA (George & Berger, 1966), in grebes and loons the ITCR is very well‐developed, approximately equal in size to the ITCA. This convergent pattern may partially result from pelvic shape. Diving birds possess a particularly long and narrow pelvis, with a thin iliac fossa but long iliac ventral rim (Johnsgard, 1987; Hinić‐Frlog & Motani, 2010). Yet, the relatively large size of the ITCR may also serve a functional role. To dive underwater grebes and loons must generate force both to power forward propulsion and oppose buoyancy. To oppose buoyancy when not descending exactly vertically, diving birds must produce a component of force directed ventrally. Yet, grebes and loons swim by paddling their feet lateral to the body, with the femur at a large splay angle (Hertel & Campbell, 2007). With the legs in this orientation, a ventrally directed force will act on the feet to adduct the limbs. If the ITCR is active during the force‐producing power stroke, analogous to stance phase in running birds (Gatesy, 1999), the ITCR would oppose limb adduction to stabilize the hip joint. Hip stabilization in diving birds is further aided through a large antitrochanter that braces against hip abduction (Hertel & Campbell, 2007). A relatively large ITCR may serve as one of several anatomical features that reduce hip flexion‐extension mobility to streamline the body.
Iliofibularis (IF)
The iliofibularis (IF) is a large superficial, triangular muscle arising from the postacetabular ilium. Distally, the IF forms a thick tendon, which passes through a tendinous loop attached to the distal femur, the ansa fibularis or IF loop, then travels distally along the tibiotarsus to insert on the fibular tubercle. The IF acts as a knee flexor and hip extensor.
In Guineafowl, surface swimmers, and cormorants, the IF inserts onto the tibiotarsus at one‐third its length distal to the knee joint. However, in highly diving birds, the insertion shifts in grebes and loons to approximately halfway down the tibiotarsal shaft. The relatively longer moment arm of the IF increases its ability to produce a flexion moment at the knee, which may aid efficient paddling. The power stroke of paddling birds primarily involves ankle extension, moving the foot from an acutely dorsiflexed position to plantarflexion (Johansson & Norberg, 2001; Carr, 2008; Provini et al. 2012). During this motion, the reaction pressure acting on the foot acts in front of the knee creating an extensor moment at the knee. If unopposed, these knee extension moments would extend the knee, driving the feet away from the body and resulting in increased drag that would resist forward motion of the body (Fish, 1993; Pennycuick et al. 1996). The flexion moments generated by the IF counteract extension at the knee joint. Therefore, shifting the insertion of the IF distally may allow highly diving birds to effectively oppose large knee extensor moments produced by strong paddling forces.
Flexor cruris lateralis (FCL)
The flexor cruris lateralis (FCL) muscle lies caudal to the IF, originating on the caudal portion of the ilium, and in some cases, ischium. Many species possess two FCL muscles: the main muscle belly, called the flexor cruris lateralis pars pelvica (FCLP), and the accessory head, the flexor cruris lateralis pars accessoria (FCLA). The FCLP inserts onto the proximal, posterior tibiotarsus shaft with connections to the flexor cruris medialis (FCM), which insert directly deep to the FCL. Near the distal end of the FCLP is a ligamentous raphe, or seam, serving as the origin of the FCLA and inserting onto the tendon of the gastrocnemius intermedia (GI). The FCLA fibers insert onto the distal, posterior femoral shaft. Through this three‐muscle linkage, the FCL acts in hip extension, knee flexion, and ankle extensor (Ellerby & Marsh, 2010).
The insertion of the FCLP shifts distally along the tibiotarsus with swimming ability. In non‐swimming Guineafowl, the FCLP inserts at the most proximal portion of the posterior tibiotarsal shaft. In surface swimmers, the insertion centers around one‐fifth or one‐fourth of the tibiotarsal shaft length distal to the knee. In highly diving birds, the FCLP inserts one‐third or halfway down the tibiotarsal shaft. A more distal FCLP insertion enhances the capacity of the muscle to produce an extension moment at the hip and flexion moment at the knee, similar to the IF as discussed above. This distal shift could be facilitated by the elongation of the postacetabular ilium in diving birds (Johnsgard, 1987; Hinić‐Frlog & Motani, 2010), resulting in a caudal shift of the FCLP origin relative to the hip. Taken to an extreme in loons, the FCLP primarily originates from the most caudal flange of the ischium (Figs 8 and 9). The evolutionary extension of the postacetabular pelvis in diving birds shifts the whole FCLP muscle distally along the tibiotarsus, increasing the FCLP moment arm around the hip and knee.
The FCLA is present broadly across the avian phylogeny, in paleognathae, Galliformes, and nearly all passerines (George & Berger, 1966). However, the accessory head has been reported absent in almost all swimming birds, except for coots (Fulica), as well as in trogons and some woodpeckers (George & Berger, 1966). In Galliformes, the FCLA and FCLP are coactive in late stance during running, especially at an upward incline, suggesting that the FCLA primarily functions to enhance hip extension for fast movements (Ellerby et al. 2005; Rubenson et al. 2006; Ellerby & Marsh, 2010). We confirm that none of the seven foot‐propelled swimming species dissected in this study possess the FCLA. This repeated evolutionary loss of the FCLA in swimming birds may result from reduced movement at the hip during swimming (see below).
Without the accessory head in swimming birds, the FCLP acts as a hip extensor and knee flexor. It is active during Mallard surface swimming from slightly before the power stroke until midway through the recovery stroke (Carr, 2008). While walking, the FCL shortens during activation; however, during swimming it contracts almost isometrically, suggesting it acts to prevent motion at the hip and knee throughout the power stroke. Reducing hip motion and maintaining a flexed knee throughout swimming preserves a relatively streamlined body shape (Fish, 1993; Pennycuick et al. 1996). Despite this important function, the FCLP is relatively small in highly diving birds. The reduced size of the muscle could result from increased FCLP hip and knee moment arms in diving birds, which permit a reduction in muscle strength while maintaining the same joint moment capacity. Although experimental evidence does not exist for FCLP function in diving birds, it most likely serves a similar role as in Mallards (Carr, 2008): resisting movement of the hip and knee throughout paddling and especially during the power stroke.
Limb musculature
Femorotibialis medius (FTM)
Three femorotibialis muscles comprise the deep thigh musculature. The femorotibialis medius (FTM) originates along the anterior surface of the femur and inserts on the patella and patellar tendon. The FTM fuses with the femorotibialis externus (FTE) along the lateral surface of the femur, splitting only at the proximal origin. The FTM acts as a knee extensor.
The FTM is typically the largest femorotibialis muscle, but reaches an extreme size in highly diving birds. Grebes and loons possess an exceptionally elongate cnemial crest, with a similarly extended patella in grebes (Beddard, 1896; Shufeldt, 1904) and a completely fused patella in loons (Shufeldt, 1913; Wilcox, 1952). Cormorants possess a somewhat expanded, bulky patella (Shufeldt, 1913; Owre, 1967) but its size is not as large as the knee structures in grebes and loons. In these highly diving birds, the FTM muscle attaches to the entire posterior surface of the patella (grebes) or cnemial crest (loons), without contributing to a patellar tendon. With both an expanded size and increased moment arm, the FTM can produce comparatively large knee extension moments in diving birds. While the activation pattern of the FTM has never been studied, the reduced flexion‐extension motion at the knee during swimming (Johnsgard, 1987) suggests that the FTM acts to reduce knee motion. The FTM may help oppose strong knee flexion moments produced by the IF and FCL, or could prevent knee flexion as the foot experiences backward drag during the recovery stroke or during steering maneuvers.
Gastrocnemius (GM, GI, GL)
The largest limb muscle is the gastrocnemius, consisting of three heads that form the common calcaneal (or Achilles) tendon. The medial head (GM) covers most of the internal shank, originating on the medial patella, cnemial crest, and proximal tibiotarsus. The lateral head (GL) originates from the posterolateral femoral shaft. The intermediate head (GI) is much smaller than the GM or GL, originating from the distal posterior femur with additional fibers originating from the proximal posterior tibia in cormorants, grebes, and loons. Guineafowl have a common gastrocnemius aponeurosis on which all three heads insert. In all other species examined here, the GI tendon combines with the GM tendon, which then combines with the LG tendon to form the calcaneal tendon and insert on the tibial cartilage overlying the hypotarsus. All three heads act as ankle plantarflexors. The GI and GL also act to flex the knee.
All three gastrocnemius heads are relatively large in diving birds. In cormorants, grebes, and loons, the GI is unusually well‐developed. In grebes and loons, the expanded GM origin extends from the tip of the medial cnemial crest to partway down the tibiotarsus shaft. In loons, the GL is especially enlarged with an origin extending across two‐thirds of the femur. With the increased size of the gastrocnemius muscles, diving birds can produce stronger plantarflexion moments to power paddling.
Size variation among the three gastrocnemius heads in diving birds may demonstrate the importance of GI and GL biarticular actions. The GL and GM heads of a Mallard produce approximately equal stress (force normalized for muscle area) while walking but not during surface swimming, which favors the GL (Biewener & Corning, 2001). This pattern may simply represent unequal recruitment under lower‐force swimming conditions (Biewener & Corning, 2001). But, since the GL acts at the knee while the GM only acts at the ankle, the unequal contributions of the two muscles during swimming could also demonstrate a preference for concurrent knee flexion with ankle plantarflexion. Two other anatomical patterns support this theory. First, the GI, which also acts as both ankle plantarflexor and knee flexor, is enlarged in diving birds. Secondly, the more distal insertions of the IF and FCL increase their capacity to produce knee flexion moments in divers, supporting the importance of knee flexion in swimming. Enlarged GI and GL heads could represent a demand for knee stabilization throughout ankle plantarflexion motion of the power stroke.
The effect of the enlarged and more powerful gastrocnemius in diving birds is augmented through an increased moment arm of the calcaneal tendon. In grebes, loons, and especially cormorants, the hypotarsus (the proximal posterior portion of the tarsometatarsus) expands posteriorly away from the ankle center of rotation (Owre, 1967; Mayr, 2016). A protruding hypotarsus increases the calcaneal tendon moment arm around the ankle joint, further enhancing the capacity of diving birds to propel their feet backward and power underwater swimming.
Digital flexor muscles (SFPPDII, SFPPDIII, SFPPDIV, FPDII, FPDIII)
Deep to the gastrocnemius, six muscles travel along the shank to insert on the plantar surface of the phalanges of the toes. The most superficial muscle on the lateral shank is the (superficialis) flexor perforans et perforatus digiti II (SFPPDII). The (superficialis) flexor perforans et perforatus digiti III (SFPPDIII) lies deep and lateral to SFPPDII. The (superficialis) flexor perforans et perforatus digiti IV (SFPPDIV) lies deep and medial to the SFPPDII, traveling along the posterior surface of the shank. Deep to these muscles, the flexor perforatus digiti III (FPDIII) muscle originates from two tendons, forming two muscle heads at one‐third to one‐half of the way down the tibiotarsus shank. The two heads fuse along the distal tibiotarsus, forming a single insertion tendon. The flexor perforatus digiti II (FPDII) also has two heads, one sometimes having a tendinous origin from the femur and the other from the common digital flexor tendon. The heads fuse proximally along the tibiotarsus and travel in between the FPDIII heads. The insertion tendon of each digital flexor muscle crosses the posterior ankle (see Garrod, 1875; Hudson, 1937; Mayr, 2016 for tendon‐hypotarsus anatomy), travels through a sheath along the posterior tarsometatarsus, and inserts onto the phalanges of the digits. These muscles act mostly as digital flexors and ankle plantarflexors, with the SFPPDIV, FPDII, and FPDIII also acting as knee flexors.
Based on our comparative analysis, we propose changing the name of the digit IV flexor and adding superficialis to the name of three digital flexors. The currently accepted nomenclature of the digital flexor muscles derives from the superficial tendons perforating the deeper tendons after crossing the MTP joint. However, digit IV only has one digital flexor muscle, automatically classifying it as a perforatus muscle and not as a perforans et perforatus muscle. Yet, the ultimate insertion of the digit IV flexor muscle onto the fourth phalanx suggests a homology with the perforans et perforatus muscles for digits II and III. To emphasize the grouping of these muscles, we propose adding superficialis to their names and identifying the digit IV flexor as the (superficialis) flexor perforans et perforatus digiti IV (SFPPDIV).
All superficial digital flexors are relatively enlarged in diving birds, particularly in loons. The relatively large size of the superficial digital flexor muscles in divers supports the importance of their role in powering underwater paddling. The bulky patella in cormorants and elongated cnemial crest in grebes and loons expand the origin for the SFPPDIII. In loons, the second head of the SFPPDII extends proximally along the anterolateral cnemial crest. Loons also possess an exceptionally large SFPPDIV, originating from half of the femur. The relatively large SFPPDIV origin could either result from the muscle being particularly well‐developed or from a disproportionately shortened femur (Raikow, 1985; Hinić‐Frlog & Motani, 2010). Femur size is likely not the only factor, since grebes also possess short femurs but do not have a similar SFPPDIV origin expansion (Hinić‐Frlog & Motani, 2010). Therefore, it is likely that the SFPPDIV origin represents an expansion particular to loons and may serve to increase the flexor moment arm at the knee. In diving birds, large digital flexors produce stronger MTP and ankle flexion forces, and help to maintain foot shape, to power effective swimming.
Unlike their closest relatives, Western Grebes exhibit tendon calcification in many distal limb muscles. Some extent of hindlimb tendon calcification is present in almost all lineages of birds (Hudson, 1937; Hutchinson, 2002; Landis & Silver, 2002). However, the closest relatives to grebes, flamingos and shorebirds, lack multiple, if any, calcified tendons (Weldon, 1883; Vanden Berge, 1970; Bennett, 1996). Past studies have either identified only two calcified tendons in grebes (Vanden Berge & Storer, 1995) or generally remarked on the presence of calcification without specifying which tendons (Bennett, 1996). Here, we present the first known record of extensive calcification in grebe tendons (Beddard, 1896; Hudson, 1937; George & Berger, 1966). In Western Grebes, the insertion tendon of every superficial digital flexor is calcified, along with the origin tendon of the FPDIII medial head.
The unusual calcification in the digital flexor tendons of Western Grebes may represent an adaptation to high forces experienced during a seasonal breeding display performed by males and females. Previous work on calcified tendons, primarily using the Turkey gastrocnemius, suggests that tendons calcify in response to strong tensile forces to prevent rupture of tendon cells (Landis & Silver, 2002). However, theoretical peak swimming force estimates for cormorants (15–20 N or 0.70–0.93 bodyweight (BW); Ribak et al. 2004), the closest available proxy for grebes, do not reach peak running forces in birds with calcified tendons (60–75 N or 1.4–2.1 BW in turkeys; Roberts et al. 1998; Birn‐Jeffrey et al. 2014). Instead, the need for tendon calcification in grebes could stem from their participation in a physically demanding breeding display involving water surface running, called rushing. During rushing, grebes completely support their bodyweight through slapping the water surface at up to 20 steps per second and with no help from flapping wings (Clifton et al. 2015). Experimental measurements of slap impulses using taxidermy grebe feet suggest that each foot may experience more than 50 N of force (6.4 BW) while slapping the water surface (an impulse of 0.25 N/s over 0.005 s, Clifton et al. 2015). These values more closely resemble running forces, suggesting that grebe calcified tendons may help mitigate high loading of the thin digital flexor tendons during rushing.
Conclusions
In this study we highlight anatomical trends related to foot‐based swimming by directly comparing the hindlimb myology of seven species of swimming birds. Our data are drawn from historical records as well as new, detailed dissections of eight bird species. We provide a convenient key that summarizes discrepancies in muscle naming from several historical texts to help promote future comparisons across these works.
Birds that excel at swimming using their feet exhibit several anatomical trends conserved across convergent evolutionary lineages. First, highly diving birds tuck the proximal portion of the hindlimb inside the abdominal wall skin, creating a streamlined body shape but reducing mobility on land. Second, the shifted insertions and therefore increased moment arms of several pelvic muscles (including the ITCR, IF, and FCL) likely help stabilize the hip and knee joints while paddling. Third, as a result of increased moment arms and less mobile hip and knee joints, many pelvic muscles are relatively reduced in divers. Lastly, highly diving birds possess especially enlarged knee structures (the patella, tibiotarsal cnemial crest, or both) increasing attachment areas for the FTM, gastrocnemius heads, and digital flexor muscles. The expanded size of these muscles demonstrates the importance of knee stabilization and strong ankle plantarflexion while paddling. Surface swimmers across a range of body sizes display intermediate stages of these trends. These conserved anatomical patterns suggest that phylogenetically divergent lineages of birds have evolved similar hindlimb muscle features to accommodate the physical demands of foot‐based swimming.
Author contributions
Conceived and designed the study: G.T.C., J.A.C., A.A.B. Performed the dissections: G.T.C., J.A.C. Analyzed the data and drafted the manuscript: G.T.C. Critical revision of the manuscript: J.A.C., A.A.B.
Supporting information
Table S1. Pelvic musculature origins and insertions. This table shows the origin and insertion of major pelvic muscles dissected in the helmeted Guineafowl (GF), Mallard (MA), Canada Goose (CG), Mute Swan (MS), Double‐crested Cormorant (DCC), Western grebe (WG), Red‐throated Loon (RTL), and Common Loon (CL). Attachment points are fleshy unless otherwise noted. GF do not swim. MA, CG, and MS are surface‐swimmers of increasing body mass. DCC, WG, RTL, and CL are divers, with the DCC representing an intermediate specialization, since it retains the ability to walk. A key for muscle abbreviations can be found in Tables 1 and 2.
Table S2. Limb musculature origins and insertions. This table shows the origin and insertion of major pelvic muscles dissected in the helmeted Guineafowl (GF), Mallard (MA), Canada Goose (CG), Mute Swan (MS), Double‐crested Cormorant (DCC), Western grebe (WG), Red‐throated Loon (RTL), and Common Loon (CL). Attachment points are fleshy unless otherwise noted. GF do not swim. MA, CG, and MS are surface‐swimmers of increasing body mass. DCC, WG, RTL, and CL are divers, with the DCC representing an intermediate specialization, since it retains the ability to walk. A key for muscle abbreviations can be found in Tables 1 and 2.
Acknowledgements
The authors would like to thank several people who helped us acquire bird carcasses: Mark Pokras, DVM, and Florina S. Tseng, DVM, at the Tufts Wildlife Clinic; Greg Mertz, DVM, and Robert Adamski, DVM, at the NE Wildlife Center; and the late Sharnelle Fee at the Wildlife Center of the North Coast. We would also like to the Jeremiah Trimble and the Ornithology Collections at the Harvard Museum of Natural History for access to specimen for drawing the hindlimb skeletons. Lastly, we are grateful to Dr. John Hutchinson, Dr. George Lauder, and Dr. Stephanie Pierce for feedback on the manuscript, in addition to two anonymous reviewers.
References
- Abourachid A (2000) Bipedal locomotion in birds: the importance of functional parameters in terrestrial adaptation in Anatidae. Can J Zool 78, 1994–1998. [Google Scholar]
- Abourachid A (2001) Kinematic parameters of terrestrial locomotion in cursorial (ratites), swimming (ducks), and striding birds (quail and guinea fowl). Comp Biochem Physiol A Mol Integr Physiol 131, 113–119. [DOI] [PubMed] [Google Scholar]
- Baumel J, Witmer LM (1993) Handbook of Avian Anatomy: Nomina Anatomica Avium. Cambridge: Nuttall Ornithological Club. [Google Scholar]
- Beddard FE (1896) On the anatomy of a Grebe (Aechmophorus major), with remarks upon the classification of some of the Schizognathous Birds. Proc Zool Soc London 64, 538–547. [Google Scholar]
- Bennett M (1996) Allometry of the leg muscles of birds. J Zool 238, 435–443. [Google Scholar]
- Biewener AA (2003) Muscles and skeletons : the building blocks of animal movement In: Animal Locomotion. pp 15–45, New York, NY:Oxford University Press. [Google Scholar]
- Biewener AA, Corning WR (2001) Dynamics of Mallard (Anas platyrhynchos) gastrocnemius function during swimming versus terrestrial locomotion. J Exp Biol 204, 1745–1756. [DOI] [PubMed] [Google Scholar]
- Birn‐Jeffrey AV, Hubicki CM, Blum Y, et al. (2014) Don't break a leg: running birds from quail to ostrich prioritize leg safety and economy on uneven terrain. J Exp Biol 217, 3786–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PJ (2004) Metabolic regulation in diving birds and mammals. Respir Physiol Neurobiol 141, 297–315. [DOI] [PubMed] [Google Scholar]
- Carr JA (2008) Muscle Function During Swimming and Running in Aquatic, Semi‐Aquatic and Cursorial Birds. Biology Dissertations. Paper 4. http://hdl.handle.net/2047/d10016144 [Google Scholar]
- Clifton GT, Hedrick TL, Biewener AA (2015) Western and Clark's Grebes use novel strategies for running on water. J Exp Biol 218, 1235–1243. [DOI] [PubMed] [Google Scholar]
- Doube M, Yen SCW, Kłosowski MM, et al. (2012) Whole‐bone scaling of the avian pelvic limb. J Anat 221, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerby DJ, Marsh RL (2010) The mechanical function of linked muscles in the Guinea Fowl hind limb. J Exp Biol 213, 2201–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerby DJ, Henry HT, Carr JA, et al. (2005) Blood flow in Guinea Fowl Numida meleagris as an indicator of energy expenditure by individual muscles during walking and running. J Physiol 564, 631–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish FE (1993) Influence of hydrodynamic design and propulsive mode on mammalian swimming energetics. Aust J Zool 42, 79–101. [Google Scholar]
- Flück M (2006) Functional, structural and molecular plasticity of mammalian skeletal muscle in response to exercise stimuli. J Exp Biol 209, 2239–48. http://jeb.biologists.org.ezp-prod1.hul.harvard.edu/content/209/12/2239 (Accessed June 28, 2017). [DOI] [PubMed] [Google Scholar]
- Forbes WA (1885) The Collected Scientific Papers of the Late William Alexander Forbes. London: Taylor and Francis. [Google Scholar]
- Gadow H, Selenka E (1891) Aves In: Dr. H.G. Bronn's Klassen und Ordnungen des Thier‐Reiches, in Wort und Bild. C. F. Winter'sche: Leipzig and Heidelberg. [Google Scholar]
- Garrod AH (1873) On certain muscles of birds and their value in classification. Part I. Proc Zool Soc London 41, 626–644. [Google Scholar]
- Garrod AH (1874) On certain muscles of birds and their value in classification. Part II. Proc Zool Soc London 42, 111–123. [Google Scholar]
- Garrod AH (1875) On the disposition of the deep plantar tendons in different birds. Proc Zool Soc London 43, 339–348. [Google Scholar]
- Garrod AH, Darwin F (1872) Notes on an ostrich lately living in the Society's collection. Proc Zool Soc London 40, 356–363. [Google Scholar]
- Gatesy SM (1994) Neuromuscular diversity in archosaur deep dorsal thigh muscles. Brain Behav Evol 43, 1–14. [DOI] [PubMed] [Google Scholar]
- Gatesy SM (1999) Guineafowl hind limb function. II: Electromyographic analysis and motor pattern evolution. J Morphol 240, 127–142. [DOI] [PubMed] [Google Scholar]
- George JC, Berger AJ (1966) Avian Myology. New York: Academic Press. [Google Scholar]
- Haughton S (1865) Notes on animal mechanics. III: On the muscular mechanism of the leg of the ostrich. Proc R Irish Acad 9, 50–61. [Google Scholar]
- Hertel F, Campbell KE Jr (2007) The antitrochanter of birds: form and function in balance. Auk 124, 789–805. [Google Scholar]
- Hinić‐Frlog S, Motani R (2010) Relationship between osteology and aquatic locomotion in birds: determining modes of locomotion in extinct Ornithurae. J Evol Biol 23, 372–385. [DOI] [PubMed] [Google Scholar]
- Hudson G (1937) Studies on the muscles of the pelvic appendage in birds. Am Midl Nat 18, 1–108. [Google Scholar]
- Hudson G, Lanzillotti P, Edwards G (1959) Muscles of the pelvic limb in galliform birds. Am Midl Nat 61, 1–67. [Google Scholar]
- Hutchinson JR (2002) The evolution of hindlimb tendons and muscles on the line to crown‐group birds. Comp Biochem Physiol Part A Mol Integr Physiol 133, 1051–1086. [DOI] [PubMed] [Google Scholar]
- Johansson L, Norberg UM (2001) Lift‐based paddling in diving Grebe. J Exp Biol 204, 1687–1696. [DOI] [PubMed] [Google Scholar]
- Johnsgard PA (1987) Diving Birds of North America. Lincoln: University of Nebraska Press. [Google Scholar]
- Kardong K (2015) Vertebrates: Comparative Anatomy, Function, Evolution, 7th edn New York: McGraw‐Hill Education. [Google Scholar]
- Landis WJ, Silver FH (2002) The structure and function of normally mineralizing avian tendons. Comp Biochem Physiol A Mol Integr Physiol 133, 1135–1157. [DOI] [PubMed] [Google Scholar]
- Lieber R, Fridén J (2001) Clinical significance of skeletal muscle architecture. Clin Orthop Relat Res 383, 140–151. [DOI] [PubMed] [Google Scholar]
- Marsh RL (1999) How muscles deal with real‐world loads: the influence of length trajectory on muscle performance. J Exp Biol 202, 3377–3385. http://jeb.biologists.org/content/202/23/3377.short (accessed 16 July 2017). [DOI] [PubMed] [Google Scholar]
- Mayr G (2016) Variations in the hypotarsus morphology of birds and their evolutionary significance. Acta Zool 97, 196–210. [Google Scholar]
- McMahon TA (1984) Muscles, Reflexes, and Locomotion. Princeton, NJ: Princeton University Press. [Google Scholar]
- Owre OT (1967) Locomoton and feeding in the Anhinga and the Double‐crested Cormorant. Ornithol Monogr 6, 1–138. [Google Scholar]
- Pennycuick CJ (1991) Adapting skeletal muscles to be efficient In: Efficiency, Economy and Related Concepts in Comparative Animal Physiology. (ed.Blake RW.). pp 33–42. New York: Cambridge University Press. [Google Scholar]
- Pennycuick CJ, Klaassen M, Kvist A, et al. (1996) Wingbeat frequency and the body drag anomaly: wind‐tunnel observations on a Thrush Nightingale (Luscinia luscinia) and a Teal (Anas crecca). J Exp Biol 199, 2757–2765. [DOI] [PubMed] [Google Scholar]
- Provini P, Goupil P, Hugel V, et al. (2012) Walking, paddling, waddling: 3D kinematics anatidae locomotion (Callonetta leucophrys). J Exp Zool 317, 275–282. [DOI] [PubMed] [Google Scholar]
- Prum RO, Berv JS, Dornburg A, et al. (2015) A comprehensive phylogeny of birds (Aves) using targeted next‐generation DNA sequencing. Nature 526, 569–573. [DOI] [PubMed] [Google Scholar]
- Raikow RJ (1973) Locomotor mechanisms in North American ducks. Wilson Bull 85, 295–307. [Google Scholar]
- Raikow RJ (1985) Locomotor system In: Form and Function in Birds, Vol. 3 (eds King A, McLelland J.), pp 57–147, London: Academic Press. [Google Scholar]
- Raikow RJ (1987) Hindlimb myology and evolution of the old world suboscine passerine birds (Acanthisittidae, Pittidae, Philepittidae, Eurylaimidae). Ornithol Monogr 41, iii–81. [Google Scholar]
- Ribak G, Swallow J (2010) Drag‐based ‘hovering’ in ducks: the hydrodynamics and energetic cost of bottom feeding. PLoS ONE 5, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribak G, Weihs D, Arad Z (2004) How do cormorants counter buoyancy during submerged swimming? J Exp Biol 207, 2101–2114. [DOI] [PubMed] [Google Scholar]
- Roberts TJ, Chen MS, Taylor CR (1998) Energetics of bipedal running II: limb design and running mechanics. J Exp Biol 201, 2753–2762. [DOI] [PubMed] [Google Scholar]
- Rodewald P. (ed.) (2015) The Birds of North America. Ithaca, NY: Cornell Laboratory of Ornithology. [Google Scholar]
- Rosser BWC, Secoy D, Riegert P (1982) The leg muscles of the American Coot (Fulica americana Gmelin). Can J Zool 60, 1236–1256. [Google Scholar]
- Rubenson J, Henry HT, Dimoulas PM, et al. (2006) The cost of running uphill: linking organismal and muscle energy use in Guinea Fowl (Numida meleagris). J Exp Biol 209, 2395–2408. [DOI] [PubMed] [Google Scholar]
- Schorger A (1947) The deep diving of the Loon and Old‐Squaw and its mechanism. Wilson Bull 59, 151–159. [Google Scholar]
- Shufeldt RW (1890) The Myology of the Raven (Corvus corax sinuatus): A Guide to the Study of the Muscular System in Birds. London: Macmillan and Company. [Google Scholar]
- Shufeldt R (1904) On the osteology and systematic position of the pygopodes. Am Nat 38, 13–49. [Google Scholar]
- Shufeldt R (1913) On the patella in the Phalacrocoracidae. Proc Zool Soc London 83, 393–402. [Google Scholar]
- Strod T, Arad Z, Izhaki I, et al. (2004) Cormorants keep their power: visual resolution in a pursuit‐diving bird under amphibious and turbid conditions. Curr Biol 14, R376–R377. [DOI] [PubMed] [Google Scholar]
- Vanden Berge JC (1970) A Comparative Study of the Appendicular Musculature of the Order Ciconiiformes. Am Midl Nat 84, 289. [Google Scholar]
- Vanden Berge JC, Storer RW (1995) Intratendinous ossification in birds: a review. J Morphol 226, 47–77. [DOI] [PubMed] [Google Scholar]
- Vanden Berge JC, Zweers G. (1993) Myologia In: Handbook of Avian Anatomy: Nomina Anatomica Avium. (eds Baumel J, King AS, Anthony S, Breazile JE, Evans HE, Berge JC Vanden.), pp. 189–247. Cambridge: Nuttall Ornithological Club. [Google Scholar]
- Vogel S (2008) Modes and scaling in aquatic locomotion. Integr Comp Biol 48, 702–712. [DOI] [PubMed] [Google Scholar]
- Weldon W (1883) On some points in the anatomy of Phoenicopterus and its allies. J Zool 51, 638–652. [Google Scholar]
- White CR, Martin GR, Butler PJ (2008) Pedestrian locomotion energetics and gait characteristics of a diving bird, the Great Cormorant, Phalacrocorax carbo . J Comp Physiol B 178, 745–754. [DOI] [PubMed] [Google Scholar]
- Wilcox HH (1952) The pelvic musculature of the Loon, Gavia immer . Am Midl Nat 48, 513–573. [Google Scholar]
- Wisdom KM, Delp SL, Kuhl E (2015) Use it or lose it: multiscale skeletal muscle adaptation to mechanical stimuli. Biomech Model Mechanobiol 14, 195–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeffer A, Johansson LC, Marmebro Å (2003) Functional correlation between habitat use and leg morphology in birds (Aves). Biol J Linn Soc 79, 461–484. [Google Scholar]
- Zinoviev AV (2011) Notes on the hindlimb myology and syndesmology of the Mesozoic toothed bird Hesperornis regalis (Aves: Hesperornithiformes). J Syst Palaeontol 9, 65–84. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Pelvic musculature origins and insertions. This table shows the origin and insertion of major pelvic muscles dissected in the helmeted Guineafowl (GF), Mallard (MA), Canada Goose (CG), Mute Swan (MS), Double‐crested Cormorant (DCC), Western grebe (WG), Red‐throated Loon (RTL), and Common Loon (CL). Attachment points are fleshy unless otherwise noted. GF do not swim. MA, CG, and MS are surface‐swimmers of increasing body mass. DCC, WG, RTL, and CL are divers, with the DCC representing an intermediate specialization, since it retains the ability to walk. A key for muscle abbreviations can be found in Tables 1 and 2.
Table S2. Limb musculature origins and insertions. This table shows the origin and insertion of major pelvic muscles dissected in the helmeted Guineafowl (GF), Mallard (MA), Canada Goose (CG), Mute Swan (MS), Double‐crested Cormorant (DCC), Western grebe (WG), Red‐throated Loon (RTL), and Common Loon (CL). Attachment points are fleshy unless otherwise noted. GF do not swim. MA, CG, and MS are surface‐swimmers of increasing body mass. DCC, WG, RTL, and CL are divers, with the DCC representing an intermediate specialization, since it retains the ability to walk. A key for muscle abbreviations can be found in Tables 1 and 2.


