Abstract
The mimic muscles are usually described as containing no muscle spindles. In the present publication the human platysma was reinvestigated concerning its content of corpuscular sensors. Serial sections through the platysma of seven donors revealed numerous muscle spindles but no Pacini corpuscules. The muscle spindles were located in the cranial two‐thirds of the platysma, and were evenly distributed with a tendency to have more spindles in the lateral part of the muscle. Immunohistochemical staining with S46 antibodies revealed a predominance of nuclear bag fibers. The results point to an extended function of the platysma as an afferent center of the lower face mimic muscles.
Keywords: distribution, immunohistochemistry, platysma, striated muscle
Introduction
Only recently, the macroscopic appearance of the human platysma muscle was reviewed using papers listed in PubMed and Scopus (Hwang et al. 2017). Variations exist in the origin and insertion, but also in the degree of interlacing muscle fibers crossing from one side to the other.
At the microscopic level, the striated muscle fibers of the platysma show some peculiarities: their mean diameter is only 50% of that of normal limb muscle fibers, increasing from medial to lateral, as do the proportion of Type I fibers (Dittert & Bardosi, 1989). The proportion between fast and slow myosin light chains could be correlated to the activity level of the muscle (Oudet et al. 1989).
The platysma is most commonly innervated by the cervical branch of the facial nerve (Hwang et al. 2017); as an exception nerve fibers may join from the cervical plexus originating from the glossopharyngeal nerve (Arakawa et al. 2008) or cervical motor nucleus (Socolovsky et al. 2008). Being part of the mimic muscles, the platysma is in general referred as being free of corpuscular muscle afferents (no data of the otherwise complete screening by Voss, 1971; Schwarting et al. 1982). Although the presence of muscle spindles in the human platysma has been mentioned once (Feinstein et al. 1955), this topic has never been studied in detail.
Therefore, the presence and characterization of muscle spindles and other corpuscular sensors in the human platysma was reinvestigated and the possible role of the platysma reconsidered.
Material and methods
Tissue preparation
Muscle specimens of the platysma muscle were collected from seven human cadavers. They were part of the donor program of the Department of Anatomy in Dresden (Germany) and had in their lifetime given a written consent to use their bodies for the purposes of science and education after death. There were three male and four female cadavers, age range 72–94 years, lacking neuro‐ or myopathies in their medical history as far as documented (see Table 1). All cadavers were fixed 2–4 days postmortem with a mixture of formalin and alcohol and remained in that solution for at least 1 year. After dissecting the epidermis and dermis and identifying the margins of the platysma, the muscle was removed with its surrounding connective tissue. It was then rolled from medial to lateral, divided into three portions, fixed with thin thread, and washed several times in phosphate‐buffered saline (PBS; pH 7.4, 0.01 M). The tissue was then processed for embedding in paraffin wax. The thread was removed just before blocking into holders. The portions were further divided in approximately 1.5‐cm‐thick sections.
Table 1.
Data of the donors and muscle spindle count of a more cranial and a more caudal transverse region of the platysma (length: 1.5 mm; whole muscle thickness)
| Age (years) | Sex | Postmortem time (days) | Side of tissue preparation | Cranial spindle count | Caudal spindle count | |
|---|---|---|---|---|---|---|
| 1 | 72 | M | 3 | Right | 7 | 0 |
| 2 | 79 | F | 4 | Left | 9 | 0 |
| 3 | 80 | F | 3 | Left | 8 | 0 |
| 4 | 81 | M | 2 | Left | 7 | 0 |
| 5 | 82 | M | 3 | Right | 6 | 2 |
| 6 | 86 | F | 2 | Right | 6 | 0 |
| 7 | 94 | F | 3 | Left | 5 | 1 |
Histology and immunohistochemistry
Serial sections (5 μm thick) of each specimen were cut, and selected sections stained with haematoxylin and eosin (H&E) to identify muscle spindles and the general morphology of the platysma.
For immunohistochemistry, consecutive sections of H&E‐stained sections containing muscle spindles at the equatorial and polar zone (regions A & B) were dewaxed, rehydrated and irradiated with microwaves in 0.01 M sodium citrate buffer (pH 6.0) for 2 × 5 min at 800 W to unmask the antigens. After washing in PBS, the sections were treated with 0.3% hydrogen peroxide for 10 min and blocked in normal mouse serum for 15 min at 37 °C followed by washing in PBS. The primary antibody S46 (specific for MyHC‐sto, monoclonal mouse IgG1, dilution 1 : 50, DSHB, University of Iowa, USA) was incubated over night at 4 °C. In humans, S46 is specific for the slow‐tonic myosin of intrafusal nuclear bag fibers and extraocular muscles (Bombardi et al. 2006; Sokoloff et al. 2007; Schiaffino & Reggiani, 2011). After washing in PBS, an appropriate biotinylated secondary antibody was added and incubated for 15 min at 37 °C, followed by washing and incubation with a Vectastain® Elite ABC mouse kit (PK 6101, PK 6102 Vector Laboratories Inc., Burlingame, CA, USA). Visualization of peroxidase activity was realised by adding 3,3‐diaminobenzidine for 8 min.
The sections were examined on a Zeiss Jenamed2 microscope (Carl Zeiss AG, Oberkochen, Germany) and images were recorded using a Digital Sight DS‐Fi1 camera (Nikon AG, Tokyo, Japan).
From one donor, the complete platysma was sectioned and the number of spindles counted. To estimate the relative abundance of the muscle spindles, several platysmata were weighted and the mean weight calculated as 30 g. The predicted number of spindles and the relative abundance of muscle spindles were calculated based on a regression analysis between muscle weight and spindle number (Banks, 2006).
From the other donors, selected serial sections (collecting period: 5‐μm‐thick sections every 50 μm) within 1.5 mm of the platysma length were stained and the number of spindles counted. The cranial transverse region was about 2–3 cm below the chin, the caudal transverse region was in the transition zone between the neck and the trunk. The number of spindles was counted in both regions.
Selected spindles were further analyzed containing their number of nuclear bag and nuclear chain fibers.
Results
The platysma could be easily identified in the subcutaneous layer (Fig. 1). It originated from the upper portion of the thorax (clavicular and infraclavicular regions) and the muscle fibers ascended towards the face. In most of the cases the fibers remained ipsilateral. Only one case showed a few muscle bundles in the upper third of the platysma interlacing to the contralateral side. The platysma inserted onto the skin of the cheek in close relation to the infero‐lateral perioral muscles.
Figure 1.
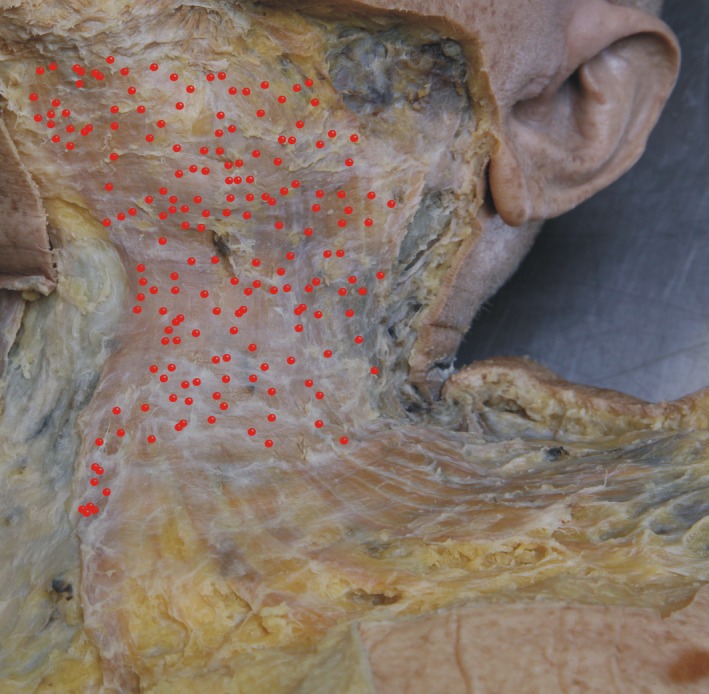
Projection of the 177 muscle spindles (red dots, representing the equatorial region of the spindles) histologically identified on a micrograph of the investigated left platysma of a 79‐year‐old donor. Note the equal distribution in the upper two‐thirds of the platysma (neck portion), whereas almost no muscle spindles were found in the lower third of the muscle (trunk portion).
Presence and distribution of muscle spindles in the platysma
In serial sections through the complete left‐sided platysma of a 79‐year‐old female donor, 177 muscle spindles could be identified. Most of the spindles were located in the cranial two‐thirds of the platysma covering the ventrolateral neck (Fig. 1). In the caudal third of the muscle only sporadic muscle spindles were identified, mainly in the medial part of the platysma. Using the mean calculated weight of 30 g for one side of the platysma, the predicted number of muscle spindles according to Banks was 108 muscle spindles and the relative abundance was 1.64.
An interindividual comparison of a more cranial and a more caudal section of the platysma in seven donors revealed a similar distribution of muscle spindles in all platysma samples (Table 1).
Absence of other corpuscular sensors than muscle spindles
Neither within the platysma muscle nor in the connective tissue around the platysma muscle were any other corpuscular sensors detected.
Pacini corpuscles, approximately 1 mm in size, should have been seen in several consecutive sections but were not present in any sample. Ruffini corpuscles, 50–150 μm in size, might have been missed due to the collecting period. In addition, no specific staining for Ruffini corpuscles was performed. However, the samples studied showed no indication of Ruffini corpuscles within or around the platysma muscle.
Characterization of muscle spindles in the platysma
The light microscopic appearance of the muscle spindles showed the main characteristics that included an inner and an outer capsule, and a neuronal and vascular supply (Fig. 2). Although the donors were all old, the spindles showed a normal appearance and only mild thickening of the capsule was noted.
Figure 2.
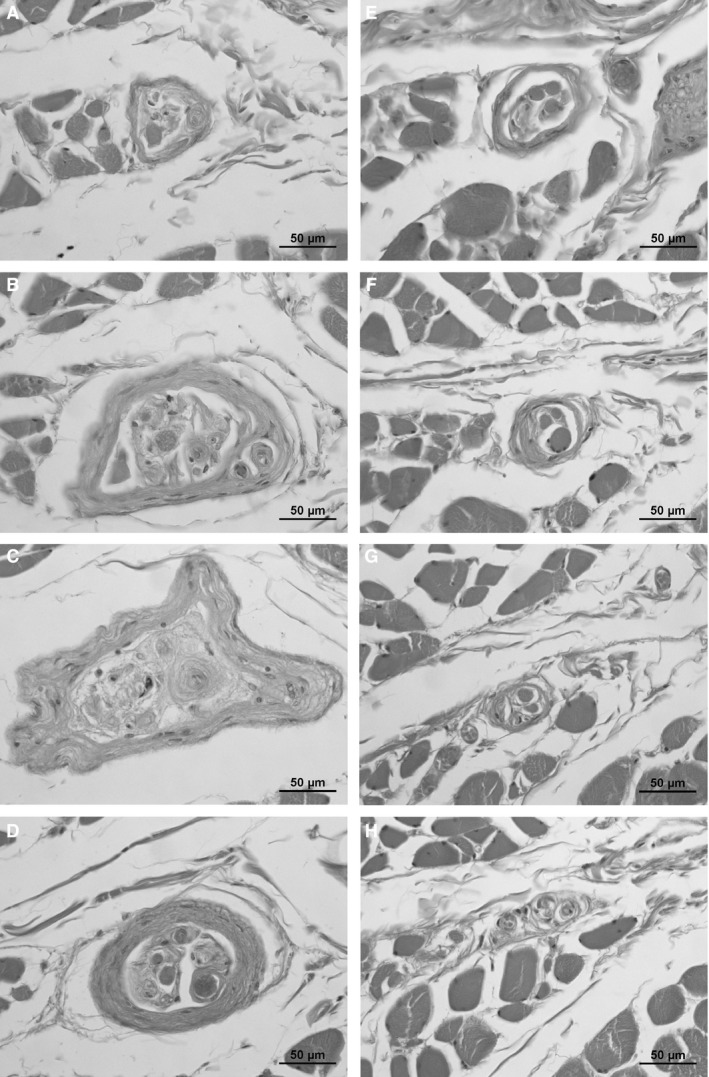
Typical muscle spindle appearance in the human platysma (one spindle, each section is 500 μm apart). Note the different appearance and fiber number in different sections.
All muscle spindles showed single encapsulations; tandem linkages as described in the literature (Cooper & Daniel, 1963) were not detected. The length of the encapsulated part of the muscle spindles varied between 1.3 and 5.4 mm (data from 66 spindles with certified margins: median 2.5 mm; mean 2.8 ± 1.3 mm); as an exception, the encapsulated part of one single muscle spindle extended 9 mm in length.
An immunohistochemical characterization of 158 intrafusal fibers of 42 (of 177) muscle spindles of a single donor revealed mainly spindles with two bag fibers (Fig. 3) but the number of nuclear chain fibers was low (Table 2). The bag fiber specific myosin marker S46 stained the contractile parts of the fibers (region B of the muscle spindle) but was absent in the central part (region A) containing the nuclei (Fig. 3).
Figure 3.
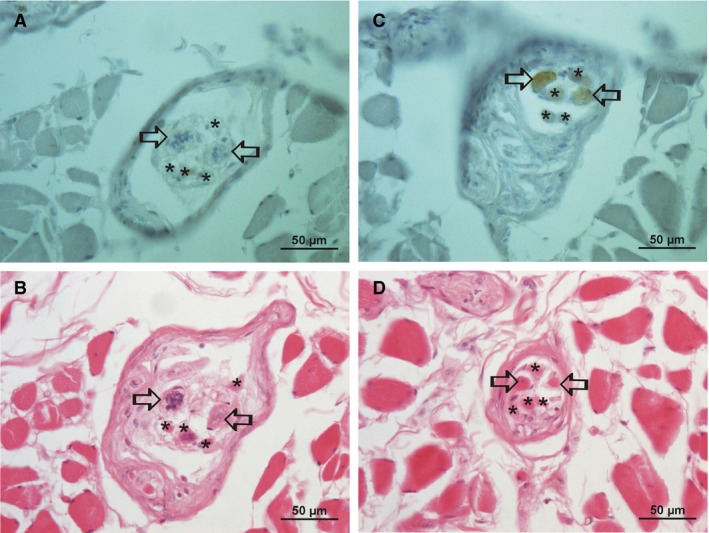
Immunohistochemical staining for S46, a marker for nuclear bag fiber myosin. (A) and (B) are in region (A); there is no specific staining in the nuclear bag fibers (open arrows) for S46 (a) due to a lack of myosin in region (A), whereas the bag‐like nuclei are clearly visible (B); nuclear chain fibers are marked with an asterisk. (C,D) Same muscle spindle in a distance of 250 μm (region B; contractile part). Note the clear staining for S46 of the two nuclear bag fibers (open arrows), whereas the four nuclear chain fibers are unstained.
Table 2.
Characterization of 42 muscle spindles (of 177) of one platysma (79‐year‐old female donor) according to their fiber content
| Total intrafusal fibers | Nuclear bag fibers | Nuclear chain fibers | Frequency | |
|---|---|---|---|---|
| 3 | 0 | 3 | 1 | |
| One‐bag spindles (n = 11) | 1 | 1 | 0 | 3 |
| 2 | 1 | 1 | 1 | |
| 3 | 1 | 2 | 5 | |
| 4 | 1 | 3 | 1 | |
| 5 | 1 | 4 | 1 | |
| Two‐bag spindles (n = 22) | 2 | 2 | 0 | 3 |
| 3 | 2 | 1 | 8 | |
| 4 | 2 | 2 | 2 | |
| 5 | 2 | 3 | 7 | |
| 6 | 2 | 4 | 1 | |
| 8 | 2 | 6 | 1 | |
| Three‐bag spindles (n = 7) | 3 | 3 | 0 | 1 |
| 4 | 3 | 1 | 2 | |
| 5 | 3 | 2 | 3 | |
| 7 | 3 | 4 | 1 | |
| 6 | 4 | 2 | 1 | |
| Ʃ | 158 | 80 | 78 | 42 |
Discussion
The platysma develops as part of the superficial musculoaponeurotic system (de la Cuadra‐Blanco et al. 2013) and is mainly described as belonging to the lower mimic muscles. In this first systematic study of muscle spindles in the platysma we show that a substantial number of muscle spindles are constantly present. Therefore, the afferent neural regulation of the platysma is different from the rest of the mimic muscles, indicating distinct functions of the platysma.
The muscle spindles are even distributed, almost entirely in the upper two‐thirds of the platysma. Sensory information about the muscle fiber length seems to be focused on the facial and cervical regions, whereas the expansion on the trunk seems of less importance. Recent clinical examinations indicate the main platysma function to be an upward movement of the neck skin rather than a downward movement of the lateral lower lip (Le Louarn, 2016), although the platysma might also influence and be influenced by the lower face mimic (de Almeida et al. 2017; Fahmi et al. 2017). Do the muscle spindles indicate additional aspects? Considering the density of muscle spindles, the platysma ranges between the mastication muscles and the hyoidal muscles (Table 3). However, since this number is from a single count, it may be too early to draw major conclusions from it. Nonetheless, the afferent sensoric information might have some relevance, although no major side effects have been described following neck rejuvenation or surgical manipulation of the platysma (Pelle‐Ceravolo et al. 2016).
Table 3.
Comparison of the platysma muscle spindle abundance with data from other related muscles (calculations from Banks, 2006)
| Muscle | Mass (g) | Actual number | Predicted number | Relative abundance |
|---|---|---|---|---|
| Temporalis | 14.73 | 217 | 91 | 2.4 |
| Pterygoideus medialis | 7.62 | 155 | 66.1 | 2.3 |
| Sternocleidomastoideus | 53.4 | 303 | 169.9 | 1.8 |
| Platysma | 30 | 177 | 108 | 1.64 |
| Sternothyroideus | 6.2 | 86 | 59.8 | 1.4 |
| Omohyoideus | 6.2 | 83 | 59.8 | 1.4 |
| Sternohyoideus | 6.1 | 41 | 59.3 | 0.69 |
| Thyrohyoideus | 2 | 12 | 34.5 | 0.35 |
| Geniohyoideus | 3.3 | 15 | 44.0 | 0.34 |
| Stylohyoideus | 1.2 | 6 | 26.9 | 0.22 |
| Digastricus | 7.2 | 7.5 | 64.3 | 0.12 |
Despite recent characterization of muscle spindles (Banks, 2015; Bewick & Banks, 2015; Thornell et al. 2015) a functional association to morphological differences is not yet established. The low number of nuclear chain fibers observed may be age‐related (Swash & Fox, 1972) and thus further investigations in younger donors are needed. Taking the preliminary counts into account, they may point to a more dynamic‐sensing role of the platysma muscle spindles.
The length of the encapsulated part of the identified muscle spindles was in the lower end of the ranges reported in the literature (Sahgal et al. 1985; Subramani et al. 1985); this may reflect a more sensitive perception of changes in platysma stretch.
No sensors other than muscle spindles were detected in our samples of the platysma. Although we can exclude the presence of Pacini corpuscles, there may be a possibility of the existence of Ruffini corpuscles, as recently described in the human zygomatic major and buccal muscles (Cobo et al. 2017). Our sections did not, however, show evidence of Ruffini corpuscles in the platysma.
At present functional considerations of the platysma muscle spindles can be only hypothesized. The influence of the lower face mimic muscles on movement of the upper neck and the concentration of muscle spindles in the upper part of the platysma might indicate that the platysma serves as a centralized sensoric region instead of containing single sensoric information of individual mimic muscles that might interfere with each other.
Author contributions
A.M. took tissue samples, performed the staining and evaluation, and prepared the manuscript. S.B. performed the staining and reviewed the manuscript. R.H.W.F. supervised and reviewed the manuscript. C.A.M. took tissue samples, supervised the staining and evaluation, and prepared the manuscript.
Acknowledgements
The late donors are especially recognized for their individual support of science.
No author has any conflicts of interest.
References
- de Almeida AR, Romiti A, Carruthers JD (2017) The facial platysma and its underappreciated role in lower face dynamics and contour. Dermatol Surg 43, 1042–1049. [DOI] [PubMed] [Google Scholar]
- Arakawa T, Terashima T, Banneheka S, et al. (2008) Nerve communication between the glossopharyngeal nerve, external carotid plexus and the superficial cervical ansa: human autopsy case. Anat Sci Int 83, 112–119. [DOI] [PubMed] [Google Scholar]
- Banks RW (2006) An allometric analysis of the number of muscle spindles in mammalian skeletal muscles. J Anat 208, 753–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks RW (2015) The innervation of the muscle spindle: a personal history. J Anat 227, 115–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewick GS, Banks RW (2015) Mechanotransduction in the muscle spindle. Pflugers Arch 467, 175–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombardi C, Grandis A, Chiocchetti R, et al. (2006) Immunohistochemical localization of alpha(1a)‐adrenoreceptors in muscle spindles of rabbit masseter muscle. Tissue Cell 38, 121–125. [DOI] [PubMed] [Google Scholar]
- Cobo JL, Abbate F, de Vicente JC, et al. (2017) Searching for proprioceptors in human facial muscles. Neurosci Lett 640, 1–5. [DOI] [PubMed] [Google Scholar]
- Cooper S, Daniel PM (1963) Muscle spindles in man; their morphology in the lumbricals and the deep muscles of the neck. Brain 86, 563–586. [DOI] [PubMed] [Google Scholar]
- de la Cuadra‐Blanco C, Peces‐Pena MD, Carvallo‐de Moraes LO, et al. (2013) Development of the platysma muscle and the superficial musculoaponeurotic system (human specimens at 8–17 weeks of development). ScientificWorldJournal 2013, 716962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittert DD, Bardosi A (1989) Distribution of different fiber types in whole cross‐sections of human platysma. A histological and morphometric study. Acta Anat (Basel) 134, 206–211. [DOI] [PubMed] [Google Scholar]
- Fahmi A, Mandai A, Mitsuyama T, et al. (2017) Bilateral platysma dystonia. Asian J Neurosurg 12, 244–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein B, Lindegard B, Nyman E, et al. (1955) Morphologic studies of motor units in normal human muscles. Acta Anat 23, 127–142. [DOI] [PubMed] [Google Scholar]
- Hwang K, Kim JY, Lim JH (2017) Anatomy of the platysma muscle. J Craniofac Surg 28, 539–542. [DOI] [PubMed] [Google Scholar]
- Le Louarn C (2016) A new approach to functional anatomy of the lower face: role of the hyoplatysmal ligament, of the platysma and of the depressor labii lateralis. Ann Chir Plat Esth 61, 101–109. [DOI] [PubMed] [Google Scholar]
- Oudet C, Petrovic A, Champy M, et al. (1989) Is the myosin type altered in the aging platysma? J Craniomaxillofac Surg 17, 190–194. [DOI] [PubMed] [Google Scholar]
- Pelle‐Ceravolo M, Angelini M, Silvi E (2016) Complete platysma transection in neck rejuvenation: a critical appraisal. Plast Reconstr Surg 138, 781–791. [DOI] [PubMed] [Google Scholar]
- Sahgal V, Subramani V, Sahgal S, et al. (1985) Morphology and morphometry of human muscle spindles In: The muscle spindle. (eds Boyd IA, Gladden MH.), pp. 107–114, New York: Macmillan. [Google Scholar]
- Schiaffino S, Reggiani C (2011) Fiber types in mammalian skeletal muscles. Physiol Rev 91, 1447–1531. [DOI] [PubMed] [Google Scholar]
- Schwarting S, Schröder M, Stennert E, et al. (1982) Enzyme histochemical and histographic data on normal human facial muscles. ORL J Otorhinolaryngol Relat Spec 44, 51–59. [DOI] [PubMed] [Google Scholar]
- Socolovsky MP, Bertelli JA, Masi GD, et al. (2008) Surgical anatomy of the platysma motor branch as a donor for transfer in brachial plexus repair. Surg Radiol Anat 30, 669–674. [DOI] [PubMed] [Google Scholar]
- Sokoloff AJ, Li H, Burkholder TJ (2007) Limited expression of slow tonic myosin heavy chain in human cranial muscles. Muscle Nerve 36, 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani V, Sahgal V, Sahgal S, et al. (1985) Distribution of muscle spindles in primate skeletal muscles In: The muscle spindle. (eds Boyd IA, Gladden MH.), pp. 89–93, New York: Macmillan. [Google Scholar]
- Swash M, Fox KP (1972) The effect of age on human skeletal muscle. Studies of the morphology and innervation of muscle spindles. J Neurol Sci 16, 417–432. [DOI] [PubMed] [Google Scholar]
- Thornell LE, Carlsson L, Eriksson PO, et al. (2015) Fibre typing of intrafusal fibres. J Anat 227, 136–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss H (1971) Tabulation of the absolute and relative muscular spindle numbers in human skeletal musculature. Anat Anz 129, 562–572. [PubMed] [Google Scholar]


