Abstract
Injury to the nerves of the aortic‐ and superior hypogastric plexuses during retroperitoneal surgery often results in significant post‐operative complications, including retrograde ejaculation and/or loss of seminal emission in males. Although previous characterizations of these plexuses have done well to provide a basis for understanding the typical anatomy, additional research into the common variations of these plexuses could further optimize nerve‐sparing techniques for retroperitoneal surgery. To achieve this, the present study aimed to document the prevalence and positional variability of the infrarenal lumbar splanchnic nerves (LSNs) through gross dissection of 26 human cadavers. In almost all cases, two LSNs were observed joining each side of the aortic plexus, with 48% (left) and 33% (right) of specimens also exhibiting a third joining inferior to the left renal vein. As expected, the position of the LSNs varied greatly between specimens. That said, the vast majority (98%) of LSNs joining the aortic plexus were found to originate from the lumbar sympathetic trunk above the level of the inferior mesenteric artery. It was also found that, within specimens, adjacent LSNs often coursed in parallel. In addition to these nerves, 85% of specimens also demonstrated retroaortic LSN(s) that were angled more inferior compared with the other LSNs (P < 0.05), and exhibited a unique course between the aorta/common iliac arteries and the left common iliac vein before joining the superior hypogastric plexus below the aortic bifurcation. These findings may have significant implications for surgeons attempting nerve‐sparing procedures of the sympathetic nerves in the infrarenal retroperitoneum such as retroperitoneal lymphadenectomies. We anticipate that the collective findings of the current study will help improve such retroperitoneal nerve‐sparing surgical procedures, which may assist in preserving male ejaculatory function post‐operatively.
Keywords: aortic plexus, intermesenteric plexus, lumbar sympathetic chain, lumbar sympathetic trunk, retroaortic lumbar splanchnic nerve, retroiliac lumbar splanchnic nerve, superior hypogastric plexus, variability
Introduction
The major source of sympathetic innervation to the pelvic organs is provided by the aortic‐ and superior hypogastric plexuses. Overlying the infrarenal abdominal aorta, the aortic plexus receives sympathetic supply from the lumbar sympathetic trunk and suprarenal preaortic plexuses via lumbar splanchnic nerves (LSNs) and intermesenteric nerves, respectively (Motoc et al. 2010; Beveridge et al. 2015a). After extending branches to form the inferior mesenteric plexus, the main cords of the aortic plexus converge near the aortic bifurcation as the superior hypogastric plexus that descends into the pelvis to innervate the smooth muscle of the urogenital organs, including the internal urethral sphincter, ductus deferens, prostate gland and seminal vesicles (Learmonth, 1931; Mitchell, 1953; Crosby et al. 1962; Duncan & Jonck, 1965; Brindley et al. 1989; Coolen et al. 2004; Paraskevas et al. 2008; Beveridge et al. 2015a). Unfortunately, given their seemingly variable positioning in the retroperitoneum and pelvis, the delicate nerves of the aortic and/or superior hypogastric plexus are at risk of damage during retroperitoneal surgery, which can result in significant post‐operative complications, including loss of seminal emission and/or retrograde ejaculation in males (Duncan & Jonck, 1965; Johnson & McGuire, 1981; Flynn & Price, 1984; Jewett et al. 1988; Jewett & Groll, 2007; Veroux et al. 2010; Heidenreich & Pfister, 2012; Hsiao et al. 2012). Although this preventable post‐operative complication is well acknowledged in the surgical literature, additional research into the common variations of the nerves comprising and contributing to the aortic‐ and superior hypogastric plexuses could further optimize nerve‐sparing techniques for retroperitoneal surgeons.
Based on cadaveric and surgical investigations, most of our understanding of the LSNs has been derived from qualitative observations (Motoc et al. 2010; Beveridge et al. 2015a, 2016b). Classical anatomical texts reference four LSNs on each side with two–four joining the aortic plexus (Mitchell, 1953; Hollinshead, 1971; Gray, 1973; Woodburne & Burkel, 1994; Mirilas & Skandalakis, 2010), and more recent cadaveric studies have supported this assertion by reporting two consistent infrarenal LSNs (with the potential for accessory fibers) joining the intermesenteric nerves of the aortic plexus (Beveridge et al. 2015a, 2016a,b). Moreover, below the aortic bifurcation, some sources indicate the possibility of a LSN(s) joining the superior hypogastric plexus (Dwight et al. 1930; Learmonth, 1931; Mitchell, 1953; Duncan & Jonck, 1965; Hollinshead, 1971; O'Rahilly, 1986; Mirilas & Skandalakis, 2010; Beveridge et al. 2015a); however, details about their prevalence and/or course are rarely discussed. Of the reports available, it remains unclear whether these fibers course: (i) anterior to the common iliac arteries (see fig. 38.7 from O'Rahilly, 1986; fig. 6–71 from Woodburne & Burkel, 1994; fig. 9 from Mirilas & Skandalakis, 2010; plate 389 from Netter, 2011); (ii) between the aortic bifurcation and the left common iliac vein (see fig. 1 from Learmonth, 1931; fig. 110 from Mitchell, 1953; Beveridge et al. 2015a); or (iii) posterior to the left common iliac vein (see fig. 1140 from Dwight et al. 1930; fig. 1 from Learmonth, 1931; fig. 9 from Mirilas & Skandalakis, 2010; plates 297 and 392 from Netter, 2011). Furthermore, inconsistencies exist with respect to whether one (Dwight et al. 1930; Hollinshead, 1971; O'Rahilly, 1986; Mirilas & Skandalakis, 2010; Beveridge et al. 2015a) or two (Learmonth, 1931; Woodburne & Burkel, 1994) LSNs are present on each side that extend to join the superior hypogastric plexus. Based on our own observations (Beveridge et al. 2015a), it is our prediction that LSN(s) joining the superior hypogastric plexus, when present, will course behind the aortic bifurcation, yet anterior to the left common iliac vein.
Figure 1.
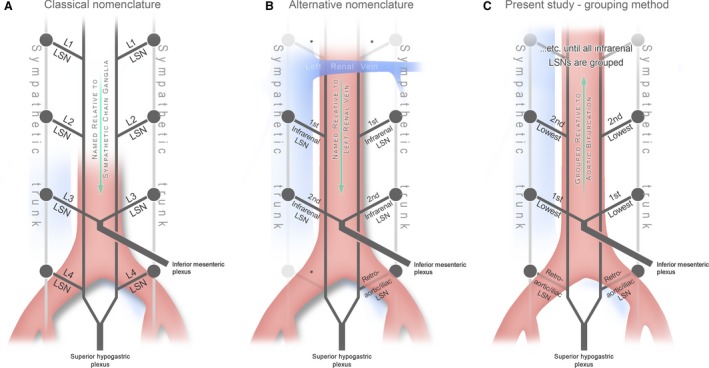
An illustration of the different nomenclatures used in the literature compared with the grouping method used in this study. (A) Illustrates how the lumbar splanchnic nerves (LSNs) are classically named as per the sympathetic trunk ganglia from which they originate (Hollinshead, 1971; Mirilas & Skandalakis, 2010). (B) Illustrates a more contemporary strategy used in the primary literature where LSNs are named in order relative to the left renal vein (Beveridge et al. 2016a,b); an asterisk (*) indicates portions of the plexus not examined in these studies. To accurately group alike LSNs for comparison in this study, the retroaortic LSNs were grouped and the remaining (above the aortic bifurcation) were grouped in ascending order (C).
Given the precise involvement of each LSN in seminal emission and antegrade ejaculation is not yet understood, further investigation is warranted to quantify the positional deviations of the LSNs in order to assist comprehensive nerve‐sparing retroperitoneal surgery. Although the position of the LSNs may vary greatly, based on our own dissections, we predict that the course of adjacent LSNs will remain similar within specimens (i.e. each LSN will course in parallel to one another within an individual). Ultimately, to complement previous observations and address the aforementioned predictions, the present study endeavored to provide the first quantification of the prevalence, positional deviations and course of the infrarenal LSNs adjoining the aortic‐ and superior hypogastric plexuses through dissection and measurement of human cadavers.
Materials and methods
This study was conducted on 26 embalmed human cadavers (16 females, 10 males; μage at death = 80.4 ± 9.8 years; range 59–97 years) over a period of two years from the HEART (Haase Education in Anatomy & Research Technologies) Lab at Western University, ON, Canada. All data were obtained in accordance with the Anatomy Act of Ontario and Western's Cadaveric Use in Research. Specimens were excluded from the present study if they exhibited evidence of previous infrarenal retroperitoneal dissection that may have altered the anatomy (n = 10), or retroperitoneal disease that may have altered the position of the aortic plexus (n = 8; i.e. abdominal aortic aneurysms greater than 3 cm, local metastases).
Dissection
The preaortic sympathetic nerves were dissected from the left renal vein to the pelvic inlet. LSNs were carefully isolated where they intersected the left/right cord of the aortic plexus or the posterior aspect of the superior hypogastric plexus, and followed posteriorly to their origin at the lumbar sympathetic trunk. Because all LSNs joining the superior hypogastric plexus below the aortic bifurcation coursed posterior to the common iliac arteries, they were referred to (and grouped for analysis) as retroaortic LSNs. Nerves were pinned during dissection in preparation for measurement.
Measurement protocol
To quantify the position of each LSN, we measured its origin (proximal position at the sympathetic trunk) and termination (distal position at the cord of the aortic plexus or superior hypogastric plexus) relative to the aortic bifurcation along the rostrocaudal axis. This positional metric was adopted based on its successful implementation in a previous study that examined the position of the lumbar arteries and veins within the infrarenal retroperitoneum (Beveridge et al. 2015b). In addition, measurements of the infrarenal abdominal aortic length, rostrocaudal position of the inferior mesenteric artery and lateral position of the superior hypogastric plexus were obtained to standardize and contextualize the data reported in the study. To determine the lateral positioning of the superior hypogastric plexus, the width of the plexus and its distance from each side of the aorta were measured at the level of the aortic bifurcation.
Inter‐ and intra‐rater reliability of the measurement protocol was performed using an intraclass correlation coefficient in a two‐way mixed model assessing absolute agreement. To assess inter‐rater reliability, two raters (Raters A and B) measured the same three specimens on different days, and were blinded to all previous measurements. Subsequently, Rater A measured a different specimen on two different days (blinded from all previous measurements) to assess intra‐rater reliability. An excellent agreement of 0.998 (0.995–0.999: 95% CI) and 0.995 (0.984–0.998: 95% CI) was determined for inter‐ and intra‐rater reliability, respectively. All measurements used in this study were obtained by Rater A because his performance underwent both inter‐ and intra‐rater reliability analyses.
Data analyses and statistics
To assess the variability of the number and positioning of LSNs, we first needed to implement a strategy that grouped alike LSNs between individuals. This was a challenging task given the potential for inaccuracy inherent to the inconsistency of the infrarenal region (Yeager & Cowley, 1948; Hollinshead, 1971; Mirilas & Skandalakis, 2010; Beveridge et al. 2016a,b). Classically, the LSNs are named by the sympathetic trunk ganglia to which they are associated (Fig. 1A). Unfortunately, this method can be unreliable (Hollinshead, 1971) due to the extreme variability present in the anatomy of the lumbar sympathetic trunk (Yeager & Cowley, 1948; Gray, 1973; Mirilas & Skandalakis, 2010; Gandhi et al. 2013). Recognizing this shortcoming of the classical nomenclature, we previously elected to refer to the LSNs joining the aortic plexus in their order relative to the left renal vein (i.e. first infrarenal LSN, etc.), as seen in Fig. 1B (Beveridge et al. 2016a,b). However, this approach proved problematic for the present study because subtle positional variations in the left renal vein inappropriately changed how nerves were grouped for subsequent statistical analysis. Thus, in the present study, we instead grouped the LSNs for analysis based on their position relative to the aortic bifurcation (i.e. the most inferior LSNs were grouped together, etc.; Fig. 1C). Note that this strategy was used to describe the LSNs joining the aortic plexus, whereas the retroaortic LSNs that joined the superior hypogastric plexus were identified and grouped based on their unique coursing pattern posterior to the aorta/common iliac arteries.
Prior to statistical analyses, the positions of the LSNs were normalized to the infrarenal length of the abdominal aorta to limit the variability associated with size differences between specimens. In most cases, normalized values were preferentially reported; however, some raw measurements were also included (see Results). To examine if the positions of the left and right LSNs were similar, we calculated the difference between where a pair of LSNs (e.g. the right and left lowest LSNs) joined the aortic/superior hypogastric plexus (Δd) using normalized values. A value of Δd equal to zero indicated perfect positional symmetry. To determine if adjacent LSNs on the same side coursed in parallel, we calculated the difference in position between the connections of each LSN with the sympathetic trunk and the aortic/superior hypogastric plexus (Δp) using normalized values. Subsequently, each side was independently compared using non‐parametric one‐way analysis of variance tests (Kruskal–Wallis H‐tests, α = 0.05). Non‐parametric statistics were used because the calculated values of Δp were not normally distributed (Shapiro–Wilk, P < 0.05). For clarity, an example of Δd and Δp values can be seen in Fig. 2C.
Figure 2.
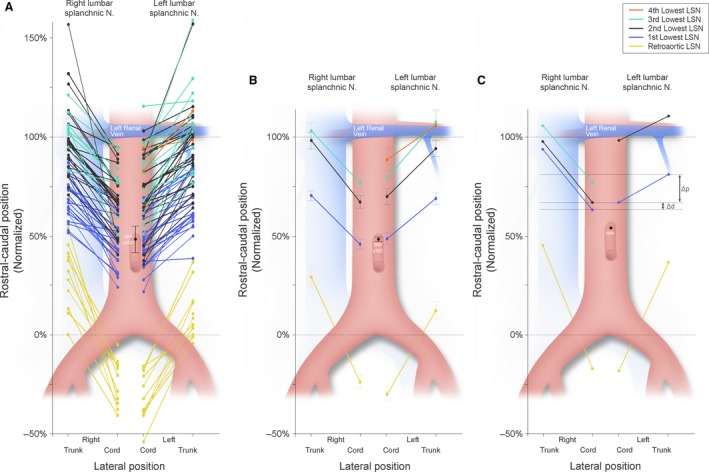
A graphic illustration showing the position of the infrarenal lumbar splanchnic nerves (LSNs) as they join the cord of the aortic plexus and the lumbar sympathetic trunk relative to the total infrarenal space (100%). (A) Each individual nerve that was measured in the study. (B) The mean position of each nerve with the associated standard error of that mean. (C) The measurements taken from a typical specimen (#1849) that illustrates, at the individual level, the position of LSNs on one side does not always predict the contralateral positions (see the pair of 2nd lowest LSNs coloured in black). Irrespective of this, consistency in the angle of projection (Δp) between ipsilateral LSNs can also be observed in this specimen (refer to Fig. 7 for statistics). Retroaortic LSN – yellow; most inferior LSN – blue; second inferior LSN – black; third inferior LSN – teal; and fourth inferior LSN – orange.
Data were handled and calculations were completed with Excel (Version 2016, Microsoft), and all statistical tests were performed using spss statistics (Version 24, IBM). Statistical power was determined using g*power v3.1.9.2. Graphical figures were generated with Excel or spss, then stylized and formatted using adobe photoshop cs6. Photos were obtained using a Nikon D80 DSLR camera, and 3D modeling of the findings was completed using blender (Version 2.78a, Blender Foundation).
Results
Of the 26 human cadavers included in this study, 21 had an intact retroperitoneum prior to examination; the remaining five specimens had a partially dissected retroperitoneum as a result of medical students’ laboratory exercises at our institution. In these five cases, only the regions of the retroperitoneal plexuses that remained untouched were examined. Therefore, the present study examined the variability in the number and position of the LSNs on the right (n = 24) and left sides (n = 25) of the aortic plexus. In our study population, the mean distance from the aortic bifurcation to the inferior mesenteric artery and inferior border of the left renal vein was 44.0 ± 6.6 mm and 91.6 ± 12.8 mm, respectively. Of the specimens with bilaterally intact/untouched anatomy around the bifurcation (n = 22), the diameter of the aorta (at the level of the aortic bifurcation) was measured to be 30.8 ± 6.8 mm. In 17/22 (77%) of these specimens, the center of the superior hypogastric plexus was shifted left of the aortic bifurcation 2.7 ± 2.4 mm; the remaining five were shifted right of the bifurcation 1.8 ± 1.4 mm. Irrespective of this predominant left‐sided shift, the superior hypogastric plexus still extended right of the bifurcation in 90.9% (n = 20/22) specimens. Overall, the superior hypogastric plexus extended 4.5 ± 3.2 mm right and 7.9 ± 4.0 mm left of the bifurcation, exhibiting an average width of 12.4 ± 4.4 mm.
A total of 142 LSNs (n LEFT = 76, n RIGHT = 66) were identified joining the cords of the aortic plexus (Fig. 2A). In most cases (n = 136, 95%), the LSNs existed as a single fiber bundle coursing at an anteroinferior angle. In a few cases (n = 7, 5%; n LEFT = 3, n RIGHT = 4), a variation was observed where a LSN originated from two points on the lumbar sympathetic trunk and converged to a common fiber prior to joining the aortic plexus. In these cases, the larger root was used as the connection with the sympathetic trunk for statistical analyses (larger root was superior in 4/7 specimens). The mean difference between the two roots was 13.1 ± 7.3 mm (range 4.1–25.6 mm). On the right side, the two roots were often seen coursing on either side of a lumbar vein.
A variable number of one to four LSNs joined the cords of the aortic plexus below the level of the left renal vein, with two most commonly present (prevalence left = 52%; right = 63%). The prevalence and location of each LSN is further reported in Table 1. As shown in Fig. 2A, there was considerable variability in the rostrocaudal position of the LSNs. Still, 118/120 (98.3%) LSNs that extended to join the aortic plexus originated from the sympathetic trunk superior to the level of the inferior mesenteric artery. Of these, 69.5% (n = 82) also joined the aortic plexus at a position superior to the inferior mesenteric artery. No LSNs were observed joining the aortic plexus along the lower 21.8% (left) and 24.1% (right) of the infrarenal abdominal aortic length. The mean position of each LSN is shown graphically in Fig. 2B, and schematically in three dimensions in Fig. 3. Although the mean difference in positions between the left and right sides of each LSN ranged from 2.6% to 2.8% (Fig. 2B), this was rarely observed at the individual level, as noted by the wide standard deviations associated with the mean Δd of each pair (mean Δd 1st lowest LSN = 3.7 ± 13.7%, mean Δd 2st lowest LSN = 1.7 ± 22.1%, mean Δd 3rd lowest LSN = 2.9 ± 13.6%). Figure 2C shows data obtained from a typical specimen (specimen #1849) that illustrates how the position of the LSNs on one side did not often mirror the contralateral positions. This relationship can also be seen in Fig. 4, where the anatomy for this specimen is shown in three dimensions, panelled in 60° increments.
Table 1.
Descriptive statistics for the LSNs joining the infrarenal aortic plexus and superior hypogastric plexus. Nerves grouped inferiorly‐superiorly (1–4) and compared relative to the total infrarenal space (100%). Reported are the total number of splanchnic nerves identified and at which level. LSN, lumbar splanchnic nerve.
| LSN order | LSN (%) | Relative proximal position (%) | Relative distal position (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |||
| Left | 4th Lowest | 2 (8) | 106.0 | 10.5 | 98.6–113.4 | 88.7 | 8.2 | 82.0–94.5 |
| 3rd Lowest | 12 (48) | 107.7 | 23.3 | 76.6–159.1 | 79.6 | 18.7 | 51.0–115.6 | |
| 2nd Lowest | 25 (100) | 94.4 | 19.8 | 64.2–157.2 | 70.1 | 19.6 | 35.6–103.1 | |
| 1st Lowest | 25 (100) | 69.1 | 13.9 | 38.6–94.5 | 48.7 | 14.3 | 21.8–82.4 | |
| Retroaortic | 12 (48) | 12.4 | 15.1 | −4.5–36.8 | −29.9 | 12.6 | −54.2– −15.0 | |
| Right | 3rd Lowest | 8 (33) | 103.2 | 11.4 | 82.9–121.3 | 77.0 | 15.4 | 50.5–94.5 |
| 2nd Lowest | 23 (96) | 98.5 | 19.6 | 70.7–157.0 | 67.3 | 15.2 | 40.4–94.6 | |
| 1st Lowest | 24 (100) | 70.6 | 11.2 | 82.9–121.3 | 46.0 | 12.2 | 24.1–68.3 | |
| Retroaortic | 11 (46) | 29.4 | 16.4 | 0.0–53.8 | −23.7 | 11.6 | −40.8– −5.0 | |
Figure 3.
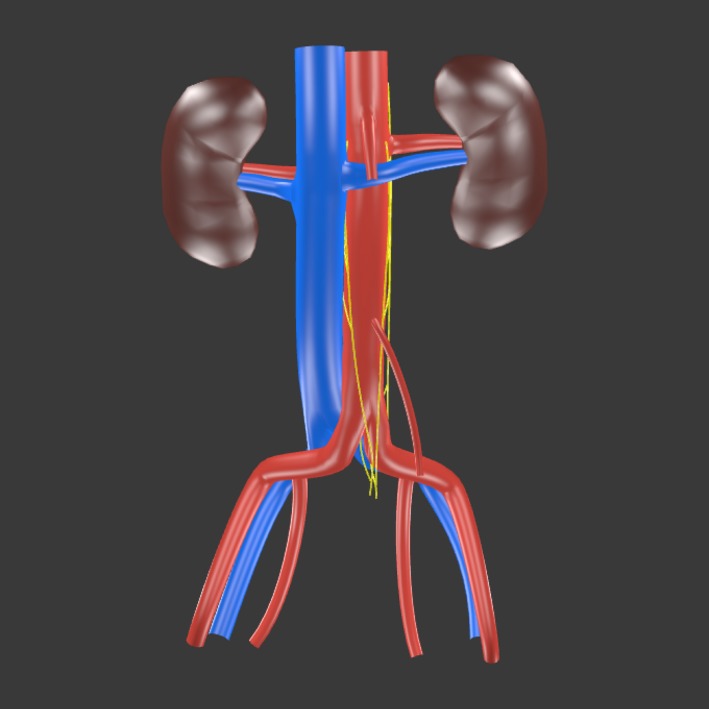
A 3D model (click to activate) showing the average position and course of the infrarenal lumbar splanchnic nerves (LSNs) as determined from the present study. The rostrocaudal positions of the LSNs are drawn to scale relative to the infrarenal length. Adobe Acrobat Reader is required to interact with the 3D model.
Figure 4.
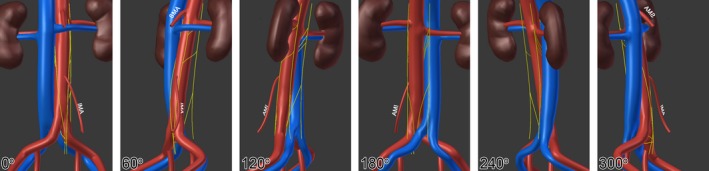
A 3D representation of the data acquired from a single representative specimen (#1849). The first panel (0°) represents an anterior view of the infrarenal region, then each subsequent panel rotates 60° clockwise around the rostrocadual axis (i.e. 180° represents the posterior view) IMA, inferior mesenteric artery; SMA, superior mesenteric artery.
In 85% (n = 22/26) of specimens, a retroaortic LSN was present joining the superior hypogastric plexus below the aortic bifurcation. They were observed bilaterally (n = 8), unilaterally (n LEFT = 8, n RIGHT = 3), or were considered to have inconclusive laterality (n LEFT = 2, n RIGHT = 1) due to a lack of contralateral examination because of exclusion due to prior trainee dissection. In the remaining 15% (n = 4/26) of specimens, retroaortic LSNs were bilaterally absent in three and not examined in one due to previous bilateral dissection in the region of interest by trainees. In contrast to the LSNs joining the aortic plexus, retroaortic LSNs invariably coursed behind the aorta/common iliac artery and anterior to the left common iliac vein to join the superior hypogastric plexus below the aortic bifurcation. Figure 5 demonstrates this relationship in the case of a right retroaortic LSN, and Fig. 6 shows the anatomy of a typical left retroaortic LSN. As seen in Fig. 2A, the retroaortic LSNs (yellow) had scant positional overlap with the LSNs that joined the aortic plexus (blue, teal and orange). When bilaterally present, the difference in position where each side joined the superior hypogastric plexus was rarely similar (mean Δd retroaortic LSN = 2.6 ± 12.9%). Additional descriptive statistics are presented in Table 1.
Figure 5.
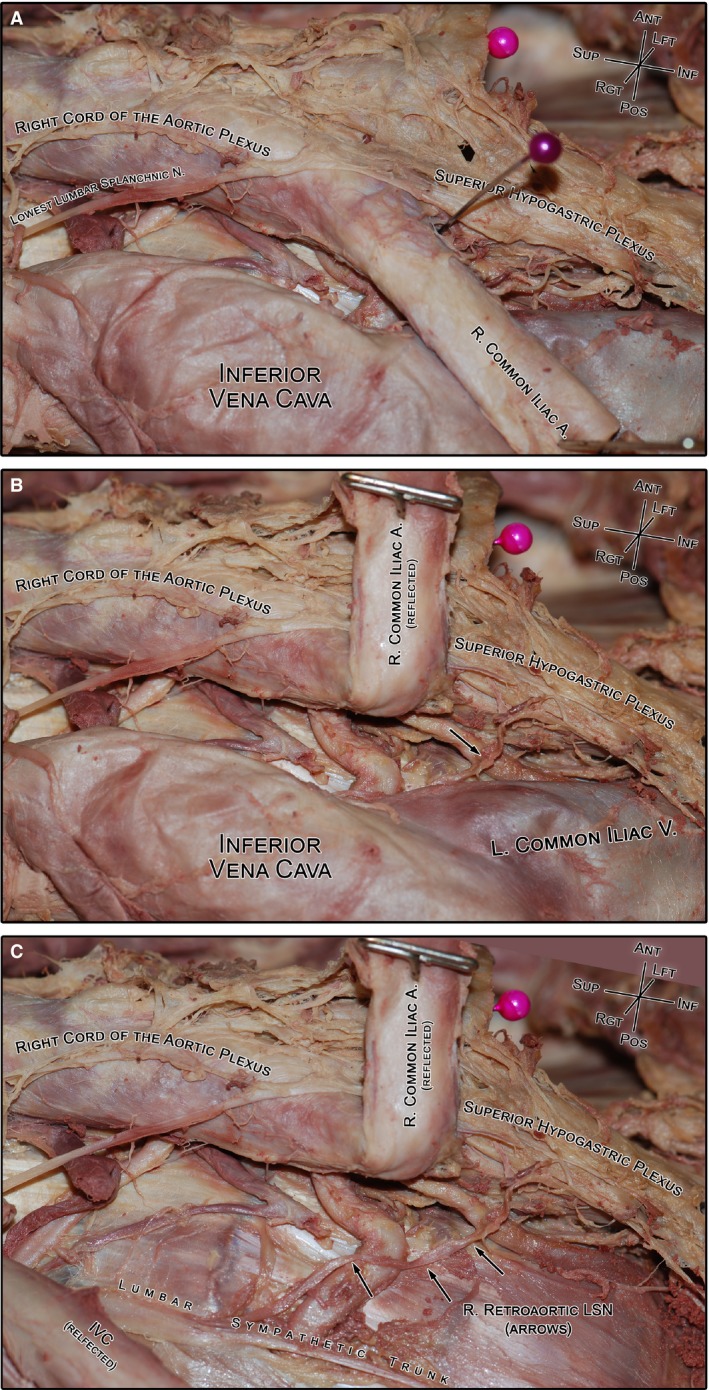
A photograph of the gross dissection of a typical right retroaortic lumbar splanchnic nerve (LSN; specimen #1854). (A) The course of the retroaortic LSN cannot be clearly observed without moving the vasculature. For reference, the purple pin indicates the bifurcation of the aorta. (B) Reflecting the right common iliac artery reveals the underlying retroaortic nerve (black arrow) and its connection with the superior hypogastric plexus. (C) Lateral translation of the inferior vena cava reveals the entire course of the retroaortic nerve from the lumbar sympathetic trunk to the superior hypogastric plexus. IVC, inferior vena cava.
Figure 6.
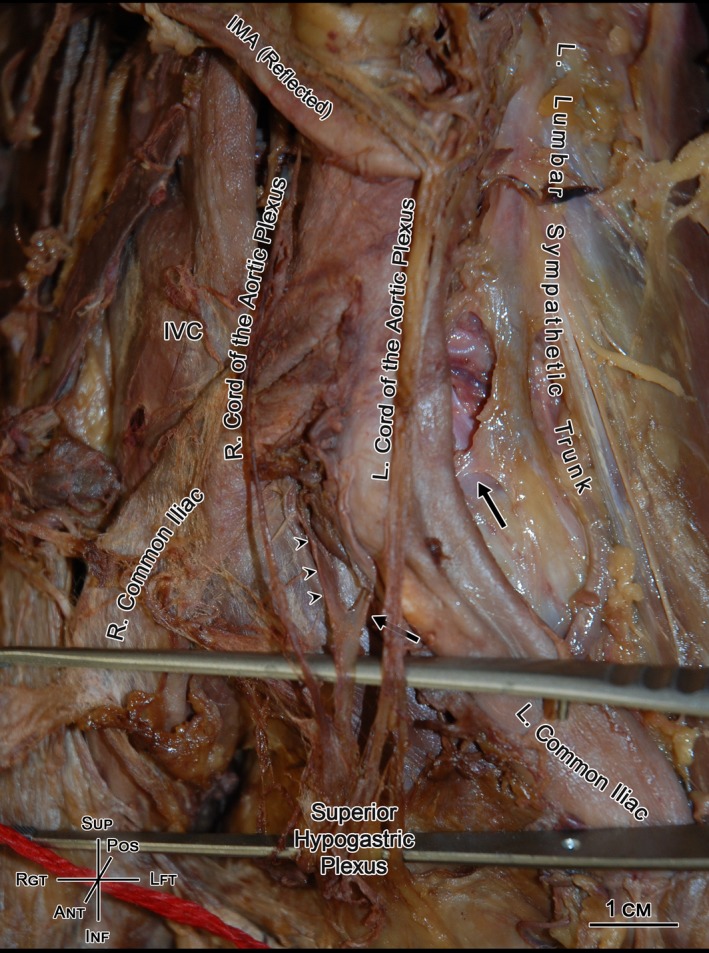
An anterior view of the infrarenal retroperitoneum showing the aortic bifurcation and superior hypogastric plexus. The overlaid superior hypogastric plexus (held up by forceps) is supplied by the right and left cords of the aortic plexus. In addition, it is supplied posteriorly by the right (arrowheads) and left (arrows) retroaortic lumbar splanchnic nerves (LSNs). Note, in this specimen, the bilateral retroaortic nerves converged into a common nerve immediately prior to joining superior hypogastric plexus; a unique observation to this study that has been briefly acknowledged in the previous literature (Learmonth, 1931). IMA, inferior mesenteric artery; IVC, inferior vena cava.
To examine our prediction that adjacent LSNs on the same side course in parallel (i.e. project at the same orientation angle), the Δp (refer to Fig. 2C) of each LSN was compared. In line with this prediction, the left and right LSNs were analyzed independent of one another. A significant difference was observed for both the right [Kruskal–Wallis H‐test; χ 2(3) = 19.8, P = 0.00] and left LSNs [Kruskal–Wallis H‐test; χ 2(3) = 10.1, P = 0.02]. Post hoc analyses indicated the Δp for the retroaortic LSNs (left mean rank Δp retroaortic LSN = 55.0; right mean rank Δp retroaortic LSN = 55.8) were significantly different from all other nerves on the right (mean rank Δp 1st lowest LSN = 25.8, mean rank Δp 2nd lowest LSN = 33.3, mean rank Δp 3rd lowest LSN = 26.6), and from the lowest two LSNs on the left (mean rank Δp 1st lowest LSN = 32.6, mean rank Δp 2nd lowest LSN = 34.4, mean rank Δp 3rd lowest LSN = 38.4). The results of the analysis are shown in Fig. 7. It is important to note that this trend was also often observed at the individual level, as illustrated in specimen #1849 (Figs 2C and 4).
Figure 7.
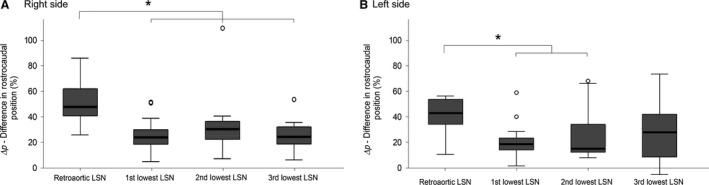
A box‐whisker plot showing the difference in rostrocaudal position (Δp) of the origin and termination of each group of infrarenal lumbar splanchnic nerves (LSNs) on the right (A) and left (B) side of the body. Outliers, calculated by spss, are represented by hollow dots. Significance at the P < 0.05 level is denoted with an asterisk. As shown, the LSNs joining the aortic plexus typically course in parallel, whereas the length and angle of the retroaortic LSNs on each side are significantly different. Note, the 3rd lowest LSN on the left was not significantly different from any other nerve.
Discussion
Variations in the LSNs adjoining the aortic and superior hypogastric plexuses may complicate complete nerve sparing during retroperitoneal surgery. As such, the present study used dissection of cadaveric specimens to quantify the prevalence, course and positional deviations of the infrarenal LSNs. In doing so, our results also clarified the inconsistency that is currently present in the literature regarding the path of the LSNs that join the superior hypogastric plexus below the bifurcation.
The LSNs joining the superior hypogastric plexus are occasionally illustrated in anatomical images, yet the course of these nerves relative to the common iliac vessels varies between authors (see Introduction). In the present study, this quandary was resolved by the fact that all LSNs joining the superior hypogastric plexus invariably coursed between the aorta/common iliac arteries and the left common iliac vein (Figs 3, 4, 5, 6). Despite exhibiting a consistent course, retroaortic LSNs were variably present, with confirmed unilateral absence in 11 (44%) specimens and complete bilateral absence in three (12%) specimens. However, when present, only one retroaortic LSN was observed per side. This contrasts drawings by Learmonth (1931) and descriptions from Woodburne & Burkel (1994) that suggest both the third and fourth LSNs join the superior hypogastric plexus. Although this arrangement was not observed in the present population, the potential for additional retroaortic fibers could very well exist in some people considering the acknowledged disorganization of lumbar sympathetic trunk ganglia that may predispose such a variation (Yeager & Cowley, 1948; Mitchell, 1953; Webber, 1958; Hollinshead, 1971; Gray, 1973; Woodburne & Burkel, 1994; Mirilas & Skandalakis, 2010; Gandhi et al. 2013).
Despite the extreme variation of the lumbar sympathetic nerves described in previous literature, recent qualitative observations of the aortic plexus have suggested that it is almost always bilateral in its organization, but not necessarily symmetrical in its positioning (Beveridge et al. 2015a, 2016a). Put another way, the aortic plexus often has the same number of LSNs on its left and right sides, but the position of these nerves may not be mirrored. Despite only minor differences between the overall mean positions of each LSN joining the aortic plexus (range Δd = 1.7–3.7%), as seen in Fig. 2B, our results show large standard deviations when averaging the positional differences of the left‐ and right‐matched LSNs within individuals (mean Δd retroaortic LSN = 2.6 ± 12.9%, mean Δd 1st lowest LSN = 3.7 ± 13.7%, mean Δd 2nd lowest LSN = 1.7 ± 22.1%, mean Δd 3rd lowest LSN = 2.9 ± 13.6%). This suggests that, on average, the positions of the contralateral LSNs are similar yet, within an individual, the position of a left LSN is rarely the same as the associated right LSN, and vice versa. These data are consistent with the suggestions and qualitative findings present in the previous literature (Beveridge et al. 2015a, 2016a).
Given this clear positional variability of the infrarenal LSNs joining the aortic plexus within individuals, landmarking these fibers during surgery may be difficult to achieve. With that said, our findings indicate that there are no LSNs adjoining the aortic plexus within the lower 21.8% (left) and 24.1% (right) of the infrarenal abdominal aorta, with 98% (n = 118) originating from the sympathetic trunk above the level of the inferior mesenteric artery. In addition, the present study saw no evidence of a ‘preaortic’ LSN joining the superior hypogastric plexus below the bifurcation. This knowledge may be welcomed by surgeons tasked with removing caudal interaortocaval masses, or those posterior to the inferior mesenteric artery. Although the lower aspect of the abdominal aorta does not have any LSNs adjoining the aortic plexus, it is important to recognize the presence of the lumbar sympathetic trunk and retroaortic LSN overlying the anterior longitudinal ligament of the vertebral column in this region. As detailed above, the present study clarified the path of these LSNs as they coursed ‘between’ the common iliac arteries and left common iliac vein (Figs 2, 5 and 6). In addition, it was noted that despite the similar course (Δp) of the rest of the LSNs, the retroaortic LSNs are significantly longer and course more inferiorly (P < 0.05), perhaps owing to their necessary descent along the anterior vertebral column before extending to join the superior hypogastric plexus. On average, the retroaortic LSNs joined the superior hypogastric plexus 29.9 ± 12.6% (left) and 23.7 ± 11.6% (right) of the infrarenal abdominal aortic length ‘inferior’ to the bifurcation. Considering the function of the retroaortic LSNs remains unknown, surgeons attempting presacral lymphandectomies should consider sparing these fibers when possible (in addition to the sacral and pelvic splanchnic nerves, more inferiorly). Furthermore, surgeries that attempt to circumnavigate the superior hypogastric plexus should be aware of these fibers given the aforementioned anatomy and possible risk for iatrogenic injury when mobilizing the superior hypogastric plexus.
In conclusion, the present study commonly observed two to three infrarenal LSNs joining the aortic plexus that varied greatly in rostrocaudal position. Within specimens, the position of the LSNs on one side rarely predicted the position of the contralateral nerves; however, adjacent nerves on the same side joining the aortic plexus typically coursed in parallel to one another. More inferiorly, retroaortic LSNs were observed in the majority (85%) of specimens, present both unilaterally and bilaterally with no obvious predominance to a particular side. Of note, the retroaortic LSNs exhibited a significantly different (more inferior) course relative to the LSNs joining the aortic plexus. Specifically, the retroaortic LSNs coursed between the common iliac arteries and the left common iliac vein before joining the superior hypogastric plexus below the bifurcation. Because the precise components of the retroperitoneal sympathetic plexuses involved in ejaculation still remain unknown, complete nerve sparing, when possible, remains the most prudent surgical approach. Ultimately, we are optimistic that the collective findings of the present study will further inform anatomists as well as benefit surgeons tasked with navigating and sparing these delicate structures during retroperitoneal and pelvic surgeries. Further studies are recommended to explore the specific role of the individual nerves described in this manuscript as they pertain to seminal emission and antegrade ejaculation to allow further refinement of nerve‐sparing techniques.
Author contributions
TSB, NEP, BLA, study conception; TSB, DEF, AG, data acquisition and analysis; TSB, DEF, figure illustrations; TSB, DEF, AG, MJ, BLA, data interpretation; TSB, DEF, BLA, writing and critical revision of the manuscript.
All authors contributed to the overall study design as well as providing edits and approval of the final version of the manuscript.
Acknowledgements
The authors would like to extend thanks to: Haley Linklater and Kevin Walker for their contributions in the HEART Anatomy Lab; Christian Buchanan‐Fraser for his help in data recording; Santiago Cobos C for his help modeling the 3D anatomy; and finally, the donors and their families – without their generosity, this research would not have been possible. The authors have no conflict of interest with the content of the manuscript.
o‐first authors.
References
- Beveridge TS, Johnson M, Power A, et al. (2015a) Anatomy of the nerves and ganglia of the aortic plexus in males. J Anat 226, 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge TS, Power A, Johnson M, et al. (2015b) The lumbar arteries and veins: quantification of variable anatomical positioning with application to retroperitoneal surgery. Clin Anat 28, 649–660. [DOI] [PubMed] [Google Scholar]
- Beveridge TS, Allman BL, Johnson M, et al. (2016a) Retroperitoneal lymph node dissection: anatomical and technical considerations from a cadaveric study. J Urol 196, 1764–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge TS, Johnson M, Power NE, et al. (2016b) Histological verification of the prehypogastric and ovarian ganglia confirms a bilaterally symmetrical organization of the ganglia comprising the aortic plexus in female human cadavers. J Anat 228, 805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley GS, Sauerwein D, Hendry WF (1989) Hypogastric plexus stimulators for obtaining semen from paraplegic men. Br J Urol 64, 72–77. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Allard J, Truitt WA, et al. (2004) Central regulation of ejaculation. Physiol Behav 83, 203–215. [DOI] [PubMed] [Google Scholar]
- Crosby EC, Humphrey T, Lauer EW (1962) Correlative Anatomy of the Nervous System. New York, NY: The Macmillan Company. [Google Scholar]
- Duncan H, Jonck L (1965) The presacral plexus in anterior fusion of the lumbar spine. S Afr J Surg 3, 93–96. [Google Scholar]
- Dwight T, McMurrich J, Hamann C (1930) The sympathetic system of nerves In: Human Anatomy. (ed. Peirsol G.), pp. 1366–1374. London: J.B. Lippincott. [Google Scholar]
- Flynn JC, Price CT (1984) Sexual complication of anterior fusion of lumbar spine. Spine 9, 489–492. [DOI] [PubMed] [Google Scholar]
- Gandhi KR, Verma VK, Chavan SK, et al. (2013) The morphology of lumbar sympathetic trunk in humans: a cadaveric study. Folia Morphol (Poland) 72, 217–222. [DOI] [PubMed] [Google Scholar]
- Gray H (1973) Sympathetic system In: Gray's Anatomy. (ed. Goss C.), pp. 1025–1036. Philadelphia, PA: Lea & Febiger. [Google Scholar]
- Heidenreich A, Pfister D (2012) Retroperitoneal lymphadenectomy and resection for testicular cancer: an update on best practice. Ther Adv Urol 4, 187–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollinshead WH (1971) Anatomy for Surgeons: Volume 2 – The Thorax, Abdomen, and Pelvis. New York, NY: Harper & Row. [Google Scholar]
- Hsiao W, Deveci S, Mulhall JP (2012) Outcomes of the management of post‐chemotherapy retroperitoneal lymph node dissection‐associated anejaculation. BJU Int 110, 1196–1200. [DOI] [PubMed] [Google Scholar]
- Jewett MA, Groll RJ (2007) Nerve‐sparing retroperitoneal lymphadenectomy. Urol Clin North Am 34, 149–158. [DOI] [PubMed] [Google Scholar]
- Jewett MA, Kong YP, Goldberg SD, et al. (1988) Retroperitoneal lymphadenectomy for testis tumor with nerve sparing for ejaculation. J Urol 139, 1220–1224. [DOI] [PubMed] [Google Scholar]
- Johnson RM, McGuire EJ (1981) Urogenital complications of anterior approaches to the lumbar spine. Clin Orthop Relat Res 154, 114–118. [PubMed] [Google Scholar]
- Learmonth JR (1931) A contribution to the neurophysiology of the urinary bladder in man. Brain 54, 147–176. [Google Scholar]
- Mirilas P, Skandalakis J (2010) Surgical anatomy of the retroperitoneal spaces part II. Am Surg 76, 253–262. [PubMed] [Google Scholar]
- Mitchell G (1953) The abdominal part of the sympathetic system. In: Anatomy of the Autonomic Nervous System. pp. 257–296. E. & S. Livingstone Ltd.: Edinburgh, Scotland. [Google Scholar]
- Motoc A, Rusu MC, Jianu AM (2010) The spermatic ganglion in humans: an anatomical update. Rom J Morphol Embryol 51, 719–723. [PubMed] [Google Scholar]
- Netter F (2011) Section 4 – autonomic nerves and ganglia of abdomen In: Altlas of Human Anatomy. (eds Hansen J, Benninger B, Brueckner J, Carmichael S, Granger N, Tubbs RS.), p. 297, 319, 390. Philadelphia, PA: Elsevier. [Google Scholar]
- O'Rahilly R (1986) The abdomen In: Anatomy: a Regional Study of Human Structure. (ed. Meier A.), pp. 434–436. Philadelphia, PA: W.B. Saunders. [Google Scholar]
- Paraskevas G, Tsitsopoulos P, Papaziogas B, et al. (2008) Variability in superior hypogastric plexus morphology and its clinical applications: a cadaveric study. Surg Radiol Anat 30, 481–488. [DOI] [PubMed] [Google Scholar]
- Veroux P, D'Arrigo G, Veroux M, et al. (2010) Sexual dysfunction after elective endovascular or hand‐assisted laparoscopic abdominal aneurysm repair. Eur J Vasc Endovasc Surg 40, 71–75. [DOI] [PubMed] [Google Scholar]
- Webber RH (1958) A contribution on the sympathetic nerves in the lumbar region. Anat Rec 24, 581–604. [DOI] [PubMed] [Google Scholar]
- Woodburne R, Burkel W (1994) The autonomic nerves of the abdomen In: Essentials of Human Anatomy. pp. 502–507. New York, NY: Oxford University Press. [Google Scholar]
- Yeager G, Cowley R (1948) Anatomical observations on the lumbar sympathetics with evaluation of sympathectomies in organic peripheral vascular disease. Ann Surg 127, 953–967. [DOI] [PMC free article] [PubMed] [Google Scholar]


