Abstract
Definitive radiotherapy is an important alternative treatment for meningioma patients who are inoperable or refuse surgery. We evaluated the efficacy and toxicity of CyberKnife-based stereotactic radiosurgery (SRS) and hypofractionated stereotactic radiotherapy (hSRT) as first-line treatments for intracranial meningiomas that were diagnosed using magnetic resonance imaging (MRI) and/or computed tomography (CT). Between February 2005 and September 2015, 41 patients with intracranial meningiomas were treated with CyberKnife-based SRS or hSRT. Eleven of those tumors were located in the skull base. The median tumor volume was 10.4 ml (range, 1.4–56.9 ml). The median prescribed radiation dose was 17 Gy (range, 13–20 Gy to the 61–88% isodose line) for SRS (n = 9) and 25 Gy (range, 14–38 Gy to the 44–83% isodose line) for hSRT (n = 32). The hSRT doses were delivered in 2 to 10 daily fractions. The median follow-up period was 49 months (range, 7–138). The 5-year progression-free survival rate (PFS) for all 41 patients was 86%. The 3-year PFS was 69% for the 14 patients with tumor volumes of ≥13.5 ml (30 mm in diameter) and 100% for the 27 patients with tumor volumes of <13.5 ml (P = 0.031). Grade >2 toxicities were observed in 5 patients (all of them had tumor volumes of ≥13.5 ml). SRS and hSRT are safe and effective against relatively small (<13.5 ml) meningiomas.
Keywords: meningioma, stereotactic radiosurgery, stereotactic radiotherapy, hypofractionated stereotactic radiotherapy, CyberKnife
Introduction
Meningioma is one of the most common intracranial benign tumors.1) Although gross total resection or subtotal resection followed by radiotherapy is the standard treatment for operable and growing or symptomatic meningiomas, three-dimensional conformal radiotherapy and normofractionated (1.8–2.0 Gy per fraction) stereotactic radiotherapy (SRT) have also been reported to be effective as primary treatments.2–8) In addition, favorable results have recently been obtained using stereotactic radiosurgery (SRS).2,3,9–14) Both SRT and SRS can be performed efficiently using the CyberKnife.
The CyberKnife is a compact, image-guided linear accelerator with a robotic manipulator designed for SRS and SRT.15) Using the CyberKnife, sufficient tumor coverage, steep dose gradients, and tight dose conformity can be achieved, and the frameless nature of the system makes it easy to administer hypofractionated regimens.16–20) Some reports have suggested that definitive radiotherapy is an important alternative treatment for meningioma patients whose disease is inoperable due to the location of the tumor or background factors and patients with medically operable disease who refuse surgery.2,9,10) Such patients, i.e., those with “imaging-diagnosed” meningiomas, could be good candidates for Cyber Knife-based SRS or hypofractionated (>3 Gy per fraction) SRT (hSRT). However, there have only been a few reports about the treatment of imaging-diagnosed meningiomas using hSRT. In the present study, we evaluated the efficacy and toxicity of Cyber Knife-based SRS and hSRT as first-line treatments for intracranial imaging-diagnosed meningiomas.
Materials and Methods
Patient characteristics
Between February 2005 and September 2015, 44 patients with imaging-diagnosed intracranial meningiomas were treated with SRS or hSRT using the CyberKnife II (Accuray, Inc., Sunnyvale, CA, USA) at Tsushima City Hospital (Aichi, Japan). All of them had slow-growing or symptomatic tumors, and none of them had previously undergone biopsy examinations of, or surgery for, their tumors. Thus, the tumors were diagnosed as meningiomas by two or more medical specialists in diagnostic radiology based on the typical computed tomography (CT) and/or magnetic resonance imaging (MRI) findings of such lesions.21,22) In asymptomatic tumors detected by chance, tumor growth was confirmed by CT or MRI performed at 3- to 6-month intervals before the treatment. Symptomatic tumors were treated immediately in order not to worsen the symptoms. Of the above-mentioned patients, 41 who had undergone follow-up examinations involving CT or MRI for 6 months or longer were evaluated in this study. Written informed consent was obtained from the patients before treatment. This study was approved by the institutional review board. One patient underwent transcatheter embolization before SRS (to achieve better local control of their tumor). The patients’ characteristics are summarized in Table 1. Eleven of them were males. Their median age was 70 years (range, 33–92 years). The median tumor volume of the SRS group was smaller than that of the hSRT group (4.6 and 11.3 ml, respectively). The locations of the tumors and the fractionation schedules used are summarized in Table 2.
Table 1.
Patients’ characteristics
| Characteristics | Value |
|---|---|
| All patients | 41 |
| Age, median (range) | 70 (33–92) |
| Male/female | 11/30 |
| Performance status, 0/1/2/3/4 | 13/21/7/0/0 |
| Follow-up (months), median (range) | 49 (7–138) |
| Total tumor volume (ml), median (range) | 10.4 (1.4–56.9) |
| SRS | |
| No. of patients | 9 |
| Tumor volume (ml), median (range) | 4.6 (1.7–29.3) |
| Dose (Gy), median (range) | 17 (13–20) |
| hSRT | |
| No. of patients | 32 |
| Tumor volume (ml), median (range) | 11.3 (1.4–56.9) |
| 2 fractions dose (Gy) | 14 |
| 3 fractions dose (Gy), median (range) | 21 (14.5–24) |
| 5 fractions dose (Gy), median (range) | 25 (18–30) |
| 10 fractions dose (Gy) | 38 |
hSRT: hypofractionated stereotactic radiotherapy, SRS: stereotactic radiosurgery.
Table 2.
Tumor location and number of fractions
| Tumor location | No. of patients | No. of fractions | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 5 | 10 | ||
| Convexity Skull base | 11 | 6 | 2 | 2 | 1 | |
| Sphenoid ridge | 4 | 1 | 3 | |||
| Cerebellopontine angle | 3 | 3 | ||||
| Olfactory groove | 2 | 1 | 1 | |||
| Middle cranial fossa | 2 | 2 | ||||
| Falx | 8 | 1 | 1 | 1 | 5 | |
| Parasagittal | 4 | 4 | ||||
| Tentorial | 3 | 1 | 2 | |||
| Petroclival | 2 | 2 | ||||
| Lateral ventricle | 1 | 1 | ||||
| Tuberculum sellae | 1 | 1 | ||||
| Total | 41 | 9 | 2 | 4 | 25 | 1 |
Treatment planning
All treatments were carried out using the CyberKnife. Our method for CyberKnife treatment was described in detail previously.16) The patient was placed in the supine position, and a thermoplastic mask was molded to the patient’s head and attached to the head support. In principle, contrast-enhanced CT images were used for contouring. Contrast-enhanced MR images were also fused to the CT images in the more recent cases. The gross tumor volume was defined as the enhanced region. The planning target volume was defined as being equal to the gross tumor volume. Dose planning was performed with the On-Target treatment planning system version 3.4.2 (Accuray Inc., Sunnyvale, CA, USA). The prescribed dose covered 95% of the planning target volume (D95). We revised the protocol in 2010. From 2005 to 2009, when the tumor volume was ≥10 ml or critical structures were located nearby, hSRT was delivered on consecutive days, except in one patient, while the other patients were treated with SRS. Thereafter, all patients were treated with hSRT to ensure that higher doses could be prescribed while maintaining low rates of adverse events. During hSRT, the radiation was delivered in 2 to 10 daily fractions. Although our basic fractionation schedule was 25–27.5 Gy in 5 fractions, the dose-fractionation schedule was determined case-by-case, taking tumor location, size, and peritumoral edema into account. Twenty-five (78%) of the 32 patients received hSRT in 5 fractions. The median prescribed dose was 17 Gy (range, 13–20 Gy to the 61–88% isodose line) for SRS (n = 9) and 25 Gy (range, 14–38 Gy to the 44–83% isodose line) for hSRT (n = 32).
Evaluation and statistics
All endpoints were calculated from the start of the SRS or hSRT. When calculating the progression-free survival rate, the dates of imaging follow-up examinations were used. Increases and reductions of ≥2 mm in the longest diameter of the tumor were considered to represent tumor progression and regression, respectively. Tumors whose diameters changed by <2 mm were labeled as unchanged. Overall and progression-free survival rates were calculated using the Kaplan-Meier method, and the significance of inter-group differences was examined using the log rank test. Toxicities were evaluated with the Common Terminology Criteria for Adverse Events, version 4.0. Statistical analyses were carried out with the statistical software package “R”/package = survival (R Development Core Team (2010). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, free download at: www.R-project.org/).
Results
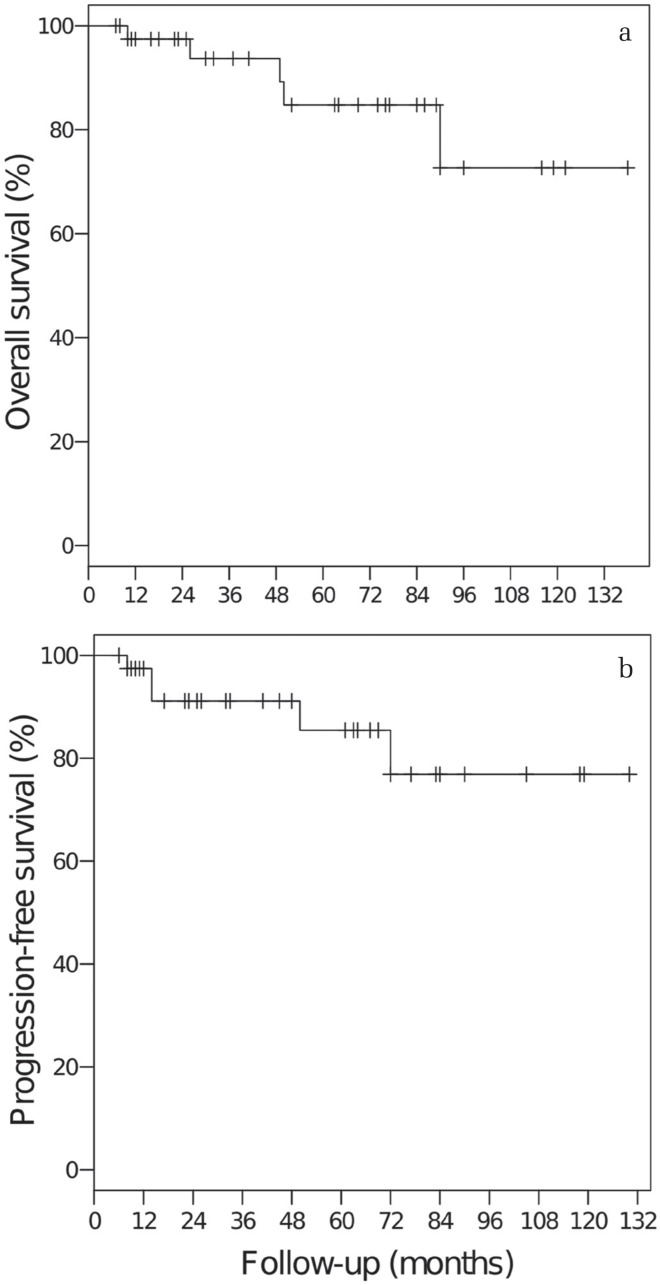
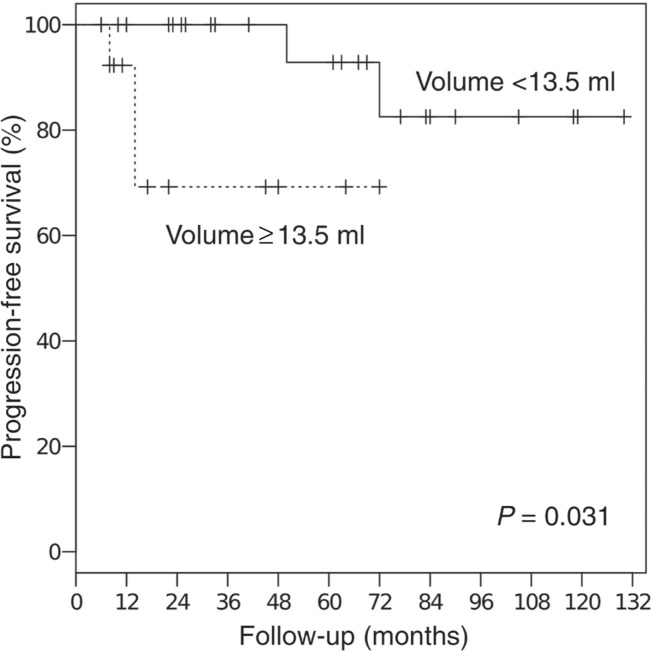
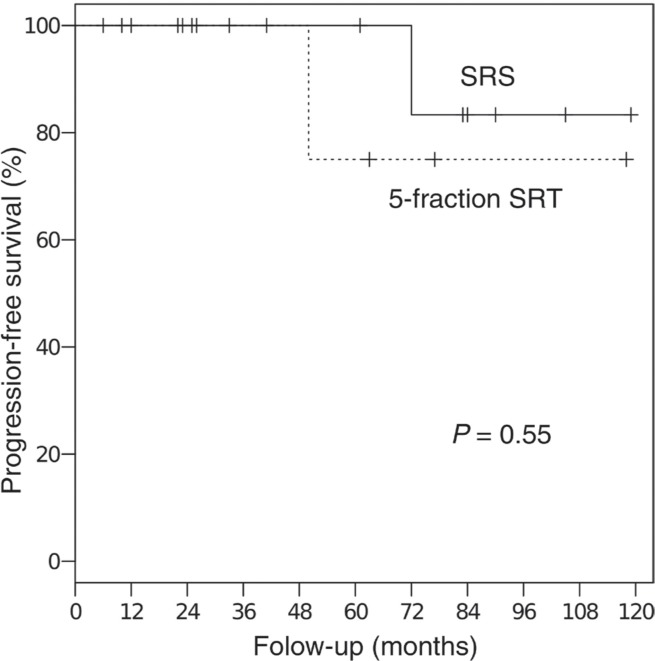
The median duration of the follow-up period was 49 months (range, 7–138 months). The 5-year overall and progression-free survival rates for all 41 patients were 85% and 86%, respectively (Fig. 1). All deaths resulted from other diseases and were unrelated to the treatment. The 3-year progression-free survival rate was 69% for the 14 patients with tumor volumes of ≥13.5 ml (30 mm diameter) and 100% for the 27 patients with tumor volumes of <13.5 ml (P = 0.031, Fig. 2). Among the patients with tumor volumes of <13.5 ml, there was no significant difference in the progression-free survival rate between the patients treated with 5-fraction SRT (n = 14, median dose: 25 Gy) and those treated with SRS (n = 8, median dose: 16.5 Gy), although the median tumor volume of the 5-fraction SRT group was larger than that of the SRS group (8.7 ml vs. 3.8 ml, P < 0.01; t test, Fig. 3).
Fig. 1.
Overall survival rate (a) and progression-free survival rate (b) for all patients.
Fig. 2.
Progression-free survival rates for patients with tumor volumes of ≥13.5 ml or <13.5 ml.
Fig. 3.
Progression-free survival rates for patients with tumor volumes of <13.5 ml that were treated with SRS or 5-fraction SRT.
The tumor volume decreased after treatment in 12 patients, remained stable in 24 patients, and increased in 5 patients (Table 3). Of the 5 patients that exhibited tumor progression, 2 underwent surgery, one underwent re-hSRT, and one underwent surgery followed by re-hSRT. The remaining patient was observed without treatment due to his high age. One of the tumors (50.8 ml, a falx tumor, progression observed at 14 months after hSRT) turned out to be an atypical meningioma (grade II according to the World Health Organization classification) during a pathological examination. Improvements in clinical symptoms, such as hearing disturbance and visual disorders, were seen in 2 patients.
Table 3.
Tumor volume change after treatment
| Initial volume | <13.5 ml | ≥13.5 ml | Total |
|---|---|---|---|
| Decreased | 10 | 2 | 12 (29%) |
| Stable | 15 | 9 | 24 (59%) |
| Increased | 2 | 3 | 5 (12%) |
The grade >2 toxicities observed in this study included a grade 4 optic nerve disorder (a tuberculum sellae tumor, 15.5 ml, 1 month after hSRT, the patient’s eyesight before the hSRT was less than 20/200), grade 4 cerebral necrosis requiring necrotomy (a middle cranial fossa tumor, 5 months after hSRT), grade 3 stroke (cerebral artery occlusion) due to cerebral edema in 2 patients (sphenoid ridge tumors; 51.5 ml and 19.1 ml, respectively; both tumors compressed the middle cerebral artery before hSRT, 3 and 1 years after hSRT, respectively), grade 3 hydrocephalus (a sphenoid ridge tumor, 19.1 ml, 10 months after hSRT), and grade 3 pyramidal tract syndrome due to cerebral edema (a convexity tumor, 21.9 ml, 6 months after hSRT). One patient experienced both a stroke and hydrocephalus. No grade >2 toxicities were observed in the patients with tumor volumes of <13.5 ml.
Discussion
Promising results have been published for imaging-diagnosed meningiomas that were treated with SRS, fractionated SRT, or hSRT (Table 4). Although the median tumor volume in this study was relatively large compared with those described in previous reports, the progression-free survival rate was comparable. In this study, it was found that large tumors (≥13.5 ml, 30 mm in diameter) were difficult to control (Fig. 2). Similar results have been reported for SRS and normofractionated SRT.5,12)
Table 4.
Summary of published studies of SRS, fSRT, and hSRT for imaging-diagnosed meningiomas
| Authors | Year | Tumors (n) | Imaging diagnosed tumors (n) | Median volume (ml) | Technique | Dose (Gy) | Follow-up (months) | 5-year PFS (%) | Toxicity (%) |
|---|---|---|---|---|---|---|---|---|---|
| Flickinger et al.9) | 2003 | 219 | 219 | 5.0 | SRS | 14 | 29 | 93 | 8.8 |
| DiBiase et al.12) | 2004 | 121 | 85 | 4.5 | SRS | 14 | 54 | 86 | 8.3 |
| Milker-Zabel et al.6) | 2005 | 317 | 97 | 33.6 | fSRT | 57.6 | 67 | 91 | 8.2 |
| 12.0 | fSRT | 55.8 | |||||||
| Henzel et al.4) | 2006 | 224 | 95 | 3.8 | hSRT | 35–50 | 36 | 97 | 8.2 |
| 1.9 | SRS | 15–18 | |||||||
| Colombo et al.11) | 2009 | 199 | 85 | 7.5* | hSRT | 18.5* | 30 | 94 | 5.3 |
| Minniti et al.7) | 2011 | 57 | 29 | 35.4 | fSRT | 50 | 42 | 93 | 5.5 |
| Morimoto et al.18) | 2011 | 32 | 17 | 6.3 | hSRT | 27.8 | 48 | 87 | 9.7 |
| Santacroce et al.13) | 2012 | 4565 | 2976 | 4.8 | SRS | 14 | 63 | 95 | 4.8 |
| 16.0 | fSRT | 55.8 | |||||||
| Fokas et al.3) | 2014 | 318 | 176 | 6.1 | hSRT | 40 | 50 | 93 | 12.0 |
| 1.84 | SRS | NA | |||||||
| Present study | 2017 | 41 | 41 | 4.6 | SRS | 17 | 49 | 86 | 12.1 |
| 11.3 | hSRT | 25 |
fSRT: normofractionated stereotactic radiotherapy, hSRT: hypofractionated stereotactic radiotherapy, NA: not available, PFS: progression-free survival rate, SRS: stereotactic radiosurgery, *mean.
The doses required to control tumors of various volumes is an important issue. Shrieve et al.23) estimated that meningiomas exhibit α/β ratios of 2.7–3.85 Gy based on the assumption that 15 Gy in one fraction and 54 Gy in 30 fractions are isoeffective at controlling benign meningiomas. Assuming that meningiomas have low α/β ratios, hypofractionated regimens might be suitable for increasing the biological effective dose (BED) while maintaining a low total dose. However, it should be noted that the linear-quadratic model overestimates the effect of a high fractional dose of radiation.24,25) The linear-quadratic model might only be applicable to fractional doses up to twice the α/β ratio.25) In practice, this study suggested that 25 Gy in 5 fractions was not inferior to 16.5 Gy in one fraction when the volume of the tumor was <13.5 ml, although, assuming an α/β ratio of 3 Gy, these regimens resulted in BED of 66.7 Gy and 107.2 Gy, respectively (Fig. 3).
Benign meningiomas rarely directly cause death. Indeed, in this study the 5-year overall survival rate was 85%, and all deaths were caused by other diseases. Thus, reducing the rates of adverse events is important during the treatment of meningioma. Therefore, SRS and hSRT are reasonable treatment strategies for imaging-diagnosed meningiomas because they are minimally invasive. However, adverse events are more likely to occur in cases in which the tumor is large or located near to organs at risk. In this study, a grade 4 optic nerve disorder and grade 3 cerebral artery occlusion were observed. The maximum doses delivered to the optic nerve and middle cerebral artery in these patients were 25 Gy and 21 Gy in 5 fractions, respectively. These doses were considered to be below or around the tolerated dose,17,19,20,26) but marked cerebral edema and severe compression of the optic nerve and medial cerebral artery by the tumors were seen prior to treatment. Moreover, Morimoto et al.18) reported that large tumor volumes (>11 ml, 2.56 cm in diameter) were associated with peritumoral edema during hSRT treatment. These factors might cause severe adverse events. In such cases, it is unclear whether the effectiveness of the planned treatment or the safety of organs at risk should be considered first. Larger tumors might require higher doses of radiation, but the dose that can be tolerated by the surrounding nerves must be considered. Since nerve tissue has a low α/β ratio,27) the estimated tolerated dose for nerve tissue is lower than those for other tissues in the setting of SRS and hSRT. For example, the administration of 25 Gy in 5 fractions will be tolerated, but the administration of 16.5 Gy in one fraction might result in excessive doses being administered to the optic nerve and chiasma.23,26) To reduce the risk of toxicities in these tissues, the administration of 25 Gy in 5 fractions might be reasonable for small tumors, but slightly reducing the dose in one session and increasing the number of fractions (e.g., 36–40 Gy in 10 fractions) or the use of normofractionated SRT (1.8–2 Gy per fraction) might be recommended for large tumors located near to these structures.6) Indeed, acceptable control rates and low rates of adverse events have been detected after normofractionated SRT was used to treat large tumors (Table 4).6,7) Taking the results of the present and previous studies together, SRS or hSRT might be indicated for cases with small tumors (<11 ml) that are not surrounded by critical organs, such as the optic nerve, chiasma, or brain stem. When the tumor is small, but located near to critical organs, hSRT or normofractionated SRT might be recommended. For large (≥13.5 ml) tumors, normofractionated SRT should be taken into consideration.
The limitations of this study include the fact that not all of the imaging-diagnosed tumors were histologically confirmed to be benign meningiomas prior to treatment. As a result, one tumor turned out to be an atypical meningioma after it recurred. Atypical meningiomas might require higher doses of radiation to bring them under control because of their high recurrence rates.6,8) Flickinger et al.9) reported that in cases of imaging-diagnosed intracranial meningioma the actuarial rate of a diagnosis other than meningioma was 2.3% at 5 and 10 years after the initial radiotherapy. It is difficult to distinguish malignant meningiomas from the benign type using conventional diagnostic imaging alone,28) so in cases involving patients with medically inoperable disease or patients with operable disease that refuse surgery and biopsy examinations there is no choice but to treat the tumor as if it were benign. Recently, D-thallium-201 chloride single-photon emission CT and MRI, including diffusion tensor imaging, were reported to be useful for distinguishing between benign and malignant meningiomas before treatment.29,30) These methods could help to ensure that the most appropriate treatments are used to treat meningioma patients. Further investigations of this topic are warranted.
Conclusion
Despite the relatively large median tumor volume seen in this study, the obtained progression-free survival rates were favorable. SRS and hSRT were found to be safe and effective treatments for small tumors (<13.5 ml, 30 mm in diameter). Taking the tumors’ locations and the patients’ conditions into account, the toxicities encountered in this study were deemed to be acceptable. For large (≥13.5 ml) tumors, a normofractionated regimen should also be considered.
Acknowledgment
We gratefully acknowledge Yasuhiro Matsushita and Tatsuya Ichihashi for treating the patients and Yuko Omiya for diagnosing the patients.
Footnotes
Conflicts of Interest Disclosure
The authors declare that they have no conflicts of interest.
References
- 1).Bondy M, Ligon BL: Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol 29: 197–205, 1996 [DOI] [PubMed] [Google Scholar]
- 2).Korah MP, Nowlan AW, Johnstone PA, Crocker IR: Radiation therapy alone for imaging-defined meningiomas. Int J Radiat Oncol Biol Phys 76: 181–186, 2010 [DOI] [PubMed] [Google Scholar]
- 3).Fokas E, Henzel M, Surber G, Hamm K, Engenhart-Cabillic R: Stereotactic radiation therapy for benign meningioma: long-term outcome in 318 patients. Int J Radiat Oncol Biol Phys 89: 569–575, 2014 [DOI] [PubMed] [Google Scholar]
- 4).Henzel M, Gross MW, Hamm K, et al. : Stereotactic radiotherapy of meningiomas: symptomatology, acute and late toxicity. Strahlenther Onkol 182: 382–388, 2006 [DOI] [PubMed] [Google Scholar]
- 5).Onodera S, Aoyama H, Katoh N, et al. : Long-term outcomes of fractionated stereotactic radiotherapy for intracranial skull base benign meningiomas in single institution. Jpn J Clin Oncol 41: 462–468, 2011 [DOI] [PubMed] [Google Scholar]
- 6).Milker-Zabel S, Zabel A, Schulz-Ertner D, Schlegel W, Wannenmacher M, Debus J: Fractionated stereotactic radiotherapy in patients with benign or atypical intracranial meningioma: long-term experience and prognostic factors. Int J Radiat Oncol Biol Phys 61: 809–816, 2005 [DOI] [PubMed] [Google Scholar]
- 7).Minniti G, Clarke E, Cavallo L, et al. : Fractionated stereotactic conformal radiotherapy for large benign skull base meningiomas. Radiat Oncol 6: 36, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Askoxylakis V, Zabel-du Bois A, Schlegel W, Debus J, Huber P, Milker-Zabel S: Patterns of failure after stereotactic radiotherapy of intracranial meningioma. J Neurooncol 98: 367–372, 2010 [DOI] [PubMed] [Google Scholar]
- 9).Flickinger JC, Kondziolka D, Maitz AH, Lunsford LD: Gamma knife radiosurgery of imaging-diagnosed intracranial meningioma. Int J Radiat Oncol Biol Phys 56: 801–806, 2003 [DOI] [PubMed] [Google Scholar]
- 10).Pollock BE, Stafford SL: Results of stereotactic radiosurgery for patients with imaging defined cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys 62: 1427–1431, 2005 [DOI] [PubMed] [Google Scholar]
- 11).Colombo F, Casentini L, Cavedon C, Scalchi P, Cora S, Francescon P: Cyberknife radiosurgery for benign meningiomas: short-term results in 199 patients. Neurosurgery 64(2 Suppl): A7–A13, 2009 [DOI] [PubMed] [Google Scholar]
- 12).DiBiase SJ, Kwok Y, Yovino S, et al. : Factors predicting local tumor control after gamma knife stereotactic radiosurgery for benign intracranial meningiomas. Int J Radiat Oncol Biol Phys 60: 1515–1519, 2004 [DOI] [PubMed] [Google Scholar]
- 13).Santacroce A, Walier M, Régis J, et al. : Long-term tumor control of benign intracranial meningiomas after radiosurgery in a series of 4565 patients. Neurosurgery 70: 32–39; discussion 39, 2012 [DOI] [PubMed] [Google Scholar]
- 14).Goto T, Ohata K: Surgical resectability of skull base meningiomas. Neurol Med Chir (Tokyo) 56: 372–378, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Adler JR, Murphy MJ, Chang SD, et al. : Image-guided robotic radiosurgery. Neurosurgery 44: 1299–1306; discussion 1306–1307, 1999 [PubMed] [Google Scholar]
- 16).Murai T, Ogino H, Manabe Y, et al. : Fractionated stereotactic radiotherapy using CyberKnife for the treatment of large brain metastases: a dose escalation study. Clin Oncol (R Coll Radiol) 26: 151–158, 2014 [DOI] [PubMed] [Google Scholar]
- 17).Demiral S, Dincoglan F, Sager O, et al. : Hypofractionated stereotactic radiotherapy (HFSRT) for who grade I anterior clinoid meningiomas (ACM). Jpn J Radiol 34: 730–737, 2016 [DOI] [PubMed] [Google Scholar]
- 18).Morimoto M, Yoshioka Y, Shiomi H, et al. : Significance of tumor volume related to peritumoral edema in intracranial meningioma treated with extreme hypofractionated stereotactic radiation therapy in three to five fractions. Jpn J Clin Oncol 41: 609–616, 2011 [DOI] [PubMed] [Google Scholar]
- 19).Conti A, Pontoriero A, Midili F, et al. : CyberKnife multisession stereotactic radiosurgery and hypofractionated stereotactic radiotherapy for perioptic meningiomas: intermediate-term results and radiobiological considerations. Springerplus 4: 37, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Iwata H, Tatewaki K, Inoue M, et al. : Single and hypofractionated stereotactic radiotherapy with CyberKnife for craniopharyngioma. J Neurooncol 106: 571–577, 2012 [DOI] [PubMed] [Google Scholar]
- 21).Saloner D, Uzelac A, Hetts S, Martin A, Dillon W: Modern meningioma imaging techniques. J Neurooncol 99: 333–340, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Goldsher D, Litt AW, Pinto RS, Bannon KR, Kricheff II: Dural “tail” associated with meningiomas on Gd-DTPA-enhanced MR images: characteristics, differential diagnostic value, and possible implications for treatment. Radiology 176: 447–450, 1990 [DOI] [PubMed] [Google Scholar]
- 23).Shrieve DC, Hazard L, Boucher K, et al. : Dose fractionation in stereotactic radiotherapy for parasellar meningiomas: radiobiological considerations of efficacy and optic nerve tolerance. J Neurosurg 101 Suppl 3: 390–395, 2004 [PubMed] [Google Scholar]
- 24).Iwata H, Matsufuji N, Toshito T, Akagi T, Otsuka S, Shibamoto Y: Compatibility of the repairable- conditionally repairable, multi-target and linear-quadratic models in converting hypofractionated radiation doses to single doses. J Radiat Res 54: 367–373, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Shibamoto Y, Otsuka S, Iwata H, Sugie C, Ogino H, Tomita N: Radiobiological evaluation of the radiation dose as used in high-precision radiotherapy: effect of prolonged delivery time and applicability of the linear-quadratic model. J Radiat Res 53: 1–9, 2012 [DOI] [PubMed] [Google Scholar]
- 26).Hiniker SM, Modlin LA, Choi CY, et al. : Dose-response modeling of the visual pathway tolerance to single-fraction and hypofractionated stereotactic radiosurgery. Semin Radiat Oncol 26: 97–104, 2016 [DOI] [PubMed] [Google Scholar]
- 27).Meeks SL, Buatti JM, Foote KD, Friedman WA, Bova FJ: Calculation of cranial nerve complication probability for acoustic neuroma radiosurgery. Int J Radiat Oncol Biol Phys 47: 597–602, 2000 [DOI] [PubMed] [Google Scholar]
- 28).Kunimatsu A, Kunimatsu N, Kamiya K, Katsura M, Mori H, Ohtomo K: Variants of meningiomas: a review of imaging findings and clinical features. Jpn J Radiol 34: 459–469, 2016 [DOI] [PubMed] [Google Scholar]
- 29).Takeda T, Nakano T, Asano K, Shimamura N, Ohkuma H: Usefulness of thallium-201 SPECT in the evaluation of tumor natures in intracranial meningiomas. Neuroradiology 53: 867–873, 2011 [DOI] [PubMed] [Google Scholar]
- 30).Toh CH, Castillo M, Wong AM, et al. : Differentiation between classic and atypical meningiomas with use of diffusion tensor imaging. AJNR Am J Neuroradiol 29: 1630–1635, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]