Abstract
With advanced understanding of molecular background and correlation with therapeutic outcomes, the revised 4th edition of World Health Organization (WHO) classification of central nervous system (CNS) tumors incorporated molecular information into the definition of diffuse gliomas. Indeed, oligodendroglioma and astrocytoma are now defined by molecular signature, with diagnosis of glioblastoma being made by histology. In parallel, numerous clinical trials are underway all over the world, and important findings are being produced every year that have an impact on patient outcomes. Moreover, novel therapies/technologies are also being actively developed; however, there are still many CNS tumors for which no effective therapy has been established except radiotherapy. In this article, the authors review the recent results of major clinical trials and present their treatment recommendations for patients with adult, supratentorial diffuse gliomas of grades II and III stratified according to the new WHO classification.
Keywords: diffuse glioma, PCV, 1p/19q, temozolomide, MGMT
Introduction
After histology-based classification for nearly a century, World Health Organization (WHO) classification of tumors of the central nervous system (CNS) has incorporated molecular parameters into the revised 4th edition.1,2) Gliomas are a prime example of tumors whose classification is now primarily based on genotype. These drastic changes entail new challenges with respect to testing and reporting; several molecular analyses required for classification are currently unavailable in many institutes/hospitals in Japan as well as in other countries, and some tumors may not fit into any of the diagnostic categories. From the therapeutic point of view, however, because the new classification seeks to define disease entities as narrowly and objectively as possible in order to establish highly biologically uniform groups,3) the integrated diagnosis incorporating important prognostic markers is more straight forward for therapeutic decision making, with decrease in clinical confusion due to biological heterogeneity in a histology-based entity. Indeed, the new classification separates astrocytoma and oligodendroglioma solely based on the presence or absence of 1p/19q codeletion. These diffuse gliomas are sharply separated from astrocytomas with circumscribed growth pattern such as pilocytic astrocytomas and pleomorphic xanthoastrocytomas more upstream of the family tree.1)
In this article, we review recent results of major clinical trials for adult, supratentorial diffuse gliomas of grades II and III and present our treatment recommendation based on these evidences stratified according to the new WHO classification. Our current practice of routine molecular tests is also presented.
Summary of Key Clinical Trials for Adult Gliomas of Grades II and III
Radiation Therapy Oncology Group (RTOG) 9802 (Grade II)
In this study, adult patients with supratentorial grade II gliomas without prior radiotherapy (RT) or chemotherapy were dichotomized into two risk groups; patients less than 40-years old (y/o) who had a neurosurgeon-determined gross total resection (GTR) were deemed to have a favorable risk, and patients ≥ 40 with any degree of resection or those < 40 who had undergone less than a GTR were deemed to have an unfavorable risk. Patients with favorable risk were only observed postoperatively (phase II), and patients with unfavorable risk were randomly assigned to RT (54 Gy/30 fractions (fr)) alone or RT followed by 6 courses of PCV (procarbazine, CCNU = lomustine, vincristine) chemotherapy (phase III).
In the phase II trial for favorable risk patients,4) the overall survival (OS) rates at 2 and 5 years were 99% and 93%, respectively; however, the progression-free survival (PFS) rates at 2 and 5 years were 82% and 48%, respectively. The criteria of GTR was based on operative reports, and the PFS rate at 5 years was 67% (estimated from Fig. 2 of reference 4) in patients with < 1 cm residual disease, versus 30% in patients with ≥ 1 cm residual disease. Preoperative tumor diameter ≥ 4 cm, astrocytoma/oligoastrocytoma histology, and post-operative residual tumor ≥ 1 cm were associated with poorer PFS. The investigators suggested that the most favorable subset of patients with grade II gliomas (< 1 cm residual tumor, tumor diameter < 4 cm, and oligodendroglioma histology) might be observed postoperatively, while all other patients became reasonable candidates for adjuvant treatment to reduce the risk of tumor progression.
In the analyses of the phase III trial for unfavorable risk patients with long-term follow-up (median follow-up of the alive patients: 11.9 y),5,6) both PFS and OS were significantly improved by the addition of PCV in a whole cohort (Table 1). The histological subtypes of the patients enrolled in the study were astrocytoma in 23%, oligodendroglioma in 45%, and mixed oligoastrocytoma in 32%. RT + PCV was superior in all histological subtypes, although the difference did not reach significance among patients with astrocytoma (PFS HR 0.58, P = 0.06; OS HR 0.73, P = 0.31). Moreover, in patients with tumoral IDH1 R132H mutation, both PFS and OS were improved by the addition of PCV (PFS HR 0.32, P < 0.001; OS HR 0.42, P = 0.02). Treatment effect was not evaluable in patients without IDH1 mutation. Although no survival analyses was provided according to 1p/19q status, this study showed that the great majority of patients with grade II gliomas benefit from the addition of PCV chemotherapy to RT, and the treatment effect appeared largest in patients with oligodendroglioma histology. The survival curves began to separate after 2 to 4 years, suggesting a delayed benefit of chemotherapy.
Table 1.
Summary of the landmark clinical trials in adult diffuse gliomas
| Clinical trial | Eligibility | Treatment | Phase | Main results | Reference |
|---|---|---|---|---|---|
| Grade II | |||||
| RTOG9802 | WHO grade II glioma < 40 & neurosurgeon-determined GTR n = 111 | Post-operative observation | II | PFS at 5 y: 48%, OS at 5 y: 93% | Shaw et al. J Neurosurg 20084) |
| WHO grade II glioma ≥ 40 or STR / PR / Biopsy n = 254 | RT vs RT + PCV 6 | III | mPFS (whole): 4.0 y vs. 10.4 y, HR 0.50, P < 0.001 mOS (whole): 7.8 y vs. 13.3 y, HR 0.59, P = 0.003 (Astro: HR 0.73, P = 0.31) (OA: HR 0.56, P = 0.05) (Oligo: HR 0.43, P = 0.009) (IDH1mut: HR 0.42, P = 0.02) | Buckner et al. N Engl J Med 20165) | |
| EORTC22033–26033 | WHO grade II glioma n = 477 | RT vs Dose-intense TMZ (21/28) | III | mPFS (whole): RT 46 m vs TMZ 39 m, HR (of TMZ vs RT) 1.16, P = 0.22 mPFS: IDHmt/codel 62 m, IDHmt/non-codel 48 m, IDHwt 20 m (IDHmt/non-codel vs IDHmt/codel: HR 1.51, P = 0.018; IDHwt vs IDHmt/codel: HR 4.08, P < 0.0001) | Baumert et al. Lancet Oncol 20167) |
| Grade III | |||||
| NOA-04 | WHO grade III glioma n = 318 | RT vs Chemotherapy (PCV or TMZ) | III | mPFS (whole): RT 30.6 m vs chemo 31.9 m, HR 1.0, P = 0.87 mTTF (whole): RT 42.7 m vs. chemo 43.8 m, HR (of chemo vs RT) 1.2, P = 0.28 | Wick et al. J Clin Oncol 200911) |
| RTOG9402 | Anaplstic oligodendroglioma / oligoastrocytoma n = 289 | RT vs PCV 4 + RT | III | mPFS (whole): RT 1.7 y vs PCV + RT 2.6 y, HR 0.69, P = 0.004 mPFS (codel): RT 2.6y vs PCV + RT not reached, HR 0.42, P = 0.001 mPFS (non-codel): RT 1 y vs PCV + RT 1.4 y, HR 0.78, P = 0.12 mOS (whole): RT 4.7 y vs PCV + RT 4.6 y, HR (PCV+RT vs RT) 0.79, 95% CI 0.60 to 1.04, P = 0.1 mOS (codel): RT 7.3 y vs PCV + RT 14.7 y, HR 0.59, P = 0.03 mOS (non-codel): RT2.7y vs PCV+RT 2.6y, HR 0.85, P = 0.39 | Cairncross et al. J Clin Oncol 200612) Cairncross et al. J Clin Oncol 201314) |
| EORTC26951 | Anaplstic oligodendroglioma / oligoastrocytoma n = 368 | RT vs RT + PCV 6 | III | mPFS (whole): RT 13.2 m vs RT + PCV 24.3 m, HR 0.66, P = 0.0003 mPFS (codel): RT 50 m vs RT + PCV 157 m, HR 0.42, P = 0.002 mPFS (non-codel): RT 9 m vs RT + PCV 15 m, HR 0.73, P = 0.026 mOS (whole): RT 30.6 m vs PCV + RT 42.3 m, HR 0.75, P = 0.018 mOS (codel): RT 112 m vs RT + PCV not reached, HR 0.56, P = 0.059 mOS (non-codel): RT 21 m vs RT + PCV 25 m, HR0.83, P = 0.185 | van den Bent et al. J Clin Oncol 200613) van den Bent et al. J Clin Oncol 201315) |
| CATNON | Anaplastic glioma without 1p/19q codeletion n = 745 | RT vs RT/TMZ vs RT + TMZ 12 vs RT/TMZ + TMZ 12 | III | adjusted OS (adjuvant TMZ): HR 0.645, P = 0.0014 | van den Bent et al. J Clin Oncol (suppl) 201618) |
m: months, mOS: median overall survival, mPFS: median progression-free survival, mTTF: median time to treatment failure, PCV: procarbazine, CCNU = lomustine, vincristine, RT: radiotherapy, TMZ: temozolomide.
European Organization for Research and Treatment of Cancer (EORTC) 22033–26033 (Grade II)
This is a phase III intergroup trial comparing RT (50.4 Gy/28 fr) alone versus dose-intense temozolomide (TMZ) monotherapy (75 mg/m2, 21/28 days schedule) in adults with supratentorial grade II glioma with at least one high-risk factor (age > 40 years, radiological progressive disease, tumor size > 5 cm, tumor crossing the midline, neurological symptoms).7) With the median follow-up of 48 months, there was no significant difference in PFS (primary endpoint) between the 2 treatment arms (Table 1). Both IDH and 1p/19q status were available in 318 cases, and three molecular subgroups (IDHmt/codel, IDHmt/non-codel, IDHwt) were significantly associated with PFS (Table 1). Patients with IDH mutation and non-codeleted tumors were associated with longer PFS with RT alone than with TMZ monotherapy (HR 1.86, P = 0.004), however, no treatment-related difference was observed in the other 2 molecular subtypes. Importantly, there was no significant difference between RT and TMZ groups in the 7 key health-related quality of life (HRQOL) scales and neurocognitive function (Mini-Mental State Examination) during 36 months of follow-up.8)
This study demonstrated that the IDH and 1p/19q–based molecular subgrouping in grade II gliomas indeed correlates with patients’ outcomes in a prospectively and uniformly treated cohort for the first time, which is consistent with previous datasets of lower grade gliomas.9,10) Although mature results for OS after long-term follow-up is awaited, the current dataset of this study suggests that initial radiotherapy is superior to upfront chemotherapy in IDHmt/con-codeleted grade II glioma (namely, diffuse astrocytoma, IDH-mutant in the new classification).
NOA-04 (Grade III)
In this phase III trial conducted in Germany, adult patients with WHO grade III gliomas were randomly assigned 2:1:1 to receive RT or PCV or TMZ.11) At disease progression, patients in the RT arm were treated with PCV or TMZ (1:1 random assignment), whereas patients in PCV or TMZ arm received RT. The primary endpoint was the time to treatment failure (TTF, treatment failure = progression after RT and one chemotherapy). There was no significant difference in either TTF or PFS between RT and chemotherapy arms in the entire cohort (Table 1). Moreover, neither TTF nor PFS differed between treatments within any of the three histologic groups (anaplastic astrocytoma, anaplastic oligoastrocytoma, anaplastic oligodendroglioma). Therefore, the treatment sequence (chemotherapy first then radiotherapy at progression, or vice versa) did not affect the prognoses of the patients with grade III glioma regardless of histological subtype. There was no difference in PFS between patients treated with PCV versus TMZ. Surprisingly, promoter methylation of the O6-methylguanine-DNA methyltransferase (MGMT) gene was associated with better PFS not only in chemotherapy arms but also in RT arm.
EORTC 26951, RTOG 9402 (Grade III)
In the 1990s, 2 phase III studies had been conducted in adult patients with newly diagnosed, supratentorial anaplastic oligodendrogliomas/oligoastrocytomas; one in Europe (EORTC) and the other in North America (RTOG).12,13) The EORTC study compared RT (59.4 Gy/33 fr) alone versus RT followed by 6 courses of PCV, and the RTOG study compared RT (59.4 Gy/33 fr) alone versus RT following 4 courses of intensified PCV. In both studies, the initial reports (median follow-up: EORTC 60 m, RTOG 5.1 y) suggested improvement of PFS but not OS by the addition of PCV for the entire cohort; patients with 1p/19q codeleted tumors showed significantly better outcomes (PFS/OS) than those with non-codeleted tumors regardless of treatment arms. Although PFS was improved by the addition of PCV in patients with codeleted tumors (but no or minimal improvement in those with non-codeleted tumors) (Table 1), this benefit disappeared in OS likely due to the efficacy of crossover chemotherapy at the time of progression. Therefore, at the time of the initial report, the results of the 2 studies were interpreted as chemotherapy being effective to improve PFS of patients with codeleted grade III gliomas, and timing of chemotherapy not being relevant to OS.
With long-term follow-up (median follow-up: EORTC 140 m, RTOG 11.3 y),14,15) both PFS and OS were improved by the addition of PCV for the entire cohort in the EORTC trial, and PFS only was significantly improved in the RTOG study (Table 1). Most importantly, in both trials, not only PFS but also OS of patients with codeleted gliomas was significantly improved by the addition of PCV (Table 1). In contrast, there was no significant difference between treatment arms in OS of patients with non-codeleted gliomas.
These trials suggested that outcomes (PFS/OS) in patients with codeleted grade III gliomas are much better than in those without codeletion regardless of treatment, and that the initial combined chemoradiotherapy is far more effective for codeleted grade III gliomas than sequential treatment, with RT first and chemotherapy at progression. It is interesting that, similar to the results of the RTOG 9802 study in grade II gliomas, the survival curves for patients with codeleted gliomas began to diverge 5–6 years after randomization, again suggesting delayed benefit of chemotherapy.
The RTOG group later analyzed whether IDH mutation status was associated with benefit of PCV within the trial cohort.16) As a result, not only patients with codeleted, mutated tumors but also those with non-codeleted, mutated tumors lived longer after PCV + RT than RT alone, suggesting that IDH mutations may also be predictive for benefit from chemotherapy (codeleted mutated tumor: PCV + RT 14.7 y vs RT 6.8 y, HR 0.49, P = 0.01; non-codeleted mutated tumor: PCV + RT 5.5 y vs RT 3.3 y, HR 0.56, P < 0.05). However, the analyses within the EORTC study cohort did not show evidence of a predictive value of IDH mutations.17)
CATNON (Grade III)
This is an intergroup phase III trial in adult patients with newly diagnosed grade III gliomas lacking 1p/19q codeletion. The patients were randomized to RT alone, RT with concurrent daily TMZ, RT followed by adjuvant TMZ (5/28-day schedule, 12 courses), and RT with both concurrent and adjuvant TMZ (Stupp regimen). The results of the first interim analysis after 219 events showed that adjuvant TMZ and MGMT methylation were significantly associated with improved OS in the multivariate analysis (adjuvant TMZ: HR 0.645, P = 0.0014; MGMT methylation: HR 0.49, P = 0.0031).18)
Treatment Recommendations for Adult Gliomas of Grades II and III According to the New WHO Classification
Treatment recommendations according to the revised WHO classification are provided based on the above evidence with our perspectives19) (Table 2).
Table 2.
Evidence-based standard of care and treatment recommendation for adult diffuse gliomas
| WHO 2016 | Evidence-based standard of care | Treatment recommendation | |
|---|---|---|---|
| Grade II | Oligodendroglioma, IDH-mutant and 1p/19q-codeleted | RT#1 → PCV 6 courses | RT#1 → PAV 4–6* courses or PAV 4 courses → RT#1 or PAV 3–6 courses#3 |
| Diffuse astrocytoma, IDH-mutant | unknown (may be) RT#1 → PCV 6 courses | RT#1 → TMZ 6–12* courses or RT#1/TMZ → TMZ 6–12* courses | |
| Grade III | Anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted | PCV 4 courses → RT#2 or RT#2 →PCV 6 courses | PAV 4 courses → RT#2 or RT#2 → PAV 4–6* courses |
| Anaplastic astrocytoma, IDH-mutant | unknown (may be) RT#2 → TMZ 12 courses or RT#2/TMZ → TMZ 12 courses | RT#2 → TMZ 6–12* courses or RT#2/TMZ → TMZ 6–12* courses |
PAV: procarbazine, ACNU = nimustine, vincristine (in Japan, lomusitne could be substituted by nimustine) (sasaki JNO),25) PCV: procarbazine, CCNU = lomustine, vincristine (cairncross 2006),12) RT/TMZ: radiotherapy and concurrent and adjuvant TMZ, TMZ: temozolomide, RT#1: radiotherapy 50–54 Gy in 1.8–2.0 Gy fraction, RT#2: radiotherapy 59.4–60 Gy in 1.8–2.0 Gy fraction, PAV 3–6 courses#3: for elderly patients or patients with minimal residual disease, *The number of treatment courses may be personalized dependent on efficacy, toxicity, and MGMT status.
Post-operative observation for grade II gliomas
EORTC 22845 was the only randomized study in low-grade glioma (grade II) comparing early treatment versus post-operative observation, and demonstrated that early RT improved PFS by about 2 years, but did not affect OS.20) Therefore, the timing of treatment intervention does not appear to affect OS in patients with grade II gliomas, and the post-operative adjuvant treatment could be deferred as long as the patient is in good condition and carefully monitored.
However, it should be noted that, as suggested by the phase II study of post-operative observation for favorable risk patients with grade II gliomas (RTOG 9802), more than half of the patients with neurosurgeon-determined GTR might suffer from tumor progression within 5 years after resection if no adjuvant treatment is prescribed.4) Therefore, clinicians should bear in mind the optimal timing of treatment if a wait-and-see policy is employed. For example, if the initial resection is insufficient, a radical second resection should be intended when progression is suggested radiologically, and adjuvant treatment may be considered after the second resection, namely, at the time when the residual tumor volume is the least.
Oligodendroglioma, IDH-mutant and 1p/19q-codeleted
The results of RTOG9802 suggested that, in the great majority of patients with grade II gliomas, initial treatment with RT + PCV is superior to the sequential treatment (initial RT and chemotherapy at the time of relapse).5) The benefit from the addition of PCV was largest in patients with oligodendroglioma histology. Thus, together with the correlation of the 1p/19q codeletion to chemotherapeutic response,14,21) the evidence-based standard treatment for oligodendroglioma, IDH-mutant and 1p/19q-codeleted is considered to be RT + PCV (Table 2). Although lomustine has not been approved in Japan unfortunately, previous case series and a meta-analysis comparing survival gain between nitrosourea drugs suggest that PAV (procarbazine, ACNU = nimustine, vincristine) or PMV (procarbazine, MCNU = ranimustine, vincristine) regimens could be substituted for PCV22–25) (Table 2).
Although it is unclear whether TMZ might be equally effective for grade II codeleted gliomas at the moment, PCV may also be replaced with TMZ with an easier schedule and better tolerance. However, it should be noted that a large retrospective study suggested the superiority of PCV over TMZ for grade III codeleted gliomas,26) and recent papers demonstrated the possible induction of hypermutator phenotype by TMZ.27) Moreover, if TMZ is used instead of PCV, it is unclear whether it should be given in an adjuvant setting or concurrently with RT or in both concurrent and adjuvant settings (Stupp regimen). Because vincristine, a part of PCV regimen, may not penetrate the blood-brain barrier,28,29) single agent nitrosourea (ACNU) might also be allowed instead of PAV.
Many neuro-oncologists may prefer chemotherapy alone as an initial treatment for 1p/19q-codeleted gliomas to avoid the risk of late RT-induced neurocognitive decline and to reserve a therapy for the time of relapse. It is unclear whether salvage RT coupled with second-line chemotherapy at the time of relapse following upfront chemotherapy is equally effective as the initial combined therapy. Indeed, previous case series showed that second-line PCV or TMZ after failure of the other were associated with a decreased response rate in comparison with first line PCV or TMZ.30–32) However, the chemotherapy alone treatment would be reasonably indicated for some cases such as elderly patients and patients with minimal residual disease.
Diffuse astrocytoma, IDH-mutant
The RTOG 9802 trial showed that the addition of PCV to RT was associated with trends toward improved PFS and OS in patients with astrocytomas defined by histology.5) Moreover, in patients with tumoral IDH1 R132H mutation, both PFS and OS were improved by the addition of PCV. However, histology-defined astrocytomas must have included both diffuse astrocytoma with IDH mutation and IDH wild-type, and IDH1-mutant tumors in the latter analysis included both codeleted (= oligodendrogliomas in the new WHO) and non-codeleted tumors (= astrocytoma in the new WHO). On the other hand, EORTC 22033–26033 showed that RT alone was associated with longer PFS than TMZ monotherapy in patients with IDH-mutant and non-codeleted low-grade gliomas.7) Therefore, RT should be included in the initial treatment for astrocytoma with IDH mutation.
Taken together, the most reasonable treatment on the basis of previous trials for these tumors would probably be RT plus chemotherapy, with either PCV or TMZ (Table 2). Because previous studies suggest that TMZ is at least equally effective as PCV for astrocytic gliomas,11,33,34) neuro-oncologists may prefer TMZ with greater tolerability. Indeed, a phase II trial with RT with concurrent and adjuvant TMZ yielded a 3-year OS rate of 73.1% for high-risk grade II patients;35) these data could be comparable to those of RTOG 9802 that was conducted in a mixed population of both high-risk and low-risk patients according to EORTC prognostic score.36) Although the optimal setting of the combination with RT is not clear, either RT followed by adjuvant TMZ or Stupp regimen might be recommended because of the recent report of the efficacy of adjuvant TMZ for grade III tumors.18) The status of the MGMT gene (methylation status or protein expression) as well as prognostic score may reasonably be taken into consideration in the use (adjuvant vs concurrent and adjuvant) and length of TMZ.
Diffuse astrocytoma, IDH-wildtype
Diffuse astrocytomas without IDH mutations are likely to comprise various entities, and should be carefully evaluated to avoid misdiagnosis of relatively indolent circumscribed tumors such as pilocytic astrocytoma and ganglioglioma.1,37) It should be noted that EORTC 22033–26033 as well as previous comprehensive studies suggest that the majority of diffuse astrocytomas without IDH mutations are aggressive tumors whose biological and molecular characteristics resemble glioblastoma without IDH mutations.7,9,10,38) Therefore, the majority of these tumors may better be treated with Stupp regimen following maximal safe resection, although treatment would be personalized considering molecular information, MRI characteristics, symptom, clinical course, etc.
Anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted
Most importantly, the results of RTOG 9402 and EORTC 26951 suggested that the administration of chemotherapy in the initial treatment is critical for those tumors,14,15) and the evidence-based standard treatment for anaplastic oligodendroglioma, IDH-mutant and 1p/19q-codeleted is PCV + RT (or RT + PCV) (Table 2). In Japan, PCV could be replaced by PAV or PMV as for grade II oligodendrogliomas.22–25) PCV could also be replaced with TMZ, however, it is unclear whether TMZ might be equally effective, and how TMZ should be paired with RT, sequentially, concurrently, or as Stupp regimen. An intergroup randomized trial is ongoing for anaplastic or high-risk low-grade gliomas with 1p/19q codeletion comparing RT with concomitant and adjuvant TMZ to RT followed by PCV (“CODEL,” NCT00887146). Preliminary data of the original CODEL trial (the design of CODEL trial was later revised) found that patients treated with TMZ alone fared worse than those treated with RT with or without TMZ.39) Therefore, in principle, chemotherapy alone treatment is not recommended for grade III oligodendrogliomas.
Anaplastic astrocytoma, IDH-mutant
As mentioned earlier, for anaplastic astrocytoma with IDH mutation or wild-type, a global phase III trial is underway (CATNON trial, NCT00626990), and the standard treatments for these tumors are undetermined yet. Importantly, the first interim analysis recently reported has shown that adjuvant TMZ was significantly associated with improved OS in the multivariate analysis,18) and, therefore, the optimal treatment for anaplastic astrocytoma with IDH mutation may be RT followed by adjuvant TMZ or Stupp regimen (Table 2). However, the optimal treatment is likely to be dependent on the status of the IDH genes and MGMT promoter, and the mature results of the trial are awaited. In daily practice, the number of adjuvant TMZ as well as whether TMZ should be administered in adjuvant setting only or in both concurrent and adjuvant settings may be personalized dependent on MGMT status and the degree of toxicity. Although the post-hoc analysis of the RTOG 9402 trial suggested the efficacy of PCV for these tumors,16) neuro-oncologists may prefer TMZ considering the equivalent efficacy and greater tolerability.11,34)
Anaplastic astrocytoma, IDH-wildtype
Most of these tumors share molecular features with glioblastoma without IDH mutations.1 Indeed, a retrospective study suggested that survival outcomes of patients with these tumors might be worse than of patients with glioblastoma with the IDH1 mutation.40) Therefore, the same treatment strategy as for patients with glioblastomas, i.e., Stupp regimen, should be considered for most patients with anaplastic astrocytomas without IDH mutation.
System of Brain Tumor Genotyping
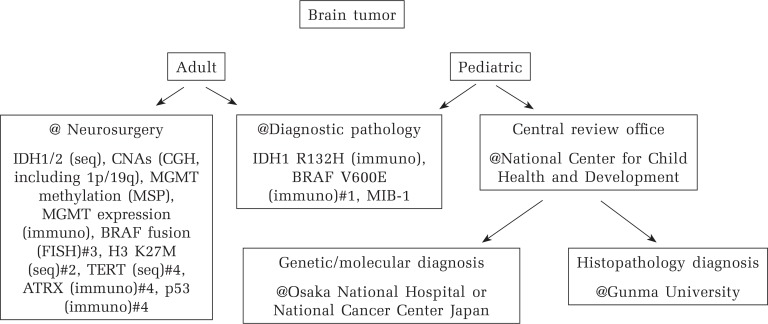
One of the challenges of the new WHO classification is the lack of availability of tumor genotyping in some centers. For example, it seems that evaluation of 1p/19q status is not yet available in many university hospitals and medical centers in Japan, and it is an urgent need to construct a system for molecular analyses required for the classification and treatment stratification. A diagnostic system of brain tumors in our hospital is shown in Fig. 1 for example. For cases of possible glioma, histology-based classification as well as immunohistochemical evaluation is performed at the division of diagnostic pathology, while molecular analyses are conducted at the department of neurosurgery in parallel with an extra fee for advanced medical technology approved by the government. Most pediatric brain tumors are currently referred to a national central review system organized by Japan Children’s Cancer Group (JCCG) (Fig. 1).
Fig. 1.
Diagnostic system of brain tumors at Keio University Hospital. #1 in cases that PXA, epithelioid GB, ganglioglioma, etc. are suspected for, #2 in cases in the midline, #3 in cases that pilocytic astrocytoma is suspected for, #4 optional.
Conclusion
This review highlights the results of recent pivotal clinical trials for diffuse gliomas of grades II and III in adults and provides treatment recommendations based on those evidences. Although compared to the enormous progress in molecular characterization, treatment for patients with diffuse glioma may be delayed, but also advancing with clinically relevant findings from clinical trials and newly developed therapies/technologies every year. We must embrace this progress, as we are expected to bring them into daily practice as quickly as possible.
Acknowledgment
The authors thank Dr. Takako Yoshioka and Dr. Keita Terashima at National Center for Child Health and Development, Dr. Koichi Ichimura at National Cancer Center Japan, Dr. Yonehiro Kanemura at Osaka National Hospital, and Dr. Junko Hirato at Gunma University for description of the diagnostic system of pediatric brain tumors.
Footnotes
Conflicts of Interest Disclosure
There is no conflict of interest regarding this article.
References
- 1).Louis DN, Ohgaki H, Wiestler OD, et al. : WHO classification of tumours of the central nervous system, Revised 4th Edition Lyon, International agency for research on cancer, 2016 [Google Scholar]
- 2).Perry A: WHO’s arrived in 2016! An updated weather forecast for integrated brain tumor diagnosis. Brain Tumor Pathol 33: 157–160, 2016 [DOI] [PubMed] [Google Scholar]
- 3).Louis DN, Perry A, Burger P, et al. International Society Of Neuropathology—Haarlem : International Society Of Neuropathology—Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol 24: 429–435, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Shaw EG, Berkey B, Coons SW, et al. : Recurrence following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma: results of a prospective clinical trial. J Neurosurg 109: 835–841, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Buckner JC, Shaw EG, Pugh SL, et al. : Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N Engl J Med 374: 1344–1355, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Shaw EG, Wang M, Coons SW, et al. : Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802. J Clin Oncol 30: 3065–3070, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Baumert BG, Hegi ME, van den Bent MJ, et al. : Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033–26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol 17: 1521–1532, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Reijneveld JC, Taphoorn MJ, Coens C, et al. : Health-related quality of life in patients with high-risk low-grade glioma (EORTC 22033–26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol 17: 1533–1542, 2016 [DOI] [PubMed] [Google Scholar]
- 9).Cancer Genome Atlas Research Network. Brat DJ, Verhaak RG, Aldape KD, et al. : Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N Engl J Med 372: 2481–2498, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Suzuki H, Aoki K, Chiba K, et al. : Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet 47: 458–468, 2015 [DOI] [PubMed] [Google Scholar]
- 11).Wick W, Hartmann C, Engel C, et al. : NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol 27: 5874–5880, 2009 [DOI] [PubMed] [Google Scholar]
- 12).Intergroup Radiation Therapy Oncology Group Trial 94021. Cairncross G, Berkey B, Shaw E, et al. : Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup radiation therapy oncology group trial 9402. J Clin Oncol 24: 2707–2714, 2006 [DOI] [PubMed] [Google Scholar]
- 13).van den Bent MJ, Carpentier AF, Brandes AA, et al. : Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol 24: 2715–2722, 2006 [DOI] [PubMed] [Google Scholar]
- 14).Cairncross G, Wang M, Shaw E, et al. : Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol 31: 337–343, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).van den Bent MJ, Brandes AA, Taphoorn MJ, et al. : Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol 31: 344–350, 2013 [DOI] [PubMed] [Google Scholar]
- 16).Cairncross JG, Wang M, Jenkins RB, et al. : Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol 32: 783–790, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).van den Bent MJ, Dubbink HJ, Marie Y, et al. : IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors: a report of the European organization for research and treatment of cancer brain tumor group. Clin Cancer Res 16: 1597–1604, 2010 [DOI] [PubMed] [Google Scholar]
- 18).van den Bent MJ, Erridge Sara, Vogelbaum MA, et al. : Results of the interim analysis of the EORTC randomized phase III CATNON trial on concurrent and adjuvant temozolomide in anaplastic glioma without 1p/19q co-deletion: An Intergroup trial. J Clin Oncol 34 (suppl; abstr LBA2000), 2016 [Google Scholar]
- 19).Komori T, Sasaki H, Yoshida K: [Revised WHO classification of tumours of the central nervous system:summary of the revision and perspective]. No Shinkei Geka 44: 625–635, 2016. (Japanese) [DOI] [PubMed] [Google Scholar]
- 20).van den Bent MJ, Afra D, de Witte O, et al. EORTC radiotherapy and brain tumor groups and the UK medical research council : Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet 366: 985–990, 2005 [DOI] [PubMed] [Google Scholar]
- 21).Ino Y, Betensky RA, Zlatescu MC, et al. : Molecular subtypes of anaplastic oligodendroglioma: implications for patient management at diagnosis. Clin Cancer Res 7: 839–845, 2001 [PubMed] [Google Scholar]
- 22).Hayashi S, Kitamura Y, Hirose Y, Yoshida K, Sasaki H: Molecular-genetic and clinicopathological prognostic factors in patients with gliomas showing total 1p19q loss: gain of chromosome 19p and histological grade III negatively correlate with patient’s prognosis. J Neurooncol 132: 119–126, 2017 [DOI] [PubMed] [Google Scholar]
- 23).Wakabayashi T, Kajita Y, Mizuno M, Nagasaka T, Yoshida J: Efficacy of adjuvant therapy with procarbazine, MCNU, and vincristine for oligodendroglial tumors. Neurol Med Chir (Tokyo) 41: 115–119; discussion 119–120, 2001 [DOI] [PubMed] [Google Scholar]
- 24).Wolff JE, Berrak S, Koontz Webb SE, Zhang M: Nitrosourea efficacy in high-grade glioma: a survival gain analysis summarizing 504 cohorts with 24193 patients. J Neurooncol 88: 57–63, 2008 [DOI] [PubMed] [Google Scholar]
- 25).Sasaki H, Hirose Y, Yazaki T, et al. : Upfront chemotherapy and subsequent resection for molecularly defined gliomas. J Neurooncol 124: 127–135, 2015 [DOI] [PubMed] [Google Scholar]
- 26).Lassman AB, Iwamoto FM, Cloughesy TF, et al. : International retrospective study of over 1000 adults with anaplastic oligodendroglial tumors. Neuro-oncology 13: 649–659, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Johnson BE, Mazor T, Hong C, et al. : Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science 343: 189–193, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Boyle FM, Eller SL, Grossman SA: Penetration of intra-arterially administered vincristine in experimental brain tumor. Neuro-oncology 6: 300–305, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Villano JL, Wen PY, Lee EQ, Nayak L, Reardon DA, Rosenfeld MR: PCV for anaplastic oligodendrogliomas: back to the future or a step backwards? A point/counterpoint discussion. J Neurooncol 113: 143–147, 2013 [DOI] [PubMed] [Google Scholar]
- 30).van den Bent MJ, Chinot O, Boogerd W, et al. : Second-line chemotherapy with temozolomide in recurrent oligodendroglioma after PCV (procarbazine, lomustine and vincristine) chemotherapy: EORTC Brain Tumor Group phase II study 26972. Ann Oncol 14: 599–602, 2003 [DOI] [PubMed] [Google Scholar]
- 31).Triebels VH, Taphoorn MJ, Brandes AA, et al. : Salvage PCV chemotherapy for temozolomide-resistant oligodendrogliomas. Neurology 63: 904–906, 2004 [DOI] [PubMed] [Google Scholar]
- 32).van den Bent MJ: Practice changing mature results of RTOG study 9802: another positive PCV trial makes adjuvant chemotherapy part of standard of care in low-grade glioma. Neuro-oncology 16: 1570–1574, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Quinn JA, Reardon DA, Friedman AH, et al. : Phase II trial of temozolomide in patients with progressive low-grade glioma. J Clin Oncol 21: 646–651, 2003 [DOI] [PubMed] [Google Scholar]
- 34).Brandes AA, Nicolardi L, Tosoni A, et al. : Survival following adjuvant PCV or temozolomide for anaplastic astrocytoma. Neuro-oncology 8: 253–260, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Fisher BJ, Hu C, Macdonald DR, et al. : Phase 2 study of temozolomide-based chemoradiation therapy for high-risk low-grade gliomas: preliminary results of Radiation Therapy Oncology Group 0424. Int J Radiat Oncol Biol Phys 91: 497–504, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Pignatti F, van den Bent M, Curran D, et al. European organization for research and treatment of cancer brain tumor cooperative group. European organization for research and treatment of cancer radiotherapy cooperative group : Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol 20: 2076–2084, 2002 [DOI] [PubMed] [Google Scholar]
- 37).Louis DN, Perry A, Reifenberger G, et al. : The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131: 803–820, 2016 [DOI] [PubMed] [Google Scholar]
- 38).Eckel-Passow JE, Lachance DH, Molinaro AM, et al. : Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med 372: 2499–2508, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Jaeckle K VM, Ballman K, Anderson SK, et al. : CODEL (Alliance-N0577; EORTC-26081/22086; NRG-1071; NCIC-CEC-2): Phase III randomized study of RT vs. RT+TMZ vs. TMZ for newly diagnosed 1p/19q-codeleted anaplastic oligodendroglial tumors. Analysis of patients treated on the original protocol design (PL02.005). Neurology 86: PL02.005, 2016 [Google Scholar]
- 40).Hartmann C, Hentschel B, Wick W, et al. : Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol 120: 707–718, 2010 [DOI] [PubMed] [Google Scholar]