Abstract
Although current treatment advances prolong patient survival, treatment for glioblastoma (GBM) in the elderly has become an emerging issue. The definition of “elderly” differs across articles; GBM predominantly occurs at an age ≥65 years, and the prognosis worsens with increasing age. Regarding molecular markers, isocitrate dehydrogenase (IDH) mutations are less common in the elderly with GBM. Meanwhile, O6-methylguanine DNA methyltransferase (MGMT) promoter methylation has been identified in approximately half of patients with GBM. Surgery should be considered as the first-line treatment even for elderly patients, and maximum safe resection is recommended if feasible. Concurrently, radiotherapy is the standard adjuvant therapy. Hypofractionated radiotherapy (e.g., 40 Gy/15 Fr) is suitable for elderly patients. Studies also supported the concurrent use of temozolomide (TMZ) with radiotherapy. In cases wherein elderly patients cannot tolerate chemoradiation, TMZ monotherapy is an effective option when MGMT promoter methylation is verified. Conversely, tumors with MGMT unmethylated promoter may be treated with radiotherapy alone to reduce the possible toxicity of TMZ. Meanwhile, the efficacy of bevacizumab (BEV) in elderly patients remains unclear. Similarly, further studies on the efficacy of carmustine wafers are needed. Based on current knowledge, we propose a treatment diagram for GBM in the elderly.
Keywords: glioblastoma, elderly, treatment, review
Introduction
Recent treatment advances have improved the survival of patients with glioblastoma (GBM), particularly since the introduction of temozolomide (TMZ) in 2006.1) However, old age remains among the most significant factors associated with poor prognosis of GBM, even after the introduction of TMZ. However, to date, no standard treatment protocol has been established due to the lack of supporting evidence because trials often exclude elderly cases. In Japan, the treatment guidelines for adults with GBM have been published by the Brain Tumor Guidelines Extension Committee centered on the Japan Society for Neuro-Oncology in July 2016 and serves as a basis for treatment of the elderly with GBM. In this review, we discuss the recent literature on GBM in the elderly.
Epidemiology of GBM in the elderly
GBM mainly occurs in patients aged >65 years. The Japan Geriatric Neurosurgery Society stipulated that patients with GBM aged ≥70 years are regarded as “elderly.” However, many studies abroad discussing GBM in the elderly include patients younger than 70 years (e.g., 60 years). An accurate definition has not been established to date. Thus, the definition of “elderly” varies across articles.
Published statistics from the Japan Brain Tumor Registry from 2001 to 2004 illustrated the following tissue histology (based on the World Health Organization (WHO) classification in 2007): GBM 10.8%, anaplastic astrocytoma 3.8%, anaplastic oligodendroglioma 0.8%, and anaplastic oligoastrocytoma 0.9%. In GBM, median overall survival (OS) was 15 months, with a 5-year OS of 9.9%. Median progression-free survival (PFS) was 8.1 months, with a 5-year PFS of 9.3%. However, the above statistics were collected before the introduction of TMZ. A large proportion of patients with GBM were elderly. Patients aged >50 years accounted for 78% of the total cases, with those aged >65 years and >75 years accounting for 42% and 11.4%, respectively. Based on the statistics, the most frequent age group of patients was 65–69 years, which accounted for 17% of the cases.2) Statistics from Kumamoto, Japan (1980–1995) demonstrated a high incidence of malignant glioma in the elderly population. The incidence of GBM in those aged <70 years was 1.42/100,000 person-years, whereas the incidence was 2.40/100,000 person-years in patients aged ≥70 years. Despite the increasing incidence of GBM in the elderly, these patients are less likely to undergo histopathological examinations. Pathological diagnosis was made in 79.7% of young patients and 69.4% of elderly patients.3) The elderly are more likely to be treated as GBM without histopathological verification.4)
With increasing age, the survival prognosis worsens without any clear borderline. Survival in elderly patients is short even in those who receive multimodal treatment with surgical resection, radiotherapy, and chemotherapy.4) Active treatment would be beneficial to elderly patients. Surgical resection prolongs survival, even in elderly patients aged ≥80 years.5) Radiotherapy with concurrent TMZ after surgery is associated with improved survival even in elderly patients aged >75 years.6) Although age is a poor prognostic factor, old age alone is suggested to have no association with poor prognosis.7,8) Radiotherapy with TMZ significantly reduced the mortality risk by 55% even in elderly patients, indicating that aggressive treatment should not be withheld because of old age.7)
Molecular Characteristics
IDH mutation
The most distinct difference in molecular characteristics of GBM between the young and elderly is CpG island methylator phenotype (G-CIMP) and isocitrate dehydrogenase (IDH) mutation. The Cancer Genome Atlas project determined the profile of promoter DNA methylation alterations in GBM. A subset of primary GBMs exhibit the glioma-G-CIMP, characterized by widespread DNA hypermethylation of a large number of CpG islands.9) G-CIMP is strongly associated with mutations of IDH1. IDH1 mutation induces DNA hypermethylation to reshaping the methylome to resemble that of the CIMP phenotype. The genome-wide genetic and epigenetic alterations resulting from mutant IDH activate key gene expression programs, characterize G-CIMP-positive proneural GBM exclusively, and are predictive of favorable survival.10) In G-CIMP-negative GBMs, age is an independent prognostic factor.11) In the WHO classification of central nervous system (CNS) tumor 2016, GBMs are subdivided according to their molecular phenotype, namely, IDH wild type and IDH mutant, based on diverse and distinct patterns of tumorigenesis and clinical significance.12) IDH mutation is considered to be an early event in tumorigenesis of low-grade glioma.13) IDH gene mutation occurs in 88% of secondary GBM, and GBM IDH mutant is more frequently diagnosed among young patients (mean age, 45 years at diagnosis) but accounts for only 5% of all GBM cases.14) Meanwhile, primary GBM, which exhibits a low frequency or absence of IDH mutation, is the most predominant GBM in the elderly. Accordingly, IDH mutation in GBM in the elderly is less commonly identified (2.4–6%).13–17) Age-adjusted multivariate analysis previously suggested that the better prognosis of secondary GBM is due to the young age rather than the difference in biological behavior of the tumor.14) Prognostic significance has recently become an issue mainly due to the predominant occurrence of IDH1 mutations in young patients.17) A meta-analyses of GBM focusing on IDH mutation demonstrated a prognostic impact of IDH on the OS of GBM patients (HR: 0.45).18)
MGMT promoter methylation status
O6-methylguanine DNA methyltransferase (MGMT) is a DNA repair enzyme targeting DNA damage, which leads to cellular resistance to alkylating chemotherapeutic agents targeting the O6 position of guanine such as TMZ. Hypermethylation of MGMT promoter downregulates the normal DNA repair mechanism by MGMT enzyme, making the tumor more susceptible to radiation or chemotherapy with an alkylating agent. Hegi et al. found that inactivation of MGMT by its promoter methylation improved the survival outcome of GBM patients treated with TMZ.19) MGMT promoter methylation is predominantly identified in astrocytic and oligodendroglial tumor with IDH mutations, although primary GBM with MGMT promoter methylation had no IDH mutation. MGMT methylation may occur as part of the G-CIMP phenotype, associating with the presence of IDH1/2 mutations. MGMT promoter methylation and IDH mutation are suggested to be among the earliest events in the tumorigenesis of low-grade glioma.20) MGMT promoter methylation was detected in approximately half (35–57.5%) of GBM in the elderly.21–23) The methylation status of the MGMT promoter was not influenced by age, sex, and Karnofsky Performance Status (KPS).20,24) A meta-analysis also indicated that MGMT promoter methylation is independent of age, with 47% in the elderly and 44% in young patients with GBM.25)
Other molecular characteristics
Analysis of collective data from systematic search of MEDLINE (1998–2010) revealed that MGMT promoter methylation and IDH mutation were prognostic factors, but known molecular players, such as epidermal growth factor receptor (EGFR), p53, CDKN2A, and PTEN were not prognostic factors in GBM.11) Molecular deregulation related to the hypoxic response and angiogenesis including vascular endothelial growth factor (VEGF) was higher in GBM in the elderly than in young patients, suggesting two different biological and clinical behaviors.26) The prognostic value of telomerase reverse transcriptase (TERT) promoter mutation in GBM has been debated. A recent study reported that altered TERT expression caused by activating mutations of the rs2853669 polymorphism within the TERT promoter region is significantly associated with poor prognosis in young patients with GBM but not in the elderly. GBM in the elderly has high TERT mRNA levels and reduced telomere lengths.24) A domestic collaborative study showed that patients with TERT mutant-MGMT unmethylated GBM have the worst prognosis, which is validated by multivariate analysis incorporating age, sex, cohort, KPS, tumor location, surgical history, TERT, and MGMT.27)
Clinical Aspects
Surgery
Surgical removal within the safety margin prolongs OS, delays tumor growth, and improves functional outcomes. Although a prospective randomized study conducted by Vuorinen et al. only enrolled a small number of patients, it is the only randomized study to discuss the extent of resection in elderly patients with malignant GBM aged >65 years. Surgical removal of the tumor prolonged survival by 2.8 times than biopsy (median OS: 171 days after the craniotomy versus 85 days after the biopsy), whereas no significant difference was observed in the time of deterioration between these two treatment arms.28) There have been many surgical series demonstrating the survival advantage of gross total resection (GTR) compared to the biopsy (Table 1).15,28–32) Almenawer investigated the optimal range of resection in patients with malignant glioma aged ≥60 years. The result of meta-analysis including 34 studies showed that surgical resection was superior to biopsy in OS (mean difference 3.88 months, 95% CI: 2.14–5.62, P < 0.001), PFS, postoperative KPS, and mortality, although the difference was unclear for morbidity. GTR of the tumor was significantly superior to subtotal resection (STR) in terms of OS (mean difference 3.77 months, 95% CI: 2.26–5.29, P < 0.001), PFS, and postoperative KPS. Surgical extension did not improve mortality and morbidity. Similar to young patients, maximum surgical resection within a safe range would result in prolonged survival, delayed tumor progression, and improved functional prognosis (Table 1).32) Epidemiological study of 20,705 adult patients with GBM in the Surveillance, Epidemiology, and End Results registry (1998–2009) illustrated a stepwise decrease of GTR with increasing age, although, GTR had a significantly better prognosis irrespective of gender and race, tumor site and size, and radiotherapy. Compared with STR, GTR extended survival by 2 months in patients aged ≥75 years.33)
Table 1.
Surgical series for elderly patients with GBM
| Author Year | N | Age | Surgery | OS (months) | 95% CI | Note |
|---|---|---|---|---|---|---|
| Vuorinen28) 2003 | 30 | >65 | Resection | 171 days | 146–278 | Randomized controlled trial |
| Biopsy | 85 days | 55–157 | ||||
| Scott29) 2011 | 206 | ≥70 | GTR | 10.7 | NA | |
| STR | 6.9 | |||||
| Biopsy | 2.8 | |||||
| Hoffermann30) 2015 | 124 | ≥65 | GTR | 15.0 | 11.4–18.7 | |
| STR | 11.0 | 7.9–14.2 | ||||
| Partial | 6.4 | 4.1–8.8 | ||||
| Biopsy | 5.6 | 3.4–7.8 | ||||
| Lombardi15) 2015 | 237 | ≥65 | GTR | 17.7 | 14.9–21.2 | |
| STR | 16.1 | 11.6–21.07 | ||||
| Babu31) 2016 | 120 | ≥65 | GTR | 14.1 | NA | |
| STR | 9.6 | |||||
| Almenawer32) 2015 | 12607 | ≥60 | GTR | 14.04 | 12.8–15.2 | Meta-analysis of 34 studies |
| STR | 8.68 | 7.87–9.48 | ||||
| Biopsy | 5.71 | 5.04–6.36 |
GTR: gross total removal, STR: subtotal removal, OS: overall survival.
The surgery is aimed at, first, achieving maximal cytoreduction of the tumor. Resection as much as possible is associated with favorable prognosis even in elderly patients with GBM. The second aim is to obtain histopathological diagnosis of GBM. Ring-shaped lesions need to be examined to rule out metastatic tumors, abscess, and inflammatory diseases because a report showed that 20% of lesions believed as GBM turned out to be other lesions.33) In addition to histological diagnosis, information regarding molecular markers, such as IDH mutation and MGMT methylation status, is necessary to develop a treatment strategy.
Radiotherapy
Radiotherapy is an effective treatment for elderly patients with GBM. The ANOCEF trial is a randomized controlled trial comparing radiotherapy (50 Gy, 1.8 Gy/day) treatment with best supportive care in patients with malignant glioma aged ≥70 years with preserved performance status (KPS ≥70). The study was terminated because the superiority of the radiotherapy group was clarified in the interim analysis (median OS: 29.1 weeks versus 16.9 weeks). Irradiation did not result in a significant difference of deterioration of quality of life (QOL) and cognitive function (Table 2).34) Roa et al. conducted a randomized controlled trial comparing standard radiotherapy (Std-RT, 60 Gy/30 Fr) with hypofractionated radiotherapy (Hypo-RT, 40 Gy/15 Fr) for 100 postsurgical patients with GBM aged ≥60 years. OS between Std-RT and Hypo-RT (5.1 and 5.6 months, respectively) was not significantly different (Tables 2, 3). No difference was also noted in KPS, but steroid use was more frequent in the Std-RT.35) In the subset analysis of International Atomic Energy Agency randomized phase III trial36) restricted to elderly and/or frail patients with GBM, further lower-dose radiotherapy of 25 Gy/5 fr demonstrated similar benefit on OS as 40 Gy/15 fr (6.8 months for 25 Gy/5 fr and 6.2 month for 40 Gy/15 fr, respectively) (Tables 2, 3).37) The Nordic trial is a randomized phase III trial comparing Std-RT (60 Gy/30 Fr), Hypo-RT (34 Gy/10 Fr), and TMZ monotherapy in elderly patients with GBM aged ≥60 years. The study revealed that Hypo-RT is an effective and reasonable treatment even for elderly patients with GBM aged >70 years (median OS: 7.0 months for Hypo-RT versus 5.2 months for Std-RT) (Table 3). No significant difference was noted in survival according to MGMT promoter methylation status when patients are treated with radiotherapy alone, which is also equivalent to TMZ treatment alone in patients with MGMT unmethylated promoter (median OS: 7.0 months for RT and 6.8 months for TMZ) (Table 2).23) Minniti et al. retrospectively studied patients with GBM aged ≥65 years treated with Std-RT (60 Gy/30 Fr) versus Hypo-RT (40 Gy/15 Fr) both with concomitant and adjuvant TMZ. Median OS and PFS did not differ between the two treatment arms (12 and 5.6 months for Std-RT, and 12.5 and 6.7 months for Hypo-RT, respectively) (Table 3). However, Std-RT with TMZ was associated with a significant increase in grade 2 and 3 neurological toxicity, decreased KPS scores, and high steroid requirement.38)
Table 2.
Randomized controlled trials for elderly patients with GBM
| Study | Age (years) | N | Treatment | Median OS (95% CI) | Hazard Ratio (95% CI) | Note |
|---|---|---|---|---|---|---|
| Surgery | ||||||
| Vuorinen 200328) | >65 | 30 | Biopsy + RT | 85 days | 2.621 (1.035–6.641) | Resection improves survival |
| Resection + RT | 171 days | |||||
| Radiotherapy | ||||||
| Keime-Guibert 200734) | ≥70 | 85 | Resection + best supportive care | 16.9 weeks | 0.47 (0.29–0.76) | ANOCEF trial RT improves survival |
| Resection + RT | 29.1 weeks | |||||
| Roa 200435) | ≥60 | 100 | Std RT (60 Gy/30 Fr) | 5.1 months | 0.89 (0.59–1.36) | Non-inferiority of Hypo-RT |
| Hypo-RT (40 Gy/15 Fr) | 5.6 months | |||||
| Roa 201536) | ≥50 + frail ≥65 | 98 | Std-RT (Hypo-RT) (40 Gy/15 Fr) | 6.4 months (5.1–7.6) | NA | IAEA E33033 trial. Frail: KPS 50–70 |
| Hypo-RT (short-course) (25 Gy/5 Fr) | 7.9 months (9.3–9.6) | |||||
| Guedes de Castro 201737) | ≥65 | 61 | Std-RT (Hypo-RT) (40 Gy/15 Fr) | 6.2 months (5.1–7.6) | NA | Subset analysis of IAEA trial |
| Hypo-RT (short-course) (25 Gy/5 Fr) | 6.8 months (9.3–9.6) | |||||
| Radiation-Chemotherapy | ||||||
| Wick 201222) | >65 | 412 | Std-RT (60 Gy/30 Fr) | 9.6 months | NOA-08 trial Non-inferiority of the dose dense TMZ. MGMT methylation associated with longer OS |
|
| Dose dense TMZ* (100 mg/m2/day, 1-week ) | 8.6 months | 1.09 (0.84–1.42) | ||||
| MGMT unmethylated | 8.2 months | |||||
| MGMT methylated | 11.9 months | 0.62 (0.42–0.91) | ||||
| Malmström 201223) | ≥60 | 342 | Std-RT (60 Gy/30 Fr) | 6.0 months | Nordic trial MGMT promoter methylation status to predict clinical benefit of TMZ but not in RT. More benefit of TMZ monotherapy in GBM aged >70 |
|
| Hypo-RT (34 Gy/10 Fr) | 7.5 months | 0.85 (0.64–1.12) | ||||
| Any radiotherapy | 7.0 months | |||||
| MGMT unmethylated | 8.2 months | 0.97 (0.69–1.38) | ||||
| MGMT methylated | ||||||
| TMZ monotherapy | 8.3 months | 0.7 (0.52–0.93) | ||||
| MGMT unmethylated | 6.8 months | |||||
| MGMT methylated | 9.7 months | 0.56 (0.34–0.93) | ||||
| Perry 201643) | ≥65 | 562 | Hypo-RT alone | 7.6 months | CCTG CE.6 trial. Improvement of OS by addition of TMZ to Hypo-RT for all cases |
|
| MGMT unmethylated | 7.9 months | |||||
| MGMT methylated | 7.7 months | |||||
| Hypo RT + TMZ (3 weeks) | 9.3 months | 0.67 (0.56–0.80) | ||||
| MGMT unmethylated | 10.0 months | 0.75 (0.56–1.01) | ||||
| MGMT methylated | 13.5 months | 0.53 (0.38–0.73) | ||||
GBM: glioblastoma, KPS: Karnofsky Performance Status, MGMT: O6-methylguanine DNA methyltransferase, MGMT unmethylated: absence of MGMT promoter methylation, MGMT methylated: presence of MGMT promoter methylation, OS: overall survival, Std-RT: Standard radiotherapy, Hypo-RT: Hypofractionated radiotherapy, RT: radiotherapy, TMZ: temozolomide.
Dose-dense TMZ: 100 mg/m2/day, 1-week on/1-week off regimen.
Table 3.
Studies comparing standard and hypofractionated radiotherapy in elderly patients with GBM
| Author Year | Age | N | OS (month) Std-RT | OS (month) Hypo-RT | Hazard ratio (95% CI) | P |
|---|---|---|---|---|---|---|
| Roa 200435) | ≥60 years | 100 | 5.1 | 5.6 | 0.89 (0.59–1.36) | 0.57 |
| Malmström 201223) | 60–70 years | 198 | 7.6 | 8.8 | 1.06 (0.73–1.54) | 0.77 |
| >70 years | 81 | 5.2 | 7.0 | 0.59 (0.37–0.93) | 0.02 | |
| Minniti 201538) | ≥65 years | 329 | 12.0 | 12.5 | 0.500 | |
| propensity matched analysis | 90 | 0.93 (0.66–1.31) | 0.70 | |||
| Guedes de Castro* 201737) | ≥65 years | 61 | 6.2 | 6.8 | NA | 0.936 |
| ≥65 years, KPS 50–70 | 40 | 6.7 | 7.5 | 0.904 | ||
| ≥65 years, KPS >80 | 21 | 8.0 | 8.0 | 0.890 |
Hypo-RT: hypofractionated radiotherapy, KPS: Karnofsky performance status, OS: overall survival, Std-RT: standard radiotherapy.
Lower-dose radiotherapy: 40 Gy/15 fr for Std-RT and 25 Gy/5 fr for Hypo-RT.
Collectively, the results of various studies indicate that Hypo-RT for elderly patients with GBM leads to similar survival benefit as that of Std-RT but with less neurotoxicity and steroid administration and short treatment time and hospitalization period. The clinical practice guideline of radiation therapy for GBM was published by the American Society for Radiation Oncology in 2016. The guidelines state that patients with GBM aged <70 years who have a reasonable performance status should receive Std-RT (e.g., 60 Gy/30 Fr) with concurrent and adjuvant TMZ. Meanwhile, elderly patients aged ≥70 years with reasonable performance status should receive Hypo-RT (e.g., 40 Gy/15 Fr). Because there is a lack of evidence that Std-RT is more efficacious than Hypo-RT.39)
Chemotherapy
1. Temozolomide
TMZ is a standard first-line chemotherapeutic agent for GBM. The EORTC-NCIC trial by Stupp et al. only included patients with GBM aged up to 70 years.1,40) Therefore, the optimal treatment of GBM in patients aged over 70 years remains unclear.
The NOA-08 trial exhibited non-inferiority of TMZ monotherapy to radiotherapy. The dose-dense TMZ monotherapy (100 mg/m2 /day; 1 week on/1 week off) demonstrated a median survival of 8.6 months with TMZ monotherapy comparable to 9.6 months with the Std-RT (HR: 1.09). OS was significantly longer in patients with MGMT promoter methylation than in MGMT unmethylated (11.9 months versus 8.2 months, respectively; HR: 0.62). The study suggested that elderly patients with MGMT promoter methylation should not be treated with radiotherapy alone (Table 2).22) In the Nordic trial, TMZ monotherapy (200 mg/m2 /day, 5 days on/23 days off) and two different schedules of radiotherapy alone were compared. TMZ monotherapy exhibited superior OS to the radiotherapy alone (8.3 months for TMZ monotherapy; 7.5 and 6.0 months for Hypo-RT and Std-RT, respectively, in GBM patients aged >60 years). In a subset of GBM patients aged >70 years, OS reached 9.0 months for TMZ monotherapy and 7.0 and 5.2 months for Hypo-RT and Std-RT, respectively. In particular, MGMT promoter methylation status predicted TMZ response, indicating median OS of 9.7 months for MGMT methylated promoter and 6.8 months for MGMT unmethylated promoter (Table 2). In the TMZ group, hematological complications of grade III/IV neutropenia and thrombocytopenia were observed, but QOL was superior while the global health status was equivalent with that of radiotherapy alone.23) A meta-analysis of 16 nonrandomized controlled trials demonstrated that radiotherapy plus TMZ decreased the mortality risk (HR: 0.59) and disease progression (HR: 0.58). The survival benefit of radiotherapy plus TMZ was evident in elderly patients with GBM with a favorable prognosis (e.g., extensive resection and favorable KPS). More frequent toxicities in radiotherapy plus TMZ were observed, particularly in hematological toxicities, although these were deemed acceptable.25) Treatment with TMZ-based chemotherapy improved OS in elderly patients with GBM with MGMT promoter methylation (HR: 0.49). The TMZ-containing regimen was superior to radiation alone in elderly patients with GBM with MGMT promoter methylation (HR: 0.48) but not in those with MGMT unmethylated promoter (HR: 1.14).41) Another meta-analysis by Zarnett concluded that TMZ monotherapy or Hypo-RT alone may be considered in elderly patients with GBM who are poor candidates to undergo radiochemotherapy. In patients with MGMT promoter methylation, TMZ monotherapy is more beneficial than radiation monotherapy. Based on this result, their recommendations are as follows: Level 1A: Either single-agent TMZ or hypofractionated radiotherapy alone may be used for the treatment of elderly patients with GBM multiforme who are not candidates for combined radiotherapy and chemotherapy. Level 1B: Elderly patients who have MGMT promoter methylation are likely to benefit from TMZ alone over radiotherapy. However, evidence to recommend either TMZ alone or radiotherapy alone in patients with MGMT unmethylated promoter are lacking.42)
The result of many trials showed MGMT promoter methylation status as a useful prognostic biomarker to predict the survival of GBM in the elderly, particularly in patients treated with TMZ, whereas survival benefit from TMZ is unclear in patients with MGMT unmethylated promoter.21) CCTG CE.6, EORTC 26062-22061, the most recently published randomized controlled trial, elucidated this issue. The study included 562 patients with GBM aged ≥65 years and compared Hypo-RT (40 Gy/15 Fr) alone versus Hypo-RT with 3 weeks of concomitant TMZ plus monthly adjuvant TMZ until progression or completion of 12 cycles. Combining TMZ with Hypo-RT was tolerable and resulted in prolonged OS and PFS in all GBM patient groups. Hypo-RT plus TMZ was superior in median OS and PFS than radiation alone (9.3 and 5.3 months versus 7.6 and 3.9 months, respectively; HR: 0.67 for OS and 0.50 for PFS). Patients with MGMT promoter methylation treated with radiotherapy plus TMZ demonstrated significantly longer survival than those treated with radiotherapy alone (13.5 months versus 7.7 months; HR: 0.53). Moreover, patients with GBM with MGMT unmethylated promoter treated with radiotherapy plus TMZ survived longer than those treated with radiotherapy alone (10.0 months versus 7.9 months; HR: 0.75). Interestingly, younger patients received less benefit by Hypo-RT with TMZ. By age group, median OS of Hypo-RT with TMZ versus Hypo-RT alone were 65–70 years: 8.7 months versus 8.3 months (HR 0.93), 71–75 years: 9.3 months versus 7.6 months (HR 0.63), and ≥76 years: 10.0 months versus 7.1 months (HR 0.53), respectively. No difference was noted in QOL, but patients in the radiotherapy plus TMZ group demonstrated high levels of nausea, vomiting, and constipation. Elderly patients with MGMT methylated tumors can expect prolonged survival from the combination of TMZ with radiotherapy. The addition of concomitant and adjuvant TMZ to Hypo-RT significantly improved OS and PFS in all elderly patients with GBM (Table 2).43)
In summary, initial treatment with TMZ combined with radiotherapy is a standard in GBM management, even in elderly patients. Reactivity to TMZ depends on the status of MGMT promoter methylation. Tumors with MGMT promoter methylation have better therapeutic response. In cases where radiochemotherapy is not feasible, TMZ monotherapy would be effective for tumors with MGMT promoter methylation. Moreover, in tumors with MGMT unmethylated promoter, adding TMZ to radiation therapy would be beneficial.
2. Bevacizumab
Evidence of efficacy of BEV use in elderly patients with GBM is limited. To date, published randomized studies of BEV focusing on elderly patients are yet to be published. Clinical benefits of bevacizumab use in elderly patients with GBM remain unclear.
Several studies proposed that BEV might affect PFS and possibly OS in selected elderly patients with a favorable prognosis, that is, GTR and preserved PS. The AVAglio trial was conducted for adult patients with GBM, including 73 patients aged ≥70 years. In this study, BEV resulted in prolonged PFS even in a subset of patients aged ≥65 years (HR: 0.68; 95% CI: 0.49–0.92), but this effect was decreased in patients aged ≥70 years (HR: 0.78; 95% CI: 0.46–1.33).44) The ANOCEF Phase II trial presented at the American Society of Clinical Oncology (ASCO) 2013 Annual Meeting included 66 newly identified patients with GBM who were diagnosed via biopsy, aged ≥70 years, and with KPS <70. TMZ monotherapy with BEV demonstrated a median OS of 24 weeks, which was quite similar to the OS for TMZ monotherapy at 25 weeks. Median PFS was 16 weeks, with 25 patients (38%) becoming transiently capable of self-care. From this result, the addition of BEV in treating elderly patients with poor performance status (PS) might not be beneficial in improving OS and PFS.45) However, adding BEV in the treatment regimen for elderly patients with preserved PS and underwent gross total excision resulted in better prognosis. In the study of surgically treated 120 GBM patients aged ≥65 years with a median KPS of 80, using BEV yielded a higher OS of 20.1 months than the 7.9 months without BEV. Multivariate stepwise analysis indicated that old age (HR: 1.06), high KPS score (HR: 0.97), and using BEV (HR: 0.51) were prognostic factors of GBM.31) The result implies that adding BEV in the treatment of selected patients with GBM might have a survival benefit.
3. Carmustine wafer
Carmustine wafer (Gliadel wafer, Eisai Co., Ltd., Tokyo, Japan) is biodegradable polymers containing 3.85% carmustine (1,3-bis[2-chlor-oethyl]-1-nitroso-urea). No published randomized controlled trials for carmustine wafer focusing on elderly patients are available.46–50) The effect of carmustine wafer on elderly patients with GBM has been unclear. Only one case control study exhibited prolonged survival due to carmustine wafer implantation in elderly GBM aged ≥65 years, with no increase of adverse events. The use of carmustine wafers resulted in significantly prolonged OS (8.7 months with carmustine wafer and 5.5 months without wafer). A subgroup analysis demonstrated significant survival advantage of carmustine wafer implantation even in patients older than 70 years (9.1 months versus 4.8 months) and 75 years (6.0 months versus 4.7 months).49) There is a lack of evidence regarding the efficacy of carmustine wafer in elderly patients with GBM. Further investigation is needed.
Conclusion
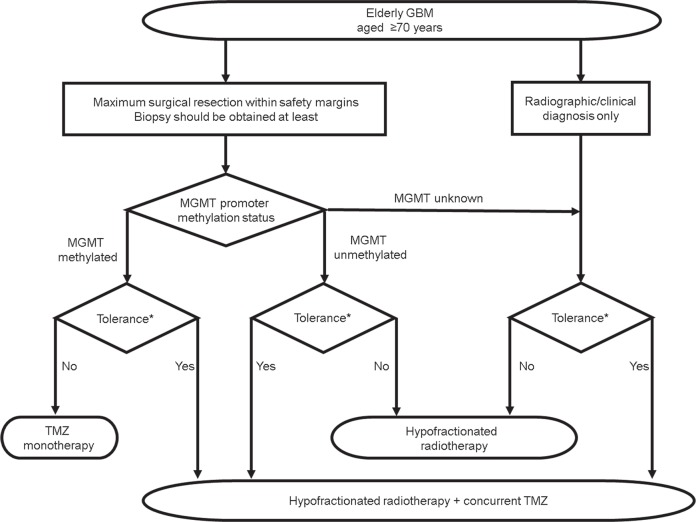
Based on current evidence in treating elderly patients with GBM, we propose the treatment diagram for elderly patients with GBM (Fig. 1). The results presented in the current study include those based on preliminary studies, particularly on the use of BEV and carmustine wafer. As such, further research is still necessary to establish the standard therapeutic regimen for elderly patients with GBM.
No accurate definition for “elderly” has been established, but an age of 70 years is one of the criteria based on the judgment of each patient’s condition.
Maximum surgical resection within the safety margins, if feasible, is recommended. Biopsy should be considered to make a histological diagnosis and verify the MGMT status.
For adjuvant treatment, hypofractionated radiotherapy and concurrent TMZ are recommended, regardless of the MGMT status.
TMZ monotherapy can be considered if the tumor is positive for MGMT promoter methylation.
Hypofractionated radiotherapy alone can be considered if the tumor has an unmethylated MGMT promoter.
Using bevacizumab might be beneficial in surgically treated patients with good performance status.
A carmustine wafer might be beneficial to both young and elderly patients, although severe toxicity in case of concomitant use with TMZ should be closely monitored.
Fig. 1.
Proposed flow chart for the treatment of elderly GBM. *Tolerance to the treatment should be judged by individual patient’s condition. (e.g. performance status, frailty and co-morbidities etc).
Footnotes
Conflicts of Interest Disclosure
All authors completed a self-declaration of the conflicts of interest (COI) to the Japan Neurosurgical Society and declare no potential COI regarding this manuscript.
References
- 1).Stupp R, Mason WP, van den Bent MJ, et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group : Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987–996, 2005 [DOI] [PubMed] [Google Scholar]
- 2).Committee of Brain Tumor Registry of Japan : Report of the Brain Tumor Registry of Japan (2001–2004). Neurol Med Chir (Tokyo) 54: 1–102, 2014 [Google Scholar]
- 3).Kuratsu J, Ushio Y: Epidemiological study of primary intracranial tumours in elderly people. J Neurol Neurosurg Psychiatr 63: 116–118, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Gulati S, Jakola AS, Johannesen TB, Solheim O: Survival and treatment patterns of glioblastoma in the elderly: a population-based study. World Neurosurg 78: 518–526, 2012 [DOI] [PubMed] [Google Scholar]
- 5).Zinn PO, Colen RR, Kasper EM, Burkhardt JK: Extent of resection and radiotherapy in GBM: A 1973 to 2007 surveillance, epidemiology and end results analysis of 21,783 patients. Int J Oncol 42: 929–934, 2013 [DOI] [PubMed] [Google Scholar]
- 6).Darefsky AS, King JT, Dubrow R: Adult glioblastoma multiforme survival in the temozolomide era: a population-based analysis of surveillance, epidemiology, and end results registries. Cancer 118: 2163–2172, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Iwamoto FM, Cooper AR, Reiner AS, Nayak L, Abrey LE: Glioblastoma in the elderly: the Memorial Sloan-Kettering Cancer Center Experience (1997–2007). Cancer 115: 3758–3766, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Gately L, Collins A, Murphy M, Dowling A: Age alone is not a predictor for survival in glioblastoma. J Neurooncol 129: 479–485, 2016 [DOI] [PubMed] [Google Scholar]
- 9).Noushmehr H, Weisenberger DJ, Diefes K, et al. Cancer Genome Atlas Research Network : Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 17: 510–522, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Turcan S, Rohle D, Goenka A, et al. : IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 483: 479–483, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Bozdag S, Li A, Riddick G, et al. : Age-specific signatures of glioblastoma at the genomic, genetic, and epigenetic levels. PLoS ONE 8: e62982, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Louis DN, Ohgaki H, Wiestler OD, et al. WHO Classification of Tumours of the Central Nervous System. WHO/IARC Classification of Tumours, 4th Edition Revised. [Google Scholar]
- 13).Hartmann C, Meyer J, Balss J, et al. : Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol 118: 469–474, 2009 [DOI] [PubMed] [Google Scholar]
- 14).Ohgaki H, Dessen P, Jourde B, et al. : Genetic pathways to glioblastoma: a population-based study. Cancer Res 64: 6892–6899, 2004 [DOI] [PubMed] [Google Scholar]
- 15).Lombardi G, Pace A, Pasqualetti F, et al. : Predictors of survival and effect of short (40 Gy) or standard-course (60 Gy) irradiation plus concomitant temozolomide in elderly patients with glioblastoma: a multicenter retrospective study of AINO (Italian Association of Neuro-Oncology). J Neurooncol 125: 359–367, 2015 [DOI] [PubMed] [Google Scholar]
- 16).Wiestler B, Claus R, Hartlieb SA, et al. Neuro-oncology Working Group (NOA) of the German Cancer Society : Malignant astrocytomas of elderly patients lack favorable molecular markers: an analysis of the NOA-08 study collective. Neuro-oncology 15: 1017–1026, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Hartmann C, Hentschel B, Wick W, et al. : Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol 120: 707–718, 2010 [DOI] [PubMed] [Google Scholar]
- 18).Cheng HB, Yue W, Xie C, Zhang RY, Hu SS, Wang Z: IDH1 mutation is associated with improved overall survival in patients with glioblastoma: a meta-analysis. Tumour Biol 34: 3555–3559, 2013 [DOI] [PubMed] [Google Scholar]
- 19).Hegi ME, Diserens AC, Godard S, et al. : Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res 10: 1871–1874, 2004 [DOI] [PubMed] [Google Scholar]
- 20).Mulholland S, Pearson DM, Hamoudi RA, et al. : MGMT CpG Island is invariably methylated in adult astrocytic and oligodendroglial tumors with IDH1 or IDH2 mutations. Int J Cancer 131: 1104–1113, 2012 [DOI] [PubMed] [Google Scholar]
- 21).Reifenberger G, Hentschel B, Felsberg J, et al. German Glioma Network : Predictive impact of MGMT promoter methylation in glioblastoma of the elderly. Int J Cancer 131: 1342–1350, 2012 [DOI] [PubMed] [Google Scholar]
- 22).Wick W, Platten M, Meisner C, et al. NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society : Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol 13: 707–715, 2012 [DOI] [PubMed] [Google Scholar]
- 23).Malmström A, Grønberg BH, Marosi C, et al. Nordic Clinical Brain Tumour Study Group (NCBTSG) : Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol 13: 916–926, 2012 [DOI] [PubMed] [Google Scholar]
- 24).Spiegl-Kreinecker S, Lötsch D, Ghanim B, et al. : Prognostic quality of activating TERT promoter mutations in glioblastoma: interaction with the rs2853669 polymorphism and patient age at diagnosis. Neuro-oncology 17: 1231–1240, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Yin AA, Zhang LH, Cheng JX, et al. : The predictive but not prognostic value of MGMT promoter methylation status in elderly glioblastoma patients: a meta-analysis. PLoS ONE 9: e85102, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Parsons DW, Jones S, Zhang X, et al. : An integrated genomic analysis of human glioblastoma multiforme. Science 321: 1807–1812, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Arita H, Yamasaki K, Matsushita Y, et al. : A combination of TERT promoter mutation and MGMT methylation status predicts clinically relevant subgroups of newly diagnosed glioblastomas. Acta Neuropathol Commun 4: 79, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Vuorinen V, Hinkka S, Färkkilä M, Jääskeläinen J: Debulking or biopsy of malignant glioma in elderly people - a randomised study. Acta Neurochir (Wien) 145: 5–10, 2003 [DOI] [PubMed] [Google Scholar]
- 29).Scott JG, Suh JH, Elson P, et al. : Aggressive treatment is appropriate for glioblastoma multiforme patients 70 years old or older: a retrospective review of 206 cases. Neuro-oncology 13: 428–436, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Hoffermann M, Bruckmann L, Kariem Mahdy A, Asslaber M, Payer F, von Campe G: Treatment results and outcome in elderly patients with glioblastoma multiforme—a retrospective single institution analysis. Clin Neurol Neurosurg 128: 60–69, 2015 [DOI] [PubMed] [Google Scholar]
- 31).Babu R, Komisarow JM, Agarwal VJ, et al. : Glioblastoma in the elderly: the effect of aggressive and modern therapies on survival. J Neurosurg 124: 998–1007, 2016 [DOI] [PubMed] [Google Scholar]
- 32).Almenawer SA, Badhiwala JH, Alhazzani W, et al. : Biopsy versus partial versus gross total resection in older patients with high-grade glioma: a systematic review and meta-analysis. Neuro-oncology 17: 868–881, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Noorbakhsh A, Tang JA, Marcus LP, et al. : Gross-total resection outcomes in an elderly population with glioblastoma: a SEER-based analysis. J Neurosurg 120: 31–39, 2014 [DOI] [PubMed] [Google Scholar]
- 34).Keime-Guibert F, Chinot O, Taillandier L, et al. Association of French-Speaking Neuro-Oncologists : Radiotherapy for glioblastoma in the elderly. N Engl J Med 356: 1527–1535, 2007 [DOI] [PubMed] [Google Scholar]
- 35).Roa W, Brasher PM, Bauman G, et al. : Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol 22: 1583–1588, 2004 [DOI] [PubMed] [Google Scholar]
- 36).Roa W, Kepka L, Kumar N, et al. : International atomic energy agency randomized phase iii study of radiation therapy in elderly and/or frail patients with newly diagnosed glioblastoma multiforme. J Clin Oncol 33: 4145–4150, 2015 [DOI] [PubMed] [Google Scholar]
- 37).Guedes de Castro D, Matiello J, Roa W, et al. : Survival outcomes with short-course radiation therapy in elderly patients with glioblastoma: data from a randomized phase 3 trial. Int J Radiat Oncol Biol Phys 98: 931–938, 2017 [DOI] [PubMed] [Google Scholar]
- 38).Minniti G, Scaringi C, Lanzetta G, et al. : Standard (60 Gy) or short-course (40 Gy) irradiation plus concomitant and adjuvant temozolomide for elderly patients with glioblastoma: a propensity-matched analysis. Int J Radiat Oncol Biol Phys 91: 109–115, 2015 [DOI] [PubMed] [Google Scholar]
- 39).Cabrera AR, Kirkpatrick JP, Fiveash JB, et al. : Radiation therapy for glioblastoma: Executive summary of an American society for radiation oncology evidence-based clinical practice guideline. Pract Radiat Oncol 6: 217–225, 2016 [DOI] [PubMed] [Google Scholar]
- 40).Stupp R, Hegi ME, Mason WP, et al. European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups. National Cancer Institute of Canada Clinical Trials Group : Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10: 459–466, 2009 [DOI] [PubMed] [Google Scholar]
- 41).Yin AA, Zhang LH, Cheng JX, et al. : Radiotherapy plus concurrent or sequential temozolomide for glioblastoma in the elderly: a meta-analysis. PLoS ONE 8: e74242, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Zarnett OJ, Sahgal A, Gosio J, et al. : Treatment of elderly patients with glioblastoma: a systematic evidence-based analysis. JAMA Neurol 72: 589–596, 2015 [DOI] [PubMed] [Google Scholar]
- 43).Perry JR, Laperriere N, O’Callaghan CJ, et al. Trial Investigators : Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N Engl J Med 376: 1027–1037, 2017 [DOI] [PubMed] [Google Scholar]
- 44).Chinot OL, Wick W, Mason W, et al. : Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370: 709–722, 2014 [DOI] [PubMed] [Google Scholar]
- 45).Reyes-Botero G, Honnorat J, Chinot OL, et al. : Temozolomide plus bevacizumab in elderly patients with newly diagnosed glioblastoma and poor performance status: An Anocef phase II trial. J Clin Oncol 30: abstr 2020, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Brem H, Piantadosi S, Burger PC, et al. : Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The polymer-brain tumor treatment group. Lancet 345: 1008–1012, 1995 [DOI] [PubMed] [Google Scholar]
- 47).Valtonen S, Timonen U, Toivanen P, et al. : Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery 41: 44–48; discussion 48–49, 1997 [DOI] [PubMed] [Google Scholar]
- 48).Westphal M, Hilt DC, Bortey E, et al. : A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-oncology 5: 79–88, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Westphal M, Ram Z, Riddle V, Hilt D, Bortey E, Executive Committee of the Gliadel Study Group : Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien) 148: 269–275; discussion 275, 2006 [DOI] [PubMed] [Google Scholar]
- 50).Chaichana KL, Zaidi H, Pendleton C, et al. : The efficacy of carmustine wafers for older patients with glioblastoma multiforme: prolonging survival. Neurol Res 33: 759–764, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]