Abstract
Purpose
To test the hypothesis that macular choroidal thickness is lower in patients with pseudoexfoliation syndrome (PXS) as compared to healthy control subjects.
Methods
In this cross-sectional, observational study, 38 non-glaucomatous PXS subjects and 37 healthy volunteers were enrolled in a tertiary care Glaucoma Clinic. The macular region was scanned with the enhanced depth imaging (EDI) protocol of a spectral domain optical coherence tomography (SD-OCT) device (Spectralis OCT, Heidelberg Engineering, Heidelberg, Germany). Macular choroidal thickness and volumes were compared in nine sectors of the Early Treatment Diabetic Retinopathy Study (ETDRS) layout profile across the central 3.45 mm zone after manual segmentation of the choroidal thickness. Linear mixed modeling was used to adjust for confounding variables.
Results
Six PXS eyes and 8 control eyes were excluded due to poor image quality leaving 32 PXS and 29 control eyes for final analyses. The average age and axial length of the PXS and control groups were 67.94 ± 7.30 vs 64.86 ± 7.04 and 22.91 ± 0.77 vs 23.24 ± 0.66 mm, respectively, (P = 0.10 and 0.20). There was no significant difference in retinal nerve fiber layer (RNFL) thickness between the two groups (P = 0.24). The choroidal thickness was significantly lower in the central subfield subfoveal area (P = 0.02) and in the inner superior (P = 0.03) and inner nasal quadrants (P = 0.03) in the PXS group compared to the control group, as was the choroidal volume (P = 0.02). No significant difference was found in macular choroidal thickness after adjusting for age, gender, and axial length. While there was a significant negative association between age and central subfield choroidal thickness in the control group (r = −0.48, P = 0.01), this association was not significant in the PXS group (r = −0.08, P = 0.68).
Conclusions
Our findings demonstrate that the choroid does not seem to be significantly altered in PXS eyes. Choroidal thickness changes need to be explored in PXS eyes with glaucoma.
Keywords: Pseudoexfoliation, Choroid, Optical coherence tomography
Introduction
Pseudoexfoliation syndrome (PXS) is a common age-related genetic disorder with a probable environmental component affecting intraocular and extraocular tissues.1, 2 A significant proportion of patients with PXS can develop glaucomatous damage that has higher intraocular pressure (IOP) and more severe fluctuation in IOP compared with primary open-angle glaucoma (POAG), revealing this type of glaucoma to have faster progression and poorer prognosis than POAG.3, 4, 5 It has been shown that at a given level of IOP, the probability of having optic nerve damage was higher in eyes with pseudoexfoliation.6 The presence of PXS has been shown to be the most important independent risk factor for progression of glaucoma in the Early Manifest Glaucoma Trial.7 Also, it has been shown that the glaucoma conversion rate was twice as high in patients with both ocular hypertension and pseudoexfoliation as in control ocular hypertension patients matched for IOP, age, and gender.8 It seems that factors other than IOP level contribute to the putting puzzle pieces into a coherent picture of PXS pathogenesis.9
Given the role of the choroidal vasculature in the blood supply of the laminar and prelaminar regions of the optic nerve head (ONH), the choroid might be a relevant target for investigation in glaucomatous patients.10, 11 Enhanced depth imaging (EDI) is a newly emerging technique that enables capturing high resolution images of the deep structures of the posterior segment, minimizing the light scattering that is encountered in regular spectral domain optical coherence tomography (SD-OCT) imaging. This technology has been used widely in evaluating of lamina cribrosa and choroid in glaucomatous eyes and revealed that these structures might be thinner in some types of glaucoma.12, 13
In this study, our goal was to compare macular choroidal thickness in non-glaucomatous pseudoexfoliative patients to normal eyes using the EDI function on the SD-OCT.
Methods
Patients
In a cross-sectional design, 38 consecutive patients with PXS whose diagnosis was established or confirmed at the Glaucoma Clinic of Farabi Eye Hospital as well as 37 normal volunteers who were examined for refractive error were enrolled. This is an extension of our previous report on peripapillary retinal nerve fiber layer (RNFL) and ONH parameters in PXS patients.14 When both eyes of the patient met the eligibility criteria, only one eye was chosen for inclusion randomly. All subjects underwent a comprehensive eye examination, including measurement of best corrected visual acuity, slit-lamp biomicroscopy, Goldmann applanation tonometry, gonioscopy, dilated stereoscopic fundus examination using a 90 or 78 diopter (D) lens, measurement of the central corneal thickness (CCT) by pachymetry (Tomey Corporation, Nagoya, Japan), standard white on white visual field [Humphrey Field Analyzer (HFA) II 750; 24-2 Swedish interactive threshold algorithm; Carl Zeiss Meditec, Dublin, CA], ocular biometry (IOLMaster; Carl Zeiss Meditec). ONH and macula (Spectralis OCT, Heidelberg Engineering, Inc., Dossenheim, Germany). Subjects also underwent ONH and macular imaging with EDI SD-OCT (Specteralis, HEYEX software 6.0 Heidelberg Engineering, Heidelberg, Jena, Germany). The inclusion criteria were as follows: 1) best corrected visual acuity of 20/40 or better with spherical equivalent within 5 D of emmetropia and cylinder correction within 3 D. 2) IOP < 22 mmHg; Exclusion criteria included: 1) any other neurologic disorders that could lead to visual field defect; 2) any history of previous ocular surgery, consistently unreliable visual fields (defined as false negative >20%; false positive >15%, and fixation losses >20%); 3) glaucoma diagnosis; or 4) history of diabetes mellitus; or 5) significant media opacity.
The normal control group had an IOP < 22 mmHg, no history of increased IOP, normal disc appearance, and a normal visual field. Absence of the glaucomatous disc appearance was characterized as an intact neuroretinal rim without cupping, notches, or localized pallor. The patients were enrolled into the PXS group if they demonstrated visible pseudoexfoliation material on the anterior lens capsule or pupillary margin after mydriasis on slit-lamp biomicroscopy and had 1) IOP less than 22 mmHg with no history of increased IOP, 2) normal optic disc head, and 3) normal visual field.
The study was conducted in accordance with the tenets of the Declaration of Helsinki. The Ethics Committee of Farabi Eye Hospital approved the study protocol and written informed was provided for all the subjects prior to enrollment.
Spectral domain optical coherence tomography
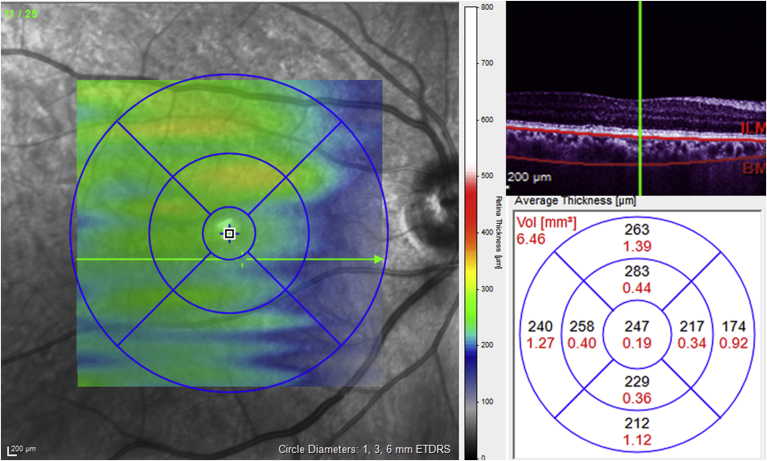
All OCT measurements were performed using SD-OCT (Heidelberg Spectralis SD-OCT; Heidelberg Engineering; Spectralis software version 5.3.2) after pupillary dilation. Scans with quality score of less than 20 were excluded from the analysis. We also excluded scans with inadequate quality as determined by poor quality fundus images, interruption of the RNFL segmentation, or those in which the posterior border of the choroid could not be delineated (Fig. 1).
Fig. 1.
An optical coherence tomography (OCT) image shows the manual segmentation of Bruch's membrane (upper red line) and sclerochoroidal border (lower red line) in a raster line of macula. Choroidal map and volume measured in nine sectors of the Early Treatment Diabetic Retinopathy Study (ETDRS) layout profile across the central 3.45 mm zone in the macula after manual segmentation of choroidal thickness.
Enhanced depth imaging spectral domain optical coherence tomography of the macular choroid
Using the Raster protocol (23 sections, each consisting of 100 averaged scans) images were obtained in a 20 × 30 rectangle centered on the fovea. The EDI images were viewed and measured with the Heidelberg Eye Explorer software (HEYEX™ Heidelberg Engineering, Dossenheim, Germany). The choroid was manually outlined for all 23 lines, with the anterior border at the basal aspect of retinal pigment epithelium and the posterior boundary (choroidoscleral interface) as a hyper-reflective line between the large vessel layer of the choroid and the sclera. Choroidal maps and volumes were measured in nine sectors of the ETDRS layout profile across the central 3.45 mm zone in the macula after manual segmentation of the choroidal thickness (Fig. 1).
To evaluate the intra-observer and inter-observer reproducibility of choroidal thickness measurements, 15 EDI-OCT macular scans were selected and analyzed by 2 independent observers. Intraclass correlation coefficients (ICC) for the measurement conducted by the two observers were calculated using a two-way mixed effect model and demonstrated excellent values for sectoral and average choroidal thickness or volume (range, 0.962–0.999).
Statistical analysis
SPSS software (version 18 for Windows; SPSS Inc., Chicago, IL, USA) was used for analyzing the data. Student t-test or Mann–Whitney U test were performed for comparisons of parametric and non-parametric continuous variables, respectively. Chi-square test was used for comparing categorical variables. Linear mixed modeling was used to adjust the choroidal thickness for age, sex, and axial length, and to account for the effect of confounders. Pearson correlation analysis was done to assess any correlation between the main choroidal parameters (central subfield choroidal thickness, choroidal volume) in each group. A P-value less than 0.05 was considered statistically significant.
Results
Of 38 PXS participants and 37 controls who met inclusion criteria, 6 (16%) PXS participants and 8 (21%) of the control eyes, were excluded because of unqualified image, and 32 pseudoexfoliation and 29 control eyes were included for final analysis. The control group comprised 13 male and 16 female patients, with a mean age of 64.8 ± 7.0 years, which was not significantly different from PXS group which included 20 male and 12 female with a mean age of 67.9 ± 7.3 years. (P = 0.10 and P = 0.52, respectively.) There was no significant differences in refractive error (0.09 ± 0.92 vs. 0.17 ± 0.86 D), IOP (14.55 ± 2.33 vs. 15.20 ± 2.67 mmHg), CCT (496.88 ± 62.51 vs. 516.96 ± 30.63 μm) and axial length (23.24 ± 0.66 vs. 22.91 ± 0.77) between control and PXS eyes (P = 0.43, P = 0.32, P = 0.38, P = 0.20, and l = 0.22, respectively).
RNFL thickness was not statistically significant different between the PXS and control eyes (Table 1). The central subfield choroidal thickness, the choroidal thickness in the superior and nasal quadrants of inner rings (3 mm), and even though choroidal volume was significantly less in the PXS group but not after adjustment for age and axial length (Table 2).
Table 1.
Retinal nerve fiber layer (RNFL) thickness comparison between the two groups with and without adjustment for age, sex, and axial length.
| RNFL | Control group (Mean ± SD) | Pseudoexfoliation group (Mean ± SD) | P-value | Adjusted P-value |
|---|---|---|---|---|
| Global retinal nerve fiber layer (μm) | 99.6 ± 12.6 | 93.1 ± 7.0 | 0.05 | 0.24 |
| Temporal retinal nerve fiber layer (μm) | 64.0 ± 13.8 | 61.8 ± 13.1 | 0.58 | 0.49 |
| Superotemporal retinal nerve fiber layer (μm) | 131.4 ± 19.6 | 122.6 ± 18.0 | 0.14 | 0.20 |
| Superonasal retinal nerve fiber layer (μm) | 114.5 ± 23.4 | 102.7 ± 18.7 | 0.07 | 0.11 |
| Nasal retinal nerve fiber layer (μm) | 78.3 ± 12.3 | 77.6 ± 9.9 | 0.85 | 0.76 |
| Inferonasal retinal nerve fiber layer (μm) | 125.4 ± 28.0 | 122.3 ± 21.7 | 0.68 | 0.40 |
| Inferotemporal retinal nerve fiber layer (μm) | 141.1 ± 24.20 | 121.7 ± 27.0 | 0.07 | 0.44 |
RNFL: Retinal nerve fiber layer.
Table 2.
Comparison of macular choroidal thickness between pseudoexfoliation and control groups with and without adjustment for age, sex and axial length.
| Control group (Mean ± SD) | Pseudoexfoliation group | P-value | Adjusted P-value | |
|---|---|---|---|---|
| Choroidal volume (μm3) | 7.79 ± 1.40 | 6.73 ± 2.04 | 0.02 | 0.16 |
| Central subfield choroidal thickness (μm) | 310.7 ± 57.5 | 270.8 ± 76.9 | 0.02 | 0.36 |
| Inner ring (Superior) (μm) | 311.2 ± 50.74 | 274.1 ± 75.14 | 0.03 | 0.32 |
| Inner ring (Nasal) (μm) | 301.8 ± 56.6 | 264.5 ± 71.46 | 0.03 | 0.36 |
| Inner ring (Inferior) (μm) | 299.3 ± 59.42 | 267.1 ± 70.71 | 0.06 | 0.37 |
| Inner ring (Temporal) (μm) | 284.2 ± 57.35 | 259.7 ± 73.86 | 0.15 | 0.39 |
| Outer ring (Superior) (μm) | 293.5 ± 51.32 | 261.4 ± 72.87 | 0.05 | 0.31 |
| Outer ring (Nasal) (μm) | 259.1 ± 53.12 | 235.5 ± 67.87 | 0.14 | 0.71 |
| Outer ring (Inferior) (μm) | 278.2 ± 58.01 | 251.9 ± 70.01 | 0.12 | 0.55 |
| Outer ring (temporal) (μm) | 237.4 ± 56.60 | 230.5 ± 76.94 | 0.69 | 0.84 |
| Inferotemporal (μm) | 145.63 ± 53.10 | 156.94 ± 65.14 | 0.56 | 0.40 |
P values less than 0.05 are shown in bold.
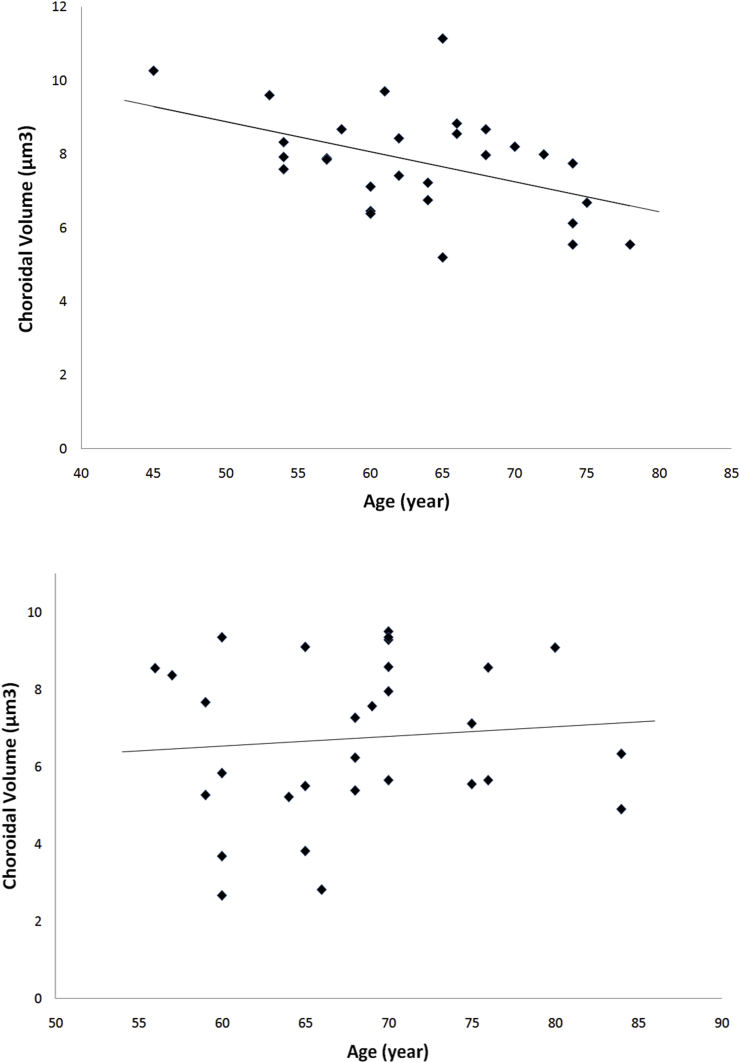
While there was significant negative correlation between age and central subfield choroidal thickness in the control eyes (r = −0.48, P = 0.01), this association was not significant in the PXS eyes (r = −0.08, P = 0.68). Similarly, we observed a significant correlation between age and choroidal volume in the control group (r = −0.46, P = 0.01). However, there was no significant association between age and choroidal volume in the PXS group (r = 0.09, P = 0.63) (Fig. 2). We also did not find any correlation between CCT, global RNFL thickness, and choroidal thickness in either PXS eyes or control eyes.
Fig. 2.
Upper image: Scatterplot showing the correlation of age and macular choroidal volume in the control group. There was a negative correlation and choroidal volume (r = −0.46, P = 0.01) and age in the control group. Lower image: Scatterplot showing the correlation of macular choroidal volume and age in the pseudoexfoliation group. No correlation was found between age and choroidal volume (r = 0.09, P = 0.63) and age in the pseudoexfoliation group.
Discussion
Recent evidence suggests that greater IOP fluctuation itself may not completely explain the poorer prognosis of PXG compared to POAG, making the dilemma of the pathogenesis of PXG like a puzzle with unrecognized pieces.7 It seems that PXS may be an independent risk factor for glaucomatous optic nerve damage.15 In this study, we evaluated the role of macular choroid as the one of possible pieces of the puzzle using EDI-SDOCT, and we found that the thickness of macular choroid is not significantly different from normal subjects.
The choroid might apply its effects through its nutritional and vascular support of the ONH. In addition, choroidal volume and its expansion throughout the day may exert an important effect on the trabecular outflow facility. Dayanir et al., have observed significant changes in ophthalmic artery hemodynamic parameters in both eyes of unilateral PXS patients, illustrating the systemic nature of the disease.16 While Maul et al have found a significantly thinner macular choroid in glaucoma patients than in glaucoma suspects, this difference was not significant when adjusted for other parameters in a multivariable model.17 In another study by Hosseini et al., investigating 43 POAG patients and 20 controls, no difference was observed in choroidal thickness in POAG eyes compared to normal eyes after adjusting for age and axial length.18 While Park et al. did not observe significant differences in choroidal thickness in patients with POAG compared to controls, peripapillary choroidal thickness was thinner in normal tension glaucoma eyes than in controls.19 In another two series, the authors found a significantly thinner choroid in PXS patients compared to controls.20 In our study, we found a significantly lower macular choroidal thickness in the PXS group compared to the control group. However, when we adjusted our result for age and axial lengths of patients, there was no longer a significant difference in macular choroidal thickness between PXS eyes and controls. Recently, Turan-Vural et al. evaluated subfoveal choroidal thickness of 35 PXS eyes and 26 controls, and reported that PXS eyes might have thinner subfoveal choroid.21 A reason for this discrepancy might be that they did not adjust their choroidal measurements for confounding factors including age and axial length. Another study has been shown that peripapillary choroid is not thinner in eyes with PXS syndrome.14 Moreover, a recent study by Ozge et al. has not shown that choroid becomes thinner in exfoliative glaucoma compared to PXS and normals.22 These studies suggest that the choroidal volume and thickness and its expansion during the different times of the day have little role in the pathogenesis of glaucomatous damage in PXG.
In another study, Kocabeyoglu et al. found that choroidal expansion during a water drinking test was not an important mechanism for IOP elevation in healthy eyes or those with PXS. Furthermore, given the similarity of IOP increase in PXS and control eyes, authors suggested that trabecular outflow facility is not impaired in eyes with PXS.23
In line with previous studies, we found that with increasing age, the central subfield choroidal thickness, choroidal volume, and peripapillary choroidal thickness decreased in the control group.14 However, we did not find a similar association in our PXS group. Pseudoexfoliation may influence on effect of age on choroidal thickness that leads to a non-significant correlation of choroidal thickness and age.
This study had some limitations that should be kept in mind. We have a small sample size, and the study may have had potential selection bias as a result of patient exclusion due to lacking image quality. Finally, although we included PXS eyes with normal visual fields and IOP, it is still possible that some of these eyes will be at the early stage of pseudoexfoliation glaucoma. However, the RNFL thickness was not significantly thinner in PXS eye.
To summarize the main conclusions of this study, macular and subfoveal choroidal thickness is not lower in eyes with PXS compared to controls. Based on anatomical measurements, the choroid does not seem to be significantly altered in PXS eyes. Although age affects choroidal thickness in healthy control eyes, we did not find any association of age and choroidal thickness in PXS eyes.
Footnotes
This is to certify that: The authors did not receive any financial support from any public or private sources. The authors have no financial or proprietary interest in any products, methods, or materials described herein.
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Anastasopoulos E., Founti P., Topouzis F. Update on pseudoexfoliation syndrome pathogenesis and associations with intraocular pressure, glaucoma and systemic diseases. Curr Opin Ophthalmol. 2015;26(2):82–89. doi: 10.1097/ICU.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 2.Dewundara S., Pasquale L.R. Exfoliation syndrome: a disease with an environmental component. Curr Opin Ophthalmol. 2015;26(2):78–81. doi: 10.1097/ICU.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koz O.G., Turkcu M.F., Yarangumeli A., Koz C., Kural G. Normotensive glaucoma and risk factors in normotensive eyes with pseudoexfoliation syndrome. J Glaucoma. 2009;18(9):684–688. doi: 10.1097/IJG.0b013e31819c4311. [DOI] [PubMed] [Google Scholar]
- 4.Konstas A.G., Quaranta L., Katsanos A. Twenty-four hour efficacy with preservative free tafluprost compared with latanoprost in patients with primary open angle glaucoma or ocular hypertension. Br J Ophthalmol. 2013;97(12):1510–1515. doi: 10.1136/bjophthalmol-2012-303026. [DOI] [PubMed] [Google Scholar]
- 5.Ritch R. Systemic associations of exfoliation syndrome. Asia Pac J Ophthalmol (Phila) 2016;5(1):45–50. doi: 10.1097/APO.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 6.Topouzis F., Harris A., Wilson M.R. Increased likelihood of glaucoma at the same screening intraocular pressure in subjects with pseudoexfoliation: the Thessaloniki Eye Study. Am J Ophthalmol. 2009;148(4) doi: 10.1016/j.ajo.2009.03.024. 606–613 e601. [DOI] [PubMed] [Google Scholar]
- 7.Leske M.C., Heijl A., Hyman L. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114(11):1965–1972. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Grodum K., Heijl A., Bengtsson B. Risk of glaucoma in ocular hypertension with and without pseudoexfoliation. Ophthalmology. 2005;112(3):386–390. doi: 10.1016/j.ophtha.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 9.Pasquale L.R., Borras T., Fingert J.H., Wiggs J.L., Ritch R. Exfoliation syndrome: assembling the puzzle pieces. Acta Ophthalmol. 2016;94(6):e505–e512. doi: 10.1111/aos.12918. [DOI] [PubMed] [Google Scholar]
- 10.Goharian I., Sehi M. Is there any role for the choroid in glaucoma? J Glaucoma. 2016;2016(5):452–458. doi: 10.1097/IJG.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banitt M. The choroid in glaucoma. Curr Opin Ophthalmol. 2013;24(2):125–129. doi: 10.1097/ICU.0b013e32835d9245. [DOI] [PubMed] [Google Scholar]
- 12.Kim S., Sung K.R., Lee J.R., Lee K.S. Evaluation of lamina cribrosa in pseudoexfoliation syndrome using spectral-domain optical coherence tomography enhanced depth imaging. Ophthalmology. 2013;120(9):1798–1803. doi: 10.1016/j.ophtha.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Lee K.M., Lee E.J., Kim T.W. Juxtapapillary choroid is thinner in normal-tension glaucoma than in healthy eyes. Acta Ophthalmol. 2016;94(8):e697–e708. doi: 10.1111/aos.13086. [DOI] [PubMed] [Google Scholar]
- 14.Moghimi S., Mazloumi M., Johari M. Evaluation of lamina cribrosa and choroid in nonglaucomatous patients with pseudoexfoliation syndrome using spectral-domain optical coherence tomography. Investig Ophthalmol Vis Sci. 2016;57(3):1293–1300. doi: 10.1167/iovs.15-18312. [DOI] [PubMed] [Google Scholar]
- 15.Aydin D., Kusbeci T., Uzunel U.D., Orsel T., Yuksel B. Evaluation of retinal nerve fiber layer and ganglion cell complex thickness in unilateral exfoliation syndrome using optical coherence tomography. J Glaucoma. 2016;25(6):523–527. doi: 10.1097/IJG.0000000000000383. [DOI] [PubMed] [Google Scholar]
- 16.Dayanir V., Topaloglu A., Ozsunar Y., Keceli M., Okyay P., Harris A. Orbital blood flow parameters in unilateral pseudoexfoliation syndrome. Int Ophthalmol. 2009;29(1):27–32. doi: 10.1007/s10792-008-9193-7. [DOI] [PubMed] [Google Scholar]
- 17.Maul E.A., Friedman D.S., Chang D.S. Choroidal thickness measured by spectral domain optical coherence tomography: factors affecting thickness in glaucoma patients. Ophthalmology. 2011;118(8):1571–1579. doi: 10.1016/j.ophtha.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hosseini H., Nilforushan N., Moghimi S. Peripapillary and macular choroidal thickness in glaucoma. J Ophthalmic Vis Res. 2014;9(2):154–161. [PMC free article] [PubMed] [Google Scholar]
- 19.Park H.Y., Lee N.Y., Shin H.Y., Park C.K. Analysis of macular and peripapillary choroidal thickness in glaucoma patients by enhanced depth imaging optical coherence tomography. J Glaucoma. 2014;23(4):225–231. doi: 10.1097/IJG.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 20.Eroglu F.C., Asena L., Simsek C., Kal A., Yılmaz G. Evaluation of choroidal thickness using enhanced depth imaging by spectral-domain optical coherence tomography in patients with pseudoexfoliation syndrome. Eye (Lond) 2015;29(6):791–796. doi: 10.1038/eye.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turan-Vural E., Yenerel N., Okutucu M., Yildiz E., Dikmen N. Measurement of subfoveal choroidal thickness in pseudoexfoliation syndrome using enhanced depth imaging optical coherence tomography. Ophthalmologica. 2015;233(3–4):204–208. doi: 10.1159/000371899. [DOI] [PubMed] [Google Scholar]
- 22.Ozge G., Koylu M.T., Mumcuoglu T. Evaluation of retinal nerve fiber layer thickness and choroidal thickness in pseudoexfoliative glaucoma and pseudoexfoliative syndrome. Postgrad Med. 2016;128(4):444–448. doi: 10.1080/00325481.2016.1170579. [DOI] [PubMed] [Google Scholar]
- 23.Kocabeyoglu S., Uzun S., Kadayifcilar S., Mocan M.C., Irkec M. The relationship between choroidal expansion and intraocular pressure rise during the water drinking test in healthy subjects and patients with exfoliation syndrome. J Glaucoma. 2016;25(4):e324–e328. doi: 10.1097/IJG.0000000000000283. [DOI] [PubMed] [Google Scholar]